Abstract
We recently demonstrated mitochondrial localization of estrogen receptor β (ERβ). We herein confirm the mitochondrial localization of ERβ by the loss of mitochondrial ERβ immunoreactivity in ERβ knockdown cells. A phenotype change characterized as an increase in resistance to oxidative stressors is associated with ERβ knockdown. ERβ knockdown results in a lower resting mitochondrial membrane potential (Δψm) and increase in resistance to hydrogen peroxide-induced Δψm depolarization in both immortal hippocampal cells and primary hippocampal neurons. ERβ knockdown cells maintained ATP concentrations despite insults that compromise ATP production and produce less mitochondrial superoxide under oxidative stress. Furthermore, similar mitochondrial phenotype changes were identified in primary hippocampal neurons derived from ERβ knock-out mice. These data demonstrate that ERβ is expressed in mitochondria and function as a mitochondrial vulnerability factor involved in Δψm maintenance, potentially through a mitochondrial transcription dependent mechanism.
Estrogens are known as the major female steroid hormones, which play a fundamental role in the female reproductive system. In recent years, estrogens have been appreciated as pleiotropic hormones that play roles in a wide variety of nonreproductive functions, such as cardiovascular function (1), memory and cognition (2), bone and mineral metabolism (3), and immune function (4). It is generally accepted that the majority of the biological effects of estrogens are mediated via two estrogen receptors (ERs)2: estrogen receptor α (ERα) and estrogen receptor β (ERβ) (5-8). Consistent with their wide biological roles in a variety of systems, both ERα and ERβ have been found to be widely distributed in different systems and tissues, including the reproductive system, central nervous system, cardiovascular system, gastrointestinal tract, urogenital tract, bone, and liver (9). ERs have been widely accepted as transcriptional factors that belong to the nuclear receptor superfamily. Classically, it is believed that estrogens could modulate the expression of nuclear estrogen-responsive genes through both ERs. Also, estrogens could elicit rapid, nonnuclear action in a number of biological processes via nongenomic mechanisms mediated by ERs (10). Consistently, extranuclear localization of both ERα and ERβ has been indicated (11-14). In fact, increasing evidence has demonstrated that ERβ, while expressed in nuclei of some cell types, is mainly localized extranuclearly (14-18).
It became clear, not long after its identification, that ERβ has biological roles distinct from those of ERα (9). ERα and ERβ are encoded by separate genes found at different chromosomal locations. While sharing a high homology in both the DNA binding and ligand binding domains, ERα and ERβ have very low sequence similarity in the N terminus (12%) and C terminus (9%), corresponding to the AF1 and AF2 domain, respectively (19). Hence, it is not a surprise that ERβ has much lower nuclear transcriptional activity when compared with ERα (20-26).
There is accumulating evidence suggesting that mitochondria are also important targets for the actions of estrogens (18, 27). Mitochondria play a fundamental role in cellular respiration, oxidative phosphorylation, and ionic homeostasis as well as synthesis of heme, lipids, amino acids, and nucleotide. Not only is endogenous estrogen synthesized in the mitochondria by aromatase, but exogenously added estrogen is also mainly transported into this organelle (27, 28). We and several other laboratories have recently reported the localization of ERβ in mitochondria in various cells, including rat primary neurons (15, 18, 29), rat primary cardiomyocytes (15), a murine hippocampal cell line (HT-22), neurons and glia in rat hippocampus (13, 14), human breast cancer cell lines (MCF-7 and MCF-10F) (16, 18), immortal human breast epithelial cells (18), human lens epithelial cell lines (nHLE and HLE-B3) (17, 30), human osteosarcoma cells (SaOS-2) (31), hepatocarcinoma cells (HepG2) (31), and human sperm (32). Similar perinuclear punctate staining of ERβ has also been reported in a murine mammary epithelial cell line (HC11) and human fetal cortical neurons (33, 34). The localization of ERβ in mitochondria suggests that ERβ may function as a mitochondrial component.
In the current paper, we demonstrate that ERβ regulates a variety of mitochondrial functions, using a murine hippocampal cell line (HT-22) with permanent knockdown of ERβ by RNA interference (siRNA) and primary hippocampal neurons derived from ERβ knock-out mice.
MATERIALS AND METHODS
Chemicals and Reagents
17β-Estradiol was obtained from Steraloids, Inc. (Newport, RI). Tissue culture materials, MitoSOX™, JC1, Slow-Fade Light Antifade reagent, and Alexa Fluor 488 goat anti-rabbit IgG were obtained from Invitrogen. Charcoal-stripped fetal bovine serum was obtained from HyClone (Logan, UT). ERβ (H-150) (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) is a rabbit polyclonal antibody raised against a recombinant protein corresponding to amino acids 1-150 mapping at the amino terminus of ERβ of human origin. ERα (MC20) (Santa Cruz Biotechnology) is a rabbit polyclonal antibody raised against a recombinant protein corresponding to the 20 amino acids mapping at the C terminus of ERα of human origin.
Cell Culture
Murine Hippocampus Cell Line—HT-22 cells (gift from Dr. David Schubert, Salk Institute, San Diego, CA), which are an immortalized murine hippocampal cell line, were maintained in Dulbecco's modified Eagle's medium supplemented with 10% charcoal-stripped fetal bovine serum and 20 μg/ml gentamycin at 37 °C in a humid atmosphere with 5% CO2.
Rat Primary Hippocampal Neuronal Culture—Sprague-Dawley rat embryos (Charles River, Wilmington, MA) of 18 days were externalized under halothane anesthesia. Hippocampi were dissected and harvested in 2 ml of preparation medium (Dulbecco's modified Eagle's medium, 4.5 g/liter glucose, 100 units/ml penicillin, 100 μg/ml streptomycin). The hippocampi were treated with trypsin. The tissue was then washed three times using washing medium (Hanks' medium, 4.5 g/liter glucose, 100 units/ml penicillin, 100 μg/ml streptomycin) and individual cells were isolated by trituration 10 times using three different sizes of fire-polished Pasteur pipettes. The cells were harvested in seeding medium (Dulbecco's modified Eagle's medium, 4.5 g/liter glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mm glutamine, 19% horse serum) and filtered through a 40-μm filter. The hippocampal cells were seeded on 25-mm coverslips. The cells were incubated in neurobasal medium (Dulbecco's modified Eagle's medium, 4.5 g/liter glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mm glutamine, B27) under normal cell culture conditions.
