Abstract
Helicobacter pylori is a Gram-negative bacterium that infects over 50% of the world’s population. This organism causes various gastric diseases such as chronic gastritis, peptic ulcer, and gastric cancer. H. pylori possesses lipopolysaccharide, which shares structural similarity to Lewis blood group antigens in gastric mucosa. Such antigenic mimicry could result in immune tolerance against antigens of this pathogen. On the other hand, H. pylori colonize gastric mucosa by utilizing adhesins, which bind Lewis blood group antigen-related carbohydrates expressed on gastric epithelial cells. In chronic gastritis, lymphocytes infiltrate the lamina propria, and such infiltration is facilitated by 6-sulfo sialyl Lewis X-capped O-glycans, peripheral lymph node addressin (PNAd), on high endothelial venule (HEV)-like vessels. The number of HEV-like vessels increases as chronic inflammation progresses. Furthermore, PNAd formed on HEV-like vessels disappear once H. pylori is eradicated. These results indicate that PNAd plays an important role in H. pylori-associated inflammation. H. pylori barely colonizes gland mucous cell-derived mucin where α1,4-GlcNAc-capped O-glycans exist. In vitro experiments show that α1,4-GlcNAc-capped O-glycans function as a natural antibiotic to inhibit H. pylori growth. We recently identified cholesterol α-glucosyltransferase (CHLαGcT) using an expression cloning strategy and showed that this enzyme is specifically inhibited by mucin-type O-glycans like those present in deeper portions of the gastric mucosa. These findings show that a battery of carbohydrates expressed in the stomach is closely associated with pathogenesis and also prevention of H. pylori-related diseases.
Keywords: Helicobacter pylori; lipopolysaccharide; Lewis blood group antigen; adhesin; 6-sulfo sialyl Lewis X-capped O-glycan; α1,4-GlcNAc-capped O-glycan; cholesteryl-α-D-glucopyranoside; cholesterol α-glucosyltransferase
1. INTRODUCTION
1.1. Impact of H. pylori Discovery
Spiral microorganisms in the stomach had been observed in the 1930’s and 1940’s [1, 2], but little attention was paid to gastric microorganisms. In 1983, Marshall and Warren in Australia first isolated and succeeded in culturing the bacterium Helicobacter pylori, originally named Campylobacter pyloridis, from the gastric mucosa of patients with chronic gastritis [3, 4]. Surprisingly, Marshall himself drank a culture of H. pylori to prove that the bacteria could infect a healthy person and cause gastritis [5]. Their epoch-making discovery revealed that H. pylori is associated with various gastric diseases such as chronic gastritis, peptic ulcer, and malignant tumors including gastric carcinoma and malignant lymphoma, and the eradication of this microorganism prevents such gastric disorders. For their achievement, Marshall and Warren won the Nobel Prize in Physiology or Medicine in 2005 [6].
1.2. Specialized Traits of H. pylori
H. pylori is a spiral-shaped, Gram-negative, and microaerophilic bacterium, measuring approximately 3–5 μm in length. H. pylori is a member of a genus of bacteria that have adapted to the ecological niche provided by gastric mucus, where there is little competition from other microorganisms [7]. Many specialized traits allow this organism to flourish in the harsh environment of the stomach. First, H. pylori elaborates a large amount of urease (10%–15% of total proteins by weight), which produces ammonia and carbon dioxide resulting from hydrolysis of endogenous urea, thereby buffering (neutralizing) gastric acid in the immediate vicinity of the organism. H. pylori also possesses numerous long flagella, the flailing movements of which allow them to swim through viscous gastric mucus with forceful screw-like movements, much like the spinning of a drill bit [8]. Finally, H. pylori binds to gastric epithelial cells via bacterial adhesins: the bacterium colonizes the gastric mucosa by adhering to mucous epithelial cells and the mucus layer lining the gastric epithelium. H. pylori possesses adhesins that enhance adhesion with gastric epithelial cells by recognizing specific carbohydrate structures, such as the Lewis b blood group antigen and sialyl dimeric Lewis X (see section 2.3. for detail).
1.3. Epidemiology of H. pylori Infection
H. pylori infection occurs worldwide and affects over 50% of the world’s population, but the prevalence of infection varies greatly from country to country. The overall prevalence is highly correlated with socioeconomic status measured by household crowding and parental income [9, 10]. Prevalence among adults is approximately 80% in many developing countries and 50% in industrialized countries [11]. The prevalence of infection increases with advancing age. In some populations, a disproportionately high rate of H. pylori infection is observed in people over 40. This seems to reflect a birth cohort effect: transmission of this chronic infection was more common in the past than it is today [12–14].
The mode of transmission has not yet been fully defined; however, it is widely believed that the organism is transmitted directly from person to person by human feces (fecal-oral spread) or gastric contents (gastric-oral spread). It is now generally accepted that most individuals acquire H. pylori infection in childhood [15]. Once the stomach is colonized and left untreated, the organism persists for decades, if not for a lifetime [16]. Frequently children are infected by a strain with a genetic fingerprint identical to that of each parent. Husbands and wives do not exchange strains, and infection is rarely transmitted to an uninfected partner [17].
1.4. H. pylori and Associated Diseases
1.4.1. Chronic Gastritis
Following H. pylori infection, a chronic, usually lifelong mucosal inflammation (gastritis) develops with concomitant appearance of serological responses against the bacterium. However, H. pylori is resistant to innate and acquired immune responses, and the immune system fails to remove the organism effectively [18]. Chronic gastritis leads eventually to mucosal atrophy characterized by a decrease in the proper gastric glands, and intestinal metaplasia marked by the replacement of gastric epithelial cells with other epithelial cells such as columnar absorptive cells and goblet cells of intestinal morphology [19].
Intestinal metaplasia has been categorized into two major types: one is the complete type, which is characterized by the presence of absorptive cells, Paneth cells, and goblet cells secreting sialomucins and corresponds to the small intestine phenotype, and the other is the incomplete type, which is characterized by the presence of columnar and goblet cells secreting sialo and/or sulfomucins [20]. These two types of intestinal metaplasia can be distinguished also by altered mucin expression patterns. While the intestinal mucin MUC2 is expressed in goblet cells of both types of intestinal metaplasia (normal gastric mucosa does not express MUC2 [21]), MUC1, MUC5AC and/or MUC6 is expressed in the incomplete type but not in the complete type [20].
1.4.2. Peptic Ulcer
Peptic ulcers are chronic, often solitary lesions that occur in gastroduodenal mucosa exposed to aggressive action of acid-peptic juices. These lesions appear to be produced by an imbalance between mucosal defense mechanisms and damaging forces. The pathogenesis of peptic ulcers appears to be multi-factorial, and the apparent role of H. pylori in peptic ulcers cannot be overemphasized. However, H. pylori infection is present in virtually all patients with duodenal ulcers and about 70% of those with gastric ulcers. Furthermore, antibiotic treatment of H. pylori infection promotes healing of ulcers and tends to prevent their recurrence [7].
1.4.3. Gastric Adenocarcinoma
Gastric adenocarcinoma is the fourth most common cancer and second leading cause of cancer-related death worldwide [22]. Gastric adenocarcinoma can be divided into two distinct histological subtypes [23], each with different epidemiological and clinicopathological features. One subtype is intestinal-type adenocarcinoma, which usually occurs at a later age and progresses through a relatively well-defined series of histological steps, namely, chronic gastritis, atrophy of pyloric glands, intestinal metaplasia, and dysplasia [24]. The other subtype is diffuse-type adenocarcinoma, which more commonly affects younger people and is not associated with intestinal metaplasia [24].
1.4.4. MALT Lymphoma
Most lymphomas of the stomach are mucosa-associated lymphoid tissue (MALT) lymphoma, a low-grade B cell lymphoma. This type of lymphoma arises in MALT, hence the name. B cells that give rise to MALT lymphomas normally reside in the marginal zones of lymphoid follicles and are increased in response to various types of chronic inflammation, including chronic gastritis due to H. pylori infection [25]. It is generally accepted that chronic infection with H. pylori leads to generation of H. pylori-reactive T cells, which, in turn, activate a polyclonal population of B cells by secreting soluble factors. In time, a monoclonal but T cell-dependent population of proliferating B cells emerges. Presumably, such monoclonal B cell proliferation subsides when the antigenic stimulus for T cells is removed by antibiotic treatment. However, if untreated, genetic mutations accumulate in these proliferating B cells, and they eventually become T cell-independent [26].
1.5. Virulence Factors
1.5.1. CagA
In the industrialized world, 60–70% of H. pylori strains possess the cytotoxin-associated antigen A (CagA), a 120–145 kDa protein [27]. The cagA gene is localized at one end of the cag pathogenicity island (cag-PAI), a 37-kb genomic fragment containing 31 genes [28, 29]. Several of these are homologous to genes encoding the type IV secretion apparatus [17]. Upon direct contact of H. pylori with gastric epithelial cells, CagA is injected from the bacterium into the host cell via the type IV secretion system [30–33]. After entering an epithelial cell, CagA is phosphorylated and binds to Src homology 2 domain-containing tyrosine phosphatase 2 (SHP-2), leading to a growth factor-like cellular response and cytokine production [34]. Deregulation of SHP-2 by CagA is an important mechanism by which CagA promotes gastric epithelial carcinogenesis. Recently, Ohnishi and co-workers generated CagA transgenic mice and found that CagA induces abnormal proliferation of gastric epithelial cells and hematopoietic cells, followed by the development of gastrointestinal carcinomas and leukemias/lymphomas in a tyrosine phosphorylation-dependent manner, revealing H. pylori CagA is the first bacterial oncoprotein that acts in mammals. [35]. CagA also elicits junctional and polarity defects in epithelial cells by interacting with and inhibiting partitioning-defective 1 (PAR1)/microtubule affinity-regulating kinase (MARK) independently of CagA tyrosine phosphorylation [36].
