Abstract
Investigators of wildlife populations often utilize demographic indicators to understand the relationship between habitat characteristics and population viability. Assessments of corticosterone may enable earlier detection of populations at risk of decline because physiological adjustments to habitat disturbance occur before reproductive diminutions. Noninvasive methods to accomplish these assesments are important in species of concern, such as the greater sage grouse (GRSG). Therefore, we validated a radioimmunoassay that measures immunoreactive corticosterone metabolites (ICM) in fecal samples and used it to characterize the adrenocortical response of 15 GRSG exposed to capture, intravenous injection of 50 IU/kg adrenocorticotrophic hormone (ACTH) or saline, and 22 h of confinement. Those animals injected with ACTH exhibited a more sustained (P = 0.0139) and less variable (P = 0.0012) response than those injected with saline, indicating different levels of adrenocortical activity. We also found that potential field-collection protocols of fecal samples did not alter ICM concentrations: samples held at 4°C for up to 16 h contained similar levels of ICM as those frozen (−20°C) immediately. This study demonstrates a multiphasic adrenocortical response that varied with the level of stimulation and indicates that the assay used to measure this phenomenon is applicable for studies of wild GRSG.
Introduction
Greater sage grouse (GRSG) abundance is estimated to have fallen by 93% from presettlement times (Braun 2006) and by a rangewide average of 33% from 1985 (Connelly and Braun 1997), while occupied habitat has declined to 56% of potential presettlement habitat (Schroeder et al. 2004). Mean rangewide GRSG productivity (ratio of juveniles to adult females in the fall harvest) between 1985 and 1995 was 25% lower than the mean productivity of the previous 21 yr (Connelly and Braun 1997). Habitat loss, fragmentation, and degradation from various sources are thought to account for this decline (Connelly and Braun 1997; Braun 2006; Walker et al. 2007). As a habitat specialist, GRSG may be more sensitive to disturbances than generalist species (MacArthur 1972; Van Tienderen 1991) and thus may serve as a sensitive indicator of the sagebrush biome’s integrity.
Investigators measure GRSG characteristics at the organismal (Barnett and Crawford 1994; Dunbar et al. 2005; Gregg et al. 2006), population, and landscape scales (Aldridge 2005) as a way of assessing population viability. Determining juvenile to hen ratios (Connelly and Braun 1997; Edelmann et al. 1998), juvenile and adult survivorship (Zablan et al. 2003), and population and possibly metapopulation trends (Akcakaya et al. 2004) yields data upon which to formulate management plans for continued monitoring and/or intervention (Connelly et al. 2000). Although informative, these studies do not detail the physiologic mechanism driving a population’s decline. Reducing the scale of the observation from demography to physiology may reduce the lag time between habitat alterations and detectable GRSG responses. This would increase our capacity to intervene through the preemptive detection of populations susceptible to decline. However, to establish biological links between habitat characteristics, physiological condition, and a population’s vital rates, the development and validation of field-deployable tools is needed because many commercially available assays are specific for laboratory models of human disease rather than wild-ranging species such as the GRSG.
Given the integrative nature of the endocrine system and its role in the regulation of allostasis (Sapolsky et al. 2000; McEwen and Wingfield 2003), accurate assessments of an animal’s endocrine status can provide an important view of the animal’s physiological and behavioral priorities (Stevenson et al. 2005; Wasser and Hunt 2005). Corticosterone (the primary glucocorticoid synthesized and secreted by avian adrenocortical tissue) manages energy use for a properly functioning “fight or flight” response. With a psychologically or physiologically perceived threat to homeostasis, an animal’s hypothalamus-pituitary-adrenal (HPA) axis responds with the secretion of corticosterone into the circulation. Although this is beneficial in the short term, with extended elevations in basal levels of circulating corticosterone, organ systems degrade (Sapolsky et al. 2000). To provide a reliable means of determining whether adrenal status may mediate the relationship between habitat quality (e.g., resource distribution) and demographic parameters, as well as a general method to quantify physiological stress, we validated an assay to noninvasively measure immunoreactive corticosterone metabolites in fecal samples from GRSG.
Accurately determining basal levels of corticosterone in plasma is difficult because of corticosterone’s rapid secretion (i.e., within minutes) upon the perception of a threat (Beuving and Vonder 1978). Fecal sampling is thought to minimize this problem because corticosterone spikes are not detectable until the metabolized hormone traverses the animal’s intestinal tract and a fecal sample is collected, effectively allowing the investigator more time to sample once an animal is captured (Wasser et al. 2000; Millspaugh and Washburn 2004; Goyman 2005; Palme 2005). Additionally, fecal samples provide an integrative rather than a stochastic measure of only the biologically available portion of circulating corticosterone, whereas plasma samples measure short-term fluctuations and reflect hormone that is both bound and unbound to corticosterone-binding globulin (Goyman 2005). For these reasons, fecal samples are thought to more accurately indicate an animal’s basal level of biologically active corticosterone than plasma samples. However, species differences in the enterohepatic processing of circulating steroids causes variation in corticosterone metabolite profiles within fecal samples as well as in the stress-response time course and the degree to which uric acid mixes with fecal pellets (Goyman 2005; Wasser and Hunt 2005). Therefore, an assay to measure metabolites of corticosterone in fecal samples must be validated for each novel species before large-scale assay deployment (Millspaugh and Washburn 2004; Möstl et al. 2005).
This study provides a detailed description of the successful validation and use of a noninvasive, field-deployable radioimmunoassay to characterize the adrenocortical response of wild-caught GRSG during 22 h of confinement using fecal samples. This scenario approximately mimics what may occur during GRSG translocations. We did this by comparing the responses of 15 wild-caught GRSG with two levels of HPA-axis stimulation in both males and females: capture plus saline injection versus capture plus ACTH injection. We stimulated the HPA axis by ACTH injection in addition to using capture and confinement because although all avian species are expected to secrete elevated levels of corticosterone after capture and confinement, we did not want our study to rely solely on this assumption. Without an ACTH injection, the interpretation of an unresponsive bird or unresponsive birds would become more difficult because one would not know whether the lack of response was due to a lack of HPA stimulation or an inadequate assay. With ACTH injection, interpretation would focus on the attributes of the assay rather than different behavioral and endocrine responses of the subjects. Furthermore, capture and confinement may lead to a wider variation in excreted metabolites because the level of HPA stimulation depends on a bird’s perception rather than a specific biochemical interaction, making the interpretation of this variation more difficult. A sensitive assay should indicate an elevated response in the ACTH-injected birds compared to the saline-injected birds because the former should experience higher levels of circulating ACTH, greater adrenal cortex stimulation, enhanced secretion of corticosterone, and, hence, increased excretion of detectable metabolites in fecal samples throughout the 22-h confinement period.
