Abstract
Purpose of review
The purpose of the present review is to describe new concepts on the role of mucins in the protection of corneal and conjunctival epithelia and to identify alterations of mucins in ocular surface diseases.
Recent findings
New evidence indicates that gel-forming and cell surface-associated mucins contribute differently to the protection of the ocular surface against allergens, pathogens, extracellular molecules, abrasive stress, and drying.
Summary
Mucins are high molecular weight glycoproteins characterized by their extensive O-glycosylation. Major mucins expressed by the ocular surface epithelia include cell surface-associated mucins MUC1, -4 and -16, and the gel-forming mucin MUC5AC. Recent advances using functional assays have allowed the examination of their roles in the protection of corneal and conjunctival epithelia. Alterations in mucin and mucin O-glycan biosynthesis in ocular surface disorders, including allergy, non-autoimmune dry eye, autoimmune dry eye, and infection, are presented.
Keywords: gel-forming mucin, cell surface-associated mucin, O-glycan, ocular surface epithelia, ocular allergy, dry eye
Introduction
The stratified, non-keratinized epithelia of the cornea and conjunctiva are continuously exposed to allergens, debris, pathogens, desiccation, injury, and rubbing [1]. Ocular surface mucins play a critical role in the protection of these epithelia. In this review, we summarize data indicating that the two classes of mucins known, secreted (both gel-forming and small soluble) and cell surface-associated, have heterogeneous functions at the ocular surface. The functions of secreted mucins include: (i) clearance of allergens, pathogens and debris, (ii) lubrication, (iii) and antimicrobial activity. The functions of cell surface-associated mucins include: (i) surface protection against abrasive stress (boundary lubrication), (ii) formation of an apical cell surface barrier, (iii) and osmosensing. In addition, both secreted and cell surface-associated mucins contribute through their hydrophilic O-glycans, to the hydrophilic character of wet-surfaced epithelia.
Definition, structure and classification of mucins
Mucins are the largest and most highly glycosylated glycoproteins known. They are characterized by the presence of multiple tandem repeats of amino acids rich in serine and threonine in the central domain of the mucin core peptide, which provide sites for O-glycosylation [2]. The number of amino acids per tandem repeat varies between different mucin genes, and the number of tandem repeats per allele is also variable, making mucin genes and the resultant proteins polymorphic [3,4]. The extensive O-glycosylation of mucins begins with the enzymatic addition of N-acetyl-galactosamine (GalNAc) to serine or threonine on the tandem repeats by a family of glycosyltransferases called polypeptide GalNAc transferases (pp-GalNAc-Ts) [5]. Elongation of the O-glycan chain by sequential addition of carbohydrates is obtained through the activity of different glycosyltransferases, that are cell-type and cell-tissue specific. As a result of the extensive O-glycosylation, approximately 55% of the ocular mucin mass is composed of O-linked glycans [6]. O-glycans also contribute to providing an extended or “bottle brush-like” conformation to the mucin protein backbone [7].
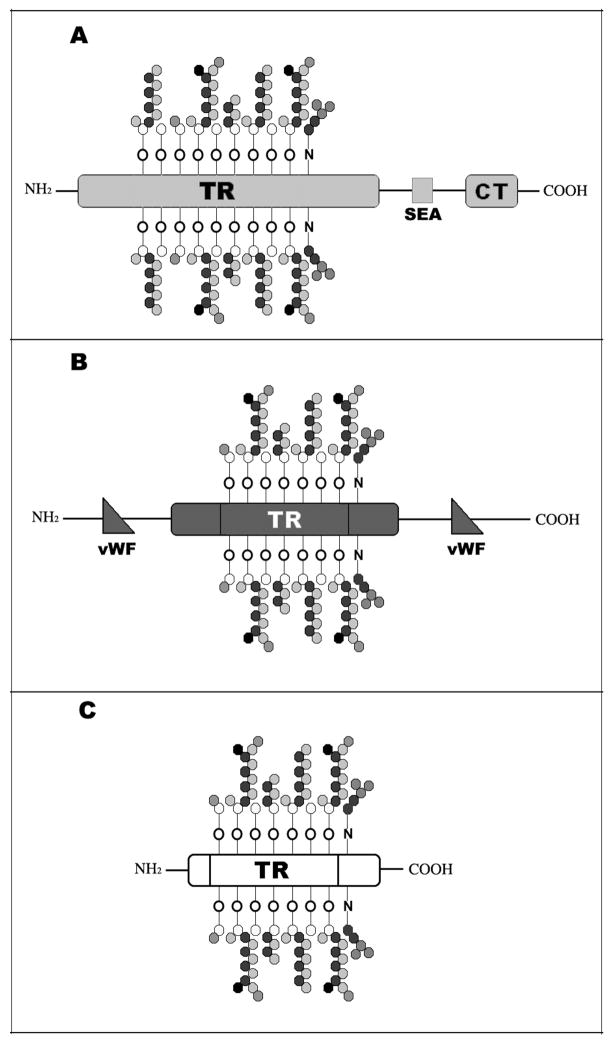
