Abstract
The assembly and secretion of transforming growth factor β superfamily ligands is dependent upon non-covalent interactions between their pro- and mature domains. Despite the importance of this interaction, little is known regarding the underlying regulatory mechanisms. In this study, the binding interface between the pro- and mature domains of the inhibin α-subunit was characterized using in vitro mutagenesis. Three hydrophobic residues near the N terminus of the prodomain (Leu30, Phe37, Leu41) were identified that, when mutated to alanine, disrupted heterodimer assembly and secretion. It is postulated that these residues mediate dimerization by interacting non-covalently with hydrophobic residues (Phe271, Ile280, Pro283, Leu338, and Val340) on the outer convex surface of the mature α-subunit. Homology modeling indicated that these mature residues are located at the interface between two β-sheets of the α-subunit and that their side chains form a hydrophobic packing core. Mutation of these residues likely disturbs the conformation of this region, thereby disrupting non-covalent interactions with the prodomain. A similar hydrophobic interface was identified spanning the pro- and mature domains of the inhibin βA-subunit. Mutation of key residues, including Ile62, Leu66, Phe329, and Pro341, across this interface was disruptive for the production of both inhibin A and activin A. In addition, mutation of Ile62 and Leu66 in the βA-propeptide reduced its ability to bind, or inhibit the activity of, activin A. Conservation of the identified hydrophobic motifs in the pro- and mature domains of other transforming growth factor β superfamily ligands suggests that we have identified a common biosynthetic pathway governing dimer assembly.
Inhibin A and B, members of the transforming growth factor β (TGFβ)3 superfamily, negatively regulate the production and secretion of follicle-stimulating hormone from the anterior pituitary (1, 2), control ovarian follicle development and steroidogenesis (3), and act as tumor suppressors in the gonads (4). Outside the hypothalamic pituitary gonadal axis, inhibins contribute to the endocrine regulation of bone metabolism (5) and play critical roles in adrenal gland growth and function (6, 7). It is recognized that inhibins regulate these processes by inhibiting the stimulatory actions of the structurally related proteins, activins (8). Inhibins are heterodimers of an 18-kDa α-subunit disulfide linked to one of two 13-kDa β-subunits (βA and βB), resulting in inhibin A or inhibin B, respectively. Activins are composed of two β-subunits: βA-βA (activin A), βA-βB (activin AB), and βB-βB (activin B). Inhibin antagonism of activin-related ligands is dependent upon interactions with betaglycan, a cell surface proteoglycan that also acts as a TGFβ2 co-receptor (9). Betaglycan binds inhibin A directly and promotes the formation of a stable high affinity complex involving activin type II receptors (10). Sequestration of type II receptors in this way prevents their interactions with signaling ligands such as activin A or activin B.
Analogous to other members of the TGFβ superfamily, inhibin subunits are synthesized as large precursor molecules. The inhibin α-subunit precursor is divided into three regions by two polyarginine cleavage sites (see Fig. 1A): the 43-amino acid proregion; the 171-amino acid αN region; and the 134-amino acid C-terminal (αC) mature region (11). The βA-subunit precursor consists of a 290-amino acid prodomain, separated by a polyarginine cleavage sequence from a 116-amino acid C-terminal mature domain (11). During the secretory process, the α- and βA-subunit mature domains fold into a disulfide-linked dimer. The large inhibin precursors are proteolytically cleaved by furin-like proprotein convertases at an RXRR consensus sequence, which separates the prodomains from the mature domains, and the mature inhibin dimers are secreted.
FIGURE 1.
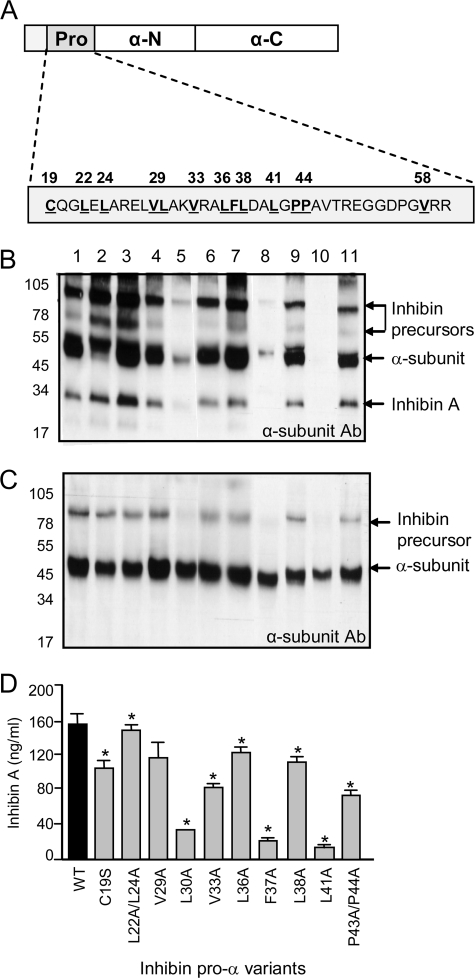
Effects of α-subunit prodomain (Pro) mutations on inhibin A biosynthesis. A, hydrophobic residues in the inhibin α-subunit prodomain were substituted with alanine using in vitro mutagenesis. To determine the effects of amino acid substitutions on inhibin A production, culture medium (B) and cell lysate (C) from CHO cells transfected with either wild type (lane 1) or mutant α-subunit (lanes 2–11), in combination with the βA-subunit, were analyzed by Western blot. Samples were detected with the R1 mAb, specific for the inhibin αC (mature) domain. The 31-kDa inhibin A dimer, 52-kDa free α-subunit, and higher molecular mass inhibin precursors forms (65 and 95 kDa) are noted. D, the effect of α-subunit prodomain mutations on inhibin A expression in CHO culture medium was also determined by ELISA (* = p < 0.05). WT, wild type.