Primary Hippocampal Neuronal Culture from ERβ Knock-out Mice—Breeding pairs of ERβ knock-out mice (homozygous male and heterozygous female) were purchased from Taconic (Hudson, NY) (35). Postnatal day 1 pups were euthanized, hippocampi were dissected separately from each pup, and neuronal cultures were produced as described above. The hippocampal cells were seeded on 25-mm coverslips and incubated in neurobasal medium. The cells were allowed to grow 4-7 days in culture, and mitochondrial membrane potential was analyzed by JC1 assay. For genotyping, total DNA was purified from the tail using a DNeasy blood and tissue kit (Qiagen, Valencia, CA). Three primers were used for PCR with the following sequences: Neo06122, 5′-GCA GCC TCT GTT CCA CAT ACA C-3′; mber-C1-2, 5′-CAT CCT TCA CAG GAC CAG ACA C-3′; mber-F2, 5′-TGG ACT CAC CAC GTA GGC TC-3′. The PCR product underwent electrophoresis on a 2% agarose gel, and the separation patterns were photographed under UV illumination. Homozygous ERβ knockouts were characterized as a single 404 bp band, while heterozygous knock-out mice were seen as two bands with 404 and 356 bp.
Knockdown of ERβ by RNA Interference
siRNA was used to permanently knock down ERβ in HT-22 cells. An ERβ siRNA gene-specific insert was designed that specified a nucleotide sequence from the ERβ cDNA (nucleotides 777-797) (gi:6978816), separated by a short spacer from the reverse complement of the same nucleotide sequence. For the construction of the expression vectors, the following oligonucleotides, that have homology sequence with ERβ, were designed. The selected sequences are homologous for humans, rats, and mice. Thus, the designed ERβ siRNA is effective for cells derived from all three species: 5′-GATCCCCtgtgaaggatgtaaggccttAGAGACATTaaggccttacatccttcacaTTTTTTGAATTCA-3′ and 5′-AGCTTGAATTCAAAAAAtgtgaaggatgtaaggccttAATGTCTCTaaggccttacatccttcacaGGG-3′. The synthetic oligonucleotides for ERβ siRNA were separately annealed, gelpurified, kinased, and ligated to HindIII and BglII-digested pBSH1RNApro to generate expression vectors of pBS-H1-ERβsiRNA (36). The insertion of the synthetic oligonucleotides for ERβ siRNA was confirmed by sequencing using the M13F promoter (SeqWright DNA Technology Services, Houston, TX). The Lipofectamine (Invitrogen) method was used to transfect HT-22 cells with pBS-H1-ERβsiRNA or vector. Following transfection, HT-22 cells were selected for neomycin (G418) resistance (250 μg/ml). G418-resistant clones were isolated and expanded for Western blotting and immunocytochemistry of ERα and ERβ to confirm the knockdown of ERβ.
Immunoblotting
The whole cell protein extracts were combined in Laemmli buffer with β-mercaptoethanol and boiled for 5 min. Mitochondrial samples (30 μg) were separated by 10% Tris-glycine polyacrylamide gel (Gradipore Ltd., Australia) and then transferred to a nitrocellulose membrane (Millipore Corp., Bedford, MA). Lanes containing Kaleidoscope prestained standards (Bio-Rad) were used to evaluate the size of the bands detected with ERα and ERβ. The membranes were blocked for 1 h with 5% nonfat dry milk in PBS and were incubated overnight at 4 °C with ERβ, ERα, or β-actin antibody. The membranes were repeatedly washed with phosphate-buffered saline (PBS) prior to incubation with secondary horseradish peroxidase-conjugated goat anti-rabbit IgG. The blots were developed with an enhanced chemiluminescence reagent.
Immunofluorescence Staining and Confocal Microscopy
Monolayer cells were washed with PBS (pH 7.4) and fixed with cold methanol for 15 min at -20 °C. Cells were rinsed several times in PBS and incubated in ice-cold Triton X-100 for 10 min to permeabilize the cells. Nonspecific sites were blocked for 1 h at room temperature with 5% normal goat serum and 5% bovine serum albumin in PBS. Cells were then incubated with an ERβ antibody overnight at 4 °C. The sections were washed three times for 10 min each in PBS and then incubated with Alexa Fluor 488 goat anti-rabbit IgG for 1 h at room temperature. After washing in PBS three times for 10 min each, the cells were stained with 100 μm 4′,6-diamidino-2-phenylindole for 5 min. Cells were mounted with SlowFade Light Antifade reagent and covered with a coverslip. Samples were analyzed with a Zeiss LSM confocal microscope.
Cell Proliferation Assay
Cells were seeded into 35-mm dishes at a density of 40,000 cells/dish under normal culture conditions. The cultures were lifted at 12, 24, 48, or 60 h after seeding and stained with trypan blue. The cell numbers were calculated using a hemocytometer.
Cell Viability Assay
For viability assays, cells were plated at a density of 5000 cells/well in 96-well plates 24 h before the initiation of experiments. Cells were exposed to various treatments for 18-24 h. For Calcein AM assays, cells were rinsed with 1× PBS (pH 7.4) after treatment, and Calcein AM (25 μm) was added. After an incubation of 15 min at 37 °C, Calcein AM fluorescence was determined at an excitation of 485 nm and an emission of 538 nm using a Biotek FL600 microplate reader (Highland Park, VT). Percentage viability was calculated by normalization of all values to the control group (equal to 100%).
Resting Mitochondrial Membrane Potential Assessment
The fluorescent potentiometric JC-1 dye exists as green monomer at low concentrations or at low Δψm. However, at higher concentrations or higher Δψm, JC-1 forms red fluorescent “J-aggregates” that exhibit a broad excitation spectrum and an emission at ∼590 nm. Thus, the emission of this cyanine dye can be used as a sensitive measure of Δψm. Cells were seeded on 25-mm coverslips and grown for 24 h. Resting Δψm was assessed by JC1 uptake. The coverslip was washed twice and mounted in a cell chamber (ALA Scientific Instruments, Westbury, NY) in HEPES buffer (145 nm NaCl, 3 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm glucose, 10 mm HEPES). Serial confocal images were taken every 5 min with a confocal scanning laser microscope (Zeiss LSM-410) with excitation at 490 nm and emission at 510 and 590 nm before and after JC1 (5 μg/ml) incubation, respectively. For detection of Δψm depolarization, the cells were incubated with JC1 (10 μg/ml) in media for 0.5 h, and then the coverslip was washed twice and mounted in a cell chamber. Serial confocal images were taken every 10 min with a confocal scanning laser microscope (×40 or ×100 objective) with excitation at 490 nm and emission at 510 and 590 nm. Collapse of Δψm was induced by adding hydrogen peroxide to a final concentration of 1 mm. Quantitative analysis of the dynamic change of the Δψm was performed by measurement of the red fluorescence intensity, using the Meta-Morph software (Carl Zeiss).