Epithelial mucins can be divided in two different classes, cell surface-associated and secreted (Figure 1). So far, 11 cell surface-associated (MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20, MUC21) and 7 secreted mucins have been identified, the latter being further divided into two subcategories, soluble (MUC7, MUC9) and gel-forming (MUC2, MUC5AC, MUC5B, MUC6, MUC19) [8–11]. Cell surface-associated mucins are single-pass transmembrane proteins characterized by a short cytoplasmic tail, a transmembrane domain, an autoproteolytic domain called SEA module, and an extended highly glycosylated extracellular domain that contributes to the formation of the glycocalyx of apical cells in wet-surfaced epithelia. Gel-forming mucins are characterized by a large central tandem repeat domain flanked by cystein-rich domains that have homology to the so-called D domains of the von Willebrand factor [2]. These domains allow polymerization of individual mucin molecules through disulfide bond formation [10]. Soluble mucins are the smallest mucins known. They lack the cysteine-rich domains and are, therefore, found as monomers.
Figure 1. Structure of mucins.
Mucins are high molecular weight glycoproteins characterized by a variable number of heavily O-glycosylated Tandem Repeats (TR) rich in serine and threonine. Cell surface-associated mucins (A) are anchored to the cell membrane and have a small cytoplasmic tail (CT). At the ocular surface, MUC1 and MUC16 contain the SEA autoproteolytic domain. Gel-forming mucins (B) are the largest glycoproteins known and can form polymers through their cystein-rich domains (von Willebrand factor-like domains, vWF). Soluble mucins (C) are the smallest mucins known and lack both the transmembrane domain and the vWF domains.
Distribution of mucins at the ocular surface epithelia
Gel-forming, cell surface-associated and small soluble mucins have been identified at the ocular surface epithelia. They are distributed throughout the corneal and conjunctival epithelia, goblet cells, and the lacrimal apparatus.
Corneal and conjunctival epithelia
The stratified corneal and conjunctival epithelia are known to express at least three membrane-associated mucins: MUC1, MUC4, and MUC16 [12–14]. These mucins are concentrated on the tips of the apical cells’ microplicae, forming a dense glycocalyx at the epithelial-tear film interface, but can also be released from the cell surface and are found in the tear film [15]. MUC1 and MUC16 are present on both corneal and conjunctival epithelia, and are known to interact, through their cytoplasmic tails, with proteins in the cytoskeleton [16**,17]. MUC4 is most prevalent in conjunctival epithelium, with an apparent diminution toward the central corneal epithelium [18]. Conjunctival epithelium also expresses the small, soluble MUC7 [19].
Conjunctival goblet cells
Goblet cells are specialized cells intercalated in conjunctival epithelium the highest number of these being found in the infero-nasal portion of the bulbar conjunctiva [20]. They produce secretory granules that contain the gel-forming mucin MUC5AC [14,21]. Recent data obtained by immunofluorescence microscopy have also shown that MUC19 is present in conjunctival goblet cells; however, this has not yet been confirmed by in situ hybridization [22]. The gel-forming mucin MUC2 has also been detected in conjunctiva, but no data has yet been published to demonstrate goblet cell-specific localization [23].
Lacrimal apparatus
In the lacrimal gland, MUC1, MUC4, MUC16, MUC5AC, MUC5B, MUC6, and MUC7, have been detected [24,25]. MUC16 has also been detected in the accessory lacrimal glands of Krause and in the nasolacrimal ducts [24,26]. MUC1, MUC2, MUC4, MUC5AC, MUC5B, MUC6, and MUC7 are expressed by epithelia of the lacrimal sac and nasolacrimal ducts [26].