It has been postulated that the prodomains of the α- and βA-subunits are necessary for the correct folding, disulfide bond formation, export, and biological activity of inhibin A (12, 13). Similar regulatory functions have been ascribed to the prodomains of other TGFβ ligands. For example, the prodomain of TGFβ1 (termed latency-associated protein; LAP) represents a functional binding partner for the mature protein (14). The N-terminal region of LAP binds mature TGFβ1 during homodimer assembly and secretion and remains associated following proteolytic cleavage (15–19). LAP binding not only blocks TGFβ1 access to its signaling receptors but also sequesters the growth factor to the extracellular matrix (via interactions with latent TGFβ-binding proteins) (20–24). Further proteolysis or conformational changes within LAP are required to release active TGFβ1 (25, 26). The other TGFβ isoforms (TGFβ2 and -β3), myostatin, and GDF11 also form latent complexes with their prodomains (27, 28).
Recent studies have indicated that complex formation between mature TGFβ ligands and their respective prodomains may be a general phenomenon within the family. Sengle et al. (29) showed that numerous bone morphogenetic proteins, including BMP-2, BMP-4, BMP-7, BMP-10, and GDF5, are secreted as stable complexes consisting of the growth factor domain non-covalently associated with two propeptides. Although prodomain binding for these ligands is not sufficient to confer latency, it is necessary to localize the growth factors to the fibrillin microfibril network within the extracellular matrix (29).
Despite the importance of prodomains in the synthesis and control of TGFβ ligands, little is known about the underlying regulatory mechanisms. In the current study, the binding interface between the pro- and mature domains of the inhibin α-subunit was characterized. Site-directed mutagenesis identified key hydrophobic residues at this interface, which are conserved across the TGFβ superfamily. Based on these investigations, we predict that a common biosynthetic pathway governs the assembly and secretion of TGFβ ligands.
EXPERIMENTAL PROCEDURES
Production of Inhibin Mutants—Mutations in the pro- and mature regions of the inhibin α- and βA-subunits were introduced using the QuikChange Lightning site-directed mutagenesis kit (Stratagene, La Jolla, CA). pCDNA3.1 (Invitrogen) vectors containing either the full-length wild type inhibin α-or βA-subunits served as the templates in these reactions. The mature region of the α-subunit also contained a mutation (N302Q) to ensure that only a 31-kDa inhibin A was produced (30). For each construct, the mutated region was confirmed by DNA sequencing. Wild type and mutant inhibin A and activin A proteins were produced by transient transfection in Chinese hamster ovary (CHO) cells using Lipofectamine (Invitrogen). Briefly, CHO cells were plated at 1 × 106 cells/well in a 6-well plate. Wild type or mutant α-subunit DNA (1.6 μg) was combined with βA-subunit DNA (3.3 μg), and Lipofectamine was added according to the manufacturer's instructions. After a 20-min incubation, DNA/Lipofectamine complexes were added directly to the plated cells and incubated in serum-free Opti-MEM medium (Invitrogen) for a further 48 h at 37 °C in 5% CO2.
The transfected CHO culture medium and cell lysate for each of the inhibin A and activin A mutants were assessed by Western blotting. At 48 h after transfection, the culture medium was removed and concentrated 50-fold using Nanosep microconcentration devices with a 10-K cut-off (Pall Life Sciences, East Hills, NY). The cells were lysed in 1% Triton X-100 in phosphate-buffered saline (pH 7.4). Non-reduced samples were loaded onto 10% SDS-PAGE and subjected to Western blotting. After electrophoresis, samples were transferred onto ECL Hybond membranes (GE Healthcare, Giles, Buckinghamshire, UK). Inhibin α- and βA-subunits were detected using the R1 and E4 antibodies, respectively.
Inhibin A ELISA—An inhibin A ELISA (Diagnostic Systems Laboratories, Webster, Texas) was used as described (31), employing kit reagents provided by Oxford Bio-Innovation Ltd. (Upper Heyford, UK). The ELISA utilized the βA-subunit antibody (E4) as capture antibody and α subunit antibody (R1) as label. The sensitivity of the assay was 2 pg/ml.
Activin A Immunofluorometric Assay—To measure the concentration of wild type and mutant activin A in the conditioned medium of CHO cells, an activin immunofluorometric assay was employed (32). The working range of the assay was 0.03–3 ng/well, and the assay sensitivity was 0.03 ng/well. Activin A assays were measured from duplicate transfections in CHO cells.
Production of the βA-Propeptide—Wild type and mutant (I62A or I62A/L66A) βA-propeptides were generated by PCR (antisense primer 5′-CTAGGAATTCCTATTTGTCGTCGTCGTCTTTGTAGTCGGCTCTCTCCCCTCCACTGGGTG-3′) and cloned into the NotI and XbaI sites of a pCDNA3.1+ vector (Invitrogen). The propeptides were truncated 5′ of the cleavage sites upstream of the mature domain so that only the prodomains were expressed. The primers incorporated a 3′-FLAG tag. The wild type and mutant βA-propeptides were produced by transient transfection in 293T cells (human renal epithelial cells) using Lipofectamine (Invitrogen). 293T cells were plated at 1.2 × 107 cells/plate in 15-cm plates. Wild type or mutant βA-propeptide DNA (75 μg) were combined with Lipofectamine according to the manufacturer's instructions. After a 20-min incubation, DNA/Lipofectamine complexes were added directly to the plates, which were incubated in serum-free Opti-MEM medium for a further 48 h at 37 °C in 5% CO2. At 48 h after transfection, the 293T conditioned medium was collected and concentrated 30-fold using Amicon Ultra-15 concentration devices with a 10-kDa regenerated cellulose membrane (Millipore, Billerica, MA). Concentrated protein was diluted in phosphate-buffered saline (pH 7.4) containing protease inhibitors (Complete protease inhibitor mixture tablets, Roche Applied Science, Basel, Switzerland).
Analysis of the Interaction between the βA-Propeptide and Mature Inhibin A/Activin A—Conditioned medium from transfected CHO cells expressing wild type and mutant βA-propeptide with a C-terminal FLAG tag was concentrated 50-fold using Nanosep microconcentration devices with a 10-K cut-off (Pall Life Sciences) and resuspended in Laemmli sample buffer. Non-reduced samples were separated by 10% SDS-PAGE and transferred onto ECL Hybond membranes (GE Healthcare). Membranes were blocked in Tris-buffered saline (TBS) with 5% milk. The blocking solution was removed by multiple washes in TBS/bovine serum albumin (3% bovine serum albumin, 0.1% Tween 20), and the membranes were probed with either 125I-inhibin A or 125I-activin A (400,000 cpm/ml) in TBS/bovine serum albumin. After incubation, the tracer was removed by multiple washes with TBS, and the membranes were exposed to film for up to 4 days at -80 °C and developed.
