Abstract
Bromodomains are acetyl lysine binding modules found in many complexes that regulate gene transcription. In budding yeast, the coactivator complex SAGA (Spt-Ada-Gcn5-acetyl-transferase) predominantly facilitates transcription of stress-activated genes and requires the bromodomain of the Gcn5 subunit for full activation of a number of these genes. This bromodomain has previously been shown to promote retention of the complex to H3 and H4 acetylated nucleosomes. Because the SAGA complex mediates histone H3 acetylation, we sought to determine to what extent the Gcn5 bromodomain directly modulates histone acetylation activity. Kinetic analysis of SAGA-mediated acetylation of nucleosomal substrates reveals that this bromodomain: 1) is required for the cooperative acetylation of nucleosomes, 2) enhances acetylation of an H3 histone tail when the other H3 tail within a nucleosome is already acetylated, and 3) augments the acetylation turnover of nucleosomes previously acetylated at lysine 16 of the histone H4 tails. These results indicate that the Gcn5 bromodomain promotes the establishment of nucleosome acetylation through multiple mechanisms and more generally show how chromatin recognition domains can modulate the enzymatic activity of chromatin modifying complexes.
The SAGA2 (Spt-Ada-Gcn5-acetyltransferase) complex in Saccharomyces cerevisiae is a 1.8-MDa complex composed of 19 or more protein subunits and is highly conserved in eukaryotes (1-3). This complex has an important role in promoting gene expression as it has been shown that, in combination with TFIID, it contributes to the transcription of nearly all yeast genes and serves especially to activate transcription of genes induced in response to cellular stress (4).
One aspect of SAGA function that is important for gene activation is the ability to act as an acetyltransferase (4). The core acetyltransferase activity of SAGA is contained in the Gcn5 subunit (1, 5), and the complex has been shown to acetylate lysine residues of histone proteins that comprise chromatin, especially the H3 histone (1). H3 acetylation occurs in the amino-terminal histone tails predominantly at lysine 14 but also occurs at lysines 9, 18, and 23 (6). Acetylation of the H3 tail is thought to facilitate transcription by promoting the localization of activators of transcription (7, 8), as well as by potentially directly changing nucleosome structure (9).
Another aspect of the SAGA complex that is important for gene activation is the bromodomain of the Gcn5 subunit as expression of a number of inducible genes relies on the presence of the subunit specifically or in combination with other bromodomain-containing proteins (8, 10). The bromodomain is a protein module that recognizes acetylated lysine resides and is found in many complexes that regulate gene transcription (11). Bromodomains recognize acetylated lysines in specific proteins through direct interactions of the bromodomain with amino acids that flank the acetylated lysine residue (12) and by the spatial arrangement of the bromodomain within the larger protein complex (8). The Gcn5 bromodomain, or a nearly identical homolog in human PCAF (p300/CBP-associated factor) protein, has been shown to directly mediate interactions with acetylated activators of transcription and with acetylated H3 and H4 histone tails (8, 12-14). In these cases, it has been proposed that binding of the Gcn5 bromodomain to acetylated targets acts to localize and increase the retention of the SAGA complex, thereby promoting its histone acetyltransferase (HAT) and HAT-independent activation functions.
Although the Gcn5 bromodomain of the SAGA complex has been shown to promote its retention on acetylated chromatin, we were interested in directly determining the effect of this bromodomain on nucleosome acetylation activity. Our previous studies have shown that detailed kinetic analysis of SAGA-mediated acetylation of nucleosomes can reveal mechanistic aspects of the establishment of nucleosome acetylation that are not readily apparent using other approaches (15-17). Using this strategy, we sought to ascertain the role of the Gcn5 bromodomain on unacetylated nucleosomes, nucleosomes homogeneously acetylated on the histone H3 tail, and nucleosomes homogeneously acetylated on lysine 16 of the H4 histone tail.
EXPERIMENTAL PROCEDURES
Histone, DNA, Octamer, and Nucleosome Preparation—Recombinant Xenopus laevis histones were expressed, purified, and characterized according to standard methods (18). Tetra-Ala H3, Tetra-Ac H3, and H4-Lys-16-Ac ligated histones were prepared and characterized as described previously (17, 19). DNA templates containing the Lytechinus veriegates 5 S rDNA sequence was generated and characterized as described previously (17). Symmetric histone octamers were assembled and quantified according to standard protocols (18). Asymmetric octamers containing ligated histone H3 were prepared and quantified as described previously (17). Mononucleosome and nucleosomal arrays were prepared by step dialysis from DNA template and purified histone octamers (20). Nucleosomes with similar saturation were used, as determined by native gel analysis of the assembled mononucleosomes or of the mononucleosomes generated by EcoRI digestion of the nucleosomal arrays (20). Mononucleosome and array concentrations were determined by DNA absorbance.
Ada2-Ada3-Gcn5 Subcomplex Expression and Purification—The plasmid containing the Ada2-Ada3-Gcn5-Y413A sequence was generated by QuikChange mutagenesis (Stratagene) from a plasmid encoding the wild-type Ada2-Ada3-Gcn5 complex (21) and confirmed by DNA sequencing. The Ada2-Ada3-Gcn5 and Ada2-Ada3-Gcn5-Y413A subcomplexes were expressed and purified as described (22). Protein concentrations were determined by comparison with known amounts of recombinant Gcn5 on 15% SDS-polyacrylamide gel, stained with Coomassie Blue. The stoichiometry of the complex is under investigation but currently unknown.