Function of mucins in the ocular surface epithelia
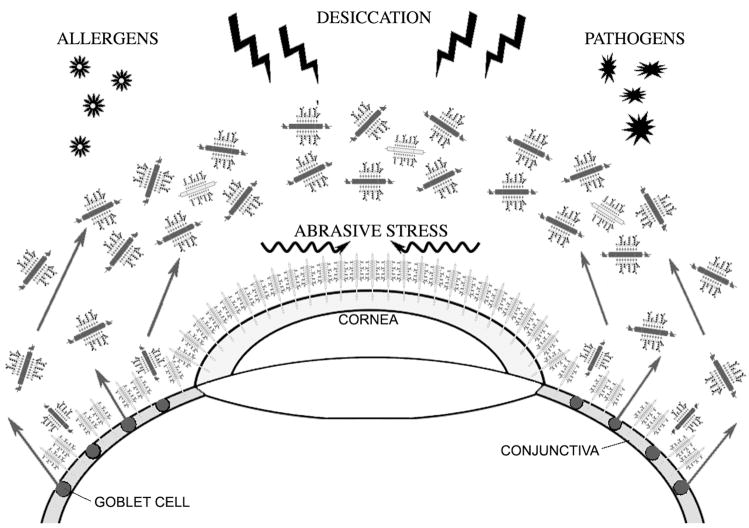
Traditionally, mucin function at the ocular surface has been ascribed to secreted gel-forming mucins acting as lubricating agents and clearing molecules. New evidence shows a role for cell surface-associated mucins in providing barrier function to corneal and conjunctival epithelia (Figure 2). This section will discuss data on the heterogeneous functions of secreted and cell surface-associated mucins at the ocular surface.
Figure 2. Proposed roles of mucins at the ocular surface.
Gel-forming mucins in the tear film, together with cell surface-associated mucins lining the epithelial glycocalyx, contribute to the formation of a protective barrier at the ocular surface. Gel-forming mucins lubricate the ocular surface and allow clearing of allergens and pathogens. Cell surface-associated mucins reduce abrasive stress and could form an apical barrier to pathogens and extracellular molecules. Through their hydrophilic O-glycans, mucins also contribute to the prevention of epithelial desiccation.
Secreted mucins
Clearing molecules
Gel-forming mucins have the capability to trap allergens and debris in order to facilitate their clearance from mucosal surfaces [27]. Electron microscopy and western blot analysis suggest that at the ocular surface these mucins tend to form polymers in the goblet cells, where they are stored, but are secreted as monomers in the tear film [15,28]. The presence of a low molecular weight mucin in tears may reflect the unique requirements of the ocular surface, where a polymerized, viscous coating of corneal and conjunctival epithelia would impair transparency and be detrimental for refractive purposes.
Gel-forming mucins can also act as counter receptors for pathogens and, therefore, contribute to their clearance from mucosal surfaces [27]. In animal models it has been shown that mucins in the tear film bind to Pseudomonas aeruginosa, thus preventing binding of the microorganism to the corneal epithelium and facilitating its clearance through the lacrimal drainage system [29].
Lubricating agents
Gel-forming mucins, through their ability to retain water, can form a highly hydrated mucus gel and contribute to the lubrication of epithelial surfaces [30]. The highly hydrophilic character of secreted mucins may be responsible for lubrication of the ocular surface and reduction of shear stress during blinking or rubbing.
Antimicrobial agents
It has been shown that a 20-mer peptide of the small, soluble mucin MUC7 has potent antifungal and antimicrobial activity in the salivary gland [31]. Although MUC7 has not been detected in tears to date [15], it is possible that it also acts as an antimicrobial agent in the lacrimal gland.
Cell surface-associated mucins
Disadhesive molecules
Cell surface-associated mucins form a thick cell surface glycocalyx that may extend up to 500 nm from the plasma membrane at the ocular surface [32]. Using static and dynamic flow assays, membrane-bound mucins have recently been shown to provide through their O-glycans a disadhesive character to the apical surface of corneal epithelial cells [33**].These results suggest that cell surface-associated mucins provide boundary lubrication and prevent adhesion of facing cell surfaces (i.e. corneal epithelium and tarsal conjunctiva) during blinking or sleeping.