Immunoprecipitation—The ability of the βA-propeptide and activin type II receptors (ActRIIA and ActRIIB) to compete for binding to mature activin A was assessed by immunoprecipitation. Increasing concentrations of ActRIIA and ActRIIB extracellular domains (25 ng–4 μg; R&D Systems, Minneapolis, MN) were added to samples containing wild type βA-propeptide (400 ng) and activin A (12.5 ng). Samples were immunoprecipitated using a FLAG M2 affinity gel (Sigma-Aldrich) directed against the βA-propeptide. Protein complexes were eluted from the resin using reducing sample buffer and separated by SDS-PAGE. After electrophoresis, samples were transferred onto an ECL Hybond membrane. The activin βA-subunit was detected using the E4 antibody, and βA-propeptides were identified using the FLAG M2 antibody.
In Vitro Bioassay—Wild type and mutant (I62A/L66A) βA-propeptides were assessed for their ability to suppress activin A bioactivity in a mouse adrenocortical cell line (7). Briefly, adrenocortical cells were plated in 48-well plates at 114,000 cells/well. After a 24-h incubation, cells were transfected with an activin responsive luciferase reporter construct (pGRAS) using Lipofectamine according to the manufacturer's protocol (Invitrogen). The cells were washed 24 h after transfection with complete medium and treated with 400 pm activin A and increasing doses of either wild type or mutant βA-propeptides (0.5–30 nm) or inhibin A (0.1–3 nm). Activin-induced luciferase activity was then determined.
Statistics—Significance (p < 0.05) was determined using one-way t tests for independent groups. In Figs. 1, 3, 4, 5, and 7, all error bars shown represent standard deviation.
FIGURE 3.
Effects of βA prodomain (pro-βA) mutations on inhibin/activin biosynthesis. A, alanine substitutions were made in the hydrophobic residues of the inhibin βA prodomain using in vitro mutagenesis. To determine the effects of these amino acid substitutions on inhibin A and activin A production, culture medium from CHO cells transfected with either wild type (lane 1) or mutant βA-subunit (lanes 2–4), in combination with the α-subunit, were analyzed by Western blot. Blots were probed with the E4 mAb, specific for the inhibin/activin mature βA domain (B) and the R1 mAb specific for the inhibin αC (mature) domain (C). D, Western blot analysis of the cell lysates of CHO cells transfected with either wild type (lane 1) or mutant βA-subunit cDNAs (lanes 2–4) is shown. The 31-kDa inhibin A dimer, 24-kDa activin A dimer, 54-kDa free βA-subunit, and higher molecular mass precursor forms of inhibin and activin are noted. The effect of βA-subunit prodomain mutations on activin A (E) and inhibin A (F) expression in CHO culture medium was also determined by ELISA (* = p < 0.05). WT, wild type.
FIGURE 4.
Analysis of the interaction between the βA-propeptide and mature inhibin and activin A dimers. A, ligand blot analysis of wild type (WT) and mutant βA-propeptide binding to 125I-inhibin A and 125I-activin A dimers. Wild type and mutant βA-propeptide (with C-terminal FLAG tag) were loaded at equivalent concentrations (as determined by Western blotting with the FLAG M2 mAb, top panel) onto SDS-PAGE and transferred to an ECL Hybond membrane. Membranes were probed with either 125I-activin A (middle panel) or 125I-inhibin A (bottom panel). B and C, the ability of the activin type II receptors (ActRIIA and ActRIIB) to compete with the βA-propeptide (proβA) for binding to mature activin A was assessed by immunoprecipitation. Increasing concentrations of ActRIIA (B) and ActRIIB (C) extracellular domains (ECD) (25 ng–4 μg; R&D Systems) were added to samples containing wild type βA-propeptide (400 ng) and activin A (12.5 ng). Samples were immunoprecipitated (IP) using FLAG M2 affinity resin and detected by immunoblot (IB) using the activin βA subunit mAb (E4). To ensure that equal amounts of activin and βA-propeptides were present in each of the samples, immunoblots using the FLAG M2 and E4 antibodies were also performed prior to immunoprecipitation. D, in vitro bioassay to assess the ability of wild type and mutant βA-propeptides to block activin signaling. Adrenocortical cells were transfected with an activin responsive luciferase reporter and treated with 400 pm activin A (Act A) and increasing doses of either wild type or mutant βA-propeptides (0.5–30 nm) (* = p < 0.05).
FIGURE 5.
Effects of αC mutations on inhibin A biosynthesis. A, hydrophobic residues in the inhibin αC (mature) domain were substituted with alanine using in vitro mutagenesis. Pro, prodomain. To determine the effects of amino acid substitutions on inhibin A production, culture medium (B) and cell lysate (C) from CHO cells transfected with either wild type (lane 1) or mutant α-subunit (lanes 2–9), in combination with the βA-subunit, were analyzed by Western blot. Samples were detected with the R1 mAb, specific for the inhibin αC domain. The 31-kDa inhibin A dimer, 52-kDa free α-subunit, and higher molecular mass inhibin precursors forms are noted. D, the effect of αC mutagenesis on inhibin A expression in CHO culture medium was also determined by ELISA (* = p < 0.05). WT, wild type.
FIGURE 7.
Effects of mutations in the mature domain of the inhibin/activin A βA-subunit on biosynthesis. A, key hydrophobic residues in the mature domain of the inhibin/activin βA-subunit were substituted with alanine using in vitro mutagenesis. To determine the effects of these amino acid substitutions on inhibin A and activin A production, culture medium from CHO cells transfected with either wild type (lane 1) or mutant βA-subunit (lanes 2–6), in combination with the α-subunit, was analyzed by Western blot. proβA, βA-prodomain. Samples were detected with the E4 mAb specific for the inhibin/activin mature βA domain (B) and the inhibin αC-specific R1 mAb (C). The 31-kDa inhibin A dimer, 24-kDa activin A dimer, 54-kDa free βA-subunit, and higher molecular mass precursor forms of inhibin and activin are noted. The effects ofβA-subunit mutations on activin A (D) and inhibin A (E) expression in CHO culture medium was also determined by ELISA (* = p < 0.05). WT, wild type.