Measurement of ATP Levels
Experiments were initiated by plating siERβ or vector-transfected HT-22 cells at a density of 1 × 106 cells/well in 12-well plates. Seventy-two h later, cells were exposed to 250 or 500 μm H2O2 for 1 h, at which time cells were still alive and attached to the plate. Cellular ATP levels were quantified using a luciferin- and luciferase-based assay. Cells were rinsed with PBS and lysed with ATP-releasing buffer containing 100 mm potassium phosphate buffer at pH 7.8, 2 mm EDTA, 1 mm dithiothreitol, and 1% Triton X-100; 10 μl of the lysate was taken for protein determination. Another 10 μl of the lysate was added to a Nunc 96-well plate. ATP concentrations in lysates were quantified using an ATP determination kit (Invitrogen) according to the manufacturer's instructions. The 96-well plates were read using a Biotek FL600 microplate reader. A standard curve was generated using solutions of known ATP concentrations. ATP levels were calculated as nm ATP/mg of protein and normalized to levels in untreated control cultures.
Measurement of Mitochondrial Superoxide
Mitochondrial superoxide was determined by a fluorescent dye, MitoSOX™. Cells were seeded on 25-mm coverslips and grown for 24 h before initiation of the experiment. Cells were loaded with MitoSOX™ at a final concentration of 50 μm for 45 min. The coverslip was washed twice and mounted in a cell chamber in HEPES buffer. Mitochondrial reactive oxygen species (ROS) production was induced by adding hydrogen peroxide. Serial confocal images were taken every 10 min with a confocal scanning laser microscope (Zeiss LSM-410) with excitation at 510 nm and emission at 580 nm. Quantitative analysis of the dynamic changes in mitochondrial superoxide was performed by measurement of the red fluorescence intensity, using the Meta-Morph software.
Mitochondria Isolation and Cytochrome c Oxidase Activity Assay
Mitochondria were isolated by differential centrifugation from permanent ERβ knockdown HT-22 cells and vector control cells (15). The absorption of cytochrome c at 550 nm changes with its oxidation state. Cytochrome c oxidase activity was determined by using a cytochrome c oxidase activity assay kit (CYTOC-OX1; Sigma). Briefly, 1 μg of isolated mitochondria preparations was resuspended in enzyme dilution buffer (10 mm Tris-HCl, pH 7.0, containing 250 mm sucrose) and added to 1× assay buffer (10 mm Tris-HCl, pH 7.0, 120 mm KCl). Fifty μl of ferrocytochrome c substrate solution was added to initiate the reaction. The absorption changes at 550 nm were measured immediately using a spectrophotometer for 45 s. The activity of enzyme was calculated using the formula, units/mg protein = (ΔA/min × dilution factor × 1.1 × 1000)/(volume of enzyme × 21.84).
Adeno-associated Virus 2 (AAV-2)-mediated Infection of Constructs into Primary Neurons
An ERβ siRNA expression cassette under a PolIII promoter was cloned into a site flanked by the AAV-2 terminal repeats. Downstream of the siRNA expression cassette, a GFP expression cassette under the PolII promoter was inserted into that construct, resulting in plasmid that can be packaged in AAV-2. Packaging of the ERβ plasmid in a recombinant AAV serotype 2 was followed by the method described in detail by Klein et al. (37). Briefly, 293 cells were transfected with the AAV-siERβ-GFP plasmid in an equimolar ratio with the plasmid pDG, which provides the AAV-2 coat protein genes, and adenovirus genes necessary for helper function in packaging (38). At 48 h after transfection, cells were collected and pelleted. The pellet was resuspended in buffer containing 50 mm Tris and 150 mm NaCl (pH 8) and freeze-thawed three times. The sample was then incubated with 125 units of benzonase (EMD Chemical, Inc., Gibbstown, NJ) per ml of supernatant for 30 min at room temperature. After benzonase treatment, the sample was centrifuged, and the supernatant was applied to 15, 25, 40, and 60% step gradients of iodixinol. The gradient was run at 65,000 rpm in a 70.1 Ti rotor (Beckman, Fullerton, CA) for 1 h at room temperature. The AAV-2 was removed and added to a heparin affinity column (Sigma), and the eluent was washed and concentrated using Amicon Ultra 24 units. The packaging of the AAV-siERβ-GFP construct into virus was confirmed by electron microscopy. The titers of the viral stocks were ∼1012 for siRNA and 1013 for GFP. For AAV-2 infection, primary neurons were treated with 9 × 107 viral particles/50,000 neurons on day 7 in culture. Four days later, cultures were assessed for Δψm and immunocytochemistry.
Statistical Analysis
All data are presented as mean ± S.E. Comparisons between two groups were performed using Student's t test. Comparisons of multiple groups were analyzed using a two-way analysis of variance followed by Tukey's multiple comparison test. For all tests, p < 0.05 was considered significant. All statistical analyses were performed using Prism software (GraphPad Software, San Diego, CA).
RESULTS
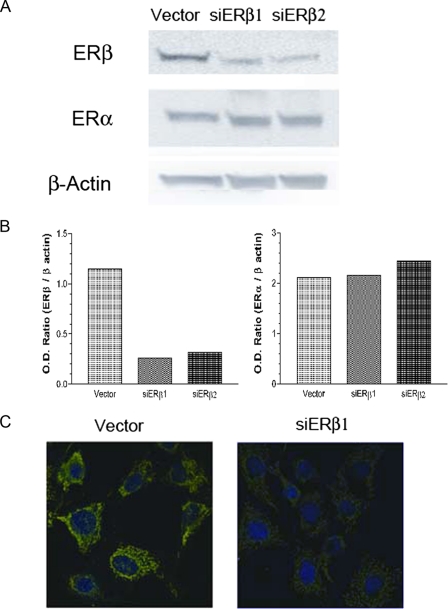
Knockdown of ERβ by RNA Interference in HT-22 Cells—In order to investigate the role of ERβ, we established two permanent siERβ clones together with a vector control clone. The clones were selected against and maintained in G418 medium. All the described experiments were conducted using these permanent cell lines within four passages. In the two permanent ERβ knockdown clones, more than 80% reductions in ERβ expression were achieved without significant changes of ERα expression, as indicated by Western blots (Fig. 1, A and B). To further confirm the immunoblotting results, we conducted immunocytochemistry on these siERβ and vector clones. In vector clones, ERβ was nearly exclusively localized in mitochondria, whereas the mitochondrial immunoreactivity for ERβ was substantially reduced in ERβ knockdown cells (Fig. 1C). siERβ1 was used to investigate the effects of ERβ knockdown in HT-22 cells, siERβ2 had similar effects in all experiments performed (data not shown).
FIGURE 1.
A, Western blots of ERβ and ERα in ERβ knockdown and vector-transfected HT-22 cells. Lane 1, vector-transfected cells; lane 2, siERβ1 knock-down cells, clone 1; lane 3, siERβ2 knock-down cells, clone 2. B, quantitative analysis of the ERα and ERβ Western blots. C, immunocytochemistry staining of ERβ in vector-transfected (Vector) and siERβ1-transfected HT-22 cells. The merged images are presented, and ERβ is shown in green, and the nucleus is shown in blue (4′,6-diamidino-2-phenylindole; DAPI).