Protective cell surface barrier
Recent evidence has shown that cell surface-associated mucins can contribute to the maintenance of the mucosal barrier integrity, preventing the penetrance of extracellular molecules onto ocular surface epithelia. In human corneal epithelial cells, the expression of membrane-bound mucins prevents penetrance of rose bengal, an anionic, organic dye used in clinical practice to evaluate epithelial damage in diseases such as dry eye [16,34]. The role of cell surface-associated mucins in providing a protective cell surface barrier has also been shown in other wet-surfaced epithelia, such as those in the gastro-intestinal tract, where abrogation of mucins and mucin O-glycans resulted in impaired mucosal integrity as shown by increased permeability to FITC-dextran, development of colitis, and systemic infection [35**,36**].
Osmosensors
Recent data from studies in yeast have shown that the mucin-like transmembrane proteins Hrk1 and Msb2 can activate the HOG signaling pathway in response to increased osmolarity [37**]. It has, therefore, been hypothesized that membrane-bound mucins in higher eukaryotic cells could have the novel function of sensing the extracellular environment through their extracellular domain, and signal through their intracellular domain [38].
Alteration of mucins in ocular surface disease
As described above, mucins are essential to maintain proper epithelial protection, and therefore a healthy wet-surface phenotype. Examples of diseases in which mucin biosynthesis and mucin O-glycosylation are altered at the ocular surface are described in this section, and include allergy, non-autoimmune dry eye, autoimmune dry eye, and infection.
Ocular allergies
Allergic diseases of the ocular surface in which alterations in both cell surface-associated and secreted mucins have been described include atopic keratoconjunctivitis (AKC) and vernal keratoconjunctivitis (VKC). AKC is a disease characterized by squamous metaplasia of the conjunctival epithelium and goblet cell loss. Patients with AKC have decreased levels of the goblet cell-specific mucin MUC5AC, which has been linked to loss of lubrication and epithelial damage [39,40]. Concomitant to the decrease in MUC5AC, there is an increase in the expression of MUC1, MUC2 and MUC4, which may represent a defense mechanism to compensate for the loss of protection provided by the goblet cell mucin MUC5AC [39].
VKC is a disease characterized by seasonal or perennial allergy symptoms that exacerbate in the spring, with intense itching, photophobia, and mucous discharge. Patients with VKC have increased numbers of conjunctival goblet cells and increased levels of MUC5AC, suggesting a protective mechanism aimed at clearing allergens from the ocular surface [41,42]. Active phases of the disease are characterized by intense ocular surface inflammation and eosinophilic infiltration [42]. Activated eosinophils are known to increase MUC5AC expression [43] and, therefore, could contribute to the mucus discharge observed in these patients. Following topical anti-allergic and anti-inflammatory treatments in VKC patients, there are reductions in conjunctival goblet cell number and MUC5AC expression towards normal levels [44,45].
Animal models have also been used to study the expression of mucins after application of defined allergens. In a mouse model of allergic conjunctivitis, repetitive application of cat dander or peptide P3-1 initially induced a decrease in the number of filled goblet cells, as well as a reduction in Muc5AC and Muc4 mRNA expression. After 24 to 48 hours, goblet cell numbers and mucin expression levels returned to normal levels [46], indicating that goblet cell differentiation and increased mucin secretion act as a defense mechanism to remove allergens.
Non-autoimmune dry eye
Dry eye, or keratoconjunctivitis sicca, is a multifactorial disease of the ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability, with potential damage to the ocular surface [47]. Dry eye can be divided in two types: aqueous-deficient including Sjögren’s and non-Sjögren’s and evaporative. In this section we will discuss non-Sjögren’s and evaporative dry eye.
Early studies on dry eye patients found alterations in carbohydrate content within goblet cells. Using gold-conjugated lectins, Versura et al. showed a decrease in sialic acid and GalNAc in secretory granules of goblet cells of patients with dry eye [48], suggesting that alterations in the glycosylation of gel-forming mucins may be responsible for the loss of hydrophilicity and lubrication, as well as a reduction in tear film stability. More recent studies on cell surface-associated mucins have shown that their carbohydrate component is also altered in dry eye. By immunofluorescence microscopy on conjunctival impression cytology samples, binding of the H185 antibody to an O-acetyl sialic acid epitope on MUC16 was altered on the apical cell surface of non-Sjögren’s dry eye patients [49,50]. In normal conjunctiva, the antibody bound apical epithelial cell membranes, whereas in dry eyes, a reduction of apical cell binding was observed concomitant to rose bengal staining of the conjunctival epithelium. Therefore, it is possible to speculate that alteration of mucin O-glycosylation in dry eye compromises the ocular surface epithelial barrier and results in increased abrasive stress and epithelial damage.