Finally, although not specifically known to be present in GRSG fecal samples, bacterial enzymes in avian (Baltic et al. 2005; Wasser and Hunt 2005) and especially mammalian (Möstl et al. 1999) feces are thought to metabolize and alter the structure of corticosterone metabolites after defecation. This could affect the cross-reactivity of a specific antibody to corticosterone metabolites in fecal samples (Millspaugh and Washburn 2004; Palme 2005). Additionally, it is not always possible to inactivate bacterial enzymes by quickly freezing fecal samples under field conditions. Variation in fecal-sample preservation techniques could complicate interpretation of results because results would be dependent on both the level of corticosterone metabolites excreted into the feces and the level of microbial enzyme activity in a given fecal sample. Therefore, we quantified the impact of a sampling protocol on concentrations of immunoreactive corticosterone metabolites in feces in order to develop a recommended sample preservation protocol and simplify the field deployment of this assay.
Material and Methods
Adrenocortical Challenge
This work was conducted according to the standards stipulated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources 1996) and Guidelines to the Use of Wild Birds in Research (Gaunt et al. 1997) and with approval from the U.S. Geological Survey National Wildlife Health Center’s Institutional Animal Care and Use Committee, protocol number EP040715-A1.
During the autumn of 2005, we performed a capture-hold experiment at Hart Mountain National Antelope Refuge in Oregon to test the validity of measuring immunoreactive corticosterone metabolites (ICM) in GRSG fecal samples. In conjunction with this assay validation, we characterized the 22-h time course of ICM excretion and assessed whether GRSG acclimate to short-term confinement and periodic disturbances such as those that may occur during translocations.
Over the course of four nights, 15 roosting birds were captured using spotlighting techniques between 1 and 4 h after sunset and individually confined for up to 22 h (Table 1). Birds were captured at this time to ensure that roosting behaviors were fully assumed so as to allow for their capture, and to ensure that they would have full crops at their time of capture, through normal feeding behaviors. Immediately after capture, a fecal sample was collected from each bird to attain a baseline value of ICM; then, alternate birds were intravenously injected in the brachial vein either with 50 IU/kg porcine ACTH (catalog no. A6303, Sigma-Aldrich, St. Louis, MO), as described by Dehnhard et al. (2003) and Washburn et al. (2003), or with 1% sterile saline. One-half of the captured birds were treated in a way that would activate adrenocortical production of corticosterone through psychological stimulation of the hypothalamus alone (capture plus saline injection; “saline group”; n = 8), whereas the other half was treated both psychologically and chemically in order to augment hypothalamic activity and to specifically enhance adrenocortical activity, respectively (capture plus ACTH injection, respectively; “ACTH group”; n = 7). Although capture and confinement was expected to augment HPA activity and thus ICM excretion, we chose to boost with ACTH because this is a standard assay validation protocol to test the hypothesis that the specific stimulation of the HPA axis increases ICM levels in fecal samples.
Table 1.
Treatment schedule for 15 greater sage grouse experimental subjects by order of capture
| Bird and Injection | Sex | Age | Weight (g) | Capture Time and Datea | Release Time and Datea | Hold Time (hours:minutes) |
|---|---|---|---|---|---|---|
| 1. Saline | Female | Yearling | 1,100 | 2100 hours on 10/7 | 1800 hours on 10/8 | 21:00 |
| 2. ACTH | Female | Juvenile | 1,050 | 2145 hours on 10/7 | 1800 hours on 10/8 | 20:45 |
| 3. Saline | Male | Adult | 2,100 | 2305 hours on 10/7 | 1800 hours on 10/8 | 18:55 |
| 4. ACTH | Male | Adult | 2,000 | 2305 hours on 10/7 | 1800 hours on 10/8 | 18:55 |
| 5. Saline | Female | Yearling | 1,100 | 2037 hours on 10/8 | 1920 hours on 10/9 | 22:43 |
| 6. ACTH | Female | Yearling | 1,050 | 2045 hours on 10/8 | 1830 hours on 10/9 | 21:45 |
| 7. Saline | Female | Adult | 1,050 | 2045 hours on 10/8 | 1830 hours on 10/9 | 21:45 |
| 8. ACTH | Male | Juvenile | 1,750 | 2120 hours on 10/8 | 1830 hours on 10/9 | 21:10 |
| 9. Saline | Male | Adult | 1,950 | 2150 hours on 10/8 | 1830 hours on 10/9 | 20:40 |
| 10. ACTH | Female | Adult | 1,150 | 2150 hours on 10/8 | 1830 hours on 10/9 | 20:40 |
| 11. Saline | Male | Adult | 1,140 | 2207 hours on 10/8 | 1830 hours on 10/9 | 20:23 |
| 12. ACTH | Male | Adult | 1,850 | 2010 hours on 10/9 | 1815 hours on 10/10 | 20:40 |
| 13. Saline | Male | Adult | 2,000 | 2020 hours on 10/9 | 1815 hours on 10/10 | 21:55 |
| 14. Saline | Male | Adult | 2,000 | 2020 hours on 10/9 | 1815 hours on 10/10 | 21:55 |
| 18. ACTH | Female | Adult | 1,150 | 2150 hours on 10/9 | 1815 hours on 10/10 | 20:50 |
All capture and release dates were in 2005.
After injection, birds were individually placed in a cardboard Natural Environmentally Secure Transporter (economy size, 0.406 m in all dimensions, 0.067 m3; Horizon Micro-Environments, Crawford, GA), which is equipped with multiple slats for air exchange. Once a night’s trapping was completed, birds were transported by automobile to a nearby (~2 km) indoor holding facility and removed from transporters only for periodic fecal sampling. This facility was used to ease the sampling process and to remove wind and intruder effects, and it was held at ambient temperature, sound, and light levels to reduce the interruption of each bird’s circadian rhythm. Birds were not allowed visual contact with the environment outside the carrier. Experimenters entered the room only to collect samples (procedure described below). Birds were offered but did not take ad lib. chopped watermelon, grapes, and sagebrush, and thus, ICM measurements are based on birds that did not feed while in captivity but were able to utilize crop content for nourishment. Fecal sampling was attempted every 30 min for the first 3 h after capture and injection and every 3 h thereafter until release. For every sampling event, each bird was placed on a clean cardboard mat inside the transporter until defecation (~10 min), after which the sample and mat were removed and the birds were then left undisturbed until the next sampling event. Each fecal sample was immediately frozen to −20°C in a 2.0-mL cryovial (catalog no. 72.694.006, Sarstedt, Newton, NC) until laboratory analysis.