Very recently, Marcos and co-workers reported that cag-PAI+ high pathogenic H. pylori strains induce expression of several genes involved in glycan biosynthesis, in particular that encoding β1,3-N-acetylglucosaminyltransferase 5 (β3GnT5) [37], a GlcNAc transferase essential for the biosynthesis of Lewis antigens on glycolipids [38]. This induction is dependent on cagA and cagE, most probably through the TNF/NF-κB pathway [38]. The study identified a novel mechanism by which H. pylori modulates the biosynthesis of the sialic acid-binding adhesin (SabA) ligand in gastric cells, thereby strengthening the epithelial attachment necessary to achieve successful colonization (see also section 2.4.2. for detail).
1.5.2. VacA
Vacuolating toxin (VacA) is a major virulence factor secreted by H. pylori and is a key component in the pathogenesis of gastric diseases [39]. Approximately 50% of H. pylori strains express the VacA protein, and that expression is correlated with expression of CagA. The most established activity of VacA is cellular vacuolation in mammalian cells [39–41]. Although the precise mechanism of VacA-induced vacuole formation is not fully understood, it involves binding and internalization of toxin. It has been proposed that vacuolation is a consequence of anion-selective channel formation in late endosomal compartments [42–45]. In addition to its vacuole formation activity, VacA causes numerous cellular events, including depolarization of the membrane [44, 46], apoptosis [47–50], interference with epithelial cell attachment [51], and inhibition of T lymphocyte activation [52].
2. GLYCOCONJUGATES ASSOCIATED WITH H. PYLORI
2.1. Glycan Structure of H. pylori LPS
The cell wall of all Gram-negative bacteria is composed of two phospholipid bilayers with a peptidoglycan layer sandwiched between them. Lipopolysaccharide (LPS) is a structural component of the outer cell wall. LPS is composed of a long-chain fatty acid anchor called lipid A, a core sugar chain, and a variable carbohydrate chain designated O antigen, which is attached to the core sugar [53]. Thus, the O antigen has the potential to exhibit enormous structural variability and is the domain determining the serological specificity of LPS [54].
Clinical isolates of H. pylori produce O antigen of a relatively constant chain length [55]. It is this region of H. pylori LPS that shares structural homology with Lewis blood group antigens in the gastric mucosa, predominantly Lewis X and Lewis Y antigens bearing type 2 blood group determinants. Serologically, 80–90% of H. pylori strains have been found to contain Lewis X and/or Lewis Y epitopes.
2.2. Putative Consequence of Lewis Expression by H. pylori
Lewis blood group antigens are present in normal human gastric mucosa, and the expression of these antigens on H. pylori LPS has important biological implications. Molecular mimicry mediated by H. pylori LPS has been suggested to camouflage the bacterium and facilitate initial colonization [56].
Additionally, H. pylori Lewis antigens undergo phase variation: specifically, random, reversible high-frequency switching of phenotype contributes to virulence. The molecular mechanisms involved in phase variation are slipped-strand mispairing in poly-C tracts and translational frameshifting by ribosomal slippage [57]. At least 5 glycosyltransferase genes are involved in generating phase variants: the genes encoding α3-fucosyltransferase (of which there are two similar but non-identical copies), α2-fucosyltransferase, β3-galactosyltransferase and β3-N-acetyl-D-glucosaminyltransferase [58]. Each of these genes can be either “on” or “off”, and thus, in any H. pylori cell population, at least 32 different glycosyltransferase gene “on-off” combinations and potentially the same number of LPS phenotypes are present [58]. Thus, any H. pylori strain can potentially express any LPS Lewis phenotype.
This antigenic mimicry may result in immune tolerance against antigens of the pathogen or in induction of autoantibodies that recognize gastric epithelial cells, which is frequently observed in patients with chronic active gastritis.
2.3. Adhesion of H. pylori to Gastric Epithelial Cells
Attachment is a prerequisite for microbial colonization of epithelial surfaces and is mediated by molecules on the bacterial surface, adhesins, which recognize proteins or glycoconjugates on the surface of eukaryotic cells. The specificity of this interaction and the limited distribution of receptors often result in a restricted range of hosts and tissues utilized for colonization, a phenomenon known as tropism. Bacteria, which are unable to adhere to epithelia, tend to be rapidly removed by shedding from surface cells and the mucus layer.
H. pylori expresses adhesins that confer intimate adherence to the gastric epithelium where the bacteria can gain easy access to nutrients from host tissues [59]. These adherence properties protect the bacteria from the extreme acidity of the gastric lumen and displacement from the stomach by forces such as those generated by peristalsis and gastric emptying [60]. Two carbohydrate structures in surface mucous cells serve as specific ligands for H. pylori adhesins: Lewis b, which binds to blood group antigen-binding adhesin (BabA), and sialyl dimeric Lewis X-bearing glycosphingolipid, which binds to sialic acid-binding adhesin (SabA). In addition, attachment of H. pylori to gastric epithelial cells can induce pedestal formation [61]. Pedestal formation describes the creation of an upright support, constructed of host cell material, beneath an attached bacterium.
2.4. H. pylori Adhesins
2.4.1. BabA
The best defined H. pylori adhesin-receptor interaction characterized to date is that between BabA, a member of a family of H. pylori outer membrane proteins, and Lewis b, H, and related ABO antigens [60]. These fucose-containing blood group antigens are found on red blood cells and in the gastrointestinal mucosa. Blood group O individuals suffer disproportionately from peptic ulcer disease [62], suggesting that bacterial adherence to H and Lewis b antigens influences severity of infection. The human population of South American Amerindians dominantly express blood group O antigen. Interestingly, BabA from this population binds blood group O antigen more efficiently than other blood group antigens [59]. BabA has 2 isoforms, babA1 and babA2. The product of babA1 gene, in contrast to that encoded by the babA2 gene, cannot interact with Lewis b; thus it does not enhance H. pylori colonization of the surface epithelium [60, 63].
2.4.2. SabA
The SabA gene encodes a 651 amino acid protein of 70 kDa and belongs to the large hop family of H. pylori outer membrane protein genes, which also includes the babA gene [64]. Sialyl dimeric Lewis X glycolipid is rarely expressed in normal gastric mucosa. However, the gastric mucosa infected by H. pylori, particularly cag-PAI+ strains, newly expresses this unique glycolipid in surface mucous cells partly facilitated by increased expression of β3GnT5 [37, 38], and its expression level is increased as inflammation progresses. The adhesion mediated by SabA binding to sialyl dimeric Lewis X glycolipid contributes to persistent H. pylori infection. Sialyl dimeric Lewis X is also expressed in leukocytes, but an “on-off” frameshift mutation of the SabA gene allows H. pylori to escape intimate contact with these inflammatory cells. Such adaptive mechanisms play an important role in the extraordinary chronicity of H. pylori infection in human gastric mucosa.
3. INDUCTION OF PNAD IN GASTRIC MUCOSA INFECTED BY H. PYLORI
3.1. Role of PNAd in Secondary Lymphoid Organs and Chronic Inflammatory Sites
In chronic inflammatory states, L-selectin and its ligands are implicated in lymphocyte recruitment in those diseases in which peripheral lymph node addressin (PNAd) is induced on high endothelial venule (HEV)-like vessels [65, 66]. Such HEV-like vessels have been observed in rheumatoid arthritis, lymphocytic thyroiditis, and inflammatory bowel diseases [67–71]. In these studies, the induction of PNAd is detected by the MECA-79 antibody [72], which decorates PNAd on HEV-like vessels. MECA-79-positive HEVs in secondary lymphoid organs play a major role in lymphocyte circulation [65]. The MECA-79 epitope has been shown to be 6-sulfo N-acetyllactosamine attached to extended core 1 O-glycans, Galβ1→4(SO3→6)GlcNAcβ1→3Galβ1→3GalNAcα1→Ser/Thr [73]. Moreover, MECA-79 antibody can also bind to its sialylated and fucosylated form that constitutes PNAd [73]. Structural studies also show that 6-sulfo sialyl Lewis X on core 2 branched O-glycans, sialic acidα2→3Galβ1→4[Fucα1→3(SO3→6)]GlcNAc β1→6(Galβ1→3)GalNAcα1→Ser/Thr, is present as a major L-selectin ligand on HEVs [73, 74].
3.2. HEV-Like Vessels Are Induced in H. pylori-Induced Inflammation
Because it has been reported that de novo formation of HEV-like vessels, which express PNAd, is associated with various chronic inflammatory diseases, we determined whether chronic inflammation caused by H. pylori infection is associated with formation of HEV-like vessels [75]. To do so, gastric mucosa from patients infected with H. pylori was stained with MECA-79 antibody and HECA-452 antibody, which reacts equally well with sialyl Lewis X and 6-sulfo sialyl Lewis X capped structure on extended core 1 and core 2 branches. Gastric mucosa derived from H. pylori-infected patients displayed HEV-like vessels expressing MECA-79 and HECA-452 antigens as well as CD31 and CD34, which are markers of vascular endothelial cells. These HEV-like vessels can potentially recruit L-selectin-expressing lymphocytes, because L-selectin•IgM chimeric protein bound to the same vessels in a calcium-dependent manner [75]. These results indicate that H. pylori-induced inflammation is associated with formation of PNAd present on HEV-like vessels.