SAGA Complex Purification—Yeast strain FY2031 encoding tandem affinity purification (TAP)-tagged Spt7p was a generous gift from Fred Winston (Harvard Medical School). Yeast strain MSK183 encoding Gch5-Y41A protein was constructed by PCR-based gene modification (23). Briefly, GCN5 was deleted by transforming strain FY2031 with URA3 PCR product composed of URA3 flanked by 40 bp of GCN5 genomic upstream and downstream sequences. The transformants were selected on Ura- plates to create strain MSK198. URA3 was then replaced by transforming strain MSK198 with Gcn5-Y413A PCR product amplified from the bacterial expression plasmid described above. Selection of 5-fluoroorotic acid-resistant colonies resulted in the desired Gcn5-Y413A mutant strain, MSK 183. Gcn5-Y413A integration was further confirmed by sequencing the PCR products obtained by using its genome as the template. The SAGA complex was isolated by tandem affinity purification as described previously (24). SAGA composition was confirmed by silver staining. SAGA concentration was determined by comparative Western blotting against known amounts of recombinant Gcn5 using anti-Gcn5 antibody (sc-9078; Santa Cruz Biotechnology).
Histone Acetyltransferase Kinetics Assays—Initial rates of nucleosome acetylation were measured using a radioactive P81 filter binding assay as described previously (17). Assays were performed at 30 °C in HAT buffer (50 mm Tris, pH 7.5, 5% glycerol, 0.125 mm EDTA, 50 mm KCl, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 10 mm sodium butyrate) with various concentrations of nucleosomal arrays or mononucleosomes, 4.0 μm acetyl-CoA (1.25 μCi/μm [3H]acetyl-CoA), and either 1.0 nm SAGA or 2.5 nm Ada2-Ada3-Gcn5 subcomplex. In addition to the gel-based quantification of the enzymes described above, equivalence of activity for WT and mutated enzymes was confirmed by acetylation assay. In these assays, for equal amounts of enzyme, the extent of acetylation after 3 min for 300 nm nucleosome was determined. For the average of three trials, it was found that mutated Ada2-Ada3-Gcn5 subcomplex and SAGA complex activities were 105 and 108% of their WT activity, respectively. Parameters describing initial rates as a function of substrate concentration were obtained by fitting the data to the cooperative saturation kinetics model.
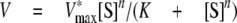 |
(Eq.1) |
Except when noted otherwise, assays were performed three times, with the reported kinetic constants being the average of the individual fits.
Characterization of Acetylation Products—The histone specificity of SAGA-mediated acetylation was determined in assays with 0.12 μm nucleosomes (1 μg of total histone), 0.83 μm acetyl-CoA (1.20 μCi/μm [3H]acetyl-CoA), and 2.0 nm SAGA complex in HAT buffer at 30 °C for 40 min. Histones were resolved by gel electrophoresis on an 18% SDS-polyacrylamide gel. The gel was then stained with Coomassie Blue to visualize histones, destained (30% methanol, 10% acetic acid, 3% glycerol), treated with ENHANCE (PerkinElmer Life Sciences) for 40 min, and incubated with cold 2% glycerol. The dried gel was analyzed by fluorography. The lysine specificity of SAGA-mediated acetylation was determined in assays with 0.58 μm nucleosomes (5 μg of total histone), 3.75 μm acetyl-CoA (1.60 μCi/μm [3H]acetyl-CoA), and 4 nm SAGA complex. Reactions proceeded for 4 min at 30 °C before being quenched with tri-chloroacetic acid (10%). Histones were resolved by gel electrophoresis on an 18% SDS-polyacrylamide gel and transferred to polyvinylidene difluoride membrane (Millipore). Histone H3 was excised from the membrane and analyzed by Edman degradation microsequencing (W. M. Keck Foundation Biotechnology Resource Laboratory, Yale University).
RESULTS
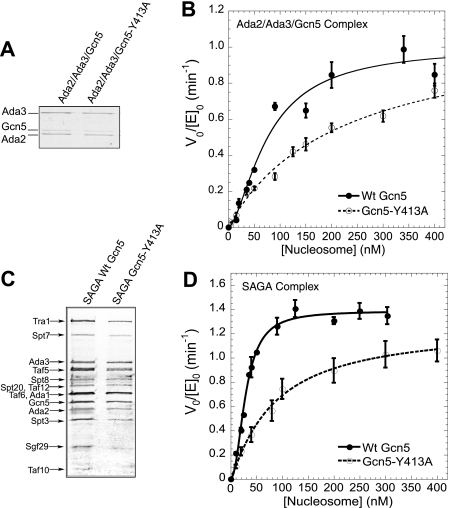
The Role of the Gcn5 Bromodomain in the Cooperative Acetylation of Nucleosomes—Previous kinetic analysis of SAGA-mediated nucleosome acetylation revealed that this process is highly cooperative, consistent with a model in which initial binding of a nucleosome promotes binding of at least one additional, non-contiguous nucleosome (17). To determine factors in the SAGA complex required for cooperative acetylation, we first sought to define subunits that were sufficient to recapitulate cooperativity. Work by Tan and co-workers (21) has shown that the majority of SAGA activity and product specificity toward peptide substrates could be reproduced with three SAGA subunits, Gcn5, Ada2, and Ada3. To test whether these subunits could also recapitulate cooperative acetylation of nucleosome substrates, they were coexpressed and purified to generate the Ada2-Ada3-Gcn5 subcomplex (Fig. 1A). For our chromatin model system, recombinant, unacetylated histone octamers were deposited onto a DNA template containing twelve 208-bp head-to-tail repeats of L. veriegates 5 S rDNA (20, 25). Total transfer of radioactive acetyl groups from acetyl-CoA to nucleosomes as a function of time was determined by a filter binding assay (26). Initial velocity kinetics of acetyl incorporation at varying nucleosomal array concentrations revealed that nucleosomal acetylation by the Ada2-Ada3-Gcn5 subcomplex also showed significant cooperativity (cooperativity constant of 1.63 ± 0.17, Fig. 1B and supplemental Fig. S1), suggesting that a required determinant of cooperative acetylation is contained within the Ada2-Ada3-Gcn5 subcomplex.