Chemical Analysis of Fecal Samples
Immunoreactive corticosterone metabolites in feces were measured with a radioimmunoassay that uses a rabbit-produced anti-corticosterone-3-carboxymethyloxime: BSA IgG polyclonal antibody (catalog no. 07-120103, MP Biomedicals, Costa Mesa, CA). This reagent has been shown to react adequately with metabolites from a wide array of animal taxa (Wasser et al. 2000; Washburn et al. 2003; Wasser and Hunt 2005). Samples were processed for analysis by first lyophilizing for 24 h to remove variation related to water content as well as to ease manual removal of indigesta and uric acid. GRSG fecal pellets occasionally contained a uric acid “cap” on the fecal front, while the remaining excreta was feces. Samples were then weighed to the nearest 0.0001 g, vortexed in 80% methanol-H2O for 30 min to suspend lipid-soluble metabolites in liquid phase, and centrifuged at 500 g for 30 min to clarify. From this suspension, 2.5-mL aliquots were placed into separate tubes and evaporated by placing in a water bath at 70°C and streaming air across the opening of each tube for 1 h. The dried extract was resuspended in 1.0 mL of steroid diluent (phosphosaline gelatin buffer, pH 7.0) and analyzed by radioimmunoassay according to the manufacturer’s protocol. The mean ± 95% confidence interval dry mass of fecal samples was 0.2480 ± 0.0129 g, and no samples were below 0.05 g dry weight.
Samples from three different birds (one of each sex and one unknown) were processed as described above, then serially diluted in steroid diluent to compare the degree to which the resulting response curve paralleled the response curve from serially diluted corticosterone standards. We performed these parallelism studies to (1) assess the linearity of the assay’s response to changing concentrations of metabolites of corticosterone in GRSG feces, (2) determine whether sample contaminants existed that would cross-react with the antibody, and (3) verify that assay reactivity did not differ by sex. Assay precision was quantified by calculating inter- and intra-assay coefficients of variation from the assay’s response to high and low corticosterone pools.
Preservation Experiment
We determined the impact of a field-sampling scenario on the detectable concentration of ICM in GRSG fecal samples in which samples were collected and then placed on ice for up to 16 h before being frozen. The impact was measured by comparing the detectable ICM in chilled (4°C) samples with the detectable ICM in samples frozen immediately (−20°C). Specifically, fecal samples gathered from 25 wild-caught GRSG were individually inserted into a 2.0-mL cryovial, then immediately placed on dry ice (−78°C) for 4 h before long-term storage in N2 vapor (−150°C) in order to cease microbial enzyme activity but preserve microbial populations. Just before experimentation, we slowly thawed the fecal samples on wet ice to reactivate microbial activity and pooled and thoroughly mixed them to equalize the ICM in the pooled sample. Next, we took seven aliquots from the pooled sample for each time point and immediately froze (−20°C) or refrigerated (4°C) each aliquot for 2, 4, 8, or 16 h before freezing to −20°C. Samples were analyzed by radioimmunoassay as descirbed above. This protocol allowed us to standardize ICM levels and microbial populations across aliquots while not changing the level of microbial enzyme activity compared to the microbial enzyme activity of fresh samples.
Statistical Analysis
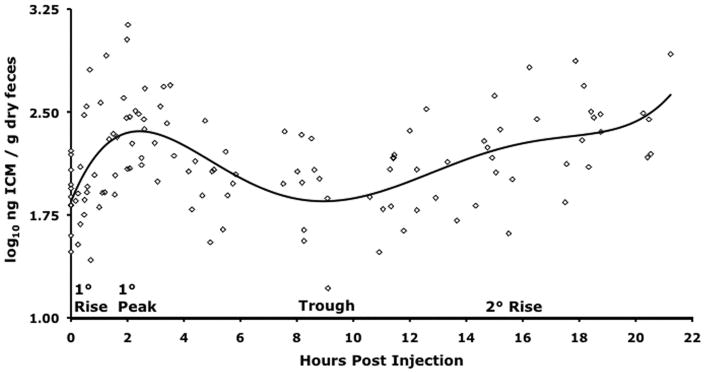
The multiphasic ICM response function was divided into four regions because after capture there was (1) a consistent primary (1°) slope that led to (2) a 1° peak in ICM followed by (3) an interim trough and (4) a secondary (2°) ICM rise (Fig. 1). Each region was treated separately for statistical analysis. We tested for differences in 1° ICM response for each sex and injection type (ACTH or saline) by calculating each bird’s 1° slope and 1° peak and then inserting these values into a mixed-effects ANCOVA with bird weight and feces dry weight as covariates. The 2° response of these same four groups was tested by a mixed-effects ANCOVA in which time after injection (hours), bird-weight, and feces dry weight were covariates. For these models, the individual bird represented the random effect, and depending on the evaluation being made, either the sex or the injection type was the fixed effect. The program SAS JMP IN 5.1.2 (SAS Institute, Cary, NC) was used for the above analyses. Immunoreactive corticosterone metabolite response curves for each injection type were graphed using linear splines to interpolate ICM values for all subjects at each measurement point. Interpolation was required for this analysis because although we attempted to collect samples at the same time points for all birds, we did not. SAS/STAT (SAS Institute, Cary, NC, USA) was used for this analysis. All means are presented as the adjusted mean ± standard error of the mean.
Figure 1.
Polynomial fit (fifth degree; R2 = 0.2957, F ratio = 9.8254, P < 0.0001) of the ICM response of 15 greater sage grouse to capture, intravenous chemical injection (saline or ACTH), and up to 22:43 h of solo confinement and periodic handling. Grouse were captured in October 2005 at Hart Mountain National Antelope Refuge and held in an indoor facility subject to ambient temperature and light conditions. From basal, a primary ICM rise initiates immediately after treatment (capture and injection) and leads to the primary ICM peak at 2:00–3:00 h postinjection (HPI), which is followed by an interim ICM trough between 4:00 and 9:30 HPI, after which a secondary ICM rise emerges (in 12 of 15 subjects) at 9:30 HPI and continues until release.
