Abstract
Group IVA cytosolic phospholipase A2 (cPLA2α) is regulated by phosphorylation and calcium-induced translocation to membranes. Immortalized mouse lung fibroblasts lacking endogenous cPLA2α (IMLF-/-) were reconstituted with wild type and cPLA2α mutants to investigate how calcium, phosphorylation, and the putative phosphatidylinositol 4,5-bisphosphate (PIP2) binding site regulate translocation and arachidonic acid (AA) release. Agonists that elicit distinct modes of calcium mobilization were used. Serum induced cPLA2α translocation to Golgi within seconds that temporally paralleled the initial calcium transient. However, the subsequent influx of extracellular calcium was essential for stable binding of cPLA2α to Golgi and AA release. In contrast, phorbol 12-myristate 13-acetate induced low amplitude calcium oscillations, slower translocation of cPLA2α to Golgi, and much less AA release, which were blocked by chelating extracellular calcium. AA release from IMLF-/- expressing phosphorylation site (S505A) and PIP2 binding site (K488N/K543N/K544N) mutants was partially reduced compared with cells expressing wild type cPLA2α, but calcium-induced translocation was not impaired. Consistent with these results, Ser-505 phosphorylation did not change the calcium requirement for interfacial binding and catalysis in vitro but increased activity by 2-fold. Mutations in basic residues in the catalytic domain of cPLA2α reduced activation by PIP2 but did not affect the concentration of calcium required for interfacial binding or phospholipid hydrolysis. The results demonstrate that Ser-505 phosphorylation and basic residues in the catalytic domain principally act to regulate cPLA2α hydrolytic activity.
Group IVA cytosolic phospholipase A2 (cPLA2α)3 specifically hydrolyzes arachidonic acid (AA) from the sn-2-position of membrane phospholipids in response to diverse cellular stimuli (1-5). In releasing AA, it performs a fundamental role in regulating signaling for a multitude of lipid-mediated pathways that control many important cell processes. Leukotrienes and prostaglandins are products of AA metabolism via 5-lipoxygenase and cyclooxygenase pathways, respectively (6). These metabolites regulate diverse processes in normal and diseased tissues, making it important to tightly regulate the release of free AA through cPLA2α. cPLA2α is widely expressed in mammalian tissues, reflecting its central role in regulating lipid mediator production in diverse cell types.
cPLA2α is regulated post-translationally by submicromolar levels of calcium and by phosphorylation (1, 2, 7). Agonist-induced increase in the [Ca2+]i leads to a loading of the C2 domain with calcium, and this mediates cPLA2α translocation to Golgi, endoplasmic reticulum, and nuclear envelope to access substrate (8-12). cPLA2α has multiple phosphorylation sites in the catalytic domain. Analysis of cPLA2α expressed in baculovirus-infected Sf9 cells revealed constitutive phosphorylation of Ser-454, Ser-437, and Ser-505 and phosphorylation on Ser-727 in response to okadaic acid (13). In mammalian cells, cPLA2α is phosphorylated on Ser-505, Ser-727, and Ser-515 by mitogen-activated protein kinases (MAPKs), MAPK-activated protein kinase MNK1 (or a related kinase), and calcium/calmodulin-dependent kinase II (CamKII), respectively (14-19). Phosphorylation of Ser-505 and Ser-727 are functionally important for regulating cPLA2α-mediated AA release from stimulated cells (14, 17). It has recently been shown that phosphorylation of cPLA2α on Ser-515 and Ser-505 is required for AA release in vascular smooth muscle cells stimulated with norepinephrine (20).
Phosphorylation of cPLA2α and physiological increases in [Ca2+]i synergistically promote the full activation of cPLA2α for releasing AA (21-23). Phosphorylation of cPLA2α on Ser-505 increases its catalytic activity (14, 15, 24); however, the role of phosphorylation in regulating calcium-induced translocation in cells has not been resolved. It has been reported that phosphorylation of cPLA2α on Ser-505 enhances the phospholipid binding affinity at low physiological calcium levels in vitro and in cells (25). This is consistent with another study showing that the inability of cPLA2α phosphorylation site mutants to release AA is overcome by inducing supraphysiological [Ca2+]i (17). However, it has previously been shown that cPLA2αS505A translocates to membrane in response to calcium ionophore, although it releases less AA (26). In addition, a direct comparison of wild type cPLA2α and phosphorylation site mutants by time lapse imaging demonstrated similar translocation properties in response to physiological increases in calcium induced by ATP (27).
Calcium binding to the cPLA2α C2 domain increases its affinity for membrane through hydrophobic interactions (28-32). This positions the catalytic domain for interaction with the membrane by hydrophobic and electrostatic mechanisms (33). A tryptophan (Trp-464) on the membrane binding face of the catalytic domain stabilizes cPLA2α and prolongs membrane binding after decreases in [Ca2+]i (34). A patch of basic residues (Lys-488, Lys-541, Lys-543, and Lys-544) in the cPLA2α catalytic domain is the site of interaction with phosphatidylinositol 4,5-bisphosphate (PIP2), which increases catalytic activity in vitro (33, 35-37). However, the role of these basic residues in regulating translocation in cells stimulated with physiological agonists has not been investigated.
The goal of this study is to investigate the role of phosphorylation and basic residues in the catalytic domain in regulating cPLA2α. We compare the interfacial binding and kinetic properties of phosphorylated and mutant forms of cPLA2α in vitro as a function of calcium concentration with the behavior of these enzymes in a cellular reconstitution model. By expressing wild type and mutant forms of cPLA2α in lung fibroblasts lacking cPLA2α, we investigated the functional role of phosphorylation and the PIP2 binding site in regulating calcium-dependent cPLA2α translocation and AA release without interference of endogenous wild type enzyme.
EXPERIMENTAL PROCEDURES
Materials—1-Palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine (PAPC), brain PIP2 (major species is 1-stearoyl-2-arachidonyl-PIP2), and dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt) were from Avanti Polar Lipids. Electrospray ionization analysis of PIP2 showed that it was not contaminated with dephosphorylated species. [14C]PAPC was from PerkinElmer Life Sciences (catalog number NEC-765). Purified water was prepared with a Milli-Q system (Millipore). [5,6,8,9,11,12,14,15-3H]AA (100 Ci/mmol) was from Amersham Biosciences. Inhibitors SB203580, U0126, wortmannin, KN93, cycloheximide (CHX), and GF109203X were purchased from Sigma. Dulbecco's modified Eagle's medium (DMEM) was from BioWhittaker. Penicillin, streptomycin, and l-glutamine were from Invitrogen. Fetal bovine serum was from Irvine Scientific. Fatty acid-free bovine serum albumin (BSA) and Fraction V BSA were from Sigma. Protease inhibitor tablets were from Roche Applied Science. The plasmid isolation kit was from Qiagen, and the site-directed mutagenesis kit was from Stratagene. FuraRed-AM was from Invitrogen, and probenecid was from Sigma. Antibody to giantin was from Abcam, and Texas Red secondary antibody was from Jackson Immunoresearch. Phosphospecific MAPK and cPLA2α antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and Cell Signaling Technology, respectively. Phorbol 12-myristate 13-acetate (PMA) potassium salt was purchased from Alexis Biochemicals. Mouse serum was from Atlanta Biologicals.
Preparation of cPLA2α—Full-length wild type and mutant forms of human cPLA2α containing the affinity peptide YHHHHHH fused to the C-terminal Ala-749 were prepared by a modification of the procedure described previously (38) (supplemental material). cPLA2α concentrations were determined from the absorbance at 280 nm (ε280 = 0.827 mg-1 ml-1 cm-1) (38). Mutagenesis of the coding region in the baculovirus transfer plasmid was carried out first by deletion of the desired region and then by insertion of the mutated region. Both steps were carried out using the QuikChange kit (Stratagene). Full coding regions were sequenced to verify the products.
Preparation of Dephosphorylated cPLA2α—Purified recombinant cPLA2α was dephosphorylated with potato acid phosphatase (PAP) by a modification of the published procedure (24) (supplemental material). cPLA2α-PAP was shown by phosphoamino analysis to lack phosphoserine (supplemental material).
Preparation of Phosphorylated Forms of cPLA2α—cPLA2α-505P and cPLA2α-515P were prepared by a modification of the previously reported methods (16, 24) (supplemental material). cPLA2α-505P/727P was prepared from activated platelets based on the method reported by Kramer (39). Full details are given as supplemental material along with data showing that the phosphorylated forms are stoichiometrically phosphorylated.
Vesicle Binding Assays—All phospholipids were stored in CHCl3 in Teflon septum-lined screw cap vials under argon at -20 °C. Concentrations of phospholipids were determined by the standard phosphate assay with ammonium molybdate(VI) tetrahydrate (for PIP2, the weight specified by the manufacturer was used). Stock solutions of phospholipids were mixed in a polypropylene microcentrifuge tube, and most of the solvent was removed (leaving ∼50 μl) with a stream of argon with the tube placed in a 37 °C bath. The tube was placed in a SpeedVac concentrator under vacuum for 1-2 h. All phospholipid mixtures used for binding studies contained 0.5 mol % lissamine rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine. Vesicles were prepared by freeze-thawing the lipid suspension (typically 4-6 mm phospholipid) in 10 mm MOPS, pH 7.2, 176 mm sucrose followed by extrusion through two 0.2-μm polycarbonate membranes (VWR catalog number 110606) using a LiposoFast device (Avestin), as described previously (40). A small aliquot of lipid suspension prior to extrusion was removed for fluorimetric analysis (see below). Extruded vesicles were submitted to a prespin procedure (1 h, 100,000 × gaverage, 21 °C) in a 1.5-ml polyallomar centrifuge tube (Beckman catalog number 357448). The supernatant was removed with a pipette and discarded, and the vesicles in the pellet were resuspended by gentle mixing (gentle up and down passage with a Pipetman, 8-10 times) in buffer A (10 mm MOPS, pH 7.2, 100 mm KCl, 0.5 mm EGTA). A small aliquot of vesicles was diluted into 50 mm Tris-HCl, pH 8.0, 1% sodium cholate and submitted to fluorometry (excitation, 550 nm; emission, 590 nm). The fluorescence value was compared with that of an aliquot of the vesicle suspension prior to extrusion to calculate the percentage yield of phospholipid, and this was used with the phospholipid concentrations in the original stock solutions to obtain the total phospholipid concentration of final vesicle suspension. Vesicles were used on the same day of preparation (stored at room temperature).
cPLA2α binding reactions were prepared in 1.5-ml polyallomar microcentrifuge tubes and contained buffer A with 0.5 mg/ml BSA containing various amounts of free calcium (see below), phospholipid (added from the calibrated, prespun stock in buffer A), and 50 ng of cPLA2α in a total volume of 1 ml. Samples were centrifuged at 100,000 × gaverage at 21 °C for 1 h, and two 100-μl aliquots of supernatant were submitted to duplicate radiometric cPLA2α assays (see below).
Buffer A containing various amounts of free calcium (0-5 μm) were prepared using EGTA/CaCl2 mixtures and calcium-binding fluorophores, as described (41) (supplemental material). The amount of cPLA2α in the supernatant above pelleted vesicles was determined using a modification of the previously reported radiometric assay (41) (supplemental material).