RESULTS
Hydrophobic Residues in the Prodomain of the Inhibin α-Subunit Regulate Heterodimer Assembly and Secretion—The inhibin α-subunit prodomain comprises a 43-amino acid proregion and a 171-amino acid N-terminal (αN) region. Based on previous studies (15, 17, 33), the proregion (Cys19–Arg61) was identified as the region most likely to be involved in non-covalent interactions with the mature α-subunit. Hydrophobic residues through this region were substituted with alanine using in vitro mutagenesis. In all, a set of 10 variants mutated at 12 different positions was generated (Fig. 1A).
Wild type and mutant proteins were expressed in CHO cells and the conditioned medium and cell lysate were collected. Western blot analysis indicated that conditioned medium from cells transfected with wild type α- and βA-subunits contained both mature (31-kDa) and precursor (65- and 95-kDa) inhibin forms, together with substantial amounts of free α-subunit (50 kDa) (Fig. 1B, lane 1). Mutation of the majority of hydrophobic residues through the proregion had little effect on the amount or the composition of the inhibin forms produced by CHO cells (Fig. 1, B and D). However, three residues (Leu30, Phe37, Leu41) were identified that, when mutated to alanine, resulted in a significant reduction (>80%) in the amount of inhibin A produced and secreted (Fig. 1, B and D). An analysis of the cell lysates from the transfected CHO cells indicated that the decrease in inhibin A production was not due to a loss of α-subunit expression as it was present at similar levels for all mutants tested (Fig. 1C). Rather, the identified point mutations (L30A, F37A, and L41A) appeared to disrupt the dimerization of the α- and βA-subunits, as evidenced by the decrease in dimeric inhibin precursor (Fig. 1C, compare lane 1 with lanes 5, 8, and 10). Together, these results suggest that Leu30, Phe37, and Leu41 are necessary to maintain the inhibin α-subunit in a conformation competent for dimerization with the βA-subunit.
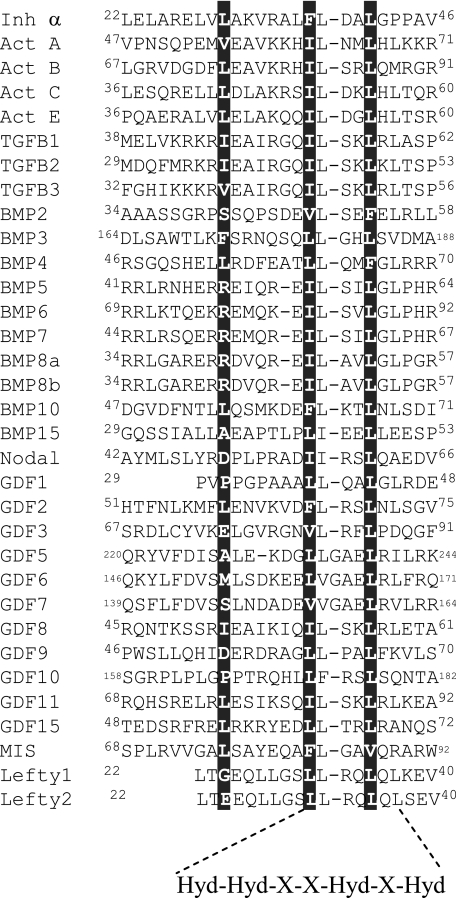
Conservation of the Hydrophobic Motif in the Prodomains of TGFβ Ligands—Sequence alignment (as determined using ClustalW) of the 33 human TGFβ family members indicated that Phe37 and Leu41 of the inhibin α-subunit form part of a hydrophobic motif (Hyd-Hyd-X-X-Hyd-X-Hyd), which is present in most family members (Fig. 2). This conservation of hydrophobicity suggests that a common mechanism governing dimer assembly may exist across the TGFβ superfamily.
FIGURE 2.
Sequence alignment of prodomains for human TGFβ ligands. The inhibin α-subunit (Inh α) prodomain was aligned with the prodomains of human TGFβ ligands using ClustalW. The residues are numbered according to the first residue of the signal peptide. The three residues determined in this study to be essential for inhibin dimer formation and secretion (Leu30, Phe37, and Leu41) are highlighted. The identified residues lie within a conserved hydrophobic motif (bottom of alignment). Act, activin.
To test this, the corresponding hydrophobic residues in the activin βA-subunit (common to both activin A and inhibin A) were substituted for alanine (Fig. 3). βA-subunit point mutations, I62A and L66A, were disruptive for activin A expression. Western blot analysis using an antibody directed against the βA-subunit (Fig. 3B), and an activin A ELISA (Fig. 3E) showed the profound effects these mutations had on activin A production and secretion. Interestingly, inhibin A levels (Fig. 3, C and F), although decreased, were not suppressed to the same extent as activin A levels. As the precursor βA-subunit was present in cell lysates at comparable levels for each of the variants (Fig. 3D), decreased activin and inhibin expression likely occurred because of a defect in folding and/or dimerization. Interestingly, mutation of Val55 of the βA-subunit (corresponding to Leu30 of the α-subunit) had no effect on inhibin A or activin A expression. This residue is upstream of the hydrophobic motif and is less well conserved across the TGFβ family.
The βA-Subunit Prodomain Interacts Directly with Activin A and Inhibin A—The nature of the conformational changes induced by mutation of βA-subunit residues, Ile62 and Leu66, were then examined. Wild type and mutant (I62A and I62A/L66A) βA-propeptides with a C-terminal FLAG tag were expressed by transient transfection in CHO cells, separated by SDS-PAGE, and transferred to ECL Hybond membranes. A Western blot with the FLAG M2 mAb confirmed that all the βA-propeptides were loaded at equivalent concentrations (Fig. 4A). Probing membranes with 125I-inhibin A or 125I-activin A indicated that both ligands bound strongly to the wild type βA-propeptide (Fig. 4A). In contrast, no detectable binding of inhibin A or activin A was observed with the βA-propeptides carrying mutations in the identified hydrophobic motif (I62A and I62A/L66A). These results indicate that the residues within the hydrophobic motif of the βA prodomain interact directly with the mature βA domain to regulate the assembly and secretion of inhibin A or activin A.