Dry eye is also a common feature of endocrine diseases. In complete androgen insensitivity syndrome a rare disease characterized by complete androgen deficiency due to mutations in the gene coding for the androgen receptor a significant reduction in the expression of both MUC1 and MUC5AC associated with dryness and reduced tear film stability, have been reported at the ocular surface [51*]. On the other hand, patients with polycystic ovary syndrome a common disease in reproductive age women characterized by hyperandrogenism show mucous discharge associated with reduced tear film stability and evaporative dry eye [52]. These patients have an increased goblet cell number and MUC5AC mRNA expression, which may contribute to the abnormal mucous discharge.
Lastly, one of the main features of dry eye syndrome is an increased tear film osmolarity [47]. Recent studies in yeast on mucin-like proteins Hkr1 and Msb2 have shown that membrane-bound mucins could also display features of osmosensors triggering signaling cascades [37**,38]. This possibility has yet to be confirmed in mammalian epithelia; however, it is possible to speculate that in dry eye, cell surface-associated mucins could function as osmosensors to induce signaling responses.
Autoimmune dry eye
Autoimmune diseases in which alterations of ocular surface mucins have been described include Sjögren’s syndrome and ocular cicatricial pemphigoid (OCP).
Sjögren’s syndrome is a systemic autoimmune disease most prevalent in women in their fourth and fifth decades of life. It is characterized by dryness of eyes, mouth, and other mucosae. At the ocular surface of patients affected by Sjögren’s dry eye, decreased levels of the goblet cell mucin MUC5AC have been detected in conjunctival epithelium and in tears [53], which correlates with decreased number of conjunctival goblet cells. Decreased levels of MUC19, another recently discovered gel-forming mucin, have also been described in conjunctival epithelium of patients with Sjögren’s dry eye [22].
OCP is another systemic autoimmune disease that causes chronic cicatrizing conjunctivitis, progressive subepithelial fibrosis of the conjunctiva and, in late stages, complete keratinization and drying of ocular surface epithelia. It has been reported that the cell-layer and cell-type-specific distribution of conjunctival pp-GalNAc-Ts is altered in these patients [54]. In early stages of OCP, there is an overproduction of pp-GalNAc-T isoenzymes on apical cells of the ocular surface, suggesting an early compensatory attempt to synthesize mucin O-glycans to maintain a wet-surfaced phenotype. This early increase in pp-GalNAc-Ts in the apical layers is reduced as keratinization and drying of the ocular surface proceeds [54].
Infectious diseases
It has been proposed that cell surface-associated mucins create a physical barrier against infection on the ocular surface epithelia, although to date no data exists with regard to human ocular surface disease. In Muc1-null mice, inoculation of the ocular surface with Corynebacteria and coagulase-negative Staphylococci increased blepharitis and conjunctival infection [55]. However, these results contrast with a later study investigating adherence of Pseudomonas aeruginosa to the ocular surface epithelia of Muc1-null mice, which showed no increase in bacterial adherence and conjunctivitis [56]. This discrepancy could be related to mouse strain variation in response to deletion of Muc1, to strain variation of pathogens in the two studies, or to different animal housing settings (in the first study, a conventional animal facility was used, while a murine pathogen-free environment was used in the second study). A recent paper describing increased binding of Staphylococcus aureus to corneal limbal epithelial cell cultures lacking MUC16, supports a role for cell surface-associated mucins in creating a physical barrier against pathogen penetrance [16**].
Conclusions
Mucins are hydrophilic molecules essential for the homeostasis of wet-surfaced epithelia. In this review, we show evidence that mucins could play multiple roles in the protection of mucosal surfaces. Gel-forming mucins are essential for lubrication and clearance of allergens, pathogens and debris. Cell surface-associated mucins provide the epithelial surface with an anti-adhesive character essential to reducing abrasive stress and could form a protective apical barrier against microorganisms and extracellular molecules.