An interesting feature of adding PIP2 to PAPC vesicles that we observed is that when the total phospholipid concentration was dropped from 200 to 25 μm, most of the PIP2 was found in the aqueous phase above vesicles that were pelleted by ultracentrifugation (see supplemental material for more details). This presumably reflects the relative high aqueous phase solubility of PIP2 compared with phospholipids that lack highly polar, phosphorylated inositol headgroups.
cPLA2α Kinetic Studies—Vesicles contained [14C]PAPC at a specific activity of 2.7 Ci/mol (made by mixing [14C]PAPC (50 Ci/mol) with PAPC) and other phospholipids as noted. Phospholipids were mixed together as CHCl3 solutions, and solvent was removed as described above. Buffer A was added to the dry lipid film, and vesicles were prepared by freeze-thawing followed by extrusion as described above. Extruded vesicles were diluted typically 20-fold into buffer A containing 0.5 mg/ml BSA and various concentrations of calcium (see above) to give a volume of 0.1 ml. Tubes were prewarmed in a 37 °C bath for 5 min, and reactions were started by adding a 2-μl aliquot of enzyme stock containing 200-250 ng of cPLA2α (enzyme stocks were made by fresh dilution into buffer A containing 1 mg/ml BSA). Reactions were quenched after 2 min, and released 14C-labeled AA was measured after extraction and silica chromatography as previously described (41).
Production of DNA Constructs and Recombinant Adenovirus—Enhanced green fluorescent protein (EGFP)-cPLA2α was inserted into AdEasy vector (Qbiogene) and titered as described previously (42). DNAs encoding monomeric (A206K) wild type enhanced yellow fluorescent protein (EYFP)- and enhanced cyan fluorescent protein (ECFP)-cPLA2α were inserted into the pVQAd5CMVK-NpA shuttle plasmid (ViraQuest, Inc.). The EYFP-cPLA2α construct was used to generate by site-directed mutagenesis the following mutants: S505A, S727A, S515A, S505A/S727A, S505A/S727A/S515A, K488N/K543N/K544N, and D43N. Constructs were confirmed by sequencing. Adenoviruses were generated by ViraQuest.
Cell Culture and AA Release Assay—Lung fibroblasts were isolated from wild type (MLF+/+) and cPLA2α knock-out (MLF-/-) mice, and SV40-transformed MLF (IMLF) were generated as described previously (42). IMLF+/+ and IMLF-/- were plated at 1.25 × 104 cells/cm2 in 250 μl of DMEM containing 10% fetal bovine serum, 0.1% nonessential amino acids, 1 mm sodium pyruvate, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.29 mg/ml glutamine (growth medium) in 48-well plates. After 18 h in 5% CO2 at 37 °C, cells were washed with and incubated in 100 μl of serum-free and antibiotic-free DMEM containing 0.1% BSA (stimulation medium) and adenoviruses. After 1.5 h, 150 μl of stimulation medium containing 0.2 μCi/ml [3H]AA was added to each well. After 26 h, cells were washed twice, and fresh stimulation medium was added. When inhibitors were tested, they were added (10 μm SB203580, 10 μm U0126, 1 μm wortmannin, 10 μm KN93, 10 μm GF109203X, and 15 ng/μl CHX) at various times before stimulation with 100 nm PMA or 10% mouse serum. Culture medium was collected after stimulation and centrifuged for 10 min at 15,000 rpm, and radioactivity was determined by scintillation counting. Cells were scraped into 50 μl of 0.1% Triton X-100 containing protease inhibitors, and the lysates were used to determine the total cellular radioactivity and to determine expression levels via immunoblotting. The amount of AA release into the culture medium was calculated as a percentage of the total radioactivity (cells plus medium) in each well.
Microscopy—IMLF+/+ and IMLF-/- were plated at 1.25 × 104 cells/cm2 in 250 μl of growth medium in glass-bottomed MatTek plates and infected with adenoviruses for expression of wild type and mutant cPLA2α as described above. After 26 h, cells used for calcium analysis were loaded with 5 μm Fura-Red-AM for 30 min at room temperature in the presence of 0.02% pleuronic acid, washed, and incubated for 30 min in Hanks' balanced salt solution containing 25 mm HEPES, pH 7.4, and 2 mm probenecid. For some microscopy, cells were fixed for 15 min with 3% paraformaldehyde in PBS containing 3% sucrose, permeabilized for 30 min with 0.1% Triton X-100 in PBS, and blocked in 5% fetal bovine serum in PBS for 1 h. Golgi was labeled using anti-giantin primary antibody (1:200 in blocking solution, 1 h), followed by a Texas Red-conjugated secondary antibody (1:100 in blocking solution; 1 h). Microscopy was conducted on an inverted Zeiss 200 M microscope driven by Intelligent Imaging Innovations Inc. (3I) software (Slidebook 4.1). Fluorescence data were calculated by subtracting background fluorescence, and correcting for differential bleaching at each wavelength. Calcium ratios were corrected for background fluorescence values.
Immunoblotting—For Western blotting, cell lysates were prepared in ice cold buffer containing 50 mm Hepes, pH 7.4, 150 mm sodium chloride, 1.5 mm magnesium chloride, 10% glycerol, 1% Triton X-100, 1 mm EGTA, and protease inhibitors. Lysates were centrifuged at 15,000 × g for 10 min at 4 °C, and protein concentration was determined using the bicinchoninic acid reagent. Lysates were diluted in Laemmli buffer and boiled for 5 min at 100 °C. Proteins were separated on 10% SDS-polyacrylamide gels, transferred to nitrocellulose, and blocked for 1 h in Tris-buffered saline containing 0.25% Tween 20 and 5% nonfat dry milk. Nitrocellulose membranes were incubated overnight with a 1:5,000 dilution of antiserum to total cPLA2α, 1:1,000 of phosphospecific cPLA2α antiserum, or 1:1,000 of anti-p38 or anti-phospho-ERK antibodies. Antibodies were diluted in blocking buffer. Immunoreactive protein was detected using the Amersham Biosciences anti-rabbit secondary antibody and ECL system.
RESULTS
Regulation of cPLA2α in IMLF+/+ Stimulated with Serum and PMA—To study the role of calcium and phosphorylation in regulating cPLA2α, we chose to use serum and PMA as agonists. They both trigger AA release and activate extracellular regulated protein kinases (ERKs) in fibroblasts (43, 44). Serum increases [Ca2+]i, but PMA has been reported to have little effect on [Ca2+]i (45). This allows us to compare the role of phosphorylation sites and the PIP2 binding residues in cPLA2α at different [Ca2+]i. In addition, cPLA2α phosphorylation may be functionally more important at low [Ca2+]i (17, 25). Serum is a complex pathophysiological fluid produced in response to tissue injury as a result of platelet aggregation. It is relevant to lung fibroblasts, since it leaks into the lung during acute lung injury and contributes to fibroproliferation (46, 47). Our first approach was to define the characteristics of endogenous cPLA2α stimulation by serum and PMA in IMLF+/+. A time course of AA release by cPLA2α in IMLF+/+ was conducted. When stimulated with 10% serum, AA was rapidly released in the first 10 min and then slowed through 80 min (Fig. 1A, right). Serum stimulated high levels of AA release that were 10% of total cell-associated radiolabeled AA at 10 min and reached 20% at 80 min. In contrast, PMA stimulated a slower mobilization of AA that increased linearly through 80 min (Fig. 1A, left). Compared with serum, PMA stimulated much less AA release, which was less than 1% of total cell-associated radiolabeled AA at 10 min and reached 4-5% at 80 min.
FIGURE 1.
Effect of PMA and serum on [Ca2+]i and AA release in IMLF+/+. A, [3H]AA-labeled IMLF+/+ were stimulated with 10% serum (right) or 100 nm PMA (left) and [3H]AA release was determined at given times and compared with unstimulated (US) cells. [3H]AA released into the medium was measured and expressed as a percentage of the total cellular radioactivity in each well. The results are the average of three experiments ± S.E. for PMA (left) and two experiments ± S.D. for serum (right). B, live cell calcium imaging of IMLF+/+ loaded with the fluorescent calcium indicator FuraRed-AM was measured after the addition (arrows) of serum, PMA, or vehicle (DMSO). Calcium changes are shown in individual cells stimulated with 10% serum without (panel 1) or with (panel 2) a 15-min preincubation in EGTA. Panel 3 shows calcium oscillations in cells stimulated with 100 nm PMA for 30 min. Panel 4 illustrates calcium changes in cells stimulated with PMA with an EGTA preincubation. Panel 5 shows control cells (incubated without EGTA) using DMSO vehicle in place of PMA. Calcium changes were determined by correcting for background fluorescence values from each cell and calculated as a ratio of bound to unbound calcium fluorescence intensities (F403/F470). Data are presented relative to time 0 (RT/R0), and starting (RT/R0) for each cell is set at 1. Each line represents data from an individual cell. Graphs are representative of a minimum of three independent experiments.
IMLF+/+ were stimulated with serum and PMA to determine the effect on [Ca2+]i. Serum caused a rapid increase in [Ca2+]i at 30 s (Fig. 1B, panel 1). The initial calcium transient decreased by 60 s, and this was followed by a sustained calcium increase characterized by low amplitude oscillations that continued for at least 30 min (data not shown). The sustained phase but not the initial spike in calcium was eliminated by incubation of the cells in medium containing EGTA to chelate extracellular calcium (Fig. 1B, panel 2). The results demonstrate that serum stimulation of IMLF+/+ induces an initial increase in [Ca2+] from intracellular stores followed by capacitative influx of extracellular calcium. In contrast, stimulation of cells with PMA did not induce an initial high amplitude increase in [Ca2+]i but promoted low amplitude oscillations in many cells that lasted at least 30 min (Fig. 1B, panel 3). The oscillations did not occur in cells incubated in medium containing EGTA (Fig. 1B, panel 4) or in control cells treated with DMSO vehicle (Fig. 1B, panel 5).
Activation of ERKs and p38 plays a role in regulation of cPLA2α-mediated AA release by many agonists in part by phosphorylation of cPLA2α on Ser-505. Western blot analysis using phospho-ERK antibodies indicated that serum and PMA rapidly activated ERKs by 0.5-1 min in IMLF+/+ (Fig. 2A). The use of antibodies that detected total ERK protein indicated equal loading of samples (data not shown). Serum also activated p38 (Fig. 2A); however, no detectable activation of p38 occurred during stimulation with PMA (not shown). Additional experiments demonstrated that activation of ERKs in response to serum and PMA and activation of p38 in response to serum were evident up to 80 min after stimulation (data not shown).
FIGURE 2.
ERK and p38 activation by serum and PMA in IMLF+/+. Cell lysates of unstimulated (US) IMLF+/+ or cells stimulated with 10% serum or 100 nm PMA were prepared at given times after stimulation. Activation of ERKs or p38 (A) or phosphorylation of cPLA2α on Ser-505 (B) was determined by Western blotting using phosphospecific antibodies. Sample loading was determined using total ERK antibodies (data not shown) or antibodies to total cPLA2α (B). Results are representative of three independent experiments. C, Western blots of lysates of serum-stimulated IMLF-/- expressing either wild type ECFP-cPLA2α (WT) or EYFP-cPLA2αS505A were probed with antibodies to total cPLA2α or phosphospecific antibodies to cPLA2α phosphorylated on Ser-505. The Western blot confirms the specificity of the phosphospecific antibodies for cPLA2α phosphorylated on Ser-505.