Activin A Binds Propeptide and Type II Receptors through Overlapping Binding Sites—It has recently been demonstrated that the BMP-7 propeptide blocks binding of mature BMP-7 to its type II receptor (34). To determine whether the binding site for the βA-propeptide on activin A overlaps with that of activin type II receptors, immunoprecipitation studies were performed. Concentrated culture medium containing βA-propeptide was combined with activin A (R&D Systems) and increasing doses of activin type II receptors (ActRIIA and ActRIIB extracellular domains, R&D Systems). Samples were immunoprecipitated using the FLAG M2 affinity resin (Sigma-Aldrich) and analyzed by Western blot using an activin A (E4) antibody. In the absence of activin type II receptors, βA-propeptide formed a complex with activin A (Fig. 4, B and C). However, increasing doses of the activin type II receptor extracellular domains decreased the amount of activin A recovered by immunoprecipitation. This suggested that ActRII/IIB can displace βA-propeptide from binding to activin A, supporting the concept that these proteins share an overlapping binding epitope on the mature activin A dimer.
The βA-Propeptide Suppresses Activin A Bioactivity in Adrenocorticol Cells—The adrenocortical cell system was used to determine whether the βA-propeptide was able to block activin biological activity. In this assay, activin A induced a 12-fold increase in luciferase response, which could be blocked with increasing doses of the antagonist, inhibin A (Fig. 4D and data not shown). High doses of wild type βA-propeptide were also able to suppress the activin-induced luciferase response (Fig. 4D), in a manner similar to that previously described for soluble activin type II receptors (35). In contrast, the I62A/L66A βA-propeptide variant was unable to inhibit activin A signaling in the adrenocortical cells. Together, these results suggest that, if present at high concentrations, the βA-propeptide may modulate activin signaling.
Characterization of the Pro- and Mature Domain Binding Interface on the Inhibin α-Subunit—The outer convex surface of the “finger” regions of TGFβ ligands bind type II receptors (36, 37) and, based on competition studies (Fig. 4) (34), likely provide the interface for interactions with propeptides. To identify the binding epitope on inhibin A for the α-subunit prodomain, residues through the finger regions of the α-subunit were substituted for alanine. In all, a set of 28 variants was generated (38). Western blot analysis of conditioned medium from CHO cells transfected with wild type or mutant α-subunit revealed that residues Phe271, Ile280, Pro283, Leu338, and Val340 in the finger region of the α-subunit are necessary for inhibin A production and secretion (Fig. 5B, lanes 2, 4, 6, 7, and 9). The identified residues are distant from the predicted inhibin α/βA dimer interface (see Fig. 8A). An inhibin A ELISA confirmed that mutation of these residues reduced inhibin A production by >80% (Fig. 5D). The reduction could not be attributed to a loss of expression of the α-subunit as it was easily identified in the cell lysates of the transfected CHO cells for all mutants tested (Fig. 5C). As these hydrophobic residues are critical for the correct folding of the α-subunit and subsequent dimerization with the βA-subunit, it is likely that they constitute the binding epitope for the prodomain residues, Leu30, Phe37, and Leu41. In support, mutation of non-hydrophobic residues (e.g. Tyr282 and His339) through this region did not affect the formation of inhibin A (Fig. 5, B and D). Moreover, conservative substitutions at positions Leu338 (L338M) and Val340 (V340L) allowed for inhibin A dimer formation (data not shown).
FIGURE 8.
Homology model of inhibin A. A, a homology model of the mature inhibin A dimer was generated in a previous study (38). The inhibin α-subunit is colored orange, whereas the inhibin βA-subunit is green. The hydrophobic residues identified in the mature domains of the inhibin α-(magenta) and βA-subunits (blue) that are required for inhibin biosynthesis were mapped onto the model. The identified residues lie on the outer convex surface of the finger regions, and the side chains of these residues form a hydrophobic packing core. Note that these residues are distant from the inhibin α/βA dimer interface. B, a model for the correct folding, dimerization, secretion, and activation of inhibin A.
Conservation of Hydrophobic Residues across the TGFβ Superfamily—Sequence alignment of the 33 human TGFβ family members indicated that the five hydrophobic α-subunit residues required for the correct folding and dimerization of inhibin A are highly conserved (Fig. 6). Pro283 (inhibin α-subunit numbering) is invariant across the family, whereas Phe271 and Ile280 are present in most family members. At other positions in the hydrophobic motif (Leu338 and Val340), conservative amino acid substitutions are noted. The corresponding hydrophobic residues in the mature region of the βA-subunit were substituted for alanine (Fig. 7A). As anticipated, in cells co-transfected with wild type α-subunit, these βA-subunit point mutations (F329A, I338A, P341A, M398A, and M400A) were disruptive for activin A expression (Fig. 7). Interestingly, inhibin A expression was only significantly reduced in the P341A and M400A βA-subunit variants (Fig. 7E), suggesting that it is less dependent on βA-subunit conformation.
FIGURE 6.
Sequence alignment of the mature domains for the human TGFβ ligands. Residues comprising finger 1 (Ser270–Ile287) and finger 2 (Met335–Tyr352) of the mature inhibin α-subunit (Inh α) were aligned with the mature domains of human TGFβ ligands using ClustalW. The residues determined in this study to be essential for inhibin dimer formation and secretion (Phe271, Ile280, Pro283, Leu338, and Val340) (highlighted) lie within the finger regions of the αC mature domain. Act, activin.
Molecular Modeling of the Inhibin A Dimer—Previously, we constructed a homology model of inhibin A (38) based on the activin A, BMP-3, and BMP-6 structures (7, 12, 21). Mapping of the inhibin α- and βA-subunit residues mutated in this study onto the modeled structure of inhibin A is shown in Fig. 8A. The α-subunit residues Phe271, Ile280, Pro283, Leu338, and Val340 are located at the interface between two β-sheets, and the side chains of these residues form a hydrophobic packing core. Disruption to this region could affect the folding or stability of mature inhibin A, which may explain the low levels of mutant proteins detected in CHO cell conditioned medium (Fig. 5). The βA-subunit residues Phe329, Ile338, Pro341, Met398, and Met400 are similarly clustered at the interface between two β-sheets. Interestingly, Pro341 of the βA-subunit is also a central component of the activin type II receptor-binding interface (36).