These results demonstrate that 6-sulfo sialyl Lewis X attached to extended core 1 O-glycans is present on HEV-like vessels, based on positive staining by MECA-79 and HECA-452 antibodies. To elaborate further the chemical nature of L-selectin ligands on these vessels, the NCC-ST-439 monoclonal antibody was used. NCC-ST-439 antibody binding has been verified for sialyl Lewis X-capped structure on Galβ1→4GlcNAcβ1→6GalNAcα1→R but not on natural core 2 branched O-glycan Galβ1→4GlcNAcβ1→6 (Gal β1→3)GalNAcα1→R [76]. Moreover, it has not been determined whether 6-sulfo sialyl Lewis X is also recognized by this antibody. To test these possibilities, we made CHO cells expressing various types of O-glycans and stained cells with NCC-ST-439 antibody. NCC-ST-439 antibody binds to CHO cells expressing non-sulfated and 6-sulfo sialyl Lewis X on core 2 branched O-glycans but barely to CHO cells expressing those capped structures on extended core 1 O-glycans. NCC-ST-439 antibody can also stain HEV-like vessels formed in the gastric mucosa. These combined results suggest that PNAd induced by H. pylori infection expresses 6-sulfo sialyl Lewis X on both extended core 1 and core 2 branched structures in the same manner as PNAd expressed in secondary lymphoid organs [73].
3.3. Increased Formation of HEV-Like Vessels Is Correlated with Progression of Inflammation
Based on the updated Sydney system, progression of inflammation initiated by H. pylori infection is ranked in four stages from least to most severe: normal, mild, moderate, and marked [77]. In moderate and marked stages, intestinal metaplasia frequently occurs, indicating an advanced stage of the disease.
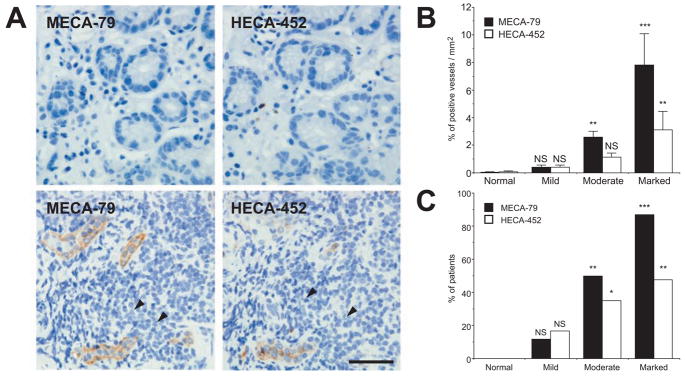
Fig. (1) A (lower panels) indicates a marked stage of inflammation in which recruitment of mononuclear cells obscures proper glands in the gastric mucosa, which contrasts with glands visible in mucosa at the mild stage (Fig. (1) A, upper panels). These observations demonstrate that lymphocyte infiltration is more prominent when HEV-like vessels are more abundant.
Fig. (1).
Gastric mucosa of different degrees of chronic inflammation and association of HEV-like vessels with progression of inflammation. (A) (Upper) Gastric mucosa at a mild stage barely expresses HEV-like vessels with minimum recruitment of lymphocytes. (Lower) Gastric mucosa at a marked stage expresses a significant number of recruited lymphocytes (arrowheads) around HEV-like vessels. (B) The number of MECA-79+ or HECA-452+ vessels is positively correlated with the progression of chronic inflammation. Each group consists of 11 (normal), 42 (mild), 67 (moderate), and 23 (marked) patients. (C) The number of patients exhibiting greater than 1% MECA-79+ or HECA-452+ vessels is highly correlated with progression of chronic inflammation. *, P<0.05; **, P<0.01;, P<0.001; NS, not significant. Bar = 50 μm. Adapted with permission from Kobayashi, M.; Mitoma, J.; Nakamura, N.; Katsuyama, T.; Nakayama, J.; Fukuda, M. Proc. Natl. Acad. Sci. U. S. A., 2004, 101(51), 17807–17812, Copyright 2004 National Academy of Science, USA.
After examining over 140 human specimens, we found that the number of HEV-like vessels, as detected by MECA-79 or HECA-452 antibody, correlates positively with the progression of inflammation (Fig. (1) B). Fig. (1) C illustrates that more patients display HEV-like vessels as inflammation progress. H. pylori was detected in 0%, 21%, 82% and 87% of patients in normal, mild, moderate, and marked stages of inflammation, respectively. Overall, HEV-like vessels were found in 79.2% of H. pylori infected patients.
3.4. Formation of HEV-Like Vessels Requires Continuous H. pylori Infection
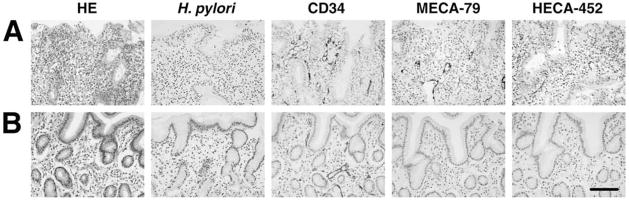
To determine whether formation of HEV-like vessels is correlated with H. pylori infection, gastric biopsies were obtained from 17 patients with chronic active gastritis before and after eradication of H. pylori by treatment with antibiotics and a proton pump inhibitor. Patients with moderate inflammation displayed both H. pylori and HEV-like vessels detected by MECA-79 and HECA-452 antibodies (Fig. (2) A). After eradication of H. pylori, the gastric mucosa of all patients no longer displayed HEV-like vessels as assessed by MECA-79 and HECA-452 staining and showed minimum lymphocyte infiltration (Fig. (2) B). These results indicate that continuous infection of H. pylori is necessary for formation and maintenance of HEV-like vessels expressing PNAd. It is tempting to speculate that bacterial components such as LPS acting through Toll-like receptor-dependent pathways in the gastric epithelium, stimulate the release of cytokines, i.e., lymphotoxin α [78]. This effect might in turn modulate gene expression in postcapillary venules in ways that could cause their biochemical, functional, and morphological transformation by up-regulating chemokines, such as CCL19 and CCL21 that act on CCR7 receptors.
Fig. (2).
Disappearance of HEV-like vessels in the gastric mucosa after eradication of H. pylori. Gastric mucosa infected with H. pylori was examined before and 2 months after treatment to eradicate H. pylori. (A) Before treatment, HEV-like vessels detected by MECA-79 and HECA-452 antibodies were abundant, and large numbers of mononuclear cells (lymphocytes) were present around these vessels. (B) After eradication of H. pylori, HEV-like vessels were no longer present and very few mononuclear cells were present. CD34 was used for a marker of vascular endothelial cells. HE, hematoxylin and eosin, Bar = 100 μm. Adapted with permission from Kobayashi, M.; Mitoma, J.; Nakamura, N.; Katsuyama, T.; Nakayama, J.; Fukuda, M. Proc. Natl. Acad. Sci. USA, 2004, 101(51), 17807–17812, Copyright 2004 National Academy of Science, USA.
3.5. HEV-Like Vessels in NSAID-Induced Gastritis
It is well established that continuous use of non-steroidal anti-inflammatory drugs (NSAIDs) results in chemical gastritis [79]. To determine whether HEV-like vessels are induced in chronic inflammatory responses caused by factors other than H. pylori infection, gastric mucosa obtained from long-term rheumatoid arthritis patients taking NSAIDs was examined. Most of the 20 patients examined exhibited chemical gastritis phenotypes and were devoid of HEV-like vessels. HEV-like vessels were found in specimens from six of the 20 patients, but three of these were also infected with H. pylori. Those three patients also had lower scores for chemical gastritis, and HEV-like vessels were likely formed by inflammation caused by H. pylori infection. In three H. pylori-free patients, HEV-like vessels were found only in 0.68%, 0.67% and 0.21% of CD34-positive vessels, and two patients displayed intestinal metaplasia, suggesting a possible prior H. pylori infection. The other patient displayed both a chemical gastritis phenotype and lymphocyte recruitment. Interestingly, MECA-79 staining was much less intense in tissues from this patient than in tissues from patients infected with H. pylori. These results indicate that chemical gastritis induces PNAd at a very low level in the gastric mucosa.
4. ANTIBIOTIC FUNCTION OF α1,4-GLCNAC-CAPPED O-GLYCANS AGAINST H. PYLORI
4.1. Two Types of Mucins Present in Gastric Mucosa
As described in section 1.3., over half the world’s population harbor H. pylori, but only a fraction of those infected develop diseases such as peptic ulcer disease, gastric adenocarcinoma, and MALT lymphoma. This observation suggests the presence of host defense mechanisms against H. pylori pathogenesis.
Gastric mucins are classified into two types based upon their histochemical properties [80]; one is a surface mucous cell-derived mucin displayed on MUC5AC core protein [21], and the other is a mucin displayed on MUC6 core protein secreted by gland mucous cells, including cardiac gland cells, mucous neck cells, and pyloric gland cells [81]. These two types of mucins form the surface mucous gel layer (SMGL), which shows an alternating laminated array.