Results
Assay Validation
Chemical
Three samples (one female, one male, one unknown) were randomly selected from wild-caught GRSG and used to check for parallelism. Response slopes resulting from serially diluted GRSG fecal samples were parallel (t-test, P > 0.05) to slopes from serially diluted standard corticosterone, and sex did not influence these results (P > 0.05). Accuracy for radio-labeled corticosterone added to samples for the parallelism procedure was 103.86% ± 2.3% (n = 6). Using fecal samples from 70 wild-caught GRSG, interassay coefficients of variation were 8.5% and 19.9%, while intra-assay coefficients of variation were 6.5% and 9.1%, for the low and high pools, respectively. There was a negative relationship between ICM and feces dry weight (F = 29.2101; R2 = −0.1945, P < 0.0001), and although all conclusions were the same, mean ICM values were reduced slightly with the inclusion of dry feces weight as a covariate.
Biological
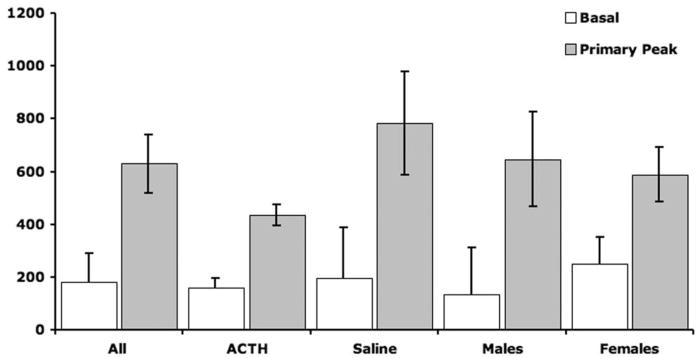
Fifteen GRSG were captured. Birds were alternately injected with ACTH and saline (i.e., every other bird was injected with ACTH). Birds were individually confined and serially fecal sampled for up to 22:43 h (throughout this article, durations are expressed as hours:minutes), in order to determine whether the effects of capture or ACTH injection (reflected by ICM) could be detected in fecal samples (Table 1). A fifth-degree polynomial fit of the response to capture and injection of all birds is plotted in Figure 1 to show the overall pattern. A primary ICM response was evident in the second fecal sample (mean collection time of 1:01 ± 0:11 h post-injection [HPI] and a response magnitude of 2.18 ± 0.37 times baseline) in 13 of 15 birds and not until the third fecal sample (collected at 1:31 and 2:36 HPI) in two of 15 birds. The ICM response peaked at 2:05 ± 0:08 HPI and was followed by a diminution to a trough at approximately 9:30 HPI and finally a secondary rise that began thereafter (Fig. 1). Both the mean primary response peak and the mean secondary maximum were significantly different from mean baseline (Fig. 2). The mean peak primary response for all birds (4.27 ± 0.72 times baseline) was calculated by dividing their mean peak primary ICM value by their mean baseline ICM value. The mean maximum secondary ICM response for all birds (3.85 ± 0.92 times baseline) was determined by dividing the maximum ICM value post-trough by the mean baseline ICM value (Table 2).
Figure 2.
Mean ± SEM primary ICM response of greater sage grouse (GRSG) to capture and chemical injection differs by injection type (ACTH or saline) but not by bird sex. The basal ICM from the first sample available after capture is compared to the mean peak primary ICM for each of the following groups, by t-test: all GRSG (n = 15), P < 0.0001; ACTH (n = 7), P = 0.0003; saline (n = 8), P = 0.0031; males (n = 8), P = 0.0002; and females (n = 7), P = 0.0068. Levene’s test was used to compare the variance of ACTH-injected to saline-injected birds’ primary ICM peak (P = 0.0012). When treatment groups were compared for differences in their mean basal and primary peak, they were not statistically different from one another (ANCOVA, P > 0.05).
Table 2.
Quantitative characterization (mean ± SEM) of the adrenocortical response of 15 greater sage grouse to capture, chemical injection (ACTH or saline), and up to 22:43 h of confinement
| Injection |
Sex |
||||
|---|---|---|---|---|---|
| All Birds | ACTH | Saline | Male | Female | |
| n | 15 | 7 | 8 | 8 | 7 |
| 1° slope (ICM/min)a | 4.25 ± 1.19 | 3.02 ± 1.74 | 5.31 ± 1.63 | 5.17 ± 1.64 | 3.19 ± 1.76 |
| 1° peak (1° peak ICM/baseline ICM) | 4.27 ± .72 | 3.74 ± 1.07 | 4.73 ± 1.00 | 5.04 ± .97 | 3.39 ± 1.04 |
| Time to 1° peak (hours:minutes) | 2:05 ± 0:08 | 2:14 ± 0:12 | 1:59 ± 0:10 | 2:17 ± 0:09b | 1:51 ± 0:11b |
| 2° slope (ICM/min) | .73 ± .16 | 1.18 ± .27c | .38 ± .13c | .96 ± .21 | .18 ± .16 |
| 2° peak (2° peak ICM/baseline ICM)d | 3.85 ± .92 | 5.24 ± 1.29 | 2.63 ± 1.21 | 5.11 ± 1.20 | 2.41 ± 1.28 |
Note. Boldface type indicates statistical significance at P < 0.05.
Units for ICM are ng ICM/g dry feces.
Sex-wise comparison of time to peak response postcapture by t-test (P = 0.0994) and one-way Wilcoxon test (P = 0.0525).
Injection-related difference in 2° response slope (mixed-effects ANCOVA, P = 0.0139 for effect “HPI × injection type”).
No statistical analyses to compare group means were performed for this category because of an inconsistent total time of captivity among birds.