FIGURE 1.
Cooperative nucleosome acetylation by the SAGA complex requires the Gcn5 bromodomain. A, characterization of recombinantly expressed yeast Ada2-Ada3-Gcn5 subcomplex containing either wild-type Gcn5 or Gcn5 with a point mutation in the bromodomain (Gcn5-Y413A). The purified complexes were analyzed by 15% SDS-PAGE and stained with Coomassie Blue. B, the initial velocity of nucleosomal array acetylation per enzyme as a function of nucleosome concentration for Ada2-Ada3-Gcn5 (solid circles) and Ada2-Ada3-Gcn5-Y413A subcomplex (open circles). At least three independent experiments were performed at each nucleosome array concentration. The average initial velocity data were fit to a cooperativity saturation model to give a cooperativity constant of 1.63 ± 0.17 for wild-type Ada2-Ada3-Gcn5 subcomplex (solid line) and 0.91 ± 0.15 for Ada2-Ada3-Gcn5-Y413A subcomplex (dashed line). For direct comparison with data in Fig. 1D, only data for nucleosome concentrations from 0 to 400 nm are shown. The full range of nucleosome concentrations is shown in supplemental Fig. S1. C, characterization of TAP-purified SAGA complex containing either wild-type Gcn5 or Gcn5-Y413A. Subunits were resolved on a gradient gel and visualized by silver staining. Known protein components were labeled according to Winston and co-workers (24). D, the initial velocity of nucleosomal array acetylation per enzyme as a function of nucleosome concentration for wild-type SAGA (solid circles) and SAGA Gcn5-Y413A (open circles). Fitting of the data gives a cooperativity constant of 1.97 ± 0.15 (wild-type SAGA, solid line) and 1.01 ± 0.22 (SAGA Gcn5-Y413A, dashed line). Wild-type data were adapted from Li and Shogren-Knaak (17).
To determine whether the bromodomain of Gcn5 has a role in cooperative acetylation, we mutated tyrosine 413 to alanine. Previous studies by Zhou and co-workers (27) have shown that in the human PCAF bromodomain, which has 95% sequence identity to the Gcn5 bromodomain, the analogous mutation completely abrogates acetyl lysine binding while maintaining the structure of the bromodomain. The formation of the Ada2-Ada3-Gcn5 subcomplex was unaffected by introduction of this point mutation (Fig. 1A). However, cooperativity of nucleosome acetylation was effectively eliminated (cooperativity constant of 0.91 ± 0.15) (Fig. 1B and supplemental Fig. S1).
To confirm that this loss in cooperativity was not somehow specific to the Ada2-Ada3-Gcn5 subcomplex, the same Gcn5-Y413A point mutation was introduced into the yeast genome. TAP tag-based purification of the endogenous SAGA complex containing the Gcn5-Y413A point mutation generated an enzyme complex with a composition identical to the TAP-tagged wild-type SAGA complex (Fig. 1C), suggesting that, similar to the subcomplex, this point mutation was not deleterious to the formation of the SAGA complex. Also similar to the subcomplex results, cooperative nucleosome acetylation was disrupted, going from a wild-type cooperativity constant of 1.97 ± 0.15 to 1.01 ± 0.22 for SAGA containing the point mutant. Thus, these results suggest that the acetyl lysine binding activity of the Gcn5 bromodomain is required for cooperative acetylation of nucleosomes.
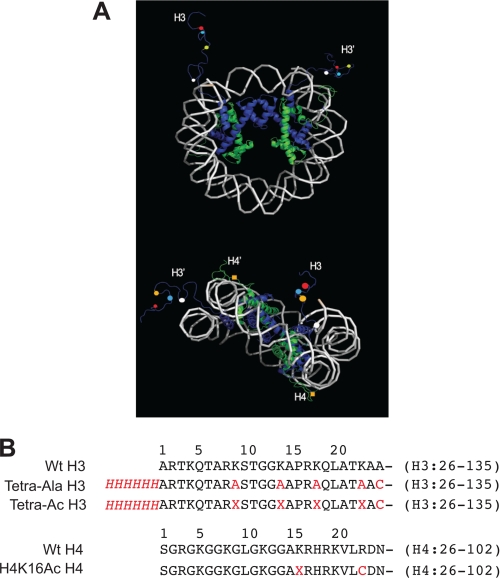
The Role of the Gcn5 Bromodomain in Acetylation of H3 Lys-9, -14, -18, and -23 Preacetylated Nucleosomes—The SAGA complex can bind nucleosomes acetylated on histone H3 in a manner dependent on the bromodomain of Gcn5 (8). Because nucleosomes contain two copies of each of the histone proteins (28), we hypothesized that the bromodomain of Gcn5 would bind acetyl lysines on one H3 tail and facilitate acetylation of the other H3 tail (Fig. 2A). To test this idea, we needed a nucleosome preacetylated on a single H3 tail. For this nucleosome, it was necessary that the single preacetylated tail be fully acetylated on all target sites of SAGA acetylation: Lys-9, -14, -18, and -23 (Fig. 2B, Tetra-Ac H3). Otherwise, it would be difficult to distinguish whether new acetylation occurred on sites within or outside a partially preacetylated H3 tail. To determine the effect of the preacetylation, an appropriate control nucleosome lacking preacetylation was also necessary. To be directly comparable, we sought a nucleosome in which a single H3 was neither acetylated nor capable of being acetylated (Fig. 2B, Tetra-Ala H3).