Phosphorylation of cPLA2α on Ser-505 was determined by Western blotting using phosphospecific antibody (Fig. 2B). cPLA2α was constitutively phosphorylated in unstimulated IMLF+/+, and phosphorylation was not significantly increased by serum or PMA. cPLA2α remained phosphorylated on Ser-505 for up to 80 min in unstimulated cells and in cells treated with serum or PMA (data not shown). Phosphorylation of cPLA2α on Ser-505 causes a decrease in electrophoretic mobility (gel shift) of cPLA2α on SDS-polyacrylamide gels (21). In unstimulated IMLF+/+, most of the cPLA2α was gel-shifted (data not shown), consistent with results of Western blots using phosphospecific antibody. This is not unexpected, since we found that cPLA2α is constitutively phosphorylated on Ser-505 in immortalized cell lines (27).4 EGFP-cPLA2α expressed in IMLF-/- was also constitutively phosphorylated on Ser-505 when assayed by Western blotting with phosphospecific antibody (data not shown). The specificity of the phosphospecific antibody was determined by comparing lysates of IMLF-/- expressing either wild type ECFP-cPLA2α or the phosphorylation site mutant ECFP-cPLA2αS505A. Western blots of cell lysates probed with antibody to total cPLA2α demonstrated that the wild type and mutant enzymes were expressed at equivalent levels (Fig. 2C). However, using the phosphospecific antibody, a signal for cPLA2α was only observed in lysates of IMLF-/- expressing wild type cPLA2α but not the S505A mutant, confirming that the antibody is specific for cPLA2α phosphorylated on Ser-505 (Fig. 2C).
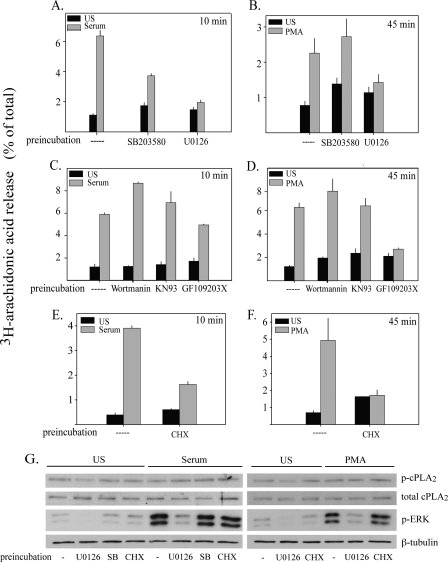
Inhibitors were used to determine if the activation of p38 and ERKs plays a role in cPLA2α-mediated AA release in IMLF+/+. Inhibition of p38 by SB203580 and of MEK1 by U0126, to block ERK activation, decreased serum-induced AA release by 64 and 91%, respectively (Fig. 3A). AA release stimulated by PMA, which does not activate p38, was not affected by SB203580, as expected. The MEK1 inhibitor blocked AA release stimulated by PMA by 83% (Fig. 3B). The results demonstrate that the MEK1/ERK pathway regulates cPLA2α-mediated AA release in response to serum and PMA, and p38 contributes to the regulation of AA release in serum-stimulated cells.
FIGURE 3.
MAPK activation is required for AA release in IMLF+/+ in response to serum and PMA. [3H]AA-labeled IMLF+/+ were preincubated with 10 μm SB203580 (15 min), 10 μm U0126 (15 min), 1 μm wortmannin (30 min), 10 μm KN93 (30 min), 10 μm GF109203X (60 min), or 15 ng/μl CHX (30 min) or no treatment followed by stimulation with serum for 10 min (A, C, and E) or PMA for 45 min (B, D, and F). [3H]AA released into the medium was measured and presented as a percentage of total cellular [3H]AA. G, IMLF+/+ were preincubated with SB203580, U0126, or CHX or no treatment followed by stimulation with serum for 10 min or PMA for 45 min, as described above. Cell lysates of unstimulated (US) or stimulated IMLF+/+ were analyzed by Western blotting to determine activation of ERKs (p-ERK) or phosphorylation of cPLA2α on Ser-505 (p-cPLA2α) using phosphospecific antibodies. Sample loading was determined using antibodies to total cPLA2α or antibodies to β-tubulin. Data represent the average of three experiments ± S.E. (A-D), representative of three independent experiments in duplicate ± S.D. (E and F) or a representative Western blot of three independent experiments (G).
In order to elucidate signaling cascades that might be involved in the activation of cPLA2α by serum and PMA, inhibitors of kinases implicated in cPLA2α activation in previous studies were employed. CaMKII regulates cPLA2α through a MAP kinase-dependent pathway involving phosphorylation of cPLA2α on Ser-515 in vascular smooth muscle cells (16, 20, 48). The CaMKII inhibitor KN93 had no affect on AA release stimulated by serum or PMA (Fig. 3, C and D). Polyphosphoinositides, including phosphatidylinositol 3,4,5-trisphosphate, activate cPLA2α in vitro and may regulate cPLA2α-mediated AA release (36). However, AA release in IMLF+/+ was not blocked by the phosphatidylinositol 3-kinase inhibitor, wortmannin (1 μm) in response to serum or PMA stimulation (Fig. 3, C and D). The ability of PMA to stimulate AA release implicates a role for PKC in regulating cPLA2α activation. To determine if PKC activation is required for serum-stimulated AA release, the general PKC inhibitor GF109203X was tested. PMA activation of cPLA2α in IMLF+/+ was inhibited by 95% by GF109203X (Fig. 3D). Serum-stimulated AA release, however, was not significantly inhibited by blocking PKC (Fig. 3C).
Several studies have shown that protein synthesis is required for AA release (49-51). To determine if protein synthesis is necessary for serum- and PMA-stimulated AA release, IMLF+/+ were treated with CHX before stimulation. CHX blocked both serum- and PMA-induced AA release by 71 and 98%, respectively, indicating that nascent protein production is needed for cPLA2α activation in IMLF+/+ (Fig. 3, E and F). CHX does not prevent translocation of cPLA2α to the Golgi in IMLF+/+ stimulated with serum (data not shown). IMLF+/+ were treated with the inhibitors (CHX, U0126, and SB203580) that blocked AA release to determine their effect on phosphorylation of Ser-505 or, particularly relevant for CHX, the effect on total cPLA2α protein levels. As shown by Western blotting (Fig. 3G), CHX had no effect on the total amount of cPLA2α in IMLF+/+. CHX also did not affect the phosphorylation of cPLA2α on Ser-505 that is observed in unstimulated IMLF+/+ or in serum- or PMA-stimulated IMLF+/+. U0126 inhibited AA release by 80-90% in response to PMA and serum, and this correlated with the inhibition of ERK activation by PMA and serum in IMLF+/+ (Fig. 3G). There was a low level of constitutive activation of ERKs in IMLF+/+ not treated with serum or PMA that was inhibited by U0126. This correlated with a decrease in the constitutive phosphorylation of cPLA2α on Ser-505 observed in unstimulated IMLF+/+. However, neither U0126 nor SB203580 inhibited the phosphorylation of Ser-505 in IMLF+/+ treated with PMA or serum. The results suggest that MAPK activation regulates cPLA2α-mediated AA release by a novel mechanism in addition to Ser-505 phosphorylation.
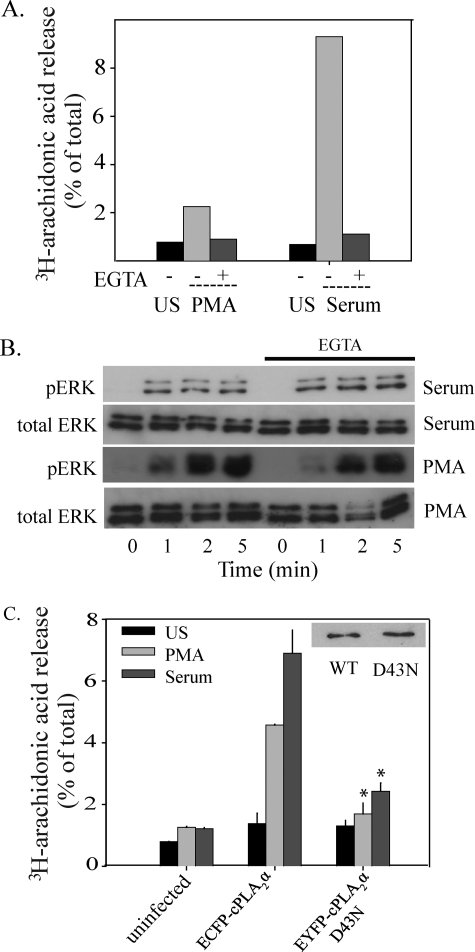
Reconstitution of AA Release in IMLF-/- by Expression of cPLA2α—To study mechanisms for cPLA2α activation by PMA and serum in more detail, wild type and mutant cPLA2α were expressed in IMLF-/- using adenovirus. Expression of fluorescent protein-tagged cPLA2α in cells lacking endogenous cPLA2α enabled us to directly compare the effect of specific mutations on AA release and on translocation of cPLA2α to Golgi when stimulated with PMA and serum. AA release, in response to serum and PMA, increases in proportion to levels of fluorescent protein-tagged cPLA2α expression up to a saturation point (Fig. 4, A and B). To determine the role of extracellular calcium on AA release, IMLF-/- expressing EGFP-cPLA2α were incubated in medium containing EGTA. Serum- and PMA-induced AA release was blocked to nearly basal levels by chelating extracellular calcium (Fig. 5A). Since activation of the MEK1/ERK pathway is required for cPLA2α-mediated AA release in response to serum and PMA in IMLF+/+, we determined if chelation of calcium by EGTA affected the activation of MAPK pathways. Western blots illustrated that chelation of extracellular calcium with EGTA does not block p38 or ERK activation (Fig. 5B).
FIGURE 4.
Reconstitution of [3H]AA release by IMLF-/- in response to serum and PMA by expression of cPLA2 α. IMLF-/- were incubated for 26 h with different amounts of adenoviruses for expression of EGFP-cPLA2α (A) and ECFP-cPLA2α (B). The adenovirus stock for expression of EGFP-cPLA2α has a lower titer than the virus stock for ECFP-cPLA2α and therefore required higher volumes to achieve similar levels of expression. Cells labeled with [3H]AA were washed and then left unstimulated (US) or treated with serum for 10 min (A) or with PMA for 45 min (B). [3H]AA released into the medium was determined and presented as a percentage of the total cellular radioactivity. Western blots of lysates collected from each well show the corresponding levels of cPLA2α expression. Results are representative of three independent experiments conducted in duplicate.
FIGURE 5.
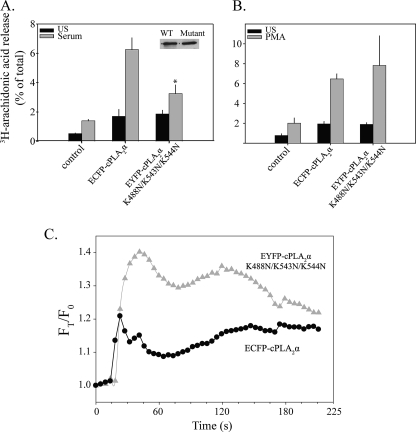
Calcium is required for AA release but not ERK and p38 activation in response to serum and PMA. A, IMLF+/+ were preincubated with 10 mm EGTA for 15 min and left unstimulated (US) or treated with serum for 10 min or PMA for 45 min. [3H]AA released into the medium was measured and presented as a percentage of total cellular radioactivity. B, IMLF+/+ were preincubated with or without EGTA and stimulated with serum or PMA for the indicated times. Immunoblots were conducted using equal protein per lane and probed with phosphospecific antibodies against phospho-ERK and phospho-p38 or with antibody to total ERKs to determine sample loading. C, [3H]AA-labeled IMLF-/- expressing either wild type ECFP-cPLA2α, EYFP-cPLA2αD43N or neither (uninfected) were stimulated with 10% serum (10 min) or 100 nm PMA (45 min), and [3H]AA release was compared with unstimulated cells. Wells with matching expression levels of cPLA2α wild type and mutant (inset) determined by Western blot analysis were used for [3H]AA release determination. Results are representative of a minimum of three independent experiments conducted in duplicate. The release of [3H]AA by the mutant was significantly less (p < 0.05) than by wild type cPLA2α (WT), as indicated (*).