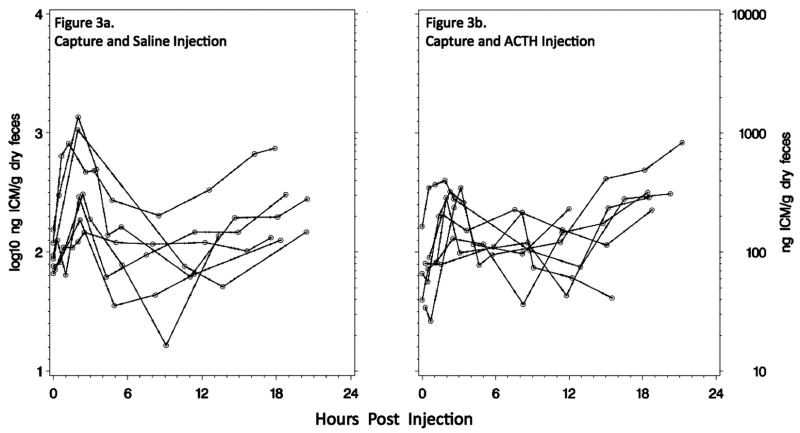
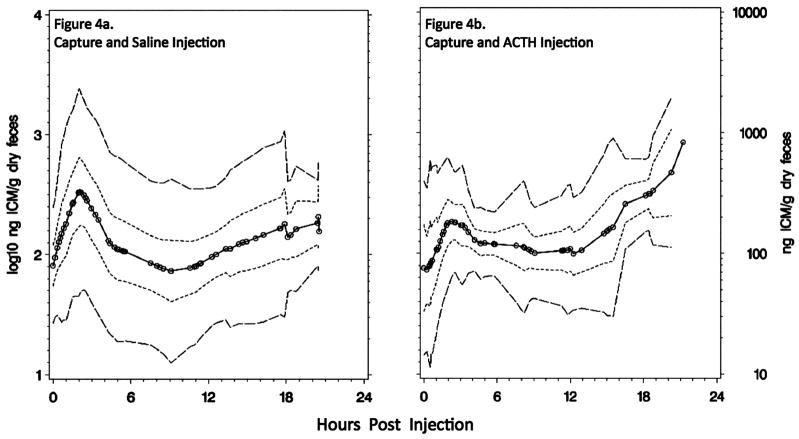
To test whether the primary response to capture and injection differed between treatment groups, we calculated for each bird the slope to maximum (ng ICM/g dry feces/min) and its maximum primary response (ng ICM/g dry feces) and averaged those values for each sex and injection type (Table 2). There were no statistically significant differences between males (n = 8) and females (n = 7) in any category assessed, although males tended to excrete their maximum level of ICM later than females (P = 0.0994; Table 2). ACTH-injected (n = 7) and saline-injected (n = 8) birds’ mean primary responses to capture and injection did not differ (Table 2; Welch ANCOVA for slope of response, P = 0.3392, and peak response, P = 0.2787), but the variance of maximum ICM was greater in saline-injected birds (SD = 462 ng ICM/g dry feces) than in ACTH-injected birds (SD = 110 ng ICM/g dry feces; Levene’s test for equal variance, P = 0.0012). The primary peak in saline-injected birds ranged from 250.9 to 2,343.8 ng/g, whereas the range for ACTH-injected birds was from 330.6 to 561.6 ng/g (Fig. 3). Saline-injected birds tended to excrete levels of ICM that were greater than baseline earlier in their response curve compared to ACTH-injected birds, but this was not a statistically significant difference (Wilcoxon test comparing treatment-related slope of ICM increase from the first to the second fecal sample, P > 0.10; Fig. 4). Both groups experienced a return to a trough approximating basal levels, but saline-injected birds again behaved more variably (Levene’s test for equal variance, P = 0.0031). Commencing at approximately 9:30 HPI, both groups of birds resumed an increase in HPA-axis activity, with ACTH-injected birds responding more robustly than saline-injected birds (mixed-effects ANCOVA to test for differences in secondary slope with the effect of injection type × HPI, P = 0.0139). Finally, 95% confidence and prediction intervals demonstrate the difference in variance and secondary rise between saline-injected and ACTH-injected birds (Fig. 4).
Figure 3.
Individual bird ICM responses to (a) capture and saline injection and up to 22:43 h of confinement (n = 8) or (b) capture and ACTH injection for up to 21:45 h of confinement (n = 7). Open circles indicate a measured ICM value, and the connecting lines indicate linear splines.
Figure 4.
Summary ICM responses to (a) capture, saline injection, and confinement (n = 8) or (b) capture, ACTH injection, and confinement (n = 7). Open circles indicate the time at which a single measurement was made. The innermost dotted line forms the mean ICM response curve for the group; the short dashed lines form 95% confidence intervals calculated with interpolated data; the outermost long dashed lines show prediction intervals calculated with interpolated data.
Sample Preservation
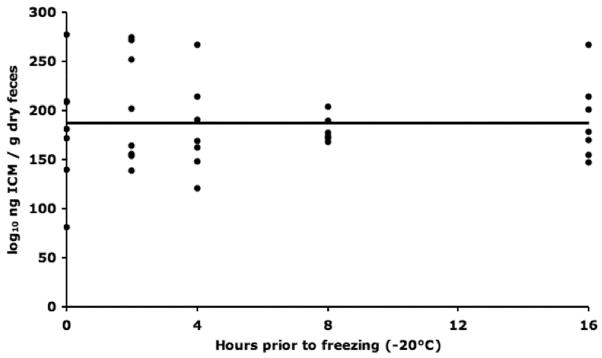
We determined the impact on the concentration of ICM from a field-sampling scenario in which samples were placed on wet ice (i.e., 4°C) for up to 16 h before freezing. We found that the amount of time for which pooled fecal samples were held at 4°C before storage at − 20°C did not impact the quantity of ICM detected (Fig. 5).
Figure 5.
Detected ICM concentration did not change over time when held at 4°C for up to 16 h before freezing (−20°C). Linear regression: ng ICM/g dry feces = 0.0152 × hours prior to freezing + 187.635 ng/g, R2 = 0.0000, F ratio = 0.0001, P = 0.9914, n = 7. Additionally, mean ICM values for each time point were not statistically different from one another (ANOVA, P = 0.8997).
Discussion
Endocrine Response
Fecal samples were an effective means of detecting changes in adrenocortical activity of these 15 wild-caught GRSG. All 15 GRSG responded to capture and confinement, as indicated by the changing concentrations of ICM in their fecal samples (Fig. 1). The mean (n = 15) adrenocortical or ICM response to capture entailed a primary ICM response that was 4.27 (range: 1.57–11.78) times baseline ICM, followed by a trough that was 1.39 (range: 0.47–4.05) times basal ICM and then a secondary response that was 3.85 (range: 0.80–14.78) times basal ICM (Table 2; Fig. 3). This multiphasic response function is not unprecedented for similarly treated birds. Both a northern spotted owl (Wasser et al. 2000) and a barred owl (Wasser and Hunt 2005) exhibited an approximately fourfold primary response at 2 h followed by a ~15-fold secondary peak at 16 HPI. Reasons for this multiphasic pattern were attributed to the sustained HPA stimulation provided by periodic sampling and handling (Wasser and Hunt 2005). It was postulated that this sustained psychological challenge promotes greater adrenal cortical secretion of corticosterone than does a onetime injection of ACTH. Given our observation that ACTH-treated birds exhibited this pattern more consistently than did saline-injected birds, we propose a more detailed mechanism in which psychological and physiological phenomena interact.