FIGURE 2.
Potential nucleosome sites recognized by the Gcn5 bromodomain. A, the relative orientation of the H3 and H4 histone tails and potential histone acetylation sites based on mononucleosome structure 1KX5 (32). Mononucleosome DNA is depicted in white. H3 and H3′ histones are shown in blue, and H4 and H4′ histones are in green. The histones H2A and H2B are omitted for clarity. In the top image, the mononucleosome is oriented to emphasize the positions of the two H3 tails. The four potential acetylation sites lysine 9 (red), lysine 14 (green), lysine 18 (yellow), and lysine 23 (white) are labeled as colored balls. In the bottom image, the mononucleosome is oriented to emphasize the relative positions of H3 tails and H4 tails. The lysine 16 residues on both H4 tails are labeled as yellow squares. B, the amino acid sequences of the histones used in this study. Deviations from unmodified, wild-type sequences are shown in red. The His6 affinity tag is denoted in red and italicized. Lysine residues that are acetylated on their side chain amine are denoted as X.
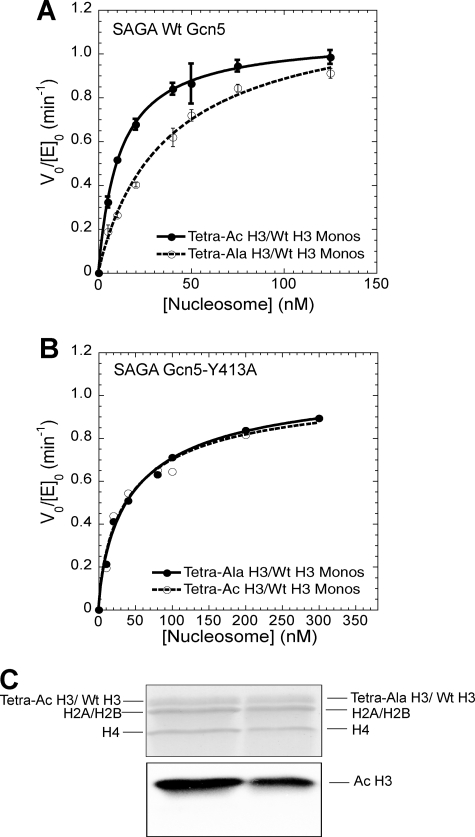
As performed previously, the appropriate homogeneously acetylated and non-acetylatable H3 histones were generated by native chemical ligation, incorporated in single copy into mononucleosomes, and subjected to initial velocity kinetic analysis with wild-type SAGA complex (17). From these experiments, it is clear that the half-saturation concentration of acetylation for the nucleosomes preacetylated on a single H3 tail is significantly lower than that for the control nucleosome (Fig. 3A), suggesting that preacetylation of the H3 tail increases SAGA binding affinity over unacetylated nucleosomes by over 3-fold (calculated from the ratio of the relevant half-saturation concentrations found in Table 1). However, this observed difference could instead be due to a decrease in binding affinity for the control substrate (alanines at H3 Lys-9, -14, -18, and -23) relative to the unacetylated lysine residues.
FIGURE 3.
When one of the two H3 tails in a nucleosome is acetylated, the Gcn5 bromodomain promotes acetylation of the other H3 tail. A, a comparison of wild-type SAGA HAT activity on mononucleosomes containing Tetra-Ac H3/WT H3 (solid circles) or Tetra-Ala H3/WT H3 (open circles) octamers. Fitting of the data gives a half-saturation concentration of 11.5 ± 2.4 nm (Tetra-Ac H3/WT H3 nucleosomes, solid line) and 38.9 ± 6.9 nm (Tetra-Ala H3/WT H3 nucleosomes, dashed line). Data were adapted from Li and Shogren-Knaak (17) B, a comparison of HAT activity of SAGA Gcn5-Y413A complex on mononucleosomes containing Tetra-Ac H3/WT H3 (open circles) and Tetra-Ala H3/WT H3 (solid circles) octamers. Two independent experiments were performed at each nucleosome concentration. The half-saturation concentrations obtained from fitting the data are 30.6 nm (Tetra-Ac H3/WT H3 nucleosomes, dashed line) and 32.8 nm (Tetra-Ala H3/WT H3 nucleosomes, solid line). C, a fluorogram showing the histone specificity of SAGA acetylation on mononucleosomes that are either preacetylated or unacetylated on one H3 histone tail (lanes 1 and 2, respectively). The Coomassie Blue-stained gel (upper panel) and corresponding fluorogram (lower panel) are shown.
TABLE 1.