To confirm a role for a functional C2 domain in cPLA2α-mediated AA release stimulated by serum and PMA, parallel cultures of IMLF-/- expressing matching amounts of wild type ECFP-cPLA2α and the C2 domain mutant EYFP-cPLA2αD43N were compared. Asp-43 interacts with both Ca2+ ions that bind the C2 domain and plays a critical role in mediating cPLA2α membrane binding (28, 52, 53). AA release from IMLF-/- expressing the D43N mutant was significantly attenuated in response to serum and PMA, indicating that calcium binding to the C2 domain is required for cPLA2α-mediated AA release in response to these agonists (Fig. 5C).
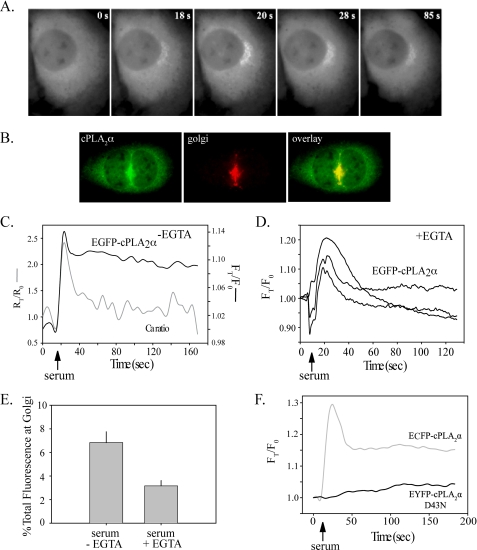
Translocation of cPLA2α in Response to Serum and PMA Stimulation—Live cell imaging of IMLF-/- expressing EGFP-cPLA2α demonstrated that translocation of cPLA2α to the perinuclear region occurred within seconds of serum stimulation (Fig. 6A). In response to serum, EGFP-cPLA2α co-localized with the Golgi marker giantin (Fig. 6B). Most IMLF are bi- or multinucleated, a characteristic of SV40 transformation (54). We observed that the Golgi apparatus is often sandwiched between the nuclei (Figs. 6B; see also Fig. 7, A and B). Translocation of EGFP-cPLA2α to Golgi occurred in parallel with the sharp increase in [Ca2+]i induced by serum (Fig. 6C). There was cell to cell variation in the amount of EGFP-cPLA2α that dissociated from the Golgi after the initial spike in calcium subsided, but most cells showed a partial (20-30%) decrease in Golgi fluorescence by 60 s after serum addition (Fig. 6C). Chelating extracellular calcium with EGTA did not block the initial calcium spike induced by serum (see Fig. 1B). However, in most cells, we did not observe translocation of cPLA2α to Golgi when cells were incubated in medium with EGTA. In a few cells incubated with EGTA, only a transient increase in cPLA2α translocation to Golgi occurred, and fluorescence returned to base line by 60 s, as shown for three cells in Fig. 6D. In contrast, in cells incubated without EGTA, a larger proportion of EGFP-cPLA2α remained associated with Golgi throughout the 160-s time course (Fig. 6C). In response to serum, ∼7% of the total cellular EGFP-cPLA2α translocated to Golgi in cells incubated without EGTA, and this decreased to 3% in the few cells where translocation was visually evident when incubated with EGTA (Fig. 6E). The data indicate that the sustained increase in [Ca2+]i induced by serum is required for stable binding of cPLA2α to Golgi and AA release. Dual imaging of IMLF-/- co-expressing wild type ECFP-cPLA2α and EYFP-cPLA2αD43N illustrated that the D43N mutant did not translocate upon serum stimulation (Fig. 6F). Thus, a sustained increase in [Ca2+]i is required for the C2 domain-dependent translocation to Golgi and AA release in serum-stimulated IMLF.
FIGURE 6.
Serum-stimulated cPLA2α translocation to Golgi correlates with [Ca2+]i increase and is dependent on a functional C2 domain. A, IMLF-/- expressing EGFP-cPLA2α were incubated in phenol red free DMEM and stimulated with 10% serum. Live cell images were collected every 3 s using an FITC filter and a ×40 oil immersion objective. Images are representative of 10 individual experiments. B, IMLF-/- expressing EGFP-cPLA2α were incubated in phenol red-free DMEM, fixed 2 min after stimulation with serum, and then probed with anti-giantin primary antibody and Texas Red secondary antibody to visualize Golgi. C, IMLF-/- expressing EGFP-cPLA2α were loaded with FuraRed-AM, and live cell images were collected using FITC, F403, and F470 filters after stimulation with serum (arrow). D, IMLF-/- expressing EGFP-cPLA2α were incubated in medium containing EGTA, and then images were collected using a FITC filter after stimulation with serum (arrow). E, translocation data from C and D were analyzed to determine the percentage of EGFP-cPLA2α bound to Golgi at the peak of serum-induced translocation (∼30 s) in cells incubated with and without extracellular EGTA. Translocation data were calculated based on average fluorescence intensity of EGFP-cPLA2α on the Golgi in each cell. Values were corrected for background fluorescence and differential bleaching at each wavelength through the duration of the imaging and expressed relative to time 0 (FT/F0). Calcium ratios (F403/F470) were calculated and corrected for background fluorescence and expressed relative to time 0 (RT/R0). Graphs are representative of 10 cells from three independent experiments. F, IMLF-/- co-expressing ECFP-cPLA2α and EYFP-cPLA2αD43N were stimulated with serum, and translocation was determined as described in A. Data are presented relative to time 0 (FT/F0). The graph is representative of 15 cells from three independent experiments.
FIGURE 7.
PMA stimulates ECFP-cPLA2α translocation to Golgi. A, IMLF-/- expressing ECFP-cPLA2α were incubated in phenol red-free DMEM and stimulated with PMA. Live cell images were collected every 30 s using a CFP filter and a ×40 oil immersion objective. Images are representative of 10 individual cells. B, IMLF-/- expressing EGFP-cPLA2α were incubated in phenol red-free DMEM, fixed 15 min after stimulation with 100 nm PMA, and then probed with anti-giantin primary antibody and Texas Red secondary antibody to visualize Golgi. C, IMLF-/- co-expressing wild type ECFP-cPLA2α and EYFP-cPLA2αD43N were stimulated with PMA (arrow), and images were collected using both a CFP and YFP filter. Translocation data were calculated based on average fluorescence intensity of a mask of the Golgi in each cell. Values were calculated by subtracting background fluorescence and correcting for differential bleaching at each wavelength through the duration of the imaging. Data are presented relative to time 0 (FT/F0). Results are representative of five independent experiments and 10 individual cells.
We next investigated the effect of PMA on translocation of ECFP-cPLA2α. In response to PMA stimulation, ECFP-cPLA2α translocated to the perinuclear region (Fig. 7A), where it co-localized with giantin at the Golgi (Fig. 7B). In contrast to serum, translocation of ECFP-cPLA2α was not detected until 8-10 min after PMA addition and reached maximal translocation ∼15 min after stimulation (Fig. 7, A and C). Dual imaging of ECFP-cPLA2α and the C2 domain mutant EYFP-cPLA2αD43N co-expressed in IMLF-/- revealed little translocation of the mutant (Fig. 7C). Collectively, the results suggest that the oscillations induced by PMA from the influx of extracellular calcium, promote the C2 domain-dependent translocation of cPLA2α to Golgi and AA release.
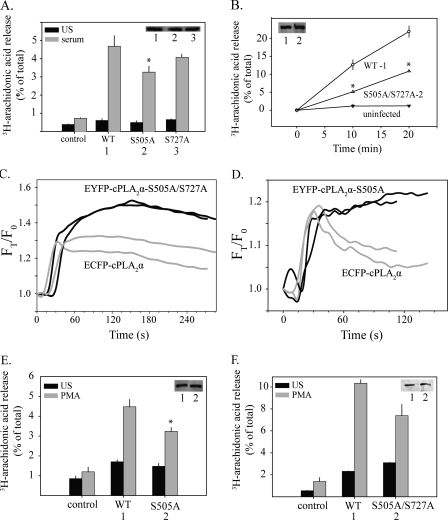
Analysis of Phosphorylation Site Mutants Expressed in IMLF-/-—The functional role of cPLA2α phosphorylation was investigated by expressing wild type cPLA2α and phosphorylation site mutants in IMLF-/- and comparing AA release and translocation. In experiments analyzing AA release, equal expression of wild type and mutant cPLA2α was achieved by expressing three dilutions of adenovirus in neighboring wells. AA release experiments were conducted on all wells, and Western blotting was used to determine the relative expression level of cPLA2α in each well for comparison of mutant and wild type cPLA2α. IMLF-/- expressing EYFP-cPLA2αS505A showed a 38% decrease in AA release in response to serum compared with IMLF-/- expressing wild type ECFP-cPLA2α (Fig. 8A). Analysis of cPLA2α phosphorylation in several cell lines has revealed that phosphorylation of Ser-505 occurs with phosphorylation of Ser-727 (13, 17, 18). Therefore, the ability of EYFP-cPLA2αS727A and EYFP-cPLA2αS505A/S727A to release AA was tested. AA release from IMLF-/- expressing EYFP-cPLA2αS727A was not significantly different from IMLF-/- expressing wild type ECFP-cPLA2α (Fig. 8A). Equal expression of wild type cPLA2α and the phosphorylation site mutant was confirmed by Western blotting (Fig. 8A, inset). IMLF-/- expressing EYFP-cPLA2αS505A/S727A released ∼50% less AA than IMLF-/- expressing equivalent levels of wild type ECFP-cPLA2α at both 10 and 20 min poststimulation by serum (Fig. 8B). AA release from IMLF-/- expressing the S505A or S505A/S727A mutants was not reduced to the extent observed using the MEK1 inhibitor U0126 (see Fig. 3A). This observation is consistent with our previous studies suggesting that ERKs also play a role in regulating cPLA2α-mediated AA release by a mechanism independent of phosphorylation on Ser-505 (27, 49). This was confirmed by data showing that U0126 inhibited the residual AA release from IMLF-/- expressing EYFP-cPLA2αS505A/S727A by ∼51% (average of two experiments) (data not shown).
FIGURE 8.
AA release and translocation of cPLA2α phosphorylation site mutants. Parallel cultures of [3H]AA-labeled IMLF-/- expressing either wild type ECFP-cPLA2α (WT) or EYFP-cPLA2α phosphorylation site mutants as indicated were stimulated for 10 min with serum (A and B) or for 45 min with PMA (E and F). [3H]AA released into the medium is expressed as a percentage of the total cellular radioactivity in each well. Immunoblotting was conducted to determine expression levels of wild type and mutant cPLA2α in each well (insets). [3H]AA release is shown from wells with matching expression levels. The release of [3H]AA by the mutants was significantly less (p < 0.05) than by wild type cPLA2α, as indicated (*). Live cell images of IMLF-/- co-expressing wild type ECFP-cPLA2α and either EYFP-cPLA2αS505A/S727A (C) or EYFP-cPLA2αS505A (D) were collected every 3 s after serum stimulation, using CFP and YFP filters and a ×40 oil immersion objective. Translocation to Golgi in cells expressing wild type (gray lines) or mutant cPLA2α (black lines) is shown for two representative cells. Values are corrected for background fluorescence and differential bleaching and are presented relative to time zero (FT/F0). Data are representative of 20 individual cells from three independent experiments.