Our experimental design augmented circulating ACTH concentrations by psychological provocation of the HPA axis via capture from the wild. We further increased circulating ACTH levels in half of the birds via exogenous injection of porcine ACTH (as in Dehnhard et al. 2003). We expected the birds receiving both of the treatments (i.e., capture plus ACTH injection) to experience more HPA-axis stimulation than those receiving only one treatment (i.e., capture plus saline injection). The supposed greater HPA-axis stimulation in the former led to two observable effects: reduced primary ICM response variation and augmented secondary ICM response (Fig. 2; Table 2). The reduced primary ICM response variance of ACTH-injected birds may have resulted from a short-loop negative-feedback mechanism (Laycock and Wise 1996) within the hypothalamic-hypophyseal portal system, a network of blood vessels that connect the capillary beds of the hypothalamus and anterior pituitary. Normally, neurohormones flow through this portal system from the hypothalamus to the anterior pituitary, but some investigators postulate reverse flow during some conditions (Laycock and Wise 1996). Perhaps artificially elevated blood ACTH provoked this short-loop negative feedback, which may have attenuated the hypothalamic secretion of coriticotropin releasing factor (CRF) and reduced variation in CRF stimulation of and therefore ACTH secretion by the anterior pituitary. This may have led to a more uniform stimulation of the adrenal cortex by ACTH and consequently a reduced variance in the secretion of corticosterone and fecal excretion of corticosterone metabolites or ICM. In addition to this, systemically increased ACTH may have directly negatively stimulated the hypothalamus and led to reduced response variance. Further experimentation is warranted to allow better understanding of the mechanism driving the difference in response variance between these differently stimulated groups.
The second observable effect of ACTH treatment was to increase the secondary ICM response curve (Table 2; Fig. 4). The increased initial concentration of circulating ACTH in ACTH-injected birds may have more uniformly and profoundly stimulated adrenocortical tissue than capture and saline injection. Vertebrate adrenal cortical cells contain a hydroxysteroid dehydrogenase and three p450 enzymes to catalyze the conversion of cholesterol to corticosterone through a four-step process (Stevens 1996; Carsia and Harvey 2000). While enzyme activity is obligate, the level of enzyme activity is facultative. Given a stressor, the enzymatic synthesis of corticosterone and its subsequent release occurs in less than 5 min (Romero and Reed 2005) upon exposure to ACTH, followed by its metabolic breakdown and excretion (biological half-life of 10–20 min; Carsia and Harvey 2000). To sustain elevated basal levels of corticosterone, upregulation of enzyme activity and enzyme synthesis occurs, the latter enabling sustained increases in hormone production but requiring several more hours to activate than the former (Laycock and Wise 1996; Carsia and Harvey 2000). We propose that the significant difference between the saline- and ACTH-injected birds’ secondary ICM rise was related to enhanced enzyme synthesis. Augmented ACTH may have promoted the synthesis of steroidogenic enzymes, such as p450side-chain cleavage, making one effect (sustained secondary ICM response) of ACTH observable only after upregulation of enzyme expression. Data from an unpublished study by J. R. Paul-Murphy and T. E. Ziegler (2001) indicate a similar delay after ACTH injection in captive pigeons (Columba livia). Paul-Murphy and Ziegler did not detect a rise in fecal ICM until 6 h after ACTH injection, perhaps demonstrating elevated enzyme synthesis rather than enzyme activity alone.
An alternative argument for an elevated secondary ICM response curve in ACTH-injected compared to saline-injected birds relies on negative feedback mechanisms rather than up-regulated enzyme synthesis. The large primary ICM response magnitude in some saline-injected birds could have caused a protracted negative feedback on HPA activity, leading to a minimal secondary response. For example, the two highest-responding saline-injected birds did not exhibit a secondary ICM response, while the remaining saline-injected birds showed primary and secondary ICM responses closer to the ICM responses of ACTH-injected birds (Fig. 3).
We do not believe the second peak to be a reflection of enterohepatic hormone cycling because those birds with a high primary response did not necessarily exhibit a high secondary response; enterohepatic cycling predicts that they would.
In total, a mechanism to explain the different multiphasic responses between the treatment groups could involve three interacting phenomena. (1) Exogenously enhanced circulating levels of ACTH in birds already undergoing an elevated HPA response from capture may have reduced variation in their primary ICM response to capture through short-loop negative feedback between the hypothalamus and the anterior pituitary. (2) ACTH may have augmented adrenal cortical enzyme synthesis, explaining the more profound secondary rise in ACTH-injected birds. (3) The high primary ICM response in some saline-injected birds (up to 2,344 ng ICM/g dry feces) may have activated a more robust negative feedback than that in ACTH-injected birds (up to 562 ng ICM/g dry feces) and thus prevented a significant secondary ICM response (Fig. 3). Whatever the mechanism, we conclude that the difference between ACTH- and saline-injected birds’ response functions (Fig. 4) indicates that the assay was sensitive in the detection of biologically relevant ICM levels and that saline injection plus capture less consistently activated extended adrenocortical activity than did ACTH injection and capture.
Sex was not a statistically significant factor when characterizing the adrenocortical response function to capture and confinement in our study. However, males tended to achieve peak ICM excretion levels later than females (2:17 vs. 1:51 HPI, respectively; P = 0.0994) and more consistently sustained a secondary ICM response curve above basal ICM levels (Table 2). The delayed primary ICM peak in males may be due to their longer gastrointestinal tract because intestinal length scales positively with mass (~mass0.33; Remington 1989), and males were an average of 1.69 times as massive as females. Although all birds were reproductively inactive as inferred from an October capture, one cannot rule out age class–associated social hierarchies as having an impact on HPA-axis reactivity to the psychological stress of capture (Creel et al. 1996, 2001; Kotrschal et al. 1998; Crawford et al. 2004). Psychological response to a threatening stimulus such as capture may differ between dominants and subordinates (Creel et al. 1996, 2001; Kotrschal et al. 1998; Walker et al. 2005) because social status is often based on an animal’s size or experience level. Our data do not support this idea because age class and weight had no impact on statistical outcomes. However, the study of stress as it relates to dominance structure during the breeding season may yield interesting discoveries in this lekking species.