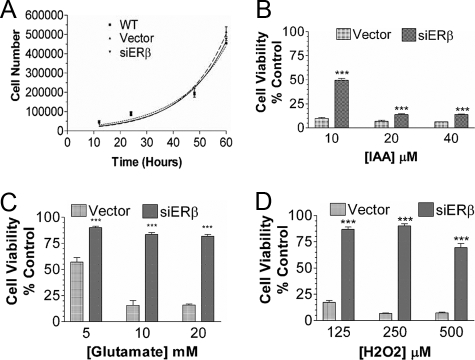
Effect of ERβ Knockdown on Cell Vulnerability—We studied the effects of ERβ knockdown on cell proliferation and cell vulnerability to various insults. There was no significant difference in the proliferation rates between the vector and siERβ clones (Fig. 2A) or between siERβ clones and wild type cells. However, ERβ knockdown significantly reduced cell death induced by iodoacetic acid treatment (Fig. 2B). Even more profound phenotype changes, characterized as an increase of resistance to glutamate and H2O2 toxicity, were demonstrated in ERβ knockdown cells (Fig. 2, C and D). Similar results were observed in repeated experiments in both permanent clones of the ERβ knockdown cell lines.
FIGURE 2.
A, effect of ERβ knockdown on HT-22 cell proliferation. No significant difference in cell proliferation was seen between wild type HT-22 cells (WT), vector-transfected HT-22 cells (Vector), and ERβ knockdown HT-22 cells (siERβ). B, effect of ERβ knockdown on cell vulnerability to various oxidative insults, including iodoacetic acid (IAA), glutamate (C), and H2O2 (D). Substantial cell death was induced by iodoacetic acid, glutamate, and H2O2 in vector cells, which was significantly diminished in ERβ knockdown cells. Results are shown as mean ± S.E. from 6-8 experiments/group. ***, p < 0.001 versus vector.
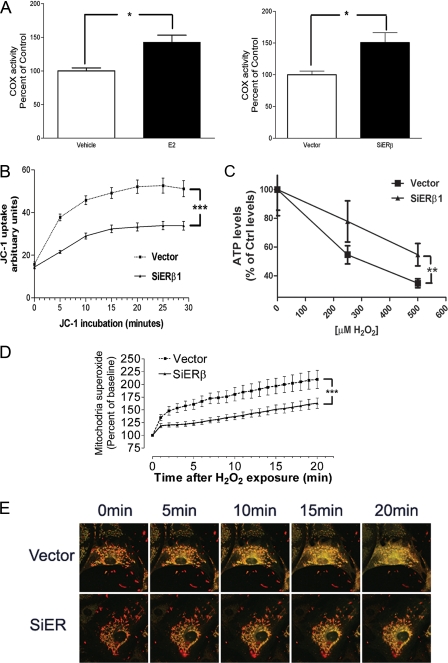
Effect of ERβ Knockdown on Mitochondrial Function—We studied the effect of ERβ knockdown on mitochondrial cytochrome c oxidase activity. As is evident from Fig. 3A, a significant increase of cytochrome c oxidase activity was seen in the ERβ knockdown cell line, compared with the vector-transfected cell line. We further determined the effect of estrogen treatment on mitochondrial cytochrome c oxidase activity in wild type HT-22 cells. HT-22 cells were treated with 17β-estradiol at the concentration of 100 nm or vehicle (DMSO) for 24 h, mitochondria were separated, and cytochrome c oxidase activity was determined. Compared with vehicle, a significant increase of cytochrome c oxidase activity was indicated in wild type HT-22 cells upon estrogen treatment (Fig. 3A).
FIGURE 3.
A, right panel, 17β-estradiol (E2) treatment increases cytochrome c oxidase activity in HT-22 cells. *, p < 0.05 versus vehicle. Left panel, quantitative analysis of cytochrome c oxidase activity in vector and siERβ cells. *, p < 0.05 versus vector. B, quantitative analysis of dynamic change of JC-1 uptake in vector-transfected (Vector) and ERβ knockdown HT-22 cells (siERβ). n = 16. ***, p < 0.001 versus vector. C, H2O2-induced decrease of ATP production in vector-transfected and ERβ knockdown HT-22 cells. n = 6. **, p < 0.01 versus vector. D, quantitative analysis of dynamic changes in H2O2-induced mitochondrial superoxide production, measured by MitoSox fluorescence, in vector and siERβ HT-22 cells. n = 16. ***, p < 0.001 versus vector. E, effect of ERβ on H2O2-induced Δψm collapse in HT-22 cells. Confocal microscopy images show the same field of cells viewed before (0 min) and at 5, 10, 15, and 20 min after H2O2 (1 mm) exposure in vector and siERβ HT-22 cells.
We studied the effect of ERβ on mitochondrial membrane potential (Δψm), using a lipophilic cationic dye, JC-1. Initially, the permanent ERβ knockdown HT-22 or permanent vector control cells were incubated with JC-1 (5 μg/ml), and the uptake of JC-1 into the mitochondria was followed. As is evident from Fig. 3B, the JC-1 uptake rate was much slower in ERβ knockdown HT-22 cells than that in the vector clone, suggesting that these mitochondria in ERβ knockdown cells have a lower resting Δψm. We further assessed ATP production under oxidative stress in siERβ and vector clones. Vector clone exhibited a robust decline in ATP production in response to H2O2 treatment, whereas siERβ cells showed an attenuated decline in ATP levels in response to H2O2 insult (Fig. 3C). Mitochondria are well known as a predominant cellular source of ROS under both physiological and pathological conditions. We assessed the role of ERβ on mitochondrial superoxide production following oxidative insults. ERβ knockdown HT-22 cells were more resistant to H2O2-induced increases in superoxide production (Fig. 3D). We also examined the effects of ERβ knockdown on oxidative stress-induced Δψm collapse in HT-22 cells. ERβ knockdown HT-22 or vector control cells were first labeled with JC-1 probe (10 μg/ml) for 0.5 h, and then Δψm collapse was induced by the treatment of H2O2 (1 mm). After exposure to H2O2, aggregated JC1 (in red) became monomeric and fluoresced green upon mitochondrial depolarization, as evidenced by the increase of yellow (merging of red and green) in the time series images. The oxidative stress-induced mitochondrial depolarization was minimized in ERβ knockdown HT-22 cells (Fig. 3E).