At the ocular surface, alteration of mucin expression and glycosylation are associated with a number of diseases. Recent advances in understanding the role of mucins in maintaining an intact mucosal barrier could contribute to the development of new therapeutic strategies.
Acknowledgments
Financial support by NIH grant R01EY014847 to Pablo Argüeso.
Abbreviations
- AKC
atopic keratoconjunctivitis
- FITC
fluorescein isothiocyanate
- GalNAc
N-acetyl-galactosamine
- HOG
high osmolarity glycerol
- MUC
human mucin
- Muc
mouse mucin
- OCP
ocular cicatricial pemphigoid
- pp-GalNAc-Ts
polypeptide N-acetyl-galactosamine transferases
- SEA
sea-urchin sperm protein, enterokinase and agrin
- VKC
vernal keratoconjunctivitis
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2007;48:4391–8. doi: 10.1167/iovs.07-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–34. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 3.Vinall LE, Hill AS, Pigny P, et al. Variable number tandem repeat polymorphism of the mucin genes located in the complex on 11p15.5. Hum Genet. 1998;102:357–66. doi: 10.1007/s004390050705. [DOI] [PubMed] [Google Scholar]
- 4.Fowler J, Vinall L, Swallow D. Polymorphism of the human MUC genes. Front Biosci. 2001;6:D1207–15. doi: 10.2741/A674. [DOI] [PubMed] [Google Scholar]
- 5.Chao CC, Vergnes JP, Brown SI. O-glycosidic linkage in glycoprotein isolates from human ocular mucus. Exp Eye Res. 1983;37:533–41. doi: 10.1016/0014-4835(83)90129-x. [DOI] [PubMed] [Google Scholar]
- 6.Chao CC, Butala SM, Herp A. Studies on the isolation and composition of human ocular mucins. Exp Eye Res. 1988;47:185–96. doi: 10.1016/0014-4835(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 7.Hanisch FG. O-glycosylation of the mucin type. Biol Chem. 2001;382:143–9. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- 8.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: Mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–78. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 10.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 11.Itoh Y, Kamata-Sakurai M, Denda-Nagai K, et al. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74–83. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]
- 12.Argüeso P, Spurr-Michaud S, Russo CL, et al. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–95. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 13.Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci. 1995;36:1818–27. [PubMed] [Google Scholar]
- 14.Inatomi T, Spurr-Michaud S, Tisdale AS, et al. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996;37:1684–92. [PubMed] [Google Scholar]
- 15.Spurr-Michaud S, Argüeso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007;84:939–50. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blalock TD, Spurr-Michaud SJ, Tisdale AS, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48(10):4509–18. doi: 10.1167/iovs.07-0430. **The authors show that MUC16 abrogation in cultured human corneal epithelial cells results in loss of cell surface protection against dye penetrance and increased S. aureus adherence, suggesting that cell surface-associated mucins contribute to the formation of a protective barrier against injury and pathogens. [DOI] [PubMed] [Google Scholar]
- 17.Pemberton LF, Rughetti A, Taylor-Papadimitriou J, Gendler SJ. The epithelial mucin MUC1 contains at least two discrete signals specifying membrane localization in cells. J Biol Chem. 1996;271:2332–40. doi: 10.1074/jbc.271.4.2332. [DOI] [PubMed] [Google Scholar]
- 18.Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379–88. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 19.Jumblatt MM, McKenzie RW, Steele PS, et al. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea. 2003;22:41–5. doi: 10.1097/00003226-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Kessing SV. Mucous gland system of the conjunctiva. A quantitative normal anatomical study. Acta Ophthalmol (Copenh) 1968;(Suppl 95):1. [PubMed] [Google Scholar]