H. pylori is associated exclusively with surface mucous cell-derived mucins and rarely colonizes deeper portions of gastric mucosa, where gland mucous cells produce mucins having terminal α1,4-linked-GlcNAc residues attached to core 2 branched O-glycans [GlcNAcα1→4Galβ1→4GlcNAcβ1→6(GlcNAcα1→4 Gal β1→3)GalNAcα→Ser/Thr] (α1,4-GlcNAc-capped O-glycans) [82]. These findings raise the possibility that α1,4-GlcNAc-capped O-glycans protect against H. pylori infection.
4.2. Effect of α1,4-GlcNAc-Capped O-Glycans on H. pylori Growth
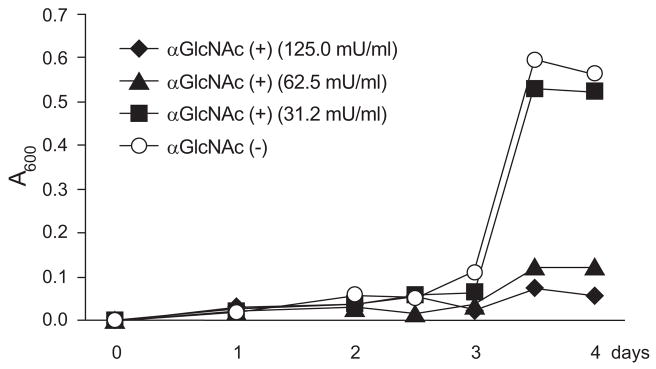
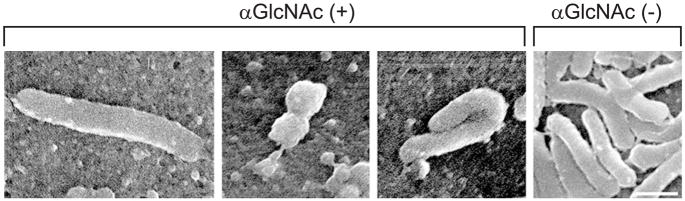
To test the above hypothesis, we generated α1,4-GlcNAc-capped O-glycans and determined their effect on H. pylori in vitro [83]. H. pylori was cultured in the presence of recombinant soluble CD43 with or without α1,4-GlcNAc-capped O-glycans secreted from transfected Lec2 cells, a mutant of Chinese hamster ovary cells defective in the CMP-sialic acid transporter [84]. As shown in Fig. (3), H. pylori cultured in the presence of control soluble CD43 lacking α1,4-GlcNAc-capped O-glycans grew rapidly, whereas soluble CD43 having α1,4-GlcNAc-capped O-glycans inhibited growth of H. pylori in a dose dependent manner. Such inhibitory effects were also demonstrated when CD34 was used as a scaffold protein instead of CD43. Moreover, GlcNAcα-p-nitrophenyl (PNP) and gastric gland mucins containing α1,4-GlcNAc-capped O-glycans also suppressed H. pylori growth. In addition to growth suppression, significant reduction of motility as well as abnormal morphology such as elongation, segmental narrowing, and folding were seen in bacteria incubated with soluble CD43 having α1,4-GlcNAc-capped O-glycans (Fig. (4)), supporting the idea that α1,4-GlcNAc-capped O-glycans have anti-H. pylori activity.
Fig. (3).
Growth curve of H. pylori incubated with soluble CD43 having α1,4-GlcNAc-capped O-glycan (αGlcNAc (+)) and soluble CD43 lacking this O-glycan (αGlcNAc (−)). One milliunit (mU) of αGlcNAc (+) is defined as 1 μg of GlcNAcα-PNP. The protein concentration of αGlcNAc (−) is the same as that of 31.2 mU/ml of αGlcNAc (+). Adapted with permission from Kawakubo, M.; Ito, Y.; Okimura, Y.; Kobayashi, M.; Sakura, K.; Kasama, S.; Fukuda, M.N.; Fukuda, M.; Katsuyama, T.; Nakayama, J. Science, 2004, 305(5686), 1003–1006.
Fig. (4).
Morphology of H. pylori incubated with 31.2 mU/ml of soluble CD43 having α1,4-GlcNAc-capped O-glycan (αGlcNAc (+)) and the same protein concentration of soluble CD43 lacking this O-glycan (αGlcNAc (−)). Bar = 1 μm. Adapted with permission from Kawakubo, M.; Ito, Y.; Okimura, Y.; Kobayashi, M.; Sakura, K.; Kasama, S.; Fukuda, M.N.; Fukuda, M.; Katsuyama, T.; Nakayama, J. Science, 2004, 305(5686), 1003–1006.
4.3. α1,4-GlcNAc-Capped O-glycan-Mediated Reduction of a Cell Wall Component, CGL
The morphological abnormalities seen in H. pylori induced by α1,4-GlcNAc-capped O-glycans are similar to those induced by antibiotics such as β-lactamase inhibitors, which disrupt biosynthesis of peptidoglycan in the cell wall [85]. Therefore, these O-glycans may inhibit cell wall biosynthesis in H. pylori. The cell wall of Helicobacter spp. characteristically contains α-cholesteryl glucosides, including cholesteryl-α-D-glucopyranoside (CGL) [86]. Mass spectrometric analysis of cell wall components, particularly CGL, from H. pylori cultured with α1,4-GlcNAc-capped O-glycans showed significant reduction of CGL compared to controls in which H. pylori was incubated with soluble CD43 without α1,4-GlcNAc-capped O-glycans [83]. Similar results were seen when sonicated H. pylori was used as an enzyme source of UDP-Glc:sterol α-glucosyltransferase, which is responsible for the biosynthesis of CGL. These results suggest that α1,4-GlcNAc capped O-glycans directly inhibit biosynthesis of CGL by H. pylori.
4.4. Role of CGL in H. pylori Viability
CGL is formed by an UDP-Glc:sterol α-glucosyltransferase, which transfers glucose (Glc) from UDP-Glc to the C3 position of cholesterol with an α-linkage. Since genes involved in cholesterol biosynthesis are not found in its genome, H. pylori cannot synthesize CGL in the absence of exogenous cholesterol. We thus created H. pylori deficient in CGL by culturing it without cholesterol for 5 days and found that growth and motility of bacteria were dramatically suppressed compared with H. pylori cultured in the presence of cholesterol [83]. Abnormal morphology was also noted in the culture, and bacteria died after 21 days in culture, indicating that CGL is crucial for H. pylori survival.
4.5. Antibiotic Function of α1,4-GlcNAc Residues
Finally, to determine whether mucous cells expressing α1,4-GlcNAc-capped O-glycans are protected against H. pylori infection, gastric adenocarcinoma AGS cells stably transfected with α1,4-N-acetylglucosaminyltransferase (α4GnT) (AGS-α4GnT) [87] were co-cultured with H. pylori [83]. With a short-term incubation of 8 hours, the microbes attached equally well to AGS-α4GnT cells and mock-transfected AGS cells. No significant morphological changes were observed in either group of cells at this time point. However, after a 24-hour incubation, mock-transfected AGS cells exhibited remarkable deterioration, such as flat morphology or shrinkage, and showed increased numbers of associated H. pylori. After three days in culture, the number of viable AGS cells was dramatically reduced. By contrast, growth of H. pylori in cultures with AGS-α4GnT cells was markedly suppressed, and evidence of cellular damage seen in mock-transfected AGS cells was barely detected in these cells. The viability of AGS-α4GnT cells was fully maintained for up to 4 days. These findings indicate that α1,4-GlcNAc-capped O-glycans have no effect on adhesion of H. pylori to GAS-α4GnT cells but protect the host cells from H. pylori infection.
Additionally, it should be noted that H. pylori is rarely observed in metaplastic glands: development of gastric intestinal metaplasia creates a microenvironment that is hostile to H. pylori colonization and generally leads to clearing of H. pylori from metaplastic glands [88, 89]. However, in contrast to the observation in normal gastric mucosa, α1,4-GlcNAc-capped O-glycans are scarcely detected in metaplastic glands, indicating that this O-glycan is not implicated in the reduction/absence of H. pylori in this particular pathologic lesion [90].
5. EXPRESSION CLONING OF CHOLESTEROL α-GLUCOSYLTRANSFERASE FROM H. PYLORI
5.1. Expression Cloning of H. pylori and H. felis CHLαGcT
As described in section 4.3., α-glucosyl cholesterol and its derivatives are major components of the cell wall of Helicobacter spp. and constitute more than 25% of total cell wall lipids of H. pylori, reaching almost 130 μM [86, 91]. In addition, it has been reported that α-glucosyl cholesterol abrogates phagocytosis of H. pylori and compromises subsequent T cell activation directed toward H. pylori [92]. Conversely, the increased amount of cholesterol resulted in increased phagocytosis of H. pylori and increased T cell responses toward H. pylori. These results demonstrate that α-glucosylation of cholesterol in H. pylori facilitates both infectivity and pathogenicity of H. pylori.
Recently, we and Lebrum et al. independently cloned genomic DNA encoding cholesterol α-glucosyltransferase (CHLαGcT) (HP0421) [93, 94]. Since a BLAST search did not reveal genomic sequences homologous to sterol β-glucosyltransferase [95] and other bacterial glucosyltransferases, we undertook expression cloning to identify a genomic DNA encoding CHLαGcT. Following expression of genomic fragments in Escherichia coli, we identified a pool of colonies directing [3H]glucose incorporation from UDP-[3H]Glc to cholesterol and eventually identified a single plasmid harboring genomic sequences for two open reading frames (designated HP0420 and HP0421 in the H. pylori genome [28]). HP0421 was responsible for CHLαGcT activity and deletion of HP0420 did not impair expression of CHLαGcT activity. H. felis CHLαGcT was identified by similar approach.