AA release in IMLF-/- expressing the EYFP-cPLA2αS515A mutant was similar to wild type, and IMLF-/- expressing EYFP-cPLA2αS515A/S505A/S727A was similar to EYFP-cPLA2αS505A/S727A, suggesting no role for Ser-515 phosphorylation for serum-stimulated AA release (data not shown). This is consistent with the lack of effect of the CAMKII inhibitor, KN93, on AA release in IMLF+/+ (see Fig. 3C).
Dual imaging was used to directly compare translocation of wild type ECFP-cPLA2α co-expressed with either EYFP-cPLA2αS505A/S727A (Fig. 8C) or EYFP-cPLA2αS505A in IMLF-/- (Fig. 8D). The initial rate of translocation of wild type ECFP-cPLA2α and EYFP-cPLA2αS505A/S727A or EYFP-cPLA2αS505A to Golgi in response to serum was similar for the first 30 s. After this time, wild type ECFP-cPLA2α did not accumulate further on the Golgi and began to slowly dissociate from the Golgi in most cells (Fig. 8, C and D). However, EYFP-cPLA2αS505A/S727A and EYFP-cPLA2αS505A continued to accumulate on Golgi over the next 1-2 min. We also compared the function of wild type cPLA2α and the phosphorylation site mutants in IMLF-/- stimulated with PMA. The release of AA in response to PMA by IMLF-/- expressing EYFP-cPLA2αS505A (Fig. 8E) or EYFP-cPLA2αS505A/S727A (Fig. 8F) was blunted by 30% compared with cells expressing wild type ECFP-cPLA2α. The results demonstrate that phosphorylation of cPLA2α at Ser-505 augments AA release but is not essential.
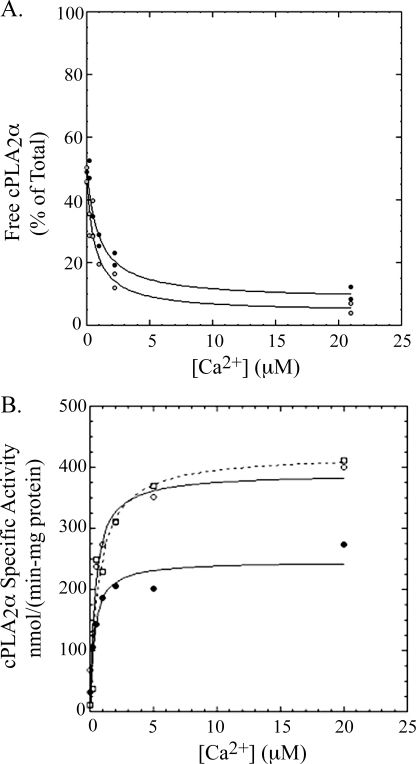
Interfacial Binding and Kinetics of cPLA2 and Its Phosphorylated Forms in Vitro—Our results show that suboptimal AA release by phosphorylation site mutants is not due to a defect in translocation to the Golgi or stable membrane binding in response to physiological increase in calcium induced by serum. These data are not consistent with a previous report showing that phosphorylation on Ser-505 increases the membrane affinity of cPLA2α in cells and in vitro (25). Therefore, we measured the interfacial kinetics and binding of different phosphorylated forms of cPLA2α in vitro. To directly measure the binding of cPLA2α to vesicles in vitro, we measured the amount of enzyme in the supernatant above vesicles that were pelleted using ultracentrifugation (vesicles were loaded with sucrose to allow them to pellet). As shown in Fig. 9A, the amount of dephosphorylated cPLA2α (cPLA2α-PAP) bound to PAPC vesicles increases as the concentration of free Ca2+ increased from 0 to 22 μm. A concentration of PAPC of 200 μm was used, which approximates the estimated concentration of phospholipid that cPLA2α encounters inside of mammalian cells (55). The buffer was chosen to give physiological pH and ionic strength. Centrifugation studies carried out in the absence of vesicles showed no loss of cPLA2α-PAP from the buffer solution at all concentrations of Ca2+ (supplemental Fig. 2); thus, depletion of enzyme from the supernatant is the result of interfacial binding to vesicles. From the data in Fig. 9A, we obtain the concentration of calcium that allows 50% of the cPLA2α-PAP to bind to vesicles. We denote this value as appKCa to reflect the fact that it is an apparent dissociation equilibrium constant that is composed of all calcium-dependent steps (56). From the same figure, we determine the fraction of enzyme bound to vesicles in the absence of Ca2+and the fraction of enzyme bound at saturating Ca2+. This interfacial binding experiment was repeated using cPLA2α that was stoichiometrically phosphorylated on Ser-505 only (cPLA2α-505P) (Fig. 9A) or on S515 (cPLA2α-515P) (supplemental Fig. 3). Results for all enzyme forms are summarized in Table 1. cPLA2α-PAP, cPLA2α-505P, and cPLA2α-515P are all about 50% bound to 200 μm PAPC vesicles in the absence of Ca2+, and increasing Ca2+ causes virtually all of the proteins to become fully vesicle-bound. Phosphorylation of cPLA2α on Ser-505 or on Ser-515 causes little, if any, statistically significant change in appKCa (Table 1).
FIGURE 9.
A, binding of cPLA2α-PAP and cPLA2αS505P to PAPC vesicles. Binding solutions contained 1 ml of buffer A, 0.5 mg/ml BSA, and 200 μm PAPC as vesicles and 50 ng of cPLA2α-PAP (filled circles) or cPLA2αS505P (open circles). After ultracentrifugation, two 100-ml aliquots of the supernatant were each submitted to the standard radiometric cPLA2α assay to determine the amount of enzyme not bound to vesicles. The latter is plotted as the percentage of enzyme added to each binding solution, where 100% corresponds to the radiometric assay signal measured for 5 ng of cPLA2α added directly from the stock solution to the radiometric assay mixture (see “Experimental Procedures”). Each experimental condition was carried out in duplicate, and both data points are plotted. B, hydrolysis of [14C]PAPC vesicles by cPLA2α-PAP, cPLA2αS505P, and cPLA2αS505P/S727P versus the concentration of calcium. Reactions contained 0.1 ml of buffer A, 0.5 mg/ml BSA, and 200 μm [14C]PAPC as vesicles and 200 ng of cPLA2α-PAP (filled circles, solid line) or cPLA2αS505P (open circles, solid line) or cPLA2αS505P/S727P (open squares, dashed line). Reactions were quenched after 2 min at 37 °C. Each experimental condition was carried out in duplicate, and both data points are plotted.
TABLE 1.
Interfacial binding of cPLA2α to vesicles
| Enzyme | Vesicle composition | appKCa | Enzyme bound without Ca2+ |
|---|---|---|---|
| μm | % | ||
| cPLA2α-PAP | 100% PAPC | 0.7 ± 0.1 | 50 |
| cPLA2α-505P | 100% PAPC | 0.9 ± 0.2 | 50 |
| cPLA2α-515P | 100% PAPC | 0.5 ± 0.1 | 44 |
| cPLA2α-PAP | 90% PAPC, 10% PIP2 | 0.4 ± 0.1 | 37 |
| cPLA2α-505P | 90% PAPC, 10% PIP2 | 0.2 ± 0.02 | 48 |
| cPLA2α-515P | 90% PAPC, 10% PIP2 | 0.3 ± 0.05 | 33 |
We also determined the Ca2+ dependence of hydrolysis of 200 μm [14C]PAPC vesicles by phosphorylated forms of cPLA2α. As shown in supplemental Fig. 4, the amount of 14C-labeled arachidonate released from [14C]PAPC vesicles rises steadily from 0 to 30 min; thus, studies carried out by quenching the reaction after a 2-min incubation provide the initial reaction velocity. As shown in Fig. 9B, the enzymatic activity of cPLA2α-PAP rises as a function of increasing concentration of Ca2+. As for interfacial binding, appKCa was obtained as the concentration of Ca2+ that causes 50% of maximal activation of enzyme. Results for cPLA2α-PAP, cPLA2α-505P, and cPLA2α-505P/727P are also shown in Fig. 9B, and those for cPLA2α-515P are shown in supplemental Fig. 5. Interfacial kinetic values are summarized in Table 2. All three enzymes show only a small amount of activity in the absence of calcium (12% or less of that seen at saturating Ca2+) and display very similar appKCa values. The specific activities of cPLA2α-505P and cPLA2α-505P/727P at saturating Ca2+ are about 2-fold higher than that of cPLA2α-PAP, whereas cPLA2α-515P shows a specific activity that is not statistically different from that for nonphosphorylated enzyme. Treatment of platelet-derived cPLA2α-505P/727P with PAP to remove phosphates yielded an enzyme that showed values of appKCa and maximum activity at saturating Ca2+ statistically indistinguishable from those of recombinant cPLA2α-PAP (data not shown), strongly suggesting that the altered properties of the enzyme isolated from platelets is due solely to phosphorylation. The fact that values of appKCa observed for interfacial binding and kinetic studies are statistically indistinguishable suggests that the sole function of Ca2+ is to support interfacial binding of enzyme. The data also indicate that phosphorylation of cPLA2α on Ser-505 functions to increase the catalytic activity but does not affect calcium-dependent membrane affinity, consistent with the results in IMLF.
TABLE 2.
Interfacial kinetics of cPLA2α on vesicles
| Enzyme | Vesicle composition | appKCa | Percentage of maximal activity without calcium | Specific activity at saturating Ca2+ |
|---|---|---|---|---|
| μm | % | |||
| cPLA2α-PAP | [14C]PAPC | 0.7 ± 0.1 | 12 | 230 ± 8 |
| cPLA2α-505P | [14C]PAPC | 0.9 ± 0.1 | 8 | 380 ± 20 |
| cPLA2α-505P/727P | [14C]PAPC | 0.7 ± 0.2 | 8 | 405 ± 30 |
| cPLA2α-515P | [14C]PAPC | 0.5 ± 0.1 | 5 | 260 ± 8 |
| cPLA2α-PAP-K488N/K543N/K544N | [14C]PAPC | 0.7 ± 0.1 | 6 | 400 ± 8 |
| cPLA2α-PAP-K271N/K273N/R274N | [14C]PAPC | 0.3 ± 0.2 | 5 | 210 ± 12 |
| cPLA2α-PAP | 90% [14C]PAPC, 10% PIP2 | 0.66 ± 0.1 | 7 | 1030 ± 43 |
| cPLA2α-505P | 90% [14C]PAPC, 10% PIP2 | 0.55 ± 0.1 | 8 | 1450 ± 44 |
| cPLA2α-515P | 90% [14C]PAPC, 10% PIP2 | 0.3 ± 0.5 | 5 | 866 ± 38 |
| cPLA2α-PAP-K488N/K543N/K544N | 90% [14C]PAPC, 10% PIP2 | 1.0 ± 0.2 | 6 | 390 ± 10 |
| cPLA2α-PAP-K271N/K273N/R274N | 90% [14C]PAPC, 10% PIP2 | 1.5 ± 0.2 | 7 | 680 ± 10 |
Analysis of Basic Residue Mutants Expressed in IMLF-/-—In addition to phosphorylation, basic residues in the catalytic domain (Lys-488, Lys-541, Lys-543, and Lys-544) are implicated in cPLA2α regulation through interaction with PIP2 (33, 37). Basic residues Lys-488, Lys-543, and Lys-544 in cPLA2α were mutated to asparagines to determine their role in regulating cPLA2α. AA release from IMLF-/- expressing the EYFP-cPLA2αK488N/K543N/K544N triple mutant was decreased by 78%, compared with cells expressing an equivalent amount of wild type ECFP-cPLA2α in response to serum (Fig. 10A). In contrast, there was no significant decrease in AA release from IMLF-/- expressing the triple mutant in response to PMA (Fig. 10B). Live cell imaging showed a similar initial rate of translocation of EYFP-cPLA2αK488N/K543N/K544N and wild type ECFP-cPLA2α in response to serum, but the mutant exhibited an increase in Golgi binding after the first 30 s (Fig. 10C). The results demonstrate that the decreased AA release by the basic residue mutant does not correlate with a defect in translocation.