Summary of kinetic constants determined in this study
Kinetic constants are the average of three independent trials, except for some of the experiments with SAGA Gcn5-Y413A, which were the average of two trials. For experiments with two trials, the results of each trial is reported in parentheses instead of reporting a standard deviation. Half-saturation concentration was calculated from K1/n. The apparent turnover rate constant, kcat,app, was determined from Vmax/[E]0. Data marked with an asterisk is from previously published work (17).
| Octamer | DNA | Enzyme | [Half-saturation] | kcat,app | Cooperativity coefficient |
|---|---|---|---|---|---|
| nm | min−1 | ||||
| Wild-type* | 208-12 Arrays | SAGA WT Gcn5 | 33.6 ± 5.0 | 1.42 ± 0.15 | 1.97 ± 0.15 |
| H4-Lys-16Ac | 208-12 Arrays | SAGA WT Gcn5 | 19.7 ± 1.7 | 2.64 ± 0.29 | 1.31 ± 0.09 |
| Wild-type | 208-12 Arrays | SAGA Gcn5-Y413A | 104.7 ± 29.7 | 1.46 ± 0.15 | 1.01 ± 0.22 |
| H4-Lys-16Ac | 208-12 Arrays | SAGA Gcn5-Y413A | 98.1 (80.3, 115.9) | 1.50 (1.39, 1.61) | 1.14 (1.13, 1.14) |
| Wild-type | 208-12 Arrays | Ada2/Ada3/WT Gcn5 | 74.0 ± 14.1 | 1.00 ± 0.07 | 1.63 ± 0.17 |
| Wild-type | 208-12 Arrays | Ada2/Ada3/Gcn5-Y413A | 201.1 ± 17.4 | 1.00 ± 0.06 | 0.91 ± 0.15 |
| Tetra-Ala H3/WT H3* | 196-1 Monos | SAGA WT Gcn5 | 38.9 ± 6.9 | 1.07 ± 0.07 | 0.95 ± 0.03 |
| Tetra-Ac H3/WT H3* | 196-1 Monos | SAGA WT Gcn5 | 11.5 ± 2.4 | 1.12 ± 0.08 | 0.99 ± 0.10 |
| Tetra-Ala H3/WT H3 | 196-1 Monos | SAGA Gcn5-Y413A | 32.8 (32.6, 32.9) | 0.96 (0.94, 0.97) | 0.91 (0.84, 0.98) |
| Tetra-Ac H3/WT H3 | 196-1 Monos | SAGA Gcn5-Y413A | 30.6 (26.4, 34.7) | 0.94 (0.89, 0.98) | 0.96 (0.89, 1.02) |
To clarify whether the observed difference in half-saturation concentration values reflected an increase in overall affinity for acetylated lysine residues or a decrease for alanine-substituted H3 histones, as well as to identify the enzymatic source of the half-saturation concentration difference, initial velocity kinetic analysis was performed on the Tetra-Ac H3/WT H3 and Tetra-Ala H3/WT H3 nucleosome substrates using the SAGA complex containing the Gcn5 bromodomain Y413A point mutation (Fig. 3B). With this mutated enzyme, the acetylation half-saturation concentrations for either the preacetylated or the non-preacetylated substrate were nearly identical, indicating that the bromodomain of Gcn5 is necessary to recognize differences in the H3 tail that is not the target of acetylation. Moreover, the acetylation half-saturation concentrations for both the preacetylated and the non-acetylated substrates were nearly 3-fold higher than that for the wild-type enzyme with preacetylated nucleosomes, showing that the interaction of the Gcn5 bromodomain with the acetylated lysine tail significantly augments overall SAGA binding to the substrate.
Although these experiments reveal that bromodomain-dependent binding of an acetylated H3 tail facilitates further nucleosome acetylation, they do not indicate the target of the additional acetylation. Because binding of the acetylated H3 tail might change the overall orientation of the SAGA complex, we sought to determine whether preacetylation of one of the H3 tails would change which histone was subsequently acetylated. To discern this, individual histones from a wild-type SAGA HAT assay were resolved by gel electrophoresis followed by fluorography to ascertain which histones showed incorporation of the radioactive acetyl group (Fig. 3C). For acetylation of both the Tetra-Ac H3/WT H3 and the Tetra-Ala H3/WT H3 nucleosome, the only apparent site of enzymatic acetylation was the H3 histone. Thus, although the Gcn5 bromodomain appears to promote binding and acetylation of nucleosomes already acetylated on a single H3 tail, it does not change which histone is acetylated.
The Role of the Gcn5 Bromodomain in Acetylation of H4 Lys-16 Preacetylated Nucleosomes—The SAGA complex can bind nucleosomes acetylated on histone H4 in a manner dependent on the bromodomain of Gcn5 (8). Like acetylated histone H3, we hypothesized that bromodomain-dependent binding of the SAGA complex to H4 acetylated nucleosomes would change subsequent nucleosome acetylation and sought to test this hypothesis in a similar fashion. Construction of a nucleosome preacetylated on histone H4 differed in several ways from the nucleosome preacetylated on histone H3. Because the SAGA complex is not known to acetylate H4 histones, we believed it would not be necessary to limit H4 preacetylation to a single histone tail, nor would we have to preacetylate all potential H4 tail lysine sites. Because the only currently known structure of the yeast Gcn5 bromodomain bound to an acetylated peptide is bound to an H4 histone peptide acetylated on lysine 16 (14) and because this mark is found on 80% of all yeast histones, especially in euchromatin (29-31), this site of acetylation was chosen for further investigation.
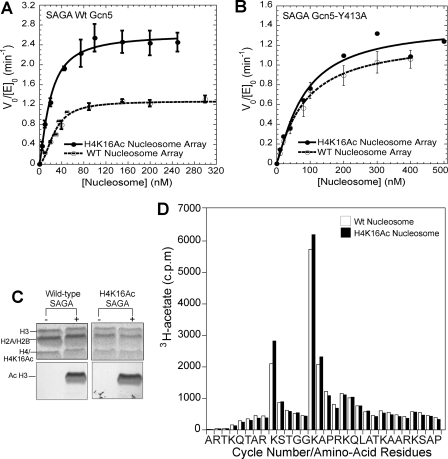
H4 histone homogeneously acetylated at lysine 16 (Fig. 2B) was generated by native chemical ligation. Initial velocity analysis of SAGA-mediated acetylation of nucleosomal arrays containing either unacetylated or H4 Lys-16 acetylated histones revealed that overall preacetylation reduced the acetylation half-saturation substrate concentration 1.7-fold and increased the apparent turnover rate constant by 1.86-fold (Fig. 4A). These results suggests that the presence of H4 Lys-16 acetylation augments nucleosome acetylation but do not indicate how because H4 Lys-16 acetylation could either facilitate Gcn5 bromodomain binding (14) or disrupt higher-order chromatin structure (19).