- 21.Paz HB, Tisdale AS, Danjo Y, et al. The role of calcium in mucin packaging within goblet cells. Exp Eye Res. 2003;77:69–75. doi: 10.1016/s0014-4835(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 22.Yu DF, Chen Y, Han JM, et al. MUC19 expression in human ocular surface and lacrimal gland and its alteration in Sjogren syndrome patients. Exp Eye Res. 2008;86:403–11. doi: 10.1016/j.exer.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie RW, Jumblatt JE, Jumblatt MM. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Invest Ophthalmol Vis Sci. 2000;41:703–8. [PubMed] [Google Scholar]
- 24.Jager K, Wu G, Sel S, et al. MUC16 in the lacrimal apparatus. Histochem Cell Biol. 2007;127:433–8. doi: 10.1007/s00418-006-0246-6. [DOI] [PubMed] [Google Scholar]
- 25.Paulsen F, Langer G, Hoffmann W, Berry M. Human lacrimal gland mucins. Cell Tissue Res. 2004;316:167–77. doi: 10.1007/s00441-004-0877-7. [DOI] [PubMed] [Google Scholar]
- 26.Paulsen FP, Corfield AP, Hinz M, et al. Characterization of mucins in the human lacrimal sac and nasolacrimal duct. Invest Ophthalmol Vis Sci. 2003;44:1807–13. doi: 10.1167/iovs.02-0744. [DOI] [PubMed] [Google Scholar]
- 27.Rubin BK. Physiology of airway mucus clearance. Respir Care. 2002 Jul;47:761–8. [PubMed] [Google Scholar]
- 28.Berry M, Ellingham RB, Corfield AP. Human preocular mucins reflect changes in surface physiology. Br J Ophthalmol. 2004;88:377–83. doi: 10.1136/bjo.2003.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleiszig SM, Zaidi TS, Ramphal R, Pier GB. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect Immun. 1994;62:1799–804. doi: 10.1128/iai.62.5.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlstedt I, Sheehan JK, Corfield AP, Gallagher JT. Mucous glycoproteins: a gel of a problem. Essays Biochem. 1985;20:40–76. [PubMed] [Google Scholar]
- 31.Bobek LA, Situ H. MUC7 20-mer: investigation of antimicrobial activity, secondary structure, and possible mechanism of antifungal action. Antimicrob Agents Chemoter. 2003;47:643–52. doi: 10.1128/AAC.47.2.643-652.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols B, Dawson CR, Togni B. Surface features of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 1983;24:570–6. [PubMed] [Google Scholar]
- 33.Sumiyoshi M, Ricciuto J, Tisdale A, et al. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:197–203. doi: 10.1167/iovs.07-1038. **Using static and dynamic flow assays, the authors demonstrate that mucin O-glycans confer an anti-adhesive character to the apical cell surface of corneal epithelial cells. These results suggest that mucin O-glycans prevent tarsal conjunctiva from adhering to corneal epithelium during blinking or sleeping, and that alteration of mucin O-glycans in ocular surface disease could induce epithelial damage by abrasive stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Argüeso P, Tisdale A, Spurr-Michaud S, et al. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 2006;47:113–9. doi: 10.1167/iovs.05-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAuley JL, Linden SK, Png CW, et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest. 2007;117:2313–24. doi: 10.1172/JCI26705. **The authors report that oral inoculation of Muc1-null mice with Campylobacter jejuni results in intestinal damage and systemic infection. Their findings suggest that Muc1 is a critical component of the mucosal barrier that protects the epithelium and prevents pathogen adherence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.An G, Wei B, Xia B, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–29. doi: 10.1084/jem.20061929. **The authors demonstrate that mice lacking core3-derived O-glycans in colon have increased intestinal permeability and a higher susceptibility to colitis, suggesting that reduction in mucin O-glycosylation may impair barrier function and enhance bacterial adherence to colonic mucosa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatebayashi K, Tanaka K, Yang HY, et al. Transmembrane mucins Hkr1 and Msb2 are putative osmosensors in the SHO1 branch of yeast HOG pathway. EMBO J. 2007;26:3521–33. doi: 10.1038/sj.emboj.7601796. **The authors show that yeast Msb2 and Hkr1, which are structurally related to mammalian membrane-bound mucins, activate the HOG pathway in response to increased extracellular osmolarity. It is, therefore, possible to speculate that membrane-bound mucins may act as osmosensors in higher eukariotes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Nadal E, Real FX, Posas F. Mucins, osmosensors in eukariotic cells? Trends Cell Biol. 2007;17:571–4. doi: 10.1016/j.tcb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Dogru M, Okada N, Asano-Kato N, et al. Atopic ocular surface disease: implications on tear function and ocular surface mucins. Cornea. 2005;24:S18–S23. doi: 10.1097/01.ico.0000178741.14212.53. [DOI] [PubMed] [Google Scholar]