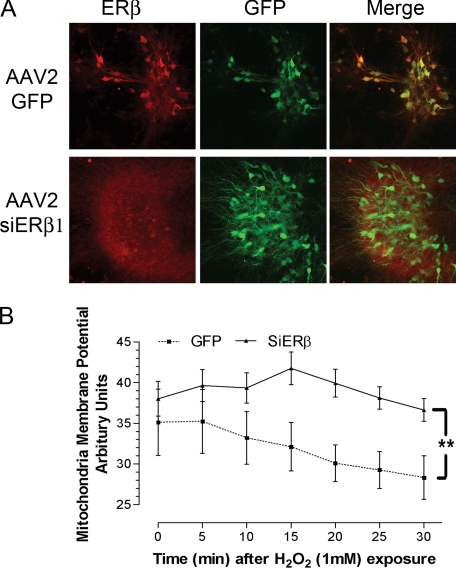
Knockdown of ERβ in Primary Hippocampal Neurons and Its Effect on Mitochondrial Membrane Potential—We assessed the effect of ERβ on Δψm in rat primary hippocampal neurons. Primary hippocampal neurons were infected with AAV-2, containing the ERβ siRNA-GFP construct or a control GFP construct. The effectiveness of infection and ERβ knockdown was confirmed by double staining of neurons with GFP and ERβ. GFP immunoreactivity was identified in both AAV2-GFP and AAV-2 ERβ primary hippocampal neurons. In AAV-2 GFP-infected neurons, a perinuclear staining of ERβ was observed, whereas the staining was absent in AAV-2 ERβ siRNA-GFP-infected neurons, which demonstrated a high effectiveness of infection and loss of ERβ immunoreactivity in the ERβ siRNA-GFP construct-infected neurons (Fig. 4A). We further determined the effect of ERβ knockdown on mitochondrial membrane potential in the primary hippocampal neurons. Infected neurons were loaded with JC-1 and were then treated with H2O2 to induce a prompt Δψm collapse. As shown in Fig. 5B, H2O2 caused the expected prompt and progressive decline in Δψm in control-infected primary neurons. In contrast, no significant effects of the high concentration of H2O2 were seen through 30 min in the ERβ siRNA-GFP construct-infected primary neurons (Fig. 4B). These data indicate that infection of primary neurons with a construct that reduces mitochondrial ERβ causes a resistance of mitochondria to prooxidant insults, which is consistent with the phenotype change in ERβ knockdown HT-22 cells.
FIGURE 4.
A, immunocytochemistry staining of ERβ (red) and GFP (green) in the AAV-2 GFP construct-infected (AAV2-GFP) and AAV-2 ERβ siRNA-GFP construct-infected (AAV2-siERβ1) primary hippocampal neuronal cultures, used for Δψm determination. B, quantitative analysis of dynamic changes in Δψm under H2O2 exposure in AAV-2 GFP construct-infected (GFP) and AAV-2 ERβ siRNA-GFP construct-infected (SiERβ) primary hippocampal neurons. A significant reduction of Δψm was observed in GFP-infected neurons after H2O2 insult but not in siERβ-AAV2-transfected neurons. Results are shown as mean ± S.E. for n = 7. *, p < 0.05 versus GFP.
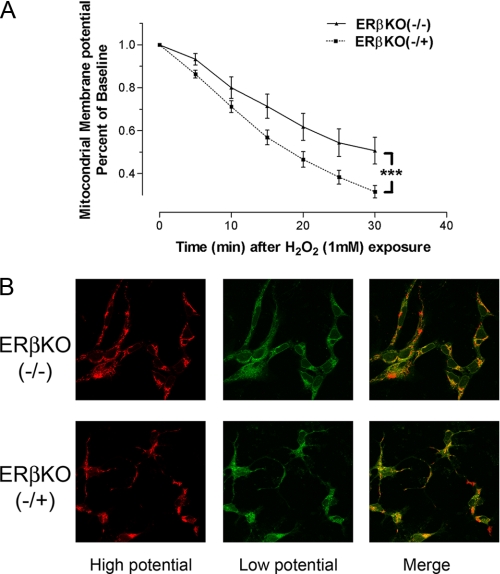
FIGURE 5.
A, quantitative analysis of dynamic changes in mitochondria membrane potential (Δψm) under H2O2 exposure in hippocampal neuronal cultures derived from homozygous (ERβKO(-/-)) and heterozygous (ERβKO(-/+)) ERβ knock-out mice. Δψm collapse was evaluated by JC-1 fluorescence intensity. ***, p < 0.001. B, representative confocal microscopy image after 30 min of H2O2 exposure.
Change of Mitochondrial Membrane Potential after Oxidative Insult in Primary Hippocampal Neurons Derived from ERβ Knock-out Mice—We assessed the effect of ERβ knock-out on Δψm in primary hippocampal neurons derived from homozygous or heterozygous ERβ knock-out mice. Primary hippocampal neurons were first labeled with JC-1 probe (10 μg/ml) for 0.5 h, and then Δψm collapse was induced with H2O2 (1 mm). Primary neurons derived from the homozygous ERβ knock-out mice were significantly more resistant to pro-oxidant insult-induced Δψm collapse than neurons from littermate heterozygous ERβ knock-out mice (Fig. 5A). Representative confocal images are shown in Fig. 5B.
DISCUSSION
The role of estrogens in growth and in the physiology of reproductive tract tissues and organs in females has been recognized for more than half a century (19). Great efforts have been invested to decipher the mechanisms underlying estrogen's physiological function. Two decades ago, the cloning of the first ER, now known as ERα (5), simplified estrogen action to one hormone, one receptor, and one system. However, a decade ago, the identification of the second ER (7), known as ERβ, has fundamentally changed the concept of estrogens and opened a new chapter in estrogen functions. Estrogens are now recognized as affecting systems beyond female reproduction. Estrogens have been found to modulate growth of tissues, bone integrity, cardiovascular tissue, the immune system, and nervous system physiology as well as male physiology (19, 39). Further, given the widespread role of estrogen in human physiology, it is not surprising that estrogen is also implicated in numerous diseases, including but not limited to various types of cancer, osteoporosis, neurodegenerative diseases, cardiovascular disease, insulin resistance, lupus erythematosus, endometriosis, and obesity (19).
In addition to exerting classical genomic actions on the expression of nuclear estrogen-responsive genes, estrogens have substantial effects on mitochondrial structure, biogenesis, and function, which were documented decades ago and recently reassessed (for reviews, see Refs. 18, 27, and 40). Four years ago, we and several other groups independently identified the localization of ERβ in the mitochondria in various types of cells, suggesting that ERβ may function as a mitochondrial component. In the present study, we investigated the role of ERβ in mitochondrial functions via the knockdown of ERβ in a murine hippocampal cell line (HT-22) through RNA interference technique. The knockdown of ERβ was confirmed by both immunoblotting and immunocytochemistry. Given the high specificity of RNA interference, the substantial reduction of ERβ immunoreactivity in the mitochondria of HT-22 cell provides further evidence to support that ERβ is localized in mitochondria. In the current studies, the phenotype change of mitochondria functions of HT-22 cells with permanent ERβ knockdown was investigated. We observed a substantial reduction in cellular vulnerability to various oxidative insults. This phenotype change was repeatedly demonstrated in two permanent clones of ERβ knockdown HT-22 cell when compared with both permanent vector-transfected and wild type HT-22 clones. We believe that the phenotype change can be attributed to the modification of mitochondrial function induced by the knockdown of ERβ.