Field Implementation of This Technique
Investigators may choose to utilize this technique in studies to address physiological or behavioral ecology questions as posed above. Those interested in GRSG conservation may utilize this assay as a way of uncovering relationships between habitat suitability, physiological health, and population viability. Others have demonstrated such potential (Wasser et al. 1997). This technique may help investigators to identify unstable GRSG populations and to track the effectiveness of habitat modifications more proximally than with demographic indicators. When used in concert, demographic and physiologic techniques can provide strong evidence for a healthy or struggling wildlife population. However, careful field deployment of this particular assay is paramount for prudent interpretation of results.
Commonly used GRSG monitoring techniques facilitate the successful use of this tool. GRSG are often captured for demographic and other studies by spotlighting and netting of individually roosting birds. This practice offers the advantage of capturing large numbers of birds during a period in which the effects of a day’s stressors (e.g., breeding or foraging related activities) have presumably subsided, potentially making results less stochastic and a better reflection of basal levels of corticosterone. This notion is substantiated by the observations that the lag time between the exposure to a stressor and maximum ICM excretion is approximately 2 h (Table 2) and that GRSG captures do not normally occur until after dusk, once GRSG have had time to assume their roosting behaviors. Additionally, trapping during new-moon cycles is thought to boost capture productivity but may also minimize the exposure to nocturnal predatory stimuli, further reducing hormone variation (Clarke 1983; Orrock et al. 2004). Furthermore, investigators commonly sample GRSG during the spring lekking season, when mean minimum temperatures are generally below 0°C (1971–2000 data from the National Climatic Data Center), reducing the activity of temperature-sensitive and potentially ICM-degrading bacteria in fecal samples.
With these sampling issues in mind, and given the myriad factors that can impact ICM levels, we developed a sampling protocol to reduce the variability of ICM results and increase the ability to measure the level of ICM before capture. Previous investigators have (Möstl et al. 1999; Millspaugh et al. 2003; Temple and Gutiérrez 2004; Palme 2005) and have not (Baltic et al. 2005) detected sampling artifacts related to sample collection and preservation (for reviews, see Millspaugh and Washburn 2004; Goyman 2005; Möstl et al. 2005; Palme 2005; Palme et al. 2005). Given the potential presence of active bacterial enzymes in GRSG fecal samples and their potential impact on ICM chemical structure and assay responses, we quantified ICM concentrations in pooled feces held at 4°C for 2, 4, 8, or 16 h before long-term freezing at −20°C. This scenario should represent typical sample treatment protocols during nocturnal captures in which investigators may carry small cool packs to maintain samples at 2°–8°C, followed by freezing of the samples to −20°C the next morning, when a freezer is available. Figure 5 shows no relationship between the time held at 4°C before freezing to −20°C and measured ICM concentrations. Therefore, maintaining samples at this temperature for up to 16 h should not impact ICM results. This finding also indicates that if present and active, fecal bacterial enzymes in GRSG feces do not alter the detectable chemical structure of ICM at 4°C. Interestingly, this result is similar to findings in the black grouse (Tetrao tetrix; Baltic et al. 2005) and agrees with the proposal that bird feces may be less laden with bacterial enzymes than mammalian feces because of the rapid fecal excretion time in birds (Wasser and Hunt 2005). In addition to proper sample preservation, an understanding of the stress response will allow investigators to more accurately interpret ICM concentrations. Calculating the stress-response time course from the 15 GRSG measured in this study yields a predicted primary ICM rise of 4.25 ± 1.19 ng ICM/g dry feces/min postcapture (Table 2). This value reflects the rate by which corticosterone is secreted from adrenocortical tissue, metabolized by liver enzymes as well as the rate at which digesta traverse the GI tract of GRSG. The earliest detectable increase in ICM from baseline occurred in two birds sampled 20 min after capture, whereas a decrease in ICM from baseline was detected in samples taken from two birds at 55 min after capture; an increase in ICM was detected in these two birds in the subsequent fecal sample. Compared to measuring corticosterone in the plasma of birds in which an increase in corticosterone after stressor can occur in as little as 1.5 min (Romero and Reed 2005), measuring corticosterone metabolites in feces provides more time after capture to attain an unbiased sample. However, the current results indicate that fecal measures of ICM in GRSG are not free from potential sampling bias. Given this finding, it is important to standardize the collection protocol across treatment groups, or one risks inferring treatment-mediated effects rather than sampling artifacts. Collecting the first fecal sample available upon capture and immediately cooling it on ice (4°C) should sufficiently limit or eliminate collection-related differences in ICM. This should not be difficult, as all 70 field-collected samples used to determine assay precision were taken within the first 5–15 min of capture. It is advisable to record the time of capture, and collection, to allow for correction while analyzing data should collection procedures differ significantly and reflect systematic sampling bias during a given study. Later trials may also consider an alternative means of normalizing ICM assay values than dry weight because ICM values decreased with grams of dry feces (R2 = − 0.19) in this study.
Measuring ICM in fecal samples will enable investigators to compare hen fecundity with adrenocortical activity and to perhaps set a “safe-range” of ICM in reproductively active hens. Physiologically based predictions of reproductive capacity show some promise (Gregg et al. 2006). However, other factors require quantification to better understand the relationship between habitat, physiological health and population viability. A wide variety of studies might integrate the current technique, using knowledge of a bird’s adrenocortical activity as one indicator of its response to the surrounding environment. Managers and scientists could incorporate measures of ICM into adaptive management experiments in which habitat modifications are applied and GRSG fecundity and ICM are monitored. For example, some research demonstrates that natural gas extraction operations negatively impact GRSG activities near gas-producing wells (Lyon and Anderson 2003; Holloran 2005; Walker et al. 2007). It is postulated that the noise and physical activity surrounding this type of energy extraction [psychologically] deters lekking GRSG (Braun 2006). Investigators could determine whether such noise impacts ICM levels in male or female GRSG, and if so, measuring ICM levels could help to guide the regularity of well placement, and the level and timing of extraction activities. Longer-term studies involving the reestablishment of a healthful landscape to optimize spatial heterogeneity (Aldridge 2005) for GRSG behavior could incorporate ICM with other physiological and demographic measures to inform managers of their progress. Significant alterations in vegetation type and structure should influence foraging efficiency and thus rates of certain physiological processes (Kitaysky et al. 2001; Kitaysky et al. 2005; Tracy et al. 2006). Therefore, monitoring hen GRSG ICM and fecundity in conjunction with manipulations of vegetation quality and distribution might achieve the benefits of higher habitat quality and a better idea of the relationship between ICM and hen productivity.