DISCUSSION
The prodomains of TGFβ family members play an important role in the biosynthesis of these ligands. As a consequence, naturally occurring mutations within the prodomains of TGFβ ligands are often associated with disease pathologies. Mutations in the proregions of GDF9 and BMP-15 have been identified in patients diagnosed with premature ovarian failure (39–41). Patients presenting with Camurati-Engelmann disease, which is characterized by alterations in bone density resulting in severe bone pain, have been found to carry mutations in the TGFβ1 prodomain (42–44). Mutations are also prevalent in the proregion of Műllerian-inhibiting substance (MIS, or anti-Műllerian hormone), which result in persistent Műllerian duct syndrome, an autosomal recessive intersex disorder (45). A greater understanding of the mechanisms by which the prodomains assist the formation and/or functions of TGFβ ligands would aid the development of future treatments for these conditions.
In this study, utilizing a site-directed mutagenesis approach, we have provided a structural basis for understanding the critical role that prodomains play in facilitating the assembly and secretion of inhibin A and related TGFβ ligands. Mutagenesis of residues in the N-terminal portion of the α-subunit prodomain had pronounced effects on inhibin A production. In particular, residues Phe37 and Leu41 and, to a lesser extent, Leu30 are critical for maintaining the α-subunit in a conformation competent for dimerization with the βA-subunit. Crystal structures are not available for the propeptides of any TGFβ ligands; however, the three identified hydrophobic residues are predicted to lie within an α-helix (determined using NNpredict, data not shown) and likely provide a binding surface for non-covalent interactions with the mature α-subunit.
Sequence alignment of the proregions of the 33 human TGFβ family members revealed that α-subunit residues Phe37 and Leu41 lie within a conserved hydrophobic motif (37Hyd-Hyd-X-X-Hyd-X-Hyd43). The conservation of hydrophobicity suggests that this region serves a common role in governing the assembly and secretion of TGFβ ligands. Several pieces of evidence support this concept. (i) Mutation of the corresponding hydrophobic residues in the βA-subunit (Ile62 and Leu66) disrupted both inhibin A and activin A dimerization (Fig. 3); (ii) deletion of residues within the identified hydrophobic motif of TGFβ1 blocked the association between the pro- and mature domains and inhibited the secretion of mature TGFβ1 (17); (iii) the TGFβ1 physiological activator, thrombospondin 1, binds to residues (54LSKL57) within the identified motif (46); and (iv) Jiang et al. (33) have mapped the inhibitory domain of the myostatin propeptide to residues 42–115, which encompasses the hydrophobic motif identified in the inhibin subunits.
Recent studies on TGFβ1, myostatin, and BMPs have indicated that after cleavage, propeptides retain the capacity to interact non-covalently with their respective dimeric growth factors (27, 28, 34). Immunoprecipitation and ligand blot studies demonstrated that this was also the case for the inhibin and activin isoforms. Isolated βA-propeptide was able to bind directly to inhibin A and activin A. Importantly, mutation of Ile62 or Leu66 within the βA-propeptide completely abrogated interactions with the mature ligands, confirming that these residues are central to the non-covalent interactions between the pro- and mature domains. For some family members, including the TGFβ isoforms, myostatin and GDF11, high affinity interactions with isolated propeptides is sufficient to confer latency (17, 27, 28). This fact has been utilized successfully for the in vivo inhibition of myostatin and, hence, muscle growth (47–49). For other ligands (e.g. BMP-7 and BMP-9), propeptides bind with lower affinity and are unable to suppress biological activity (34, 50). In the current study, bioactivity assays indicated that the βA-propeptide could inhibit activin activity, but only at high concentrations. Thus, the affinity of the βA-propeptide for activin is presumably less than the affinity of LAP for TGFβ1 but greater than the affinity of the BMP-7 propeptide for mature BMP-7.
The βA-propeptide reduced activin signaling because at high concentrations, it was capable of displacing activin A from binding to type II receptors (ActRII/IIB). In similar experiments, Sengle et al. (34) recently demonstrated that the BMP-7 propeptide competes with BMPRII for binding to the mature ligand. In addition, the sequence 94RKPK97 in the receptor-binding region of mature TGFβ1 has been implicated in binding LAP (17). Together, these studies suggest that the binding epitopes for prodomains and type II receptors overlap on TGFβ ligands (i.e. both bind to the outer convex surface of the finger regions). Using this information as a guide, we set out to identify the residues in the mature domains of the inhibin A subunits that form non-covalent interactions with their respective prodomains. After extensive mutagenesis, it was found that alanine substitution of a number of hydrophobic residues (Phe271, Ile280, Pro283, Leu338, and Val340) in the finger regions of the α-subunit were disruptive for the formation of inhibin A dimers in vitro. The identified residues are located at the interface between two β-sheets of the α-subunit, and the side chains of these residues form a hydrophobic pocket (Fig. 8). It is likely that mutation of some of these residues (Phe271, Ile280, and Leu338) perturbs the local conformation of the mature domain, thereby hindering the ability of the prodomain to bind. However, for the surface-exposed residues, Pro283 and Val340, mutations may directly disrupt hydrophobic interactions with the prodomain. In support, mutation of these residues has previously been shown to disrupt inhibin A binding to its co-receptor, betaglycan (38).
Mutations of the corresponding residues within the βA-subunit were also found to abrogate the expression of activin A. Interestingly, these mutations were significantly less disruptive for inhibin A expression, suggesting that the α-subunit drives inhibin production. Sequence alignment of the mature domains of the 33 human TGFβ ligands revealed that the identified hydrophobic residues are highly conserved across the family, suggesting that this region plays a common structural role in the formation of these ligands. In support, a naturally occurring mutation (V477A) in the mature region of MIS, which corresponds to Ile280 in the inhibin α-subunit, has been identified in patients with persistent Műllerian duct syndrome. The V477A mutation in MIS disrupts protein production, reducing the circulating levels of MIS by 90% (45).