Mitochondria produce the majority of cellular energy in the form of ATP by the process of oxidative phosphorylation. Furthermore, mitochondrial oxidative phosphorylation generates the majority of ROS. Δψm is an electrochemical gradient, which is generated by transportation of electrons, born of NAD+ and succinate in the tricarboxylic acid cycle through electron transport chain components. Mitochondria also play a critical role in the initiation of both necrotic and apoptotic cell death (41). Collapse of the Δψm is one of the early indicators of cell death. We determined the effects of ERβ on resting Δψm, using a lipophilic cationic dye, JC-1, a potentiometric dye that accumulates in mitochondria in accordance with the Nernst equation. The knockdown of ERβ led to a mitochondrial phenotype change similar to mitochondrial uncoupling, a lower Δψm condition in which electron transport is disconnected from the production of ATP. Investigations of endogenous uncoupling proteins have provided evidence to support a beneficial role of mitochondrial uncoupling. Consistently, exposure of HT-22 cells to an uncoupling agent, carbonylcyanide-p-trifluoromethoxyphenylhydrazone, resulted in a reduction of cell death induced by glutamate (data not shown). We further determined the effect of ERβ knockdown on oxidative stress-induced Δψm collapse. As predicted, oxidative insults induced a prompt collapse of Δψm in vector-transfected HT-22 cells, which was minimized in the ERβ knockdown HT-22 cells. Consistently, ERβ knockdown HT-22 cells were more resistant to oxidative stress-induced ATP depletion. It is noteworthy that the basal ATP levels in the ERβ knockdown HT-22 cells were lower than that in vector-transfected cells (data not shown). The lower basal ATP production levels in ERβ knockdown cells parallels the lower resting Δψm in these cells, since Δψm is the direct driving force for ATP production (41). Oxidative phosphorylation in mitochondria is the major endogenous source of ROS. We then determined mitochondrial superoxide production under oxidative stress. Consistent with mitochondrial uncoupling, a reduction of mitochondrial superoxide generation under oxidative stress was seen in ERβ knockdown HT-22 cells.
We speculated that the functions of ERβ, as a mitochondrial component, were not cell-specific, since localization of ERβ in mitochondria has been identified in various cell types, including but not limited to primary neurons, rat and human primary cardiomyocytes, human breast cancer cell lines, immortal human breast epithelial cells, human lens epithelial cell lines, human osteosarcoma cells, hepatocarcinoma cells, and human sperm. We knocked down ERβ expression in primary hippocampal neurons by RNA interference. Consistent with the phenotype change of HT-22 cells, ERβ knockdown in primary neurons rendered them resistant to oxidative stress-induced Δψm collapse. Furthermore, a similar mitochondrial phenotype change was also demonstrated in primary hippocampal neurons derived from ERβ knock-out mice.
Our findings may provide important insight into the widely demonstrated neuroprotective effect of estrogens. The abrupt decrease in ovarian estrogens at the time of menopause may contribute to neuronal vulnerability (42-44), and the present data suggest that this is due to a state of unliganded ERβ. The cytoprotective actions of estrogens are demonstrated in a variety of cells, including neurons, glia, vascular endothelium (42, 45), and cardiomyocytes (46). The pancellular protective effect of estrogens and the profound effects of estrogens on a wide range of cell signaling indicate that estrogens could target very upstream signals induced by a variety of insults, converging upon regulation of mitochondrial function (40, 45, 47). Indeed, estrogens have fundamental effects on mitochondria that are similar to those seen with ERβ knockdown. Estrogen has been shown to reduce mitochondrial Ca2+ influx in response to mitochondrial toxins, oxidative stress, or glutamate excitotoxicity (48-50). We have shown that estrogen maintains ATP production in the presence of mitochondrial toxins as well as pro-oxidants that would otherwise reduce ATP levels (48, 49). Estrogens and a variety of nonfeminizing estrogen analogues demonstrate protection of Δψm from collapse induced by mitochondrial overload, mitochondrial toxin, or prooxidants (48, 49). In the present study, we demonstrated that knockdown of ERβ renders decreased vulnerability of cells to various insults, which could be achieved by estrogen treatment under normal ERβ expression circumstances. In addition, our study demonstrated an increase of cytochrome c oxidase activity in ERβ knockdown cells, which was also indicated in wild type cells upon estrogen treatment. These data suggest that estrogens cause a loss of function of ERβ, which attenuates the negative function of mitochondrial ERβ. As such, the depletion of estrogens could uncover the default function of mitochondrial ERβ, which causes an increased vulnerability to oxidative damage in the organism.
Our findings are also significant in view of cancer development and progression. Localization of ERβ has been identified in breast cancer cells (16, 18). Various studies have shown decreased expression of ERβ in many cancers, including breast, ovary, colon, prostate, and glioma (51-54). Thus, ERβ has been suggested as a cancer brake. The proapoptotic properties of ERβ have been suggested in various cells. In the absence of ERβ, there was a decrease in apoptosis in colonic epithelium (55). In both prostate cancer cells and breast cancer cells, the introduction of ERβ caused a strong inhibition of cell proliferation due to the increase of apoptosis through the mitochondrial pathway (56-58). The ERβ-selective agonist, 2,3-bis(4-hydroxyphenyl)-propoinitrile, inhibited cell growth and induced apoptosis in a mammary epithelial cell line (34). Our study suggests that down-regulation of ERβ in cancer cells could decrease vulner-ability of mitochondria to various oxidative insults and hence decrease apoptosis and promote cancer progression.
In the present study, knockdown of ERβ in a hippocampal cell line did not change the proliferation rate. On the other hand, signs of hyperproliferation of bladder epithelium, epithelium of the dorsal prostate, the coagulation glands, and the urethra have been identified in ERβ knock-out mice, which indicates that ERβ may have some kind of inhibitory effect against hyperproliferation (9). The inconsistent effect of ERβ on proliferation in hippocampal cells and epithelium suggests that the antiproliferative effect of ERβ could be cell type-dependent.