FIGURE 4.
H4-Lys-16 (H4K16Ac) acetylation promotes H3 tail acetylation turnover in a bromodomain-dependent manner. A, a comparison of HAT activity of wild-type SAGA complex on nucleosome arrays containing H4-Lys-16 acetylation (solid circles) and wild-type nucleosome arrays (open circles). From the fitting of the data, for H4-Lys-16 acetylated nucleosomal arrays (solid line), the half-saturation concentration is 19.7 ± 1.7 nm, and the kcat, app is 2.64 ± 0.29 min-1. For the arrays that were not preacetylated (dashed lines), the half-saturation concentration is 33.6 ± 5.0 nm with a kcat, app of 1.42 ± 0.15 min-1. B, a comparison of HAT activity of the SAGA Gcn5-Y413A complex on nucleosome arrays containing H4-Lys-16 acetylation (solid circles) and wild-type nucleosome arrays (open circles). Two independent experiments were performed for H4-Lys-16 acetylated nucleosomes at each nucleosome concentration. Fitting of the data give a half-saturation concentration and kcat, app of 98.1 nm and 1.50 min-1 for the H4-Lys-16 acetylated nucleosome arrays (solid line) and 104.7 ± 29.7 nm and 1.46 ± 0.15 min-1 for the wild-type nucleosome arrays (dashed line). C, a comparison of the histone specificity of the SAGA complex for wild-type (lane 2) and H4-Lys-16 acetylated (lane 4) nucleosome arrays. Histones are resolved by SDS-PAGE. Staining with Coomassie Blue is shown in the upper panel, with the corresponding fluorogram shown in the lower panel. D, a comparison of the sites of SAGA-mediated acetylation for nucleosomal arrays that are not preacetylated (white column) and for nucleosome arrays that are preacetylated at H4-Lys-16 (black column). The amount of [3H]acetyl incorporation at a given position of the H3 tail was determined by microsequencing. To more clearly compare the relative site selectivity between nucleosome substrates, the data for the H4-Lys-16 preacetylated substrate have been normalized so that its total H3 histone acetylation is equivalent to the total H3 acetylation for substrate that was not preacetylated.
Additional kinetic experiments indicated that differences in acetylation rates between H4 Lys-16 preacetylated and non-acetylated substrates were almost entirely lost with mutation of the Gcn5 bromodomain (Fig. 4B). This loss not only indicates that the augmented acetylation kinetics of H4 Lys-16 acetylated substrates requires the Gcn5 bromodomain but also suggests that chromatin condensation differences are not responsible because the presence of H4 Lys-16 acetylation itself is not sufficient to augment the rate of nucleosome acetylation (Fig. 4B).
To further support the idea that changes in chromatin condensation are not responsible for differences in the acetylation kinetics, experiments were performed with mononucleosomes, which cannot undergo chromatin condensation. In these experiments, the H4 Lys-16 acetylated mononucleosomes show a bromodomain-dependent increase in the nucleosome acetylation (supplemental Fig. S2). Because this result corresponds well with the comparable experiments with nucleosomal arrays, they suggest that H4-Lys-16-Ac-mediated disruption of chromatin condensation is not necessary to achieve enhancement of nucleosome acetylation.
Because the histone H4 tails protrude from the nucleosome in a different orientation than the H3 tails (Fig. 2A), we wondered whether H4 tail binding by the Gcn5 bromodomain would alter the location of subsequent acetylation. Fluoro-graphic analysis of the products of enzymatic acetylation revealed that histone H3 was the predominant site of SAGA-mediated acetylation, regardless of whether histone H4 was acetylated on lysine 16 or not (Fig. 4C). Because multiple sites of acetylation are known to occur within the H3 histone tail (6), we also determined whether the pattern of acetylation within the H3 histone tail was altered by H4 Lys-16 acetylation. Microsequencing analysis of the products of enzymatic acetylation showed that no absolute change in the order of acetylation site utilization resulted from the presence of H4 Lys-16 acetylation, i.e. H3 Lys-14 was still the primary site of acetylation followed by Lys-9, -18, and -23 (Fig. 4D).
DISCUSSION
Our results show that in the SAGA complex, the bromodomain of Gcn5 promotes the establishment of nucleosomal acetylation in multiple, distinct ways. For nucleosomes that begin unacetylated, the bromodomain is required for cooperative nucleosome acetylation. Our previous studies suggest that this cooperativity allows the SAGA complex to bind multiple, non-contiguous nucleosomes (17). In such a model, the bromodomain might help to bridge these nucleosomes, potentially through acetylated histone tail interactions.
Although the Gcn5 bromodomain is necessary for enzyme cooperativity, it may not be sufficient. Other domains within the Ada2-Ada3-Gcn5 subcomplex could also be required. Further, the cooperativity of the Ada2-Ada3-Gcn5 subcomplex is slightly less than that of the full SAGA complex (Table 1). This suggests that SAGA subunits outside of the core subcomplex may play a role in promoting enzyme cooperativity.