The amino acid sequences of H. pylori and H. felis CHLαGcT are shown in Fig. (5). Corresponding sequences for H. hepaticus [96], H. mustelae [97], and H. acinonychis [98], based on their whole or partial genome sequences, are also shown. CHLαGcT from H. pylori has 52–93% identity with that from other Helicobacter spp. CHLαGcT does not resemble a typical eukaryotic glycosyltransferase since it lacks DXD motif, which is found all the eukaryotic glycosyltransferases cloned to date [99]. A BLAST search did not identify other proteins exhibiting significant homology to the cloned CHLαGcT, strongly suggesting that the identified enzyme is solely responsible for α-glucosylation of cholesterol in H. pylori and other Helicobacter spp.
Fig. (5).
Comparison of amino acid sequences of cloned H. pylori CHLαGcT (Hp), H. felis CHLαGcT (Hf), and putative CHLαGcTs from H. hepaticus (Hh), H. mustelae (Hm), and H. acinonychis (Ha). Identical amino acid residues are indicated by closed boxes. Adapted from Lee, H.; Kobayashi, M.; Wang, P.; Nakayama, J.; Seeberger, P.H.; Fukuda, M. Biochem. Biophys. Res. Commun., 2006, 349(4), 1235–1241.
5.2. Expression of H. pylori CHLαGcT
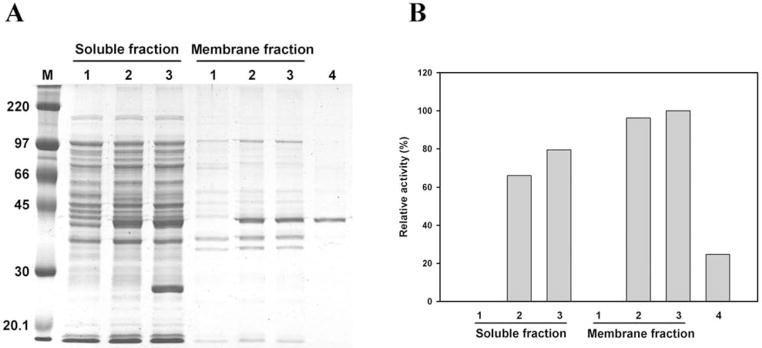
To characterize H. pylori CHLαGcT, the enzyme was expressed as a fusion protein with a C-terminal 6x His-tag. CHLαGcT expression in E. coli was driven by an amylolytic enzyme promoter from Bacillus licheniformis. After bacterial cells were treated with lysozyme and sonicated, approximately half of the enzyme was released into the soluble fraction (Fig. (6) B) and purified using a Ni-NTA column (Fig. (6) A, lane 4). The apparent molecular weight of the purified protein was slightly smaller than the calculated molecular weight (46,030 Da), indicating that the enzyme exhibits anomaly mobility upon SDS-gel electrophoresis.
Fig. (6).
Expression and purification of CHLαGcT. (A) SDS-polyacrylamide gel electrophoresis of proteins in both soluble and membrane fractions (lanes 1–3: pTKNd6xH vector only; 2, pTKNd6xH-CHLαGcT; 3, pTKNd6xH-CHLαGcT + pRARE (Novagen), and of protein purified from the soluble fraction using Ni-NTA column (lane 4). (B) Distribution of CHLαGcT activity in samples shown in (A). The amount of protein in lane 4 was approximately one-third of that in lane 3 of the membrane fraction. Adapted from Lee, H.; Kobayashi, M.; Wang, P.; Nakayama, J.; Seeberger, P.H.; Fukuda, M. Biochem. Biophys. Res. Commun., 2006, 349(4), 1235–1241.
A product obtained using the expressed CHLαGcT and cholesterol was susceptible to α-glucosidase but not β-glucosidase, confirming that the product was α-glucosyl cholesterol.
5.3. Inhibition of CHLαGcT Activity by Mucin-Type O-Glycans
As described in section 4.2., H. pylori growth is inhibited by mucin-type O-glycans, particularly those containing α1,4-GlcNAc capped structures [83]. To determine if CHLαGcT activities reflect in vivo effects of oligosaccharides, inhibition by mucin-type O-glycans was tested (Table 1). O-glycans resembling those present in gastric mucin were potent inhibitors for CHLαGcT activity, while non-sialylated O-glycans were relatively weak inhibitors. The results also indicate that the α1,4-GlcNAc capped structure is the most efficient inhibiting structure as predicted from inhibition of H. pylori grown in culture [83].
Table 1. Inhibition of Cholesterol α-Glucosyltransferase by Various O-Linked Oligosaccharides. The Concentration of UDP-Glc and Cholesterol was 3.6 μM and 400 μM, Respectively.
Adapted from Lee, H.; Wang, P.; Hoshino, H.; Ito, Y.; Kobayashi, M.; Nakayama, J.; Seeberger, P.H.; Fukuda, M. Glycobiology, 2008, 18(7), 549–558.
| O-linked oligosaccharide | IC50 (mM) |
|---|---|
| Galβ1→3GalNAcα1→p-nitrophenol | 7.49 |
| GlcNAcβ1 ↓ 6 Galβ1→3GalNAcα1→p-nitrophenol |
2.21 |
| NeuAcα2→3Galβ1→3GalNAcα1→octyl | 1.09 |
| NeuAcα2→3Galβ1→4GlcNAcβ1 ↓ 6 Galβ1→3GalNAcα1→octyl |
0.75 |
| GlcNAcα1→4Galβ1→4GlcNAcβ1 ↓ 6 Galβ1→3GalNAcα1→octyl |
0.47 |
5.4. CHLαGcT Acts in an Ordered Bi-Bi Manner
To determine how CHLαGcT acts on acceptor and donor substrates, enzyme activity was measured at various UDP-Glc concentrations, while fixed amounts of UDP or cholesterol were added as inhibitors [100]. The Lineweaver-Burk plot of the inhibition profile of UDP in the presence of UDP-Glc intersected at the Y-axis, indicating competitive inhibition. In contrast, cholesterol did not exhibit competitive inhibition in the presence of UDP-Glc and showed mixed-type inhibition. We then measured CHLαGcT activity at different fixed concentrations of cholesterol. Cholesterol affected CHLαGcT activity in a mixed-type manner, consistent with the above results.
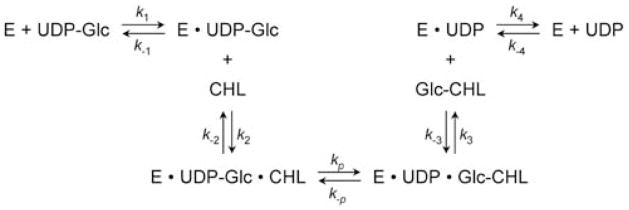
To delineate further how the enzyme acts on donor and acceptor substrates, inhibition of CHLαGcT by α-glucosyl cholesterol was examined. CHLαGcT activity was measured at various UDP-Glc concentrations in the presence of 5 μM cholesterol. α-Glucosyl cholesterol was then added as an inhibitor. The Lineweaver-Burk plot showed that α-glucosyl cholesterol inhibited CHLαGcT in a non-competitive, mixed-type manner. Similarly, α-glucosyl cholesterol inhibited CHLαGcT activity in a mixed type manner when the cholesterol concentration was varied. These results combined indicate that CHLαGcT acts in an ordered Bi-Bi manner, and strongly suggest that cholesterol is added to the enzyme/substrate complex after a complex forms between the enzyme and UDP-Glc (Fig. (7)). By this mechanism, the enzyme-UDP-Glc complex presumably induces a conformational change such that flexible loops form to accommodate more favorable binding to an acceptor substrate.
Fig. (7).
Catalytic mechanisms of CHLαGcT. The kinetic data are consistent with an ordered Bi-Bi reaction mechanism. UDP-Glc binds to CHLαGcT (E) prior to cholesterol binding, and α-glucosyl cholesterol (Glc-CHL) is released prior to UDP release from the enzyme-UDP complex. The arrows indicate the directions of reactions. kp represents a kinetic constant to form a product, Glc-CHL. Adapted from Lee, H.; Wang, P.; Hoshino, H.; Ito, Y.; Kobayashi, M.; Nakayama, J.; Seeberger, P.H.; Fukuda, M. Glycobiology, 2008, 18(7), 549–558.