In conclusion, our data indicate that a common biosynthetic pathway governs the production and secretion of TGFβ ligands. In this model (Fig. 8B), hydrophobic residues within the N-terminal portion of the prodomain and the finger regions of the mature domain interact non-covalently, maintaining the molecule in a conformation competent for dimerization (the actual dimerization interface for the two monomers, close to the cysteine knot motif, is well removed from this prodomain-binding site). Dimeric precursors are cleaved by furin-like proconvertases at RXXR sites that separate the propeptides from the mature domains. The dimeric, mature ligands are then secreted from the cell non-covalently associated with their respective prodomains. For inhibin A, the α- and βA-propeptides are readily displaced by betaglycan and activin type II receptors, respectively, ensuring that this hormone is secreted in an active state. Other ligands (e.g. TGFβ1 and myostatin) have higher affinities for their prodomains and are secreted in a latent form.
This work was supported by National Health and Medical Research Council of Australia Project Grants 388920 (to K. L. W.) and 494804 (to C. A. H.) and Program Grant 241000 (to D. M. R.).
Footnotes
The abbreviations used are: TGFβ, transforming growth factor β; ELISA, enzyme-linked immunosorbent assay; CHO, Chinese hamster ovary; BMP, bone morphogenetic protein; mAb, monoclonal antibody; LAP, latency-associated protein; ActRII, activin type II receptor; Hyd, hydrophobic residue; MIS, Műllerian-inhibiting substance.
References
- 1.Robertson, D. M., Giacometti, M. S., and de Kretser, D. M. (1986) Mol. Cell. Endocrinol. 46 29-36 [DOI] [PubMed] [Google Scholar]
- 2.Woodruff, T. K., Krummen, L. A., Lyon, R. J., Stocks, D. L., and Mather, J. P. (1993) Endocrinology 132 2332-2341 [DOI] [PubMed] [Google Scholar]
- 3.Woodruff, T. K., Lyon, R. J., Hansen, S. E., Rice, G. C., and Mather, J. P. (1990) Endocrinology 127 3196-3205 [DOI] [PubMed] [Google Scholar]
- 4.Matzuk, M. M., Finegold, M. J., Su, J. G., Hsueh, A. J., and Bradley, A. (1992) Nature 360 313-319 [DOI] [PubMed] [Google Scholar]
- 5.Perrien, D. S., Akel, N. S., Edwards, P. K., Carver, A. A., Bendre, M. S., Swain, F. L., Skinner, R. A., Hogue, W. R., Nicks, K. M., Pierson, T. M., Suva, L. J., and Gaddy, D. (2007) Endocrinology 148 1654-1665 [DOI] [PubMed] [Google Scholar]
- 6.Farnworth, P. G., Harrison, C. A., Leembruggen, P., Chan, K. L., Stanton, P. G., Ooi, G. T., Rahman, N. A., Huhtaniemi, I. T., Findlay, J. K., and Robertson, D. M. (2001) Mol. Cell. Endocrinol. 180 63-71 [DOI] [PubMed] [Google Scholar]
- 7.Farnworth, P. G., Wang, Y., Leembruggen, P., Ooi, G. T., Harrison, C., Robertson, D. M., and Findlay, J. K. (2006) J. Endocrinol. 188 451-465 [DOI] [PubMed] [Google Scholar]
- 8.Vale, W., Rivier, J., Vaughan, J., McClintock, R., Corrigan, A., Woo, W., Karr, D., and Spiess, J. (1986) Nature 321 776-779 [DOI] [PubMed] [Google Scholar]
- 9.Lewis, K. A., Gray, P. C., Blount, A. L., MacConell, L. A., Wiater, E., Bilezikjian, L. M., and Vale, W. (2000) Nature 404 411-414 [DOI] [PubMed] [Google Scholar]
- 10.Wiater, E., and Vale, W. (2003) J. Biol. Chem. 278 7934-7941 [DOI] [PubMed] [Google Scholar]
- 11.Mason, A. J., Farnworth, P. G., and Sullivan, J. (1996) Mol. Endocrinol. 10 1055-1065 [DOI] [PubMed] [Google Scholar]
- 12.Gray, A. M., and Mason, A. J. (1990) Science 247 1328-1330 [DOI] [PubMed] [Google Scholar]
- 13.Russell, D. L., Doughton, B. W., Tsonis, C. G., and Findlay, J. K. (1994) J. Reprod. Fertil. 100 115-122 [DOI] [PubMed] [Google Scholar]
- 14.Gentry, L. E., Webb, N. R., Lim, G. J., Brunner, A. M., Ranchalis, J. E., Twardzik, D. R., Lioubin, M. N., Marquardt, H., and Purchio, A. F. (1987) Mol. Cell. Biol. 7 3418-3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sha, X., Yang, L., and Gentry, L. E. (1991) J. Cell Biol. 114 827-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Crescenzo, G., Grothe, S., Zwaagstra, J., Tsang, M., and O'Connor-McCourt, M. D. (2001) J. Biol. Chem. 276 29632-29643 [DOI] [PubMed] [Google Scholar]
- 17.Young, G. D., and Murphy-Ullrich, J. E. (2004) J. Biol. Chem. 279 38032-38039 [DOI] [PubMed] [Google Scholar]
- 18.Bottinger, E. P., Factor, V. M., Tsang, M. L., Weatherbee, J. A., Kopp, J. B., Qian, S. W., Wakefield, L. M., Roberts, A. B., Thorgeirsson, S. S., and Sporn, M. B. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 5877-5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon, G. A., Dignam, J. D., and Gentry, L. E. (1996) Biochem. J. 313 343-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taipale, J., Saharinen, J., Hedman, K., and Keski-Oja, J. (1996) J. Histochem. Cytochem. 44 875-889 [DOI] [PubMed] [Google Scholar]
- 21.Maeda, S., Dean, D. D., Gomez, R., Schwartz, Z., and Boyan, B. D. (2002) Calcif. Tissue Int 70 54-65 [DOI] [PubMed] [Google Scholar]
- 22.Hyytiainen, M., Penttinen, C., and Keski-Oja, J. (2004) CRC Crit. Rev. Clin. Lab. Sci. 41 233-264 [DOI] [PubMed] [Google Scholar]