For nucleosomes that are already acetylated on either the H3 or the H4 histone tail, the bromodomain promotes acetylation of additional H3 tails. Specifically, for nucleosomes already fully acetylated on one of the two nucleosomal H3 tails, the Gcn5 bromodomain significantly reduces the half-saturation concentration of further acetylation. Mechanistically, this acetylation could occur within the same bound nucleosome or on a nucleosome not bound by the Gcn5 bromodomain. In either case, the behavior may help to ensure the acetylation of H3 tails within a nucleosome or between nucleosomes. This cross-tail templating mechanism might also promote the maintenance of nucleosomal H3 tail acetylation in the face of various pathways that result in loss of acetylation on H3 tails.
For nucleosomes already acetylated on lysine 16 of the H4 tails, overall substrate binding and turnover are elevated. Although these effects are relatively modest, it is likely that our experiments under-represent their magnitude. This is because acetylation of the H3 tails without H4-Lys-16-Ac binding is likely to occur simultaneously, making our observations an average of both H4-Lys-16-Ac-dependent and H4-Lys-16-Ac-independent pathways. Similarly, these competing pathways might mask changes in lysine site utilization. Although the overall pattern of H3 lysine acetylation is similar for nucleosomes preacetylated and non-acetylated on H4-Lys-16, subtle changes in acetylation intensity suggest that H4-Lys-16 acetylation might bias acetylation toward lysines residues that are more amino-terminal (supplemental Fig. S3).
Mechanistically, the changes in half-saturation concentration and potential acetylation site utilization may be due to the Gcn5 bromodomain binding of the H4 Lys-16 acetyl lysine positioning the Gcn5 in an orientation still capable of mediating H3 acetylation but favoring interactions with more amino-terminal residues. The increase in product turnover could potentially be due to either stimulation of the chemical step or product release. Again, changes in the orientation of the SAGA complex could potentially facilitate either of these possibilities.
Although the interaction between the Gcn5 bromodomain and histone acetylation acts to promote nucleosome acetylation, it does not appear to decrease the rate of product release. A property of the experimentally measured turnover parameter, kcat, is that it cannot be faster than any first-order rate constant on the forward reaction pathway. Thus, if the slowest mechanistic step in the forward reaction involves the dissociation of bromodomain-acetyl lysine interaction, then the turnover number would be reduced when both a functional bromodomain and acetylation are present. In experiments where the only difference is the presence of either histone tail acetylation or a functional bromodomain, no significant reduction in the turnover number is observed (Table 1). This suggests that the rate-limiting forward step must be something else, such as the chemical step or a product release step independent from the dissociation of the bromodomain.
Because the Gcn5 bromodomain modulates the acetyltransferase activity of SAGA in multiple ways, the combination of these functions in vivo is likely to be complicated. Still, because each of these pathways increases the rate of H3 histone acetylation in vitro, it is predicted that loss of cellular Gcn5 bromodomain function would ultimately lead to a decrease in the efficiency of H3 histone acetylation in vivo. To date, relatively few experiments have shown a correlation between Gcn5 bromodomain activity and H3 acetylation. This may be due, in part, to the fact that the bromodomain only modulates the inherent enzymatic activity of Gcn5 and may only ultimately dictate the rate, but not the extent, of histone acetylation. In such a case, careful time-dependent monitoring of H3 acetylation might be necessary. Furthermore, because the SAGA complex is predominantly involved in expression of genes that are induced under stress conditions (4) and because Gcn5 bromodomain function might not be utilized for all of them, effects on histone acetylation may only be readily discerned on particular nucleosomes of specific promoters under appropriate induction conditions. Indeed, one such example of the effect of the Gcn5 bromodomain on histone acetylation comes from detailed analysis of the progress of histone acetylation following SUC2 gene activation (33). In this study, the deletion of the Gcn5 bromodomain resulted in the reduction of H3 acetylation for several nucleosomes in the SUC2 promoter, with one nucleosome exhibiting a 2-fold reduction in H3 acetylation.
Such an effect on H3 acetylation, in combination with the general ability of the Gcn5 bromodomain to promote retention of SAGA on acetylated nucleosomes (8), may explain most of the known phenotypes that result from loss of the Gcn5 bromodomain (8, 10). Further studies that employ assays that are sensitive to changes in the rate of transcriptional induction, as well as subtle changes in the level of expression, are likely to uncover new genes that exploit the acetylation-promoting activity of the Gcn5 bromodomain.
Supplementary Material
Acknowledgments
We greatly appreciate the gift of the yeast strain containing TAP-tagged SAGA from Dr. Fred Winston (Harvard Medical School, Boston, MA).
This work was supported, in whole or in part, by National Institutes of Health Grant GM79663 (to M. S. K.).
The on-line version of this article (available at http://www.jbc.org) contains three supplemental figures.
Footnotes
The abbreviations used are: SAGA, Spt-Ada-Gcn5 acetyltransferase; HAT, histone acetyltransferase; TAP, tandem affinity purification; acetyl-CoA, acetyl-coenzyme A; WT, wild-type; CBP, CREB-binding protein; CREB, cAMP-response element-binding protein.