5.5. α1, 4-GlcNAc-Capped Core 2 O-Glycan Inhibits H. pylori Growth
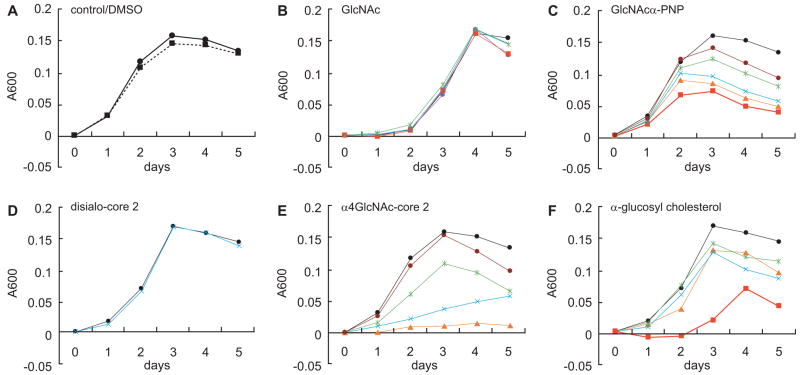
As described in section 4.2., recombinant CD43 expressing α1,4-GlcNAc residues inhibits H. pylori growth [83]; however, we had not determined whether oligosaccharides containing α1,4-GlcNAc residues inhibit H. pylori growth. We thus evaluated the effect of α1,4-GlcNAc-capped core 2 O-glycan, sialylated core 2 O-glycan, monosaccharides, or α-glucosyl cholesterol on H. pylori growth. Monosaccharide GlcNAc had no effect on H. pylori growth, but higher concentrations of GlcNAcα-PNP inhibited microbial growth (Fig. (8) B and C). H. pylori growth was significantly inhibited by 0.5 mM α1,4-GlcNAc-capped core 2 O-glycan (Fig. (8) E). Inhibition by α1,4-GlcNAc-capped core 2 O-glycan was more robust than that by α-glucosyl cholesterol, the product of CHLαGcT (Fig. (8) F). We also tested sialylated core 2 O-glycan, since this O-glycan is the second best inhibitor for CHLαGcT in vitro among the oligosaccharides tested [93], and represents a major O-glycan in mucins [101, 102]. Interestingly, sialylated core 2 O-glycan without an α1,4-GlcNAc-capped structure (disialo-core 2) had no inhibitory activity (Fig. (8) D). These combined results indicate that an α1,4-GlcNAc-capped core 2 O-glycan most efficiently inhibits H. pylori growth, and an α1,4-GlcNAc residue is essential for that inhibition.
Fig. (8).
Inhibition of H. pylori growth by synthetic oligosaccharides and monosaccharides. H. pylori was cultured for 5 days in Mueller-Hinton broth supplemented with 5.5% horse serum containing various amounts of synthetic oligosaccharides and monosaccharides. Bacterial growth was measured at O.D. 600 nm, and the absorbance for control experiments at time 0 was subtracted from absorbance at later time points. Oligosaccharide and monosaccharide concentrations are 1 mM (red), 0.75 mM (orange), 0.5 mM (blue), 0.25 mM (green), 0.125 mM (brown), and control (closed circle). Two mM GlcNAc was also added in B (magenta). Oligosaccharides and monosaccharides were initially dissolved in DMSO, and the final DMSO concentration in the culture medium was 1%. The growth curve in the absence of DMSO is shown as a dotted line (A). Adapted from Lee, H.; Wang, P.; Hoshino, H.; Ito, Y.; Kobayashi, M.; Nakayama, J.; Seeberger, P.H.; Fukuda, M. Glycobiology, 2008, 18(7), 549–558.
The discovery of CHLαGcT inhibitors will be important because of their function as antibiotics against H. pylori. Since CHLαGcT is present only in Helicobacter spp., inhibition of this enzyme acts as a Helicobacter-specific antibiotic, therefore, with minimal side effects. Future studies are important to develop α1,4-GlcNAc-capped core 2-based drug and identifying an inhibitor of low molecular weight for the treatment of H. pylori infection.
6. CONCLUSION
In this review, we have shown that a battery of O-glycans such as 6-sulfo sialyl Lewis X and α-1,4-GlcNAc-capped O-glycans expressed in the HEV-like vessels and gland mucous cells, respectively, play pivotal roles on pathogenesis of chronic active gastritis and on protection of the gastric mucosa from H. pylori, respectively. These discoveries allow us to not only understand the pathogenesis of chronic active gastritis but also to develop new carbohydrate-based therapy or prevention to H. pylori infection.
Acknowledgments
Experiments reported in this review were supported by Grant CA33000 and CA71932 from the National Institutes of Health (to M.F.), and by Grant-in-Aid for Scientific Research on Priority Area 14082201 (to J.N.) and for Young Scientists B-18790240 (to M.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Dr. Elise Lamar for critical reading of the manuscript.
ABBREVIATIONS
- α4GnT
α1,4-N-acetylglucosaminyltransferase
- BabA
Blood group antigen-binding adhesin
- β3GnT5
β1,3-N-acetylglucosaminyltransferase 5
- CagA
Cytotoxin-associated antigen A
- CGL
Cholesteryl-α-D-glucopyranoside
- CHLαGcT
Cholesterol α-glucosyltransferase
- DMSO
Dimethyl sulfoxide
- HEV
High endothelial venule
- LPS
Lipopolysaccharide
- MALT
Mucosa-associated lymphoid tissue
- MARK
Microtubule affinity-regulating kinase
- NSAID
Non-steroidal anti-inflammatory drug
- PAR1
Partitioning-defective 1
- PNAd
Peripheral lymph node addressin
- PNP
p-nitrophenyl
- SabA
Sialic acid-binding adhesin
- SHP-2
Src homology 2 domain-containing tyrosine phosphatase 2
- SMGL
Surface mucous gel layer
- VacA
Vacuolating toxin
References
- 1.Doenges JL. Spirochaetes in gastric glands of macacus rhesus and humans without definite history of related disease. Proc Soc Exp Biol Med. 1938;38:536–538. [Google Scholar]
- 2.Freedburg AS, Barron LE. The presence of spirochaetes in human gastric mucosa. Am J Dig Dis. 1940;7:443–445. [Google Scholar]
- 3.Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;321(8336):1273–1275. [PubMed] [Google Scholar]
- 4.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;323(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 5.Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch’s postulates for pyloric Campylobacter Med. J Aust. 1985;142(8):436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- 6.Megraud F. A humble bacterium sweeps this year’s Nobel Prize. Cell. 2005;123(6):975–976. doi: 10.1016/j.cell.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Crawford JM. In: Robbins and Cortran Pathologic Basis of Disease. 7. Kumar V, Abbas AK, Fausto N, editors. Elsevier Saunders; Philadelphia: 2005. pp. 797–875. [Google Scholar]
- 8.Suzuki H, Mori M, Seto K, Miyazawa M, Kai A, Suematsu M, Yoneta T, Miura S, Ishii H. Polaprezinc attenuates the Helicobacter pylori-induced gastric mucosal leucocyte activation in Mongolian gerbils—a study using intravital videomicroscopy. Aliment Pharmacol Ther. 2001;15(5):715–725. doi: 10.1046/j.1365-2036.2001.00960.x. [DOI] [PubMed] [Google Scholar]
- 9.The EUROGAST Study Group. Epidemiology of risk factors for Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut. 1993;34(12):1672–1676. doi: 10.1136/gut.34.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins RJ, Russell RG, O’Donnoghue JM, Wasserman SS, Lefkowitz A, Morris JG., Jr Seroprevalence of Helicobacter pylori in Seventh-Day Adventists and other groups in Maryland. Lack of association with diet. Arch Intern Med. 1990;150(11):2347–2348. [PubMed] [Google Scholar]
- 11.Farinha P, Gascoyne RD. Helicobacter pylori and MALT lymphoma. Gastroenterology. 2005;128(6):1579–1605. doi: 10.1053/j.gastro.2005.03.083. [DOI] [PubMed] [Google Scholar]
- 12.Parsonnet J, Blaser MJ, Perez-Perez GI, Hargrett-Bean N, Tauxe RV. Symptoms and risk factors of Helicobacter pylori infection in a cohort of epidemiologists. Gastroenterolgy. 1992;102(1):41–46. doi: 10.1016/0016-5085(92)91782-y. [DOI] [PubMed] [Google Scholar]
- 13.Banatvala N, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori. J Infect Dis. 1993;168(1):219–221. doi: 10.1093/infdis/168.1.219. [DOI] [PubMed] [Google Scholar]
- 14.Cullen DJ, Collins BJ, Christiansen KJ, Epis J, Warren JR, Surveyor I, Cullen KJ. When is Helicobacter pylori infection acquired? Gut. 1993;34(12):1681–1682. doi: 10.1136/gut.34.12.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumagai T, Malaty HM, Graham DY, Hosogaya S, Misawa K, Furihata K, Ota H, Sei C, Tanaka E, Akamatsu T, Shimizu T, Kiyosawa K, Katsuyama T. Acquisition versus loss of Helicobacter pylori infection in Japan: results from an 8-year birth cohort study. J Infect Dis. 1998;178(3):717–721. doi: 10.1086/515376. [DOI] [PubMed] [Google Scholar]
- 16.Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29(3):559–578. doi: 10.1016/s0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- 17.Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284(5418):1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 18.Sipponen P, Hyvarinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl. 1993;196:3–6. doi: 10.3109/00365529309098333. [DOI] [PubMed] [Google Scholar]
- 19.Sipponen P, Kekki M, Haapakoski J, Ihamaki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35(2):173–177. doi: 10.1002/ijc.2910350206. [DOI] [PubMed] [Google Scholar]
- 20.Reis CA, David L, Correa P, Carneiro F, de Bolos C, Garcia E, Mandel U, Clausen H, Sobrinho-Simoes M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59(5):1003–1007. [PubMed] [Google Scholar]
- 21.Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simoes M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;74(1):112–121. doi: 10.1002/(sici)1097-0215(19970220)74:1<112::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 23.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 24.Sipponen P, Marshall BJ. Gastritis and gastric cancer. Western countries. Gastroenterol Clin North Am. 2000;29(3):579–592. doi: 10.1016/s0889-8553(05)70131-x. [DOI] [PubMed] [Google Scholar]
- 25.Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer. 1984;53(11):2515–2524. doi: 10.1002/1097-0142(19840601)53:11<2515::aid-cncr2820531125>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 26.Kumar V, Abbas A, Fausto N. In: Robbins and Cortran Pathologic Basis of Disease. 7. Kumar V, Abbas A, Fausto N, editors. Elsevier Saunders; Philadelphia: 2005. pp. 269–342. [Google Scholar]