FIGURE 10.
Role of basic residues in cPLA2α catalytic domain in regulating translocation and AA release in response to serum and PMA. [3H]AA-labeled IMLF-/- expressing either wild type ECFP-cPLA2α or EYFP-cPLA2αK488N/K543N/K544N were stimulated for 10 min with serum (A) or 45 min with PMA (B), and [3H]AA release was measured and expressed as a percentage of the total cellular radioactivity in each well. The release of [3H]AA by the mutant was significantly less (p < 0.05) than by wild type cPLA2α, as indicated (*). Cell lysates were made from each well, and immunoblotting for cPLA2α was conducted to determine expression levels (inset) of wild type and mutant cPLA2α in each well. C, IMLF-/- co-expressing wild type ECFP-cPLA2α and EYFP-cPLA2αK488N/K543N/K544N were stimulated with serum, and images were collected using both a CFP and YFP filter and a ×40 oil immersion objective. Translocation data were calculated based on average fluorescence intensity of a mask of the Golgi in each cell. Values were calculated by subtracting background fluorescence and correcting for differential bleaching at each wavelength throughout the imaging. Data are presented relative to time 0 (FT/F0). Data are representative of 10 individual cells from three independent experiments.
Effect of PIP2 on the Interfacial Properties of cPLA2α in Vitro—As a correlate to the cellular experiments, we studied the effect of PIP2 on interfacial binding and kinetics of wild type cPLA2α and cPLA2α containing mutations in basic residues in the catalytic domain. The role of phosphorylation on cPLA2α activation by PIP2 was also investigated. The results are summarized in Tables 1 and 2 (binding and kinetic curves are presented as supplemental Figs. 6 and 7). The extent of interfacial binding of wild type cPLA2α to PAPC vesicles containing 10 mol % PIP2 in the absence of Ca2+ is similar to that seen with vesicles that lack PIP2 (compare supplemental Figs. 6 and 7 with Fig. 9). The presence of PIP2 in vesicles led to a modest, ∼2-fold decrease in values of appKCa (Table 1). Similar trends were seen with nonphosphorylated and various phosphorylated cPLA2α proteins (Table 1).
Interfacial kinetic studies summarized in Table 2 show that inclusion of 10 mol % PIP2 in PAPC vesicles led to a ∼5-fold increase in the specific activity of cPLA2α-PAP and all of its phosphorylated forms in the presence of saturating Ca2+ (see also supplemental Figs. 5 and 8). Values of appKCa were similar in the presence and absence of PIP2 in PAPC vesicles.
We also studied cPLA2α mutants in which basic residues possibly involved in interaction with PIP2 were mutated to asparagine. Enzyme activity data versus the concentration of Ca2+ for cPLA2α-PAP-K488N/K543N/K544N and cPLA2α-PAP-K271N/K273N/R274N are summarized in Table 2 (kinetic curves are shown in supplemental Fig. 8). cPLA2α-PAP-K488N/K543N/K544N showed an ∼2-fold increase in specific activity compared with wild type enzyme on PAPC vesicles, and the mutant did not show rate enhancement when 10 mol % PIP2 was added to PAPC vesicles (virtually identical results were obtained with cPLA2α-PAP-K541N/K543N/K544N, a second triple site mutant involving basic residues occupying a similar region of the enzyme's surface; data not shown). The mutant cPLA2α-PAP-K271N/K273N/R274N displayed the same activity as wild type enzyme on PAPC vesicles and showed a rate enhancement by 10 mol % PIP2 about half of that seen for wild type enzyme. Values of appKCa for all mutants were similar to those for wild type enzymes with both PAPC and PAPC/PIP2 vesicles (Table 2). The results of the in vitro experiments support the cellular studies and implicate a role for the basic residues in the catalytic domain of cPLA2α in regulating hydrolytic activity through interaction with anionic phospholipids and not by enhancing calcium-dependent membrane binding.
DISCUSSION
cPLA2α activity is controlled by complex post-translational mechanisms. The importance of lipid mediators produced as a result of cPLA2α activation necessitates tight multidimensional regulation (6). Since cPLA2α is expressed in most normal tissues, its function must be controlled in response to diverse stimuli by means appropriate to that cell type and circumstance. Reflecting this, AA release is stimulated by a variety of agonists that increase [Ca2+]i and activate MAPKs, thereby initiating the signaling pathways for activation of cPLA2α (1, 3, 7). In this study, we shed light on the mechanisms of cPLA2α regulation by calcium, phosphorylation, and basic residues in the catalytic domain in fibroblasts stimulated with serum and PMA. The results are supported by our findings studying the role of phosphorylation and PIP2 in regulating calcium-dependent interfacial binding and kinetics of cPLA2α in vitro.
Changes in [Ca2+]i play a fundamental role in regulating cPLA2α by promoting C2 domain-mediated translocation to membrane. Comparing translocation and AA release in response to serum and PMA allowed us to dissect out how cPLA2α is regulated by differences in the mode of calcium mobilization. Serum induces a typical capacitative increase in [Ca2+]i. A rapid, transient increase in [Ca2+]i from intracellular stores is followed by the influx of extracellular calcium, resulting in a sustained, low amplitude [Ca2+]i increase (57, 58). We have previously suggested that PMA stimulates AA release without increasing [Ca2+]i in macrophages (22, 49). However, we found that PMA induces low amplitude [Ca2+]i oscillations in IMLF+/+ that were only detected by analyzing a large number of individual cells using live cell imaging, revealing considerable cell-to-cell heterogeneity in the extent of calcium mobilization. Our findings demonstrate that the low amplitude sustained phase of [Ca2+]i due to influx of extracellular calcium is essential for AA release triggered by both serum and PMA in IMLF+/+. This is supported by results showing that mutation of a residue (Asp-43) in the C2 domain essential for binding calcium compromises the ability of cPLA2α to translocate and release AA in response to serum and PMA in IMLF. In addition, chelating extracellular calcium with EGTA abolished AA release.
The initial high amplitude calcium transient induced by serum promotes the rapid translocation of cPLA2α to Golgi and AA release. The maximal amount of cPLA2α bound to Golgi occurred ∼20 s after adding serum and represents ∼7% of the total cellular ECFP-cPLA2α. In contrast to physiological agonists, such as serum, calcium ionophores used at concentrations that induce a high, sustained influx of calcium promote translocation of a much larger proportion of cPLA2α to membrane (10). As we previously reported, the release of calcium from intracellular stores promotes more rapid translocation of cPLA2α to Golgi than does influx of extracellular calcium, suggesting the importance of local high calcium increases released from intracellular membrane stores for rapid translocation (10, 34). Chelating extracellular calcium did not block the initial increase in [Ca2+]i induced by serum but prevented AA release. Translocation of cPLA2α to Golgi in response to serum was only briefly evident in a few cells when incubated in medium containing EGTA. This indicates that the influx of extracellular calcium triggered by store depletion is required to promote stable binding of cPLA2α to membrane, which is necessary for mediating AA release. This is consistent with previous reports demonstrating the important role for capacitative calcium influx and the duration of calcium elevation in regulating cPLA2α-mediated AA release (59-61). Thus, the two phases of calcium mobilization induced by serum serve to induce rapid and stable binding of cPLA2α to membrane.
We also demonstrate that the low amplitude sustained calcium oscillations induced by PMA promote translocation of cPLA2α in the absence of a rapid calcium transient from intracellular stores. This occurs more slowly, taking several minutes for cPLA2α to accumulate on the Golgi. There was considerable variation in the extent of cPLA2α translocation in cells stimulated with PMA, reflecting the cell-to-cell variability in the extent of PMA-induced [Ca2+]i oscillations. In contrast to PMA-stimulated cells, translocation of cPLA2α was evident in over 90% of the serum-stimulated cells. Also, serum stimulates a greater proportion of cellular cPLA2α to translocate to Golgi due to the rapid high amplitude release of calcium from intracellular stores. This may account for the higher levels of AA release induced by serum compared with PMA.
Previous studies identified a role for CaMKII activation and phosphorylation of cPLA2α on Ser-515 in regulating AA release in smooth muscle cells stimulated with norepinephrine (16, 20, 48). However, we found no evidence for this pathway in regulating AA release in IMLF+/+. The CaMKII inhibitor had no effect on AA release from IMLF+/+, and AA release from IMLF-/- expressing the S515A mutant was the same as in cells expressing wild type cPLA2α. Therefore, the regulation of cPLA2α by CaMKII is cell type-specific. We could not demonstrate a change in specific activity or appKCa after stoichiometric phosphorylation of cPLA2α-PAP on Ser-515 by CaMKII. In a previous study, it was shown that treatment of cPLA2α with CaMKII in vitro led to a 2-3-fold increase in specific activity (16). The reason for this discrepancy is not known. Therefore, it remains unclear how phosphorylation of cPLA2α on Ser-515 in cells functions to regulate cPLA2α, especially since it has been shown that phosphorylation of Ser-515 or Ser-505 does not regulate cPLA2α translocation in smooth muscle cells stimulated with norepinephrine (20).
Serum- and PMA-stimulated AA release is dependent on activation of MAPKs in IMLF+/+, as observed in many cell types (1). Activation of MAPKs by serum and PMA was not blocked by extracellular EGTA, indicating that it is not dependent on the influx of calcium. cPLA2α is constitutively phosphorylated on Ser-505 in IMLF-/-, as we have observed in other cells lines (27).4 This may be a consequence of culturing cells in the presence of serum, which activates MAPKs, resulting in cPLA2α phosphorylation. We found that after overnight serum starvation, a protocol commonly used to quiesce cells, MAPKs exhibit only weak activation and can be strongly activated upon subsequent serum addition. However, cPLA2α remains phosphorylated on Ser-505 during serum starvation, indicating that phosphorylation at Ser-505 is very stable. This is consistent with our previous data in macrophages showing that activation of ERKs in response to CSF-1 is very transient but leads to stable phosphorylation of cPLA2α on Ser-505 (22). Our results in IMLF also confirm that phosphorylation on Ser-505 is not sufficient for cPLA2α to mediate AA release, which requires an increase in [Ca2+]i for translocation to membrane. Although MAPKs play a role in regulating cPLA2α by phosphorylation of Ser-505, they clearly play an additional novel role in regulating cPLA2α, since agonist-induced AA release is blocked by MAPK inhibitors without affecting Ser-505 phosphorylation. We have previously made this observation in other cell types, suggesting that it is a commonly used regulatory pathway, although the mechanism involved remains to be determined.