The crystal structure of ERβ has been well demonstrated. ERβ shares a highly conserved structure with other nuclear receptors, such as ERα. Although ERα and ERβ have nearly identical DNA-binding domains, increasing evidence has indicated that they regulate the expression of a totally distinct set of genes (59, 60). Currently, most of the studies have been focused on the nuclear transcription regulation. Consistently, most of the genes modified in ERβ knock-out mice are mitochondrial structural proteins related to oxidative phosphorylation (60). We predict that this distinction could be partly due to different compartmentation of ERα and ERβ. Indeed, recent studies demonstrated that ERβ is localized in the mitochondrial matrix, hence enabled its access to the mitochondrial genome (18). Therefore, both the ERβ structure and matrix localization provide ERβ the capacity to regulate mitochondrial gene expression. Indeed, recently published studies have found that ERβ could directly interact with mitochondrial genes to modulate cytochrome c oxidase subunit expression (18). Consistently, we have demonstrated that ERβ is involved in the regulation of cytochrome c oxidase. This notion was further supported by the results generated in the primary hippocampal neurons derived from ERβ knock-out mice. The ERβ knock-out mice used in the present study were created by insertion of a neomycin resistance gene into exon 3, corresponding to the coding sequence for the DNA binding domain of ERβ (35). Splicing variants missing sequences that encode the essential first zinc finger of the DNA binding domain have been found in these ERβ knock-out mice (35). The consistency of the mitochondrial phenotype change obtained by ERβ knockdown in HT-22 cells, rat hippocampal neurons, and ERβ knock-out hippocampal neurons derived from these ERβ knock-out mice suggests that ERβ could function as a mitochondrial transcription factor and that it is involved in mitochondrial membrane potential maintenance. In addition, many other possibilities could also contribute to the phenotype change seen when ERβ levels are decreased. The mitochondrial genome only codes 13 of the mitochondrial structural proteins, with all others coded by the nuclear genome. Mitochondrial function change could affect nuclear gene expression through mitochondria nucleus cross-talking. Thus, it would not be a surprise that nuclear actions could also play a role in the dramatic mitochondrial phenotype change induced by ERβ knockdown.
In summary, our data demonstrate that ERβ knockdown leads to a mild mitochondrial uncoupling phenotype characterized by a lower resting Δψm. These cells are resistant to provocative insults, as evident by their resistance to oxidative stressor-induced Δψm depolarization, ATP depletion, and ROS generation. These data suggest that ERβ could function as a mitochondrial vulnerability factor that is involved in mitochondrial membrane potential maintenance and mitochondrial vulnerability, potentially through a mitochondria transcription-dependent mechanism.
This work was supported, in whole or in part, by National Institutes of Health, NINDS, Grants NS054651 (to S. Y.), NS054687 (to S. Y.), AG10485 (to J. W. S.), AG22550 (to J. W. S.), and AG27956 (to J. W. S.). This work was also supported by a grant from the American Heart Association, Inc., Texas Affiliate (to S. Y.).
Footnotes
The abbreviations used are: ER, estrogen receptor; ERα, estrogen receptor α; ERβ, estrogen receptor β; Δψm, mitochondrial membrane potential; siRNA, small interfering RNA; PBS, phosphate-buffered saline; ROS, reactive oxygen species; AAV-2, adeno-associated virus 2; GFP, green fluorescent protein.
References
- 1.Stevenson, J. C. (2000) J. Steroid Biochem. Mol. Biol. 74 387-393 [DOI] [PubMed] [Google Scholar]
- 2.Sherwin, B. B. (1999) J. Psychiatry Neurosci. 24 315-321 [PMC free article] [PubMed] [Google Scholar]
- 3.Compston, J. E. (2001) Physiol. Rev. 81 419-447 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed, S. A., Hissong, B. D., Verthelyi, D., Donner, K., Becker, K., and Karpuzoglu-Sahin, E. (1999) Environ. Health Perspect. 107 Suppl. 5, 681-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene, G. L., Gilna, P., Waterfield, M., Baker, A., Hort, Y., and Shine, J. (1986) Science 231 1150-1154 [DOI] [PubMed] [Google Scholar]
- 6.Mosselman, S., Polman, J., and Dijkema, R. (1996) FEBS Lett. 392 49-53 [DOI] [PubMed] [Google Scholar]
- 7.Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S., and Gustafsson, J. A. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 5925-5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tata, J. R. (2002) Nat. Rev. Mol. Cell Biol. 3 702-710 [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson, J. A. (1999) J. Endocrinol. 163 379-383 [DOI] [PubMed] [Google Scholar]
- 10.Levin, E. R. (2005) Mol. Endocrinol. 19 1951-1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen, B., Akama, K., Alves, S., Brake, W. G., Bulloch, K., Lee, S., Li, C., Yuen, G., and Milner, T. A. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 7093-7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milner, T. A., McEwen, B. S., Hayashi, S., Li, C. J., Reagan, L. P., and Alves, S. E. (2001) J. Comp. Neurol. 429 355-371 [PubMed] [Google Scholar]
- 13.Milner, T. A., Ayoola, K., Drake, C. T., Herrick, S. P., Tabori, N. E., McEwen, B. S., Warrier, S., and Alves, S. E. (2005) J. Comp. Neurol. 491 81-95 [DOI] [PubMed] [Google Scholar]
- 14.Herrick, S. P., Waters, E. M., Drake, C. T., McEwen, B. S., and Milner, T. A. (2006) Brain Res. 1121 46-58 [DOI] [PubMed] [Google Scholar]
- 15.Yang, S. H., Liu, R., Perez, E. J., Wen, Y., Stevens, S. M., Jr., Valencia, T., Brun-Zinkernagel, A. M., Prokai, L., Will, Y., Dykens, J., Koulen, P., and Simpkins, J. W. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 4130-4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, J. Q., Delannoy, M., Cooke, C., and Yager, J. D. (2004) Am. J. Physiol. 286 E1011-E1022 [DOI] [PubMed] [Google Scholar]
- 17.Cammarata, P. R., Chu, S., Moor, A., Wang, Z., Yang, S. H., and Simpkins, J. W. (2004) Exp. Eye Res. 78 861-871 [DOI] [PubMed] [Google Scholar]