- 40.Dogru M, Okada N, Asano-Kato N, et al. Alterations of the ocular surface epithelial mucins 1, 2, 4 and the tear functions in patients with atopic keratoconjunctivitis. Clin Exp Allergy. 2006;36:1556–65. doi: 10.1111/j.1365-2222.2006.02581.x. [DOI] [PubMed] [Google Scholar]
- 41.Aragona P, Romeo GF, Puzzolo D, et al. Impression cytology of the conjunctival epithelium in patients with vernal conjunctivitis. Eye. 1996;10:82–5. doi: 10.1038/eye.1996.12. [DOI] [PubMed] [Google Scholar]
- 42.Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye. 2004;18:345–51. doi: 10.1038/sj.eye.6700675. [DOI] [PubMed] [Google Scholar]
- 43.Burgel PR, Lazarus SC, Tam DC, et al. Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation. J Immunol. 2001;167:5948–54. doi: 10.4049/jimmunol.167.10.5948. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Matsumoto Y, Dogru M, et al. The differences of tear function and ocular surface findings in patients with atopic keratoconjunctivitis and vernal keratoconjunctivitis. Allergy. 2007;62:917–25. doi: 10.1111/j.1398-9995.2007.01414.x. [DOI] [PubMed] [Google Scholar]
- 45.Corum I, Yeniad B, Bilgin LK, Ilhan R. Efficiency of Olopatadine hydrochloryde 0.1% in treatment of vernal keratoconjunctivitis and goblet cell density. J Ocul Pharm Ther. 2005;21:400–5. doi: 10.1089/jop.2005.21.400. [DOI] [PubMed] [Google Scholar]
- 46.Kunert KS, Keane-Myers AM, Spurr-Michaud S, et al. Alteration in goblet cell numbers and mucin gene expression in a mouse model of allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2001;42:2483–9. [PubMed] [Google Scholar]
- 47.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. 2007. [DOI] [PubMed] [Google Scholar]
- 48.Versura P, Maltarello MC, Cellini M, et al. Detection of mucus glycoconjugates in human conjunctiva by using the lectin-colloidal gold technique in TEM. II. A quantitative study in dry-eye patients. Acta Opthalmol (Copenh) 1986;64:451–5. doi: 10.1111/j.1755-3768.1986.tb06952.x. [DOI] [PubMed] [Google Scholar]
- 49.Argüeso P, Sumiyoshi M. Characterization of a carbohydrate epitope defined by the monoclonal antibody H185: sialic acid O-acetylation on epithelial cell-surface mucins. Glycobiology. 2006;16:1219–28. doi: 10.1093/glycob/cwl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danjo Y, Watanabe H, Tisdale AS, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–9. [PubMed] [Google Scholar]
- 51.Mantelli F, Moretti C, Micera A, Bonini S. Conjunctival mucin deficiency in complete androgen insensitivity syndrome (CAIS) Graefes Arch Clin Exp Ophthalmol. 2007;245:899–902. doi: 10.1007/s00417-006-0452-x. *The authors report decreased levels of conjunctival MUC1 and MUC5AC in complete androgen insensitivity syndrome. These findings are associated to reduced tear film stability and dry eye symptoms, and suggest that androgens play a role in the maintenance of tear film homeostasis. [DOI] [PubMed] [Google Scholar]
- 52.Bonini S, Mantelli F, Moretti C, et al. Itchy-dry eye associated with polycystic ovary syndrome. Am J Ophthalmol. 2007;143:763–71. doi: 10.1016/j.ajo.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 53.Argüeso P, Balaram M, Spurr-Michaud S, et al. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–11. [PubMed] [Google Scholar]
- 54.Argüeso P, Tisdale A, Mandel U, et al. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Invest Ophthalmol Vis Sci. 2003;44:86–92. doi: 10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- 55.Kardon R, Price RE, Julian J, et al. Bacterial conjunctivitis in Muc1 null mice. Invest Ophthalmol Vis Sci. 1999;40:1328–35. [PubMed] [Google Scholar]
- 56.Danjo Y, Hazlett LD, Gipson IK, et al. C57BL/6 mice lacking Muc1 show no ocular surface phenotype. Invest Ophthalmol Vis Sci. 2000;41:4080–4. [PubMed] [Google Scholar]