Phosphorylation of cPLA2α on Ser-505 enhances its activity in vitro and its ability to release AA in cells (14, 15, 17, 24). We found that IMLF-/- expressing EYFP-cPLA2αS505A released ∼38% less AA than cells expressing wild type ECFP-cPLA2α, indicating that phosphorylation on Ser-505 augments AA release but is not essential in IMLF. AA release in IMLF-/- expressing the double phosphorylation site mutant S505A/S727A was attenuated to a similar extent (50%). A comparison of the translocation properties of wild type ECFP-cPLA2α and the double EYFP-cPLA2αS505A/S727A and single EYFP-cPLA2αS505A phosphorylation site mutants showed similar initial rates of translocation of the mutants to Golgi in response to serum as wild type cPLA2α that correlated with the initial rise in [Ca2+]i. The phosphorylation site mutants tended to accumulate on Golgi to a greater extent than wild type cPLA2α. The reason for this is not known, but despite greater levels of the mutants on Golgi, their ability to release AA is not as efficient as that of wild type cPLA2α. Our data demonstrate that phosphorylation on Ser-505 does not augment translocation of cPLA2α in response to a capacitative calcium increase (serum stimulation) or in response to a low oscillatory rise (PMA stimulation) that is not preceded by a high amplitude transient increase in calcium from intracellular stores. Thus, we find no evidence that phosphorylation on Ser-505 affects the calcium-dependent membrane binding affinity of cPLA2α.
We found that mutating the phosphorylation site Ser-727 to alanine did not affect cPLA2α translocation or AA release in cells. Also, Ser-727 phosphorylation did not influence cPLA2α binding or catalytic activity in vitro. Unlike the role of Ser-505 phosphorylation in enhancing cPLA2α catalytic activity, a recent study has identified a novel function for Ser-727 phosphorylation in regulating protein-protein interactions (62). Previous studies had identified a cPLA2α-binding protein, p11 (also called S100-A10 or calpactin I light chain) that forms a complex with annexin A2 in cells (63, 64). p11 was reported to bind to the catalytic domain of cPLA2α and inhibit its activity (63). A recent study found that the p11-annexin A2 complex binds to the hydroxyl group of Ser-727 and prevents binding of cPLA2α to membrane (62). The interaction of cPLA2α with the p11-annexin A2 complex is disrupted by phosphorylation of cPLA2α on Ser-727, thus relieving inhibition. Mutating Ser-727 to alanine also disrupts interaction of cPLA2α with the p11-annexin A2 complex and mimics phosphorylation of Ser-727 (62). Our results showing that cPLA2αS727A behaves similarly to wild type cPLA2α when expressed in IMLF-/- suggest that wild type cPLA2α is constitutively phosphorylated on Ser-727 (as observed for Ser-505) or phosphorylated on Ser-727 in response to serum and PMA and relieved from potential inhibition by endogenous p11.
Our findings do not corroborate a report suggesting that phosphorylation of cPLA2α on Ser-505 increases the membrane binding affinity of cPLA2α particularly at low calcium concentrations induced by adding ionomycin (25). The reasons for the different findings are not known. However, our results in IMLF-/- correlate well with results of our in vitro experiments. Phosphorylation of cPLA2α at Ser-505 and at Ser-505/Ser-727 (from platelets) leads to a 2-fold increase in the specific activity of the enzyme acting on PAPC vesicles, which is in line with the level of activation reported previously (e.g. see Refs. 24 and 39). We see no evidence that phosphorylation of cPLA2α at Ser-505 dramatically alters the concentration of Ca2+ needed to support interfacial binding. It has been reported that the Kd for the nonphosphorylated S505A mutant of cPLA2α is ∼60-fold lower than that for wild type cPLA2α purified from Sf9 cells (which is shown to be mainly phosphorylated on Ser-505) or the S505E mutant, which is suggested to be a mimic of Ser-505-phosphorylated cPLA2α (25). Even if we use the same vesicles and buffer reported in this previous study, we fail to see a significant effect of cPLA2α phosphorylation on the amount of calcium required to support interfacial binding. The basis for this discrepancy between the two studies is not known. Our values of appKCa have been measured by two independent methods (centrifugation and initial velocity versus [Ca2+]). A calculation shown in the supplemental material shows that our values of appKCa are close in magnitude to those reported in the earlier study (25).
Phosphorylation of cPLA2α has been suggested to influence the conformation of the cPLA2α catalytic domain on the membrane for optimal interaction with phospholipid substrate, thus augmenting the hydrolytic activity of cPLA2α (65). Conformational effects may also be the basis for enhanced activity of cPLA2α by interaction of the basic residues (Lys-488/Lys-541/Lys-543/Lys-544) in the catalytic domain with PIP2 (33). We found that the addition of PIP2 (10 mol %) to PAPC (200 μm) resulted in a 4-fold decrease in appKCa and ∼8-fold increase in the maximal activity of wild type cPLA2α in the presence of saturating calcium but did not activate cPLA2α-K488N/K543N/K544N. Our results are more in line with data showing 3.5-8-fold stimulation of cPLA2α activity with PIP2 using PC vesicles (33, 35) and not the 120-fold increase observed using PC/Triton X-100 mixed micelles (36). In the absence of calcium, we found that PIP2 did not change the amount of cPLA2α catalytic activity, which remained low at about 7-10% of the amount of cPLA2α bound to vesicles. It has been reported that the inclusion of PIP2 in PAPC/Triton X-100 mixed micelles substrate allows the enzyme to act in a calcium-independent manner, yet the enzyme showed no activity in the absence of calcium when PIP2 was omitted from mixed micelles (36, 37). Since it is thought that the main role of calcium is to allow interfacial binding of cPLA2α, this result would suggest that PIP2 allows the enzyme to bind to PAPC/Triton X-100 mixed micelles with similar affinity in the presence and absence of calcium. It is clear from the results of our work and a previous study that calcium-independent activation of cPLA2α by PIP2 does not occur with phosphatidylcholine liposomes (33). This suggests that with an aggregate that is predominantly composed of a neutral detergent, Triton X-100, and only a small amount of PAPC, small amounts of anionic phospholipid, such as PIP2, can induce calcium-independent binding, presumably via interactions of the PIP2 with cationic residues in the catalytic domain of cPLA2α. Perhaps binding of the C2 domain to detergent micelles is not favorable compared with binding of this domain to phospholipid vesicles. In this case, electrostatic interaction of cationic residues of cPLA2α and anionic PIP2 in detergent micelles becomes a dominant mechanism for interfacial binding. It would seem that the use of phospholipid vesicles is more physiologically relevant than the use of detergent micelles.
Our results confirm that Lys-488/Lys-543/Lys-544 are important for regulating cPLA2α activation in cells in response to serum stimulation. Expression of EYFP-cPLA2αK488N/K543N/K544N in IMLF-/- resulted in a 78% decrease in AA release in response to serum compared with cells expressing wild type cPLA2α. The triple mutant and wild type cPLA2α exhibit a similar initial rate of translocation to Golgi in response to serum-induced [Ca2+]i increase. Surprisingly, the triple mutant accumulated on Golgi to a greater extent than wild type cPLA2α. This may explain our results and a previous report that the basic residue mutant has ∼2-fold greater activity in vitro using PC vesicles (33). The cellular data suggest that interaction of the basic residues with anionic components on the membrane regulates catalytic activity and not calcium-dependent membrane binding, consistent with our in vitro experiments. The cell results do not establish that PIP2 is the endogenous component in membranes that activates cPLA2α; nor does it establish whether the component is constitutively present or rapidly increased in response to serum. It has not been confirmed that PIP2 is the endogenous activator of cPLA2α in cells. It has recently been demonstrated that cPLA2α-mediated AA release is stimulated when intracellular levels of polyphosphoinositides are increased by feeding cells either PIP2 or phosphatidylinositol 3,5-bisphosphate, suggesting that the response is not specific to PIP2, but again this does not establish that phosphorylated phosphatidylinositol is the endogenous activator of cPLA2α (66). cPLA2α preferentially targets Golgi, which contains very low levels of PIP2 compared with the plasma membrane (67). A role for other anionic lipids or binding proteins in regulating cPLA2α through interaction with basic residues in the catalytic domain remains a possibility. We found that blocking nascent protein synthesis using CHX diminished cPLA2α-mediated AA release in IMLF+/+, supporting previous reports that an unidentified, rapidly turning over protein aids in cPLA2α-mediated AA release (49-51). CHX did not block calcium mobilization or translocation of cPLA2α to Golgi, suggesting that the rapidly turning over protein plays a role in enhancing cPLA2α activity once it has translocated to membrane.
In summary, our ability to reconstitute IMLF lacking endogenous cPLA2α with wild type and mutant forms of functionally active, fluorescent protein-tagged cPLA2α allowed us to directly compare for the first time translocation and AA release in response to the physiological agonist serum in the absence of endogenous wild type cPLA2α. The results demonstrate that phosphorylation of cPLA2α and the interaction of basic residues in the catalytic domain with membrane components largely act to regulate catalytic activity and not calcium-dependent membrane binding.
Supplementary Material
Acknowledgments
We thank Dr. S. Burchett for the production of mutant constructs.
This work was supported by National Institutes of Health Grants HL61378 (to C. C. L.), HL34303 (to C. C. L.), and HL50040 (to M. H. G.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1-8.
Footnotes
The abbreviations used are: cPLA2, cytosolic phospholipase A2; PAPC, 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine; cPLA2α-505P, cPLA2α phosphorylated at Ser-505; cPLA2α-505P/727P, cPLA2α phosphorylated at Ser-505 and Ser-727; cPLA2α-515P, cPLA2α phosphorylated at Ser-515; PAP, potato acid phosphatase; cPLA2α-PAP, cPLA2α fully dephosphorylated by treatment with PAP; DMEM, Dulbecco's modified Eagle's medium; BSA, bovine serum albumin; MLF, mouse lung fibroblast(s); IMLF, immortalized mouse lung fibroblast(s); AA, arachidonic acid; CaMKII, calcium/calmodulin-dependent protein kinase II; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated protein kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PMA, phorbol 12-myristate 13-acetate; EGFP, enhanced green fluorescent protein; YFP, yellow fluorescent protein; EYFP, enhanced yellow fluorescent protein; CFP, cyan fluorescent protein; ECFP, enhanced cyan fluorescent protein; CHX, cycloheximide; MOPS, 4-morpholinepropanesulfonic acid; [Ca2+]i, intracellular calcium concentration.