- 23.Saika, S., Yamanaka, O., Baba, Y., Kawashima, Y., Shirai, K., Miyamoto, T., Okada, Y., Ohnishi, Y., and Ooshima, A. (2001) Graefe's Arch. Clin. Exp. Ophthalmol. 239 234-241 [DOI] [PubMed] [Google Scholar]
- 24.Taipale, J., Miyazono, K., Heldin, C. H., and Keski-Oja, J. (1994) J. Cell Biol. 124 171-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentry, L. E., Lioubin, M. N., Purchio, A. F., and Marquardt, H. (1988) Mol. Cell. Biol. 8 4162-4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munger, J. S., Harpel, J. G., Gleizes, P. E., Mazzieri, R., Nunes, I., and Rifkin, D. B. (1997) Kidney Int. 51 1376-1382 [DOI] [PubMed] [Google Scholar]
- 27.Ge, G., Hopkins, D. R., Ho, W. B., and Greenspan, D. S. (2005) Mol. Cell. Biol. 25 5846-5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thies, R. S., Chen, T., Davies, M. V., Tomkinson, K. N., Pearson, A. A., Shakey, Q. A., and Wolfman, N. M. (2001) Growth Factors 18 251-259 [DOI] [PubMed] [Google Scholar]
- 29.Sengle, G., Charbonneau, N. L., Ono, R. N., Sasaki, T., Alvarez, J., Keene, D. R., Bachinger, H. P., and Sakai, L. Y. (2008) J. Biol. Chem. 283 13874-13888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makanji, Y., Harrison, C. A., Stanton, P. G., Krishna, R., and Robertson, D. M. (2007) Endocrinology 148 2309-2316 [DOI] [PubMed] [Google Scholar]
- 31.Robertson, D. M., Stephenson, T., Pruysers, E., Burger, H. G., McCloud, P., Tsigos, A., Groome, N., Mamers, P., McNeilage, J., Jobling, T., and Healy, D. (2002) Mol. Cell. Endocrinol. 191 97-103 [DOI] [PubMed] [Google Scholar]
- 32.Harrison, C. A., Chan, K. L., and Robertson, D. M. (2006) Endocrinology 147 2744-2753 [DOI] [PubMed] [Google Scholar]
- 33.Jiang, M. S., Liang, L. F., Wang, S., Ratovitski, T., Holmstrom, J., Barker, C., and Stotish, R. (2004) Biochem. Biophys. Res. Commun. 315 525-531 [DOI] [PubMed] [Google Scholar]
- 34.Sengle, G., Ono, R. N., Lyons, K. M., Bachinger, H. P., and Sakai, L. Y. (2008) J. Mol. Biol. 381 1025-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.del Re, E., Sidis, Y., Fabrizio, D. A., Lin, H. Y., and Schneyer, A. (2004) J. Biol. Chem. 279 53126-53135 [DOI] [PubMed] [Google Scholar]
- 36.Thompson, T. B., Woodruff, T. K., and Jardetzky, T. S. (2003) EMBO J. 22 1555-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreuder, H., Liesum, A., Pohl, J., Kruse, M., and Koyama, M. (2005) Biochem. Biophys. Res. Commun. 329 1076-1086 [DOI] [PubMed] [Google Scholar]
- 38.Makanji, Y., Walton, K. L., Wilce, M. C., Chan, K. L., Robertson, D. M., and Harrison, C. A. (2008) J. Biol. Chem. 283 16743-16751 [DOI] [PubMed] [Google Scholar]
- 39.Dixit, H., Rao, L. K., Padmalatha, V. V., Kanakavalli, M., Deenadayal, M., Gupta, N., Chakrabarty, B., and Singh, L. (2006) Hum. Genet. 119 408-415 [DOI] [PubMed] [Google Scholar]
- 40.Di Pasquale, E., Rossetti, R., Marozzi, A., Bodega, B., Borgato, S., Cavallo, L., Einaudi, S., Radetti, G., Russo, G., Sacco, M., Wasniewska, M., Cole, T., Beck-Peccoz, P., Nelson, L. M., and Persani, L. (2006) J. Clin. Endocrinol. Metab. 91 1976-1979 [DOI] [PubMed] [Google Scholar]
- 41.Di Pasquale, E., Beck-Peccoz, P., and Persani, L. (2004) Am. J. Hum. Genet. 75 106-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campos-Xavier, B., Saraiva, J. M., Savarirayan, R., Verloes, A., Feingold, J., Faivre, L., Munnich, A., Le Merrer, M., and Cormier-Daire, V. (2001) Hum. Genet 109 653-658 [DOI] [PubMed] [Google Scholar]
- 43.Janssens, K., Vanhoenacker, F., Bonduelle, M., Verbruggen, L., Van Maldergem, L., Ralston, S., Guanabens, N., Migone, N., Wientroub, S., Divizia, M. T., Bergmann, C., Bennett, C., Simsek, S., Melancon, S., Cundy, T., and Van Hul, W. (2006) J. Med. Genet. 43 1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu, S., Liang, S., Yan, Y., Wang, Y., Li, F., Deng, Y., Huang, W., Yuan, W., Luo, N., Zhu, C., Wang, Y., Li, Y., Liu, M., and Wu, X. (2007) Bone 40 1630-1634 [DOI] [PubMed] [Google Scholar]
- 45.Belville, C., Van Vlijmen, H., Ehrenfels, C., Pepinsky, B., Rezaie, A. R., Picard, J. Y., Josso, N., di Clemente, N., and Cate, R. L. (2004) Mol. Endocrinol. 18 708-721 [DOI] [PubMed] [Google Scholar]
- 46.Young, G. D., and Murphy-Ullrich, J. E. (2004) J. Biol. Chem. 279 47633-47642 [DOI] [PubMed] [Google Scholar]
- 47.Yang, J., Ratovitski, T., Brady, J. P., Solomon, M. B., Wells, K. D., and Wall, R. J. (2001) Mol. Reprod. Dev. 60 351-361 [DOI] [PubMed] [Google Scholar]
- 48.Sunada, Y. (2006) Rinsho Shinkeigaku 46 942-944 [PubMed] [Google Scholar]
- 49.Bogdanovich, S., Perkins, K. J., Krag, T. O., Whittemore, L. A., and Khurana, T. S. (2005) FASEB J. 19 543-549 [DOI] [PubMed] [Google Scholar]
- 50.Brown, M. A., Zhao, Q., Baker, K. A., Naik, C., Chen, C., Pukac, L., Singh, M., Tsareva, T., Parice, Y., Mahoney, A., Roschke, V., Sanyal, I., and Choe, S. (2005) J. Biol. Chem. 280 25111-25118 [DOI] [PubMed] [Google Scholar]