- 18.Chen, J. Q., Yager, J. D., and Russo, J. (2005) Biochim. Biophys. Acta 1746 1-17 [DOI] [PubMed] [Google Scholar]
- 19.Ascenzi, P., Bocedi, A., and Marino, M. (2006) Mol. Aspects Med. 27 299-402 [DOI] [PubMed] [Google Scholar]
- 20.Cowley, S. M., Hoare, S., Mosselman, S., and Parker, M. G. (1997) J. Biol. Chem. 272 19858-19862 [DOI] [PubMed] [Google Scholar]
- 21.Pettersson, K., Grandien, K., Kuiper, G. G., and Gustafsson, J. A. (1997) Mol. Endocrinol. 11 1486-1496 [DOI] [PubMed] [Google Scholar]
- 22.Ogawa, S., Eng, V., Taylor, J., Lubahn, D. B., Korach, K. S., and Pfaff, D. W. (1998) Endocrinology 139 5070-5081 [DOI] [PubMed] [Google Scholar]
- 23.Cowley, S. M., and Parker, M. G. (1999) J. Steroid Biochem. Mol. Biol. 69 165-175 [DOI] [PubMed] [Google Scholar]
- 24.Yi, P., Bhagat, S., Hilf, R., Bambara, R. A., and Muyan, M. (2002) Mol. Endocrinol. 16 1810-1827 [DOI] [PubMed] [Google Scholar]
- 25.Lubahn, D. B., Moyer, J. S., Golding, T. S., Couse, J. F., Korach, K. S., and Smithies, O. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 11162-11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtis Hewitt, S., Couse, J. F., and Korach, K. S. (2000) Breast Cancer Res. 2 345-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felty, Q., and Roy, D. (2005) J. Carcinog. 4 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moats, R. K., II, and Ramirez, V. D. (1998) Biol. Reprod. 58 531-538 [DOI] [PubMed] [Google Scholar]
- 29.Mehra, R. D., Sharma, K., Nyakas, C., and Vij, U. (2005) Brain Res. 1056 22-35 [DOI] [PubMed] [Google Scholar]
- 30.Cammarata, P. R., Flynn, J., Gottipati, S., Chu, S., Dimitrijevich, S., Younes, M., Skliris, G., and Murphy, L. C. (2005) Exp. Eye Res. 81 165-175 [DOI] [PubMed] [Google Scholar]
- 31.Solakidi, S., Psarra, A. M., and Sekeris, C. E. (2005) Biochim. Biophys. Acta 1745 382-392 [DOI] [PubMed] [Google Scholar]
- 32.Solakidi, S., Psarra, A. M., Nikolaropoulos, S., and Sekeris, C. E. (2005) Hum. Reprod. 20 3481-3487 [DOI] [PubMed] [Google Scholar]
- 33.Fried, G., Andersson, E., Csoregh, L., Enmark, E., Gustafsson, J. A., Aanesen, A., and Osterlund, C. (2004) Eur. J. Neurosci. 20 2345-2354 [DOI] [PubMed] [Google Scholar]
- 34.Helguero, L. A., Faulds, M. H., Gustafsson, J. A., and Haldosen, L. A. (2005) Oncogene 24 6605-6616 [DOI] [PubMed] [Google Scholar]
- 35.Krege, J. H., Hodgin, J. B., Couse, J. F., Enmark, E., Warner, M., Mahler, J. F., Sar, M., Korach, K. S., Gustafsson, J. A., and Smithies, O. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 15677-15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar, S. N., and Das, H. K. (2003) J. Neurochem. 87 333-343 [DOI] [PubMed] [Google Scholar]
- 37.Klein, R. L., Hamby, M. E., Sonntag, C. F., Millard, W. J., King, M. A., and Meyer, E. M. (2002) Methods 28 286-292 [DOI] [PubMed] [Google Scholar]
- 38.Zolotukhin, S., Potter, M., Zolotukhin, I., Sakai, Y., Loiler, S., Fraites, T. J., Jr., Chiodo, V. A., Phillipsberg, T., Muzyczka, N., Hauswirth, W. W., Flotte, T. R., Byrne, B. J., and Snyder, R. O. (2002) Methods 28 158-167 [DOI] [PubMed] [Google Scholar]
- 39.Koehler, K. F., Helguero, L. A., Haldosen, L. A., Warner, M., and Gustafsson, J. A. (2005) Endocr. Rev. 26 465-478 [DOI] [PubMed] [Google Scholar]
- 40.Nilsen, J., and Brinton, R. D. (2004) Curr. Drug Targets CNS Neurol. Disord. 3 297-313 [DOI] [PubMed] [Google Scholar]
- 41.Kroemer, G., and Reed, J. C. (2000) Nat. Med. 6 513-519 [DOI] [PubMed] [Google Scholar]
- 42.Behl, C. (2002) Nat. Rev. Neurosci. 3 433-442 [DOI] [PubMed] [Google Scholar]
- 43.Wise, P. M., Smith, M. J., Dubal, D. B., Wilson, M. E., Rau, S. W., Cashion, A. B., Bottner, M., and Rosewell, K. L. (2002) Recent Prog. Horm. Res. 57 235-256 [DOI] [PubMed] [Google Scholar]
- 44.Wise, P. M. (2006) Neuroscience 138 831-83516310320 [Google Scholar]
- 45.Yang, S. H., Liu, R., Perez, E. J., Wang, X., and Simpkins, J. W. (2005) Curr. Drug Targets CNS Neurol. Disord. 4 169-177 [DOI] [PubMed] [Google Scholar]
- 46.Patten, R. D., and Karas, R. H. (2006) Trends Cardiovasc. Med. 16 69-75 [DOI] [PubMed] [Google Scholar]
- 47.Simpkins, J. W., Wang, J., Wang, X., Perez, E., Prokai, L., and Dykens, J. A. (2005) Curr. Drug Targets CNS Neurol. Disord. 4 69-83 [DOI] [PubMed] [Google Scholar]
- 48.Wang, J., Green, P. S., and Simpkins, J. W. (2001) J. Neurochem. 77 804-811 [DOI] [PubMed] [Google Scholar]
- 49.Wang, X., Simpkins, J. W., Dykens, J. A., and Cammarata, P. R. (2003) Invest. Ophthalmol. Vis. Sci. 44 2067-2075 [DOI] [PubMed] [Google Scholar]
- 50.Nilsen, J., and Brinton, R. D. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 10506-10511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Signoretti, S., and Loda, M. (2001) Am. J. Pathol. 159 13-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bardin, A., Boulle, N., Lazennec, G., Vignon, F., and Pujol, P. (2004) Endocr. Relat. Cancer 11 537-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Batistatou, A., Stefanou, D., Goussia, A., Arkoumani, E., Papavassiliou, A. G., and Agnantis, N. J. (2004) J. Cancer Res. Clin. Oncol. 130 405-410 [DOI] [PubMed] [Google Scholar]
- 54.Lazennec, G. (2006) Cancer Lett. 231 151-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wada-Hiraike, O., Imamov, O., Hiraike, H., Hultenby, K., Schwend, T., Omoto, Y., Warner, M., and Gustafsson, J. A. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 2959-2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazennec, G., Bresson, D., Lucas, A., Chauveau, C., and Vignon, F. (2001) Endocrinology 142 4120-4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng, J., Lee, E. J., Madison, L. D., and Lazennec, G. (2004) FEBS Lett. 566 169-172 [DOI] [PubMed] [Google Scholar]
- 58.Treeck, O., Pfeiler, G., Horn, F., Federhofer, B., Houlihan, H., Vollmer, A., and Ortmann, O. (2007) Mol. Cell. Endocrinol. 264 50-60 [DOI] [PubMed] [Google Scholar]
- 59.Katzenellenbogen, B. S., and Katzenellenbogen, J. A. (2000) Breast Cancer Res. 2 335-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Lone, R., Knorr, K., Jaffe, I. Z., Schaffer, M. E., Martini, P. G., Karas, R. H., Bienkowska, J., Mendelsohn, M. E., and Hansen, U. (2007) Mol. Endocrinol. 21 1281-1296 [DOI] [PubMed] [Google Scholar]