- 27.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, Rappuoli R. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, Nelson K, Quackenbush J, Zhou L, Kirkness EF, Peterson S, Loftus B, Richardson D, Dodson R, Khalak HG, Glodek A, McKenney K, Fitzegerald LM, Lee N, Adams MD, Hickey EK, Berg DE, Gocayne JD, Utterback TR, Peterson JD, Kelley JM, Cotton MD, Weidman JM, Fujii C, Bowman C, Watthey L, Wallin E, Hayes WS, Borodovsky M, Karp PD, Smith HO, Fraser CM, Venter JC. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388(6642):539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 29.Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, Carmel G, Tummino PJ, Caruso A, Uria-Nickelsen M, Mills DM, Ives C, Gibson R, Merberg D, Mills SD, Jiang Q, Taylor DE, Vovis GF, Trust TJ. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397(6715):176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 30.Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci U S A. 1999;96(25):14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M, Suzuki T, Sasakawa C. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191(4):593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287(5457):1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 33.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci U S A. 2000;97(3):1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295(5555):683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 35.Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, Higashi H, Musashi M, Iwabuchi K, Suzuki M, Yamada G, Azuma T, Hatakeyama M. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A. 2008;105(3):1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A, Ohno S, Hatakeyama M. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447(7142):330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 37.Togayachi A, Akashima T, Ookubo R, Kudo T, Nishihara S, Iwasaki H, Natsume A, Mio H, Inokuchi J, Irimura T, Sasaki K, Narimatsu H. Molecular cloning and characterization of UDP-GlcNAc:lactosylceramide β1,3-N-acetylglucosaminyltransferase (β3Gn-T5), an essential enzyme for the expression of HNK-1 and Lewis X epitopes on glycolipids. J Biol Chem. 2001;276(25):22032–22040. doi: 10.1074/jbc.M011369200. [DOI] [PubMed] [Google Scholar]
- 38.Marcos NT, Magalhaes A, Ferreira B, Oliveira MJ, Carvalho AS, Mendes N, Gilmartin T, Head SR, Figueiredo C, David L, Santos-Silva F, Reis CA. Helicobacter pylori induces β3GnT5 in human gastric cell lines, modulating expression of the SabA ligand sialyl Lewis x. J Clin Invest. 2008;118(6):2325–2336. doi: 10.1172/JCI34324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267(15):10570–10575. [PubMed] [Google Scholar]
- 40.Catrenich CE, Chestnut MH. Character and origin of vacuoles induced in mammalian cells by the cytotoxin of Helicobacter pylori. J Med Microbiol. 1992;37(6):389–395. doi: 10.1099/00222615-37-6-389. [DOI] [PubMed] [Google Scholar]
- 41.Papini E, Bugnoli M, De Bernard M, Figura N, Rappuoli R, Montecucco C. Bafilomycin A1 inhibits Helicobacter pylori-induced vacuolization of HeLa cells. Mol Microbiol. 1993;7(2):323–327. doi: 10.1111/j.1365-2958.1993.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 42.Papini E, Zoratti M, Cover TL. In search of the Helicobacter pylori VacA mechanism of action. Toxicon. 2001;39(11):1757–1767. doi: 10.1016/s0041-0101(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 43.Czajkowsky DM, Iwamoto H, Cover TL, Shao Z. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc Natl Acad Sci USA. 1999;96(5):2001–2006. doi: 10.1073/pnas.96.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szabo I, Brutsche S, Tombola F, Moschioni M, Satin B, Telford JL, Rappuoli R, Montecucco C, Papini E, Zoratti M. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 1999;18(20):5517–5527. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tombola F, Carlesso C, Szabo I, de Bernard M, Reyrat JM, Telford JL, Rappuoli R, Montecucco C, Papini E, Zoratti M. Helicobacter pylori vacuolating toxin forms anion-selective channels in planar lipid bilayers: possible implications for the mechanism of cellular vacuolation. Biophys J. 1999;76(3):1401–1409. doi: 10.1016/S0006-3495(99)77301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schraw W, Li Y, McClain MS, van der Goot FG, Cover TL. Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts. J Biol Chem. 2002;277(37):34642–34650. doi: 10.1074/jbc.M203466200. [DOI] [PubMed] [Google Scholar]
- 47.Willhite DC, Cover TL, Blanke SR. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J Biol Chem. 2003;278(48):48204–48209. doi: 10.1074/jbc.M304131200. [DOI] [PubMed] [Google Scholar]
- 48.Galmiche A, Rassow J, Doye A, Cagnol S, Chambard JC, Contamin S, de Thillot V, Just I, Ricci V, Solcia E, Van Obberghen E, Boquet P. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19(23):6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuck D, Kolmerer B, Iking-Konert C, Krammer PH, Stremmel W, Rudi J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect Immun. 2001;69(8):5080–5087. doi: 10.1128/IAI.69.8.5080-5087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peek RM, Jr, Blaser MJ, Mays DJ, Forsyth MH, Cover TL, Song SY, Krishna U, Pietenpol JA. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 1999;59(24):6124–6131. [PubMed] [Google Scholar]
- 51.Fujikawa A, Shirasaka D, Yamamoto S, Ota H, Yahiro K, Fukada M, Shintani T, Wada A, Aoyama N, Hirayama T, Fukamachi H, Noda M. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat Genet. 2003;33(3):375–381. doi: 10.1038/ng1112. [DOI] [PubMed] [Google Scholar]
- 52.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301(5636):1099–1102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 53.McAdam AJ, Sharpe AH. In: Robbins and Cortran Pathologic Basis of Disease. 7. Kumar V, Abbas AK, Fausto N, editors. Elsevier Saunders; Philadelphia: 2005. pp. 343–414. [Google Scholar]
- 54.Moran AP, Prendergast MM. Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: contribution of gastrointestinal infections to autoimmunity. J Autoimmun. 2001;16(3):241–256. doi: 10.1006/jaut.2000.0490. [DOI] [PubMed] [Google Scholar]
- 55.Moran AP. Cell surface characteristics of Helicobacter pylori. FEMS Immunol Med Microbiol. 1995;10(3–4):271–280. doi: 10.1111/j.1574-695X.1995.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 56.Amano K, Hayashi S, Kubota T, Fujii N, Yokota S. Reactivities of Lewis antigen monoclonal antibodies with the lipopolysaccharides of Helicobacter pylori strains isolated from patients with gastroduodenal diseases in Japan. Clin Diagn Lab Immunol. 1997;4(5):540–544. doi: 10.1128/cdli.4.5.540-544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang G, Ge Z, Rasko DA, Taylor DE. Lewis antigens in Helicobacter pylori: biosynthesis and phase variation. Mol Microbiol. 2000;36(6):1187–1196. doi: 10.1046/j.1365-2958.2000.01934.x. [DOI] [PubMed] [Google Scholar]
- 58.Appelmelk BJ, Monteiro MA, Martin SL, Moran AP, Vandenbroucke-Grauls CM. Why Helicobacter pylori has Lewis antigens. Trends Microbiol. 2000;8(12):565–570. doi: 10.1016/s0966-842x(00)01875-8. [DOI] [PubMed] [Google Scholar]
- 59.Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, Vikstrom S, Sjostrom R, Linden S, Backstrom A, Lundberg C, Arnqvist A, Mahdavi J, Nilsson UJ, Velapatino B, Gilman RH, Gerhard M, Alarcon T, Lopez-Brea M, Nakazawa T, Fox JG, Correa P, Dominguez-Bello MG, Perez-Perez GI, Blaser MJ, Normark S, Carlstedt I, Oscarson S, Teneberg S, Berg DE, Boren T. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305(5683):519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 60.Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Boren T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279(5349):373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 61.Segal ED, Falkow S, Tompkins LS. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci U S A. 1996;93(3):1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ikehara Y, Nishihara S, Yasutomi H, Kitamura T, Matsuo K, Shimizu N, Inada K, Kodera Y, Yamamura Y, Narimatsu H, Hamajima N, Tatematsu M. Polymorphisms of two fucosyltransferase genes (Lewis and Secretor genes) involving type I Lewis antigens are associated with the presence of anti-Helicobacter pylori IgG antibody. Cancer Epidemiol Biomarkers Prev. 2001;10(9):971–977. [PubMed] [Google Scholar]
- 63.Gerhard M, Lehn N, Neumayer N, Boren T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci U S A. 1999;96(22):12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahdavi J, Sonden B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA, Altraja S, Wadstrom T, Kersulyte D, Berg DE, Dubois A, Petersson C, Magnusson KE, Norberg T, Lindh F, Lundskog BB, Arnqvist A, Hammarstrom L, Boren T. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297(5581):573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 66.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343(14):1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 67.Duijvestijn AM, Kerkhove M, Bargatze RF, Butcher EC. Lymphoid tissue- and inflammation-specific endothelial cell differentiation defined by monoclonal antibodies. J Immunol. 1987;138(3):713–719. [PubMed] [Google Scholar]