References
- 1.Grant, P. A., Duggan, L., Cote, J., Roberts, S. M., Brownell, J. E., Candau, R., Ohba, R., Owen-Hughes, T., Allis, C. D., Winston, F., Berger, S. L., and Workman, J. L. (1997) Genes Dev. 11 1640-1650 [DOI] [PubMed] [Google Scholar]
- 2.Baker, S. P., and Grant, P. A. (2007) Oncogene 26 5329-5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee, K. K., and Workman, J. L. (2007) Nat. Rev. Mol. Cell Biol. 8 284-295 [DOI] [PubMed] [Google Scholar]
- 4.Huisinga, K. L., and Pugh, B. F. (2004) Mol. Cell 13 573-585 [DOI] [PubMed] [Google Scholar]
- 5.Brownell, J. E., Zhou, J., Ranalli, T., Kobayashi, R., Edmondson, D. G., Roth, S. Y., and Allis, C. D. (1996) Cell 84 843-851 [DOI] [PubMed] [Google Scholar]
- 6.Grant, P. A., Eberharter, A., John, S., Cook, R. G., Turner, B. M., and Workman, J. L. (1999) J. Biol. Chem. 274 5895-5900 [DOI] [PubMed] [Google Scholar]
- 7.Agalioti, T., Chen, G., and Thanos, D. (2002) Cell 111 381-392 [DOI] [PubMed] [Google Scholar]
- 8.Hassan, A. H., Prochasson, P., Neely, K. E., Galasinski, S. C., Chandy, M., Carrozza, M. J., and Workman, J. L. (2002) Cell 111 369-379 [DOI] [PubMed] [Google Scholar]
- 9.Wang, X., and Hayes, J. J. (2008) Mol. Cell. Biol. 28 227-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbaric, S., Reinke, H., and Horz, W. (2003) Mol. Cell. Biol. 23 3468-3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mujtaba, S., Zeng, L., and Zhou, M. M. (2007) Oncogene 26 5521-5527 [DOI] [PubMed] [Google Scholar]
- 12.Zeng, L., Zhang, Q., Gerona-Navarro, G., Moshkina, N., and Zhou, M. M. (2008) Structure (Camb.) 16 643-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mujtaba, S., He, Y., Zeng, L., Farooq, A., Carlson, J. E., Ott, M., Verdin, E., and Zhou, M. M. (2002) Mol. Cell 9 575-586 [DOI] [PubMed] [Google Scholar]
- 14.Owen, D. J., Ornaghi, P., Yang, J. C., Lowe, N., Evans, P. R., Ballario, P., Neuhaus, D., Filetici, P., and Travers, A. A. (2000) EMBO J. 19 6141-6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shogren-Knaak, M. A., Fry, C. J., and Peterson, C. L. (2003) J. Biol. Chem. 278 15744-15748 [DOI] [PubMed] [Google Scholar]
- 16.Fry, C. J., Shogren-Knaak, M. A., and Peterson, C. L. (2004) Cold Spring Harbor Symp. Quant. Biol. 69 219-226 [DOI] [PubMed] [Google Scholar]
- 17.Li, S., and Shogren-Knaak, M. A. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 18243-18248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luger, K., Rechsteiner, T. J., and Richmond, T. J. (1999) Methods Enzymol. 304 3-19 [DOI] [PubMed] [Google Scholar]
- 19.Shogren-Knaak, M. A., Ishii, H., Sun, J., Pazin, M. J., Davie, J. R., and Peterson, C. L. (2006) Science 311 844-847 [DOI] [PubMed] [Google Scholar]
- 20.Carruthers, L. M., Tse, C., Walker, K. P., III, and Hansen, J. C. (1999) Methods Enzymol. 304 19-35 [DOI] [PubMed] [Google Scholar]
- 21.Balasubramanian, R., Pray-Grant, M. G., Selleck, W., Grant, P. A., and Tan, S. (2002) J. Biol. Chem. 277 7989-7995 [DOI] [PubMed] [Google Scholar]
- 22.Barrios, A., Selleck, W., Hnatkovich, B., Kramer, R., Sermwittayawong, D., and Tan, S. (2007) Methods (Amst.) 41 271-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longtine, M. S., McKenzie, A., III, Demarini, D. J., Shah, N. G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J. R. (1998) Yeast 14 953-961 [DOI] [PubMed] [Google Scholar]
- 24.Wu, P. Y., Ruhlmann, C., Winston, F., and Schultz, P. (2004) Mol. Cell 15 199-208 [DOI] [PubMed] [Google Scholar]
- 25.Simpson, R. T., Thoma, F., and Brubaker, J. M. (1985) Cell 42 799-808 [DOI] [PubMed] [Google Scholar]
- 26.Eberharter, A., John, S., Grant, P. A., Utley, R. T., and Workman, J. L. (1998) Methods (Amst.) 15 315-321 [DOI] [PubMed] [Google Scholar]
- 27.Dhalluin, C., Carlson, J. E., Zeng, L., He, C., Aggarwal, A. K., and Zhou, M. (1999) Nature 399 491-496 [DOI] [PubMed] [Google Scholar]
- 28.Luger, K., Mader, A. W., Richmond, R. K., Sargent, D. F., and Richmond, T. J. (1997) Nature 389 251-260 [DOI] [PubMed] [Google Scholar]
- 29.Smith, C. M., Gafken, P. R., Zhang, Z., Gottschling, D. E., Smith, J. B., and Smith, D. L. (2003) Anal. Biochem. 316 23-33 [DOI] [PubMed] [Google Scholar]
- 30.Suka, N., Luo, K., and Grunstein, M. (2002) Nat. Genet. 32 378-383 [DOI] [PubMed] [Google Scholar]
- 31.Kimura, A., Umehara, T., and Horikoshi, M. (2002) Nat. Genet. 32 370-377 [DOI] [PubMed] [Google Scholar]
- 32.Davey, C. A., Sargent, D. F., Luger, K., Maeder, A. W., and Richmond, T. J. (2002) J. Mol. Biol. 319 1097-1113 [DOI] [PubMed] [Google Scholar]
- 33.Boukaba, A., Georgieva, E. I., Myers, F. A., Thorne, A. W., Lopez-Rodas, Crane-Robinson, C., and Franco, L. (2004) J. Biol. Chem. 279 7678-7684 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.