C. C. Leslie, unpublished observation.
References
- 1.Clark, J. D., Schievella, A. R., Nalefski, E. A., and Lin, L.-L. (1995) J. Lipid Mediat. Cell Signal. 12 83-117 [DOI] [PubMed] [Google Scholar]
- 2.Leslie, C. C. (2004) Prostaglandins Leukot. Essent. Fatty Acids 70 373-376 [DOI] [PubMed] [Google Scholar]
- 3.Leslie, C. C. (1997) J. Biol. Chem. 272 16709-16712 [DOI] [PubMed] [Google Scholar]
- 4.Hanel, A. M., Schüttel, S., and Gelb, M. H. (1993) Biochemistry 32 5949-5958 [DOI] [PubMed] [Google Scholar]
- 5.Alonso, F., Henson, P. M., and Leslie, C. C. (1986) Biochim. Biophys. Acta 878 273-280 [DOI] [PubMed] [Google Scholar]
- 6.Funk, C. D. (2001) Science 294 1871-1875 [DOI] [PubMed] [Google Scholar]
- 7.Ghosh, M., Tucker, D. E., Burchett, S. A., and Leslie, C. C. (2006) Prog. Lipid Res. 45 487-510 [DOI] [PubMed] [Google Scholar]
- 8.Clark, J. D., Lin, L.-L., Kriz, R. W., Ramesha, C. S., Sultzman, L. A., Lin, A. Y., Milona, N., and Knopf, J. L. (1991) Cell 65 1043-1051 [DOI] [PubMed] [Google Scholar]
- 9.Nalefski, E. A., Sultzman, L. A., Martin, D. M., Kriz, R. W., Towler, P. S., Knopf, J. L., and Clark, J. D. (1994) J. Biol. Chem. 269 18239-18249 [PubMed] [Google Scholar]
- 10.Evans, J. H., Spencer, D. M., Zweifach, A., and Leslie, C. C. (2001) J. Biol. Chem. 276 30150-30160 [DOI] [PubMed] [Google Scholar]
- 11.Channon, J., and Leslie, C. C. (1990) J. Biol. Chem. 265 5409-5413 [PubMed] [Google Scholar]
- 12.Glover, S., de Carvalho, M. S., Bayburt, T., Jonas, M., Chi, E., Leslie, C. C., and Gelb, M. H. (1995) J. Biol. Chem. 270 15359-15367 [DOI] [PubMed] [Google Scholar]
- 13.de Carvalho, M. G. S., McCormack, A. L., Olson, E., Ghomashchi, F., Gelb, M. H., Yates, J. R., III, and Leslie, C. C. (1996) J. Biol. Chem. 271 6987-6997 [DOI] [PubMed] [Google Scholar]
- 14.Lin, L.-L., Wartmann, M., Lin, A. Y., Knopf, J. L., Seth, A., and Davis, R. J. (1993) Cell 72 269-278 [DOI] [PubMed] [Google Scholar]
- 15.Nemenoff, R. A., Winitz, S., Qian, N.-X., Van Putten, V., Johnson, G. L., and Heasley, L. E. (1993) J. Biol. Chem. 268 1960-1964 [PubMed] [Google Scholar]
- 16.Muthalif, M. M., Hefner, Y., Canaan, S., Harper, J., Zhou, H., Parmentier, J.-H., Aebersold, R., Gelb, M. H., and Malik, K. U. (2001) J. Biol. Chem. 276 39653-39660 [DOI] [PubMed] [Google Scholar]
- 17.Hefner, Y., Borsch-Haubold, A. G., Murakami, M., Wilde, J. I., Pasquet, S., Schieltz, D., Ghomashchi, F., Yates, J. R., 3rd, Armstrong, C. G., Paterson, A., Cohen, P., Fukunaga, R., Hunter, T., Kudo, I., Watson, S. P., and Gelb, M. H. (2000) J. Biol. Chem. 275 37542-37551 [DOI] [PubMed] [Google Scholar]
- 18.Börsch-Haubold, A. G., Bartoli, F., Asselin, J., Dudler, T., Kramer, R. M., Apitz-Castro, R., Watson, S. P., and Gelb, M. H. (1998) J. Biol. Chem. 273 4449-4458 [DOI] [PubMed] [Google Scholar]
- 19.Kramer, R. M., Roberts, E. F., Um, S. L., Börsch-Haubold, A. G., Watson, S. P., Fisher, M. J., and Jakubowski, J. A. (1996) J. Biol. Chem. 271 27723-27729 [DOI] [PubMed] [Google Scholar]
- 20.Pavicevic, Z., Leslie, C. C., and Malik, K. U. (2008) J. Lipid Res. 49 724-737 [DOI] [PubMed] [Google Scholar]
- 21.Lin, L.-L., Lin, A. Y., and Knopf, J. L. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 6147-6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu, Z.-H., Gijón, M. A., de Carvalho, M. S., Spencer, D. M., and Leslie, C. C. (1998) J. Biol. Chem. 273 8203-8211 [DOI] [PubMed] [Google Scholar]
- 23.Schalkwijk, C. G., van der Heijden, M. A. G., Bunt, G., Maas, R., Tertoolen, L. G. J., van Bergen en Henegouwen, P. M. P., Verkleij, A. J., van den Bosch, H., and Boonstra, J. (1996) Biochem. J. 313 91-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayburt, T., and Gelb, M. H. (1997) Biochemistry 36 3216-3231 [DOI] [PubMed] [Google Scholar]
- 25.Das, S., Rafter, J. D., Kim, K. P., Gygi, S. P., and Cho, W. (2003) J. Biol. Chem. 278 41431-41442 [DOI] [PubMed] [Google Scholar]
- 26.Schievella, A. R., Regier, M. K., Smith, W. L., and Lin, L.-L. (1995) J. Biol. Chem. 270 30749-30754 [DOI] [PubMed] [Google Scholar]
- 27.Evans, J. H., Fergus, D. J., and Leslie, C. C. (2002) BMC Biochem. 3 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perisic, O., Fong, S., Lynch, D. E., Bycroft, M., and Williams, R. L. (1998) J. Biol. Chem. 273 1596-1604 [DOI] [PubMed] [Google Scholar]
- 29.Perisic, O., Paterson, H. F., Mosedale, G., Lara-González, S., and Williams, R. L. (1999) J. Biol. Chem. 274 14979-14987 [DOI] [PubMed] [Google Scholar]
- 30.Davletov, B., Perisic, O., and Williams, R. L. (1998) J. Biol. Chem. 273 19093-19096 [DOI] [PubMed] [Google Scholar]
- 31.Nalefski, E. A., and Falke, J. J. (1998) Biochemistry 37 17642-17650 [DOI] [PubMed] [Google Scholar]
- 32.Bittova, L., Sumandea, M., and Cho, W. (1999) J. Biol. Chem. 274 9665-9672 [DOI] [PubMed] [Google Scholar]
- 33.Das, S., and Cho, W. (2002) J. Biol. Chem. 277 23838-23846 [DOI] [PubMed] [Google Scholar]
- 34.Evans, J. H., and Leslie, C. C. (2004) J. Biol. Chem. 279 6005-6016 [DOI] [PubMed] [Google Scholar]
- 35.Leslie, C. C., and Channon, J. Y. (1990) Biochim. Biophys. Acta 1045 261-270 [DOI] [PubMed] [Google Scholar]
- 36.Mosior, M., Six, D. A., and Dennis, E. A. (1998) J. Biol. Chem. 273 2184-2191 [DOI] [PubMed] [Google Scholar]
- 37.Six, D. A., and Dennis, E. A. (2003) J. Biol. Chem. 278 23842-23850 [DOI] [PubMed] [Google Scholar]
- 38.Hixon, M. S., Ball, A., and Gelb, M. H. (1998) Biochemistry 37 8516-8526 [DOI] [PubMed] [Google Scholar]
- 39.Kramer, R. M., Roberts, E. F., Manetta, J. V., Hyslop, P. A., and Jakubowski, J. A. (1993) J. Biol. Chem. 268 26796-26804 [PubMed] [Google Scholar]
- 40.Bezzine, S., Bollinger, J. G., Singer, A. G., Veatch, S. L., Keller, S. L., and Gelb, M. H. (2002) J. Biol. Chem. 277 48523-48534 [DOI] [PubMed] [Google Scholar]
- 41.Ghomashchi, F., Schuttel, S., Jain, M. K., and Gelb, M. H. (1992) Biochemistry 31 3814-3824 [DOI] [PubMed] [Google Scholar]
- 42.Stewart, A., Ghosh, M., Spencer, D. M., and Leslie, C. C. (2002) J. Biol. Chem. 277 29526-29536 [DOI] [PubMed] [Google Scholar]
- 43.Frost, J. A., Geppert, T. D., Cobb, M. H., and Feramisco, J. R. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 3844-3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghosh, M., Stewart, A., Tucker, D. E., Bonventre, J. V., Murphy, R. C., and Leslie, C. C. (2004) Am. J. Respir. Cell Mol. Biol. 30 91-100 [DOI] [PubMed] [Google Scholar]
- 45.McNeil, P. L., McKenna, M. P., and Taylor, D. L. (1985) J. Cell Biol. 101 372-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bitterman, P. B. (1992) Am. J. Med. 92 39S-43S [DOI] [PubMed] [Google Scholar]
- 47.Burkhardt, A. (1989) Am. Rev. Respir. Dis. 140 513-524 [DOI] [PubMed] [Google Scholar]
- 48.Muthalif, M. M., Benter, I. F., Uddin, M. R., and Malik, K. U. (1996) J. Biol. Chem. 271 30149-30157 [DOI] [PubMed] [Google Scholar]
- 49.Gijón, M. A., Spencer, D. M., Siddiqi, A. R., Bonventre, J. V., and Leslie, C. C. (2000) J. Biol. Chem. 275 20146-20156 [DOI] [PubMed] [Google Scholar]
- 50.Bonney, R. J., Wightman, P. D., Dahlgren, M. E., Davies, P., Kuehl, Jr., F. A., and Humes, J. L. (1980) Biochim. Biophys. Acta 633 410-421 [DOI] [PubMed] [Google Scholar]
- 51.Aderem, A. A., Scott, W. A., and Cohn, Z. A. (1986) J. Exp. Med. 163 139-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gijón, M. A., Spencer, D. M., Kaiser, A. L., and Leslie, C. C. (1999) J. Cell Biol. 145 1219-1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu, G.-Y., McDonagh, T., Hsiang-Ai, Y., Nalefski, E. A., Clark, J. D., and Cumming, D. A. (1998) J. Mol. Biol. 280 485-500 [DOI] [PubMed] [Google Scholar]
- 54.Kurimura, T., and Hirano, A. (1980) J. Gen. Virol. 46 237-242 [DOI] [PubMed] [Google Scholar]
- 55.Corbin, J. A., Evans, J. H., Landgraf, K. E., and Falke, J. J. (2007) Biochemistry 46 4322-4336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain, M. K., Yu, B. Z., Rogers, J., Ranadive, G. N., and Berg, O. G. (1991) Biochemistry 30 7306-7317 [DOI] [PubMed] [Google Scholar]
- 57.Lewis, R. S. (2007) Nature 446 284-287 [DOI] [PubMed] [Google Scholar]
- 58.Berridge, M. J., Bootman, M. D., and Roderick, H. L. (2003) Nat. Rev. Mol. Cell. Biol. 4 517-529 [DOI] [PubMed] [Google Scholar]
- 59.Hirabayashi, T., Kume, K., Hirose, K., Yokomizo, T., Iino, M., Itoh, H., and Shimizu, T. (1999) J. Biol. Chem. 274 5163-5169 [DOI] [PubMed] [Google Scholar]
- 60.Rzigalinski, B. A., Blackmore, P. F., and Rosenthal, M. D. (1996) Biochim. Biophys. Acta 1299 342-352 [DOI] [PubMed] [Google Scholar]
- 61.Chang, W.-C., and Parekh, A. B. (2004) J. Biol. Chem. 279 29994-29999 [DOI] [PubMed] [Google Scholar]
- 62.Tian, W., Wijewickrama, G. T., Kim, J. H., Das, S., Tun, M. P., Gokhale, N., Jung, J. W., Kim, K. P., and Cho, W. (2008) J. Biol. Chem. 283 3960-3971 [DOI] [PubMed] [Google Scholar]
- 63.Yao, X.-L., Cowan, M. J., Gladwin, M. T., Lawrence, M. M., Angus, C. W., and Shelhamer, J. H. (1999) J. Biol. Chem. 274 17202-17208 [DOI] [PubMed] [Google Scholar]
- 64.Wu, T., Angus, C. W., Yao, X.-L., Logun, C., and Shelhamer, J. H. (1997) J. Biol. Chem. 272 17145-17153 [DOI] [PubMed] [Google Scholar]
- 65.Dessen, A., Tang, J., Schmidt, H., Stahl, M., Clark, J. D., Seehra, J., and Somers, W. S. (1999) Cell 97 349-360 [DOI] [PubMed] [Google Scholar]
- 66.Casas, J., Gijón, M. A., Vigo, A. G., Sanchez Crespo, M., Balsinde, J., and Balboa, M. A. (2006) Mol. Biol. Cell 17 155-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watt, S. A., Kular, G., Fleming, I. N., Downes, C. P., and Lucocq, J. M. (2002) Biochem. J. 363 657-666 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.