- 68.Kabel PJ, Voorbij HA, de Haan-Meulman M, Pals ST, Drexhage HA. High endothelial venules present in lymphoid cell accumulations in thyroids affected by autoimmune disease: a study in men and BB rats of functional activity and development. J Clin Endocrinol Metab. 1989;68(4):744–751. doi: 10.1210/jcem-68-4-744. [DOI] [PubMed] [Google Scholar]
- 69.van Dinther-Janssen AC, Pals ST, Scheper R, Breedveld F, Meijer CJ. Dendritic cells and high endothelial venules in the rheumatoid synovial membrane. J Rheumatol. 1990;17(1):11–17. [PubMed] [Google Scholar]
- 70.Salmi M, Granfors K, MacDermott R, Jalkanen S. Aberrant binding of lamina propria lymphocytes to vascular endothelium in inflammatory bowel diseases. Gastroenterology. 1994;106(3):596–605. doi: 10.1016/0016-5085(94)90691-2. [DOI] [PubMed] [Google Scholar]
- 71.Suzawa K, Kobayashi M, Sakai Y, Hoshino H, Watanabe M, Harada O, Ohtani H, Fukuda M, Nakayama J. Preferential induction of peripheral lymph node addressin on high endothelial venule-like vessels in the active phase of ulcerative colitis. Am J Gastroenterol. 2007;102(7):1499–1509. doi: 10.1111/j.1572-0241.2007.01189.x. [DOI] [PubMed] [Google Scholar]
- 72.Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107(5):1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB, Fukuda M. Novel sulfated lymphocyte homing receptors and their control by a core1 extension β1,3-N-acetylglucosaminyltransferase. Cell. 2001;105(7):957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 74.Hemmerich S, Leffler H, Rosen SD. Structure and the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J Biol Chem. 1995;270(20):12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- 75.Kobayashi M, Mitoma J, Nakamura N, Katsuyama T, Nakayama J, Fukuda M. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc Natl Acad Sci U S A. 2004;101(51):17807–17812. doi: 10.1073/pnas.0407503101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumamoto K, Mitsuoka C, Izawa M, Kimura N, Otsubo N, Ishida H, Kiso M, Yamada T, Hirohashi S, Kannagi R. Specific detection of sialyl Lewis X determinant carried on the mucin GlcNAcβ1→6GalNAcα core structure as a tumor-associated antigen. Biochem Biophys Res Commun. 1998;247(2):514–517. doi: 10.1006/bbrc.1998.8824. [DOI] [PubMed] [Google Scholar]
- 77.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 78.Pablos JL, Santiago B, Tsay D, Singer MS, Palao G, Galindo M, Rosen SD. A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-α/β and TNF-α in cultured endothelial cells. BMC Immunol. 2005;6(1):6. doi: 10.1186/1471-2172-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frezza M, Gorji N, Melato M. The histopathology of non-steroidal anti-inflammatory drug induced gastroduodenal damage: correlation with Helicobacter pylori, ulcers, and haemorrhagic events. J Clin Pathol. 2001;54(7):521–525. doi: 10.1136/jcp.54.7.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ota H, Katsuyama T, Ishii K, Nakayama J, Shiozawa T, Tsukahara Y. A dual staining method for identifying mucins of different gastric epithelial mucous cells. Histochem J. 1991;23(1):22–28. doi: 10.1007/BF01886504. [DOI] [PubMed] [Google Scholar]
- 81.Reis CA, David L, Carvalho F, Mandel U, de Bolos C, Mirgorodskaya E, Clausen H, Sobrinho-Simoes M. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem. 2000;48(3):377–388. doi: 10.1177/002215540004800307. [DOI] [PubMed] [Google Scholar]
- 82.Hidaka E, Ota H, Hidaka H, Hayama M, Matsuzawa K, Akamatsu T, Nakayama J, Katsuyama T. Helicobacter pylori and two ultrastructurally distinct layers of gastric mucous cell mucins in the surface mucous gel layer. Gut. 2001;49(4):474–480. doi: 10.1136/gut.49.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305(5686):1003–1006. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- 84.Deutscher SL, Nuwayhid N, Stanley P, Briles EI, Hirschberg CB. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984;39(2):295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- 85.Horii T, Mase K, Suzuki Y, Kimura T, Ohta M, Maekawa M, Kanno T, Kobayashi M. Antibacterial activities of β-lactamase inhibitors associated with morphological changes of cell wall in Helicobacter pylori. Helicobacter. 2002;7(1):39–45. doi: 10.1046/j.1523-5378.2002.00054.x. [DOI] [PubMed] [Google Scholar]
- 86.Hirai Y, Haque M, Yoshida T, Yokota K, Yasuda T, Oguma K. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J Bacteriol. 1995;177(18):5327–5333. doi: 10.1128/jb.177.18.5327-5333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakayama J, Yeh JC, Misra AK, Ito S, Katsuyama T, Fukuda M. Expression cloning of a human α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1→4Galβ→R, a glycans specifically expressed in the gastric gland mucous cell-type mucin. Proc Natl Acad Sci USA. 1999;96(16):8991–8996. doi: 10.1073/pnas.96.16.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Craanen ME, Blok P, Dekker W, Ferwerda J, Tytgat GN. Subtypes of intestinal metaplasia and Helicobacter pylori. Gut. 1992;33(5):597–600. doi: 10.1136/gut.33.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teixeira A, David L, Reis CA, Costa J, Sobrinho-Simoes M. Expression of mucins (MUC1, MUC2, MUC5AC, and MUC6) and type 1 Lewis antigens in cases with and without Helicobacter pylori colonization in metaplastic glands of the human stomach. J Pathol. 2002;197(1):37–43. doi: 10.1002/path.1083. [DOI] [PubMed] [Google Scholar]
- 90.Ferreira B, Marcos NT, David L, Nakayama J, Reis CA. Terminal α1,4-linked N-acetylglucosamine in Helicobacter pylori-associated intestinal metaplasia of the human stomach and gastric carcinoma cell lines. J Histochem Cytochem. 2006;54(5):585–591. doi: 10.1369/jhc.5A6836.2006. [DOI] [PubMed] [Google Scholar]
- 91.Haque M, Hirai Y, Yokota K, Mori N, Jahan I, Ito H, Hotta H, Yano I, Kanemasa Y, Oguma K. Lipid profile of Helicobacter spp.: presence of cholesteryl glucoside as a characteristic feature. J Bacteriol. 1996;178(7):2065–2070. doi: 10.1128/jb.178.7.2065-2070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, Zahringer U, Mollenkopf HJ, Heinz E, Meyer TF. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12(9):1030–1038. doi: 10.1038/nm1480. [DOI] [PubMed] [Google Scholar]
- 93.Lee H, Kobayashi M, Wang P, Nakayama J, Seeberger PH, Fukuda M. Expression cloning of cholesterol α-glucosyltransferase, a unique enzyme that can be inhibited by natural antibiotic gastric mucin O-glycans, from Helicobacter pylori. Biochem Biophys Res Commun. 2006;349(4):1235–1241. doi: 10.1016/j.bbrc.2006.08.145. [DOI] [PubMed] [Google Scholar]
- 94.Lebrun AH, Wunder C, Hildebrand J, Churin Y, Zahringer U, Lindner B, Meyer TF, Heinz E, Warnecke D. Cloning of a cholesterol-α-glucosyltransferase from Helicobacter pylori. J Biol Chem. 2006;281(38):27765–27772. doi: 10.1074/jbc.M603345200. [DOI] [PubMed] [Google Scholar]
- 95.Warnecke D, Erdmann R, Fahl A, Hube B, Muller F, Zank T, Zahringer U, Heinz E. Cloning and functional expression of UGT genes encoding sterol glucosyltransferases from Saccharomyces cerevisiae, Candida albicans, Pichia pastoris, and Dictyostelium discoideum. J Biol Chem. 1999;274(19):13048–13059. doi: 10.1074/jbc.274.19.13048. [DOI] [PubMed] [Google Scholar]
- 96.Fox JG, Dewhirst FE, Tully JG, Paster BJ, Yan L, Taylor NS, Collins MJ, Jr, Gorelick PL, Ward JM. Helicobacter hepaticus sp. nov and a microaerophilic bacterium isolated from livers, intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32(5):1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andrutis KA, Fox JG, Schauer DB, Marini RP, Li X, Yan L, Josenhans C, Suerbaum S. Infection of the ferret stomach by isogenic flagellar mutant strains of Helicobacter mustelae. Infect Immun. 1997;65(5):1962–1966. doi: 10.1128/iai.65.5.1962-1966.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eppinger M, Baar C, Linz B, Raddatz G, Lanz C, Keller H, Morelli G, Gressmann H, Achtman M, Schuster SC. Who ate whom? Adaptive Helicobacter genomic changes that accompanied a host jump from early humans to large felines. PloS Genet. 2006;2(7):e120. doi: 10.1371/journal.pgen.0020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taniguchi N, Honke K, Fukuda M, editors. Handbook of Glycosyltransferases and Related Genes. Springer; Tokyo: 2001. [Google Scholar]
- 100.Lee H, Wang P, Hoshino H, Ito Y, Kobayashi M, Nakayama J, Seeberger PH, Fukuda M. α1,4GlcNAc-capped mucin-type O-glycan inhibits cholesterol α-glucosyltransferase from Helicobacter pylori and suppresses H. pylori growth. Glycobiology. 2008;18(7):549–558. doi: 10.1093/glycob/cwn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Capon C, Laboisse CL, Wieruszeski JM, Maoret JJ, Augeron C, Fournet B. Oligosaccharide structures of mucins secreted by the human colonic cancer cell line CL. 16E. J Biol Chem. 1992;267(27):19248–19257. [PubMed] [Google Scholar]
- 102.Lloyd KO, Burchell J, Kudryashov V, Yin BW, Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J Biol Chem. 1996;271(52):33325–33334. doi: 10.1074/jbc.271.52.33325. [DOI] [PubMed] [Google Scholar]