We have shown that this assay provides a way to assess HPA activity in GRSG by comparing the 22-hour adrenocortical response to two different levels of stimulation. Considering only those injected with saline, we have shown that capture and periodic handling, as may occur during translocation activities, consistently affects these birds in the short-term but less consistently over the period held, as some did not develop a secondary ICM rise (Fig. 4a). This is not to say, however, that translocation-type activities are stress-free; rather, this finding indicates that some of the birds may have acclimated to periodic handling (every 3 h) and short-term confinement. We suggest that this assay could be used to guide improvements to translocation procedures by monitoring GRSG’s adrenocortical response to different levels of enrichment, for example. Furthermore, we have recommended standardized sampling procedures to facilitate range-wide comparisons of GRSG HPA activity. We advise that comparisons be made in a way that controls for the effects of the time of day and season, as these variables are known to impact basal adrenal activity (Laycock and Wise 1996; Millspaugh and Washburn 2004; Nelson and Demas 2004; Wasser and Hunt 2005). And, given that the primary ICM rise is observable shortly after capture, collection protocols must be standardized within and preferably across experiments. Combining this technique with other physiological measures and demographic parameters should further inform researchers and managers of the physiological constraints imposed by different types of habitat. Prudent application of this technique will lead to deeper knowledge of basic biological and ecological phenomena and perhaps to successful physiologically based adaptive-conservation of GRSG.
Acknowledgments
Financial support for this work was provided by the USGS Biological Resources Discipline through the Great Lakes–Northern Forest Cooperative Ecosystems Study Unit, University of Wisconsin–Madison award 02HQAG0112, and the Wisconsin National Primate Research Center grant RR000167. We also acknowledge the National Research Service Award Predoctoral Traineeship awarded to M.D.J., National Institutes of Environmental Health Sciences Training Grant T32 ES07015, Molecular and Environmental Toxicology Center, University of Wisconsin–Madison. We acknowledge the U.S. Fish and Wildlife Service staff at Hart Mountain National Antelope Refuge for their logistical support. Finally, we thank D. Goldberg and M. Lund of the USGS National Wildlife Health Center; C. Hagen of the Oregon Department of Fish and Wildlife (ODFW) and C. Hummel, a volunteer for the ODFW; and T. Fitzhenry of the Oregon Parks and Recreation Department for their assistance in the capturing and sampling of the animal subjects used for this study.
Literature Cited
- Akcakaya HR, V, Radeloff C, Mladenoff DJ, He HS. Integrating landscape and metapopulation modeling approaches: viability of the sharp-tailed grouse in a dynamic landscape. Conserv Biol. 2004;18:526–537. [Google Scholar]
- Aldridge CL. PhD diss. University of Alberta; Edmonton: 2005. Identifying Habitats for Persistence of Greater Sage-Grouse (Centrocercus urophasianus) in Alberta, Canada. [Google Scholar]
- Baltic M, Jenni-Eiermann S, Arlettaz R, Palme R. A noninvasive technique to evaluate human-generated stress in the black grouse. Ann N Y Acad Sci. 2005;1046:81–95. doi: 10.1196/annals.1343.008. [DOI] [PubMed] [Google Scholar]
- Barnett JK, Crawford JA. Pre-laying nutrition of sage grouse hens in Oregon. J Range Manag. 1994;47:114–118. [Google Scholar]
- Beuving G, Vonder GM. Effect of stressing factors on corticosterone levels in the plasma of laying hens. Gen Comp Endcrinol. 1978;35:153–159. doi: 10.1016/0016-6480(78)90157-0. [DOI] [PubMed] [Google Scholar]
- Braun CE. A Blueprint for Sage-Grouse Conservation and Recovery. Grouse; Tucson, AZ: 2006. [Google Scholar]
- Carsia RV, Harvey S. Adrenals. In: Whittow GC, editor. Sturkie’s Avian Physiology. 5. Academic Press; San Diego, CA: 2000. pp. 489–537. [Google Scholar]
- Clarke JA. Moonlight’s influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus) Behav Ecol Sociobiol. 1983;13:205–209. [Google Scholar]
- Connelly JW, Braun CE. Long-term changes in sage-grouse Centrocercus urophasianus populations in western North America. Wildl Biol. 1997;3:229–234. [Google Scholar]
- Connelly JW, Schroeder MA, Sands AR, Braun CE. Guidelines to manage sage grouse populations and their habitats. Wildl Soc Bull. 2000;28:967–985. [Google Scholar]
- Crawford JA, Olson RA, West NE, Mosley JC, Schroeder MA, Whitson TD, Miller RF, Gregg MA, Boyd CS. Ecology and management of sage-grouse and sage-grouse habitat. J Range Manag. 2004;57:2–19. [Google Scholar]
- Creel S. Social dominance and stress hormones. Trends Ecol Evol. 2001;16:491–497. [Google Scholar]
- Creel S, Marushacreel N, Monfort SL. Social stress and dominance. Nature. 1996;379:212. [Google Scholar]
- Dehnhard M, Schreer A, Krone O, Jewgenow K, Krause M, Grossman R. Measurement of plasma corticosterone and fecal glucocorticoid metabolites in the chicken (Gallus domesticus), the great cormorant (Phalacrocorax carbo), and the goshawk (Accipiter gentilis) Gen Comp Endocrinol. 2003;131:345–352. doi: 10.1016/s0016-6480(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Dunbar MR, Gregg MA, Crawford JA, Giordano MR, Tornquist SJ. Normal hematologic and biochemical values for prelaying greater sage-grouse (Centrocercus urophasianus) and their influence on chick survival. J Zoo Wildl Med. 2005;36:422–429. doi: 10.1638/04-065.1. [DOI] [PubMed] [Google Scholar]
- Edelmann FD, Ulliman MJ, Wisdom MJ, Reese KP, Connelly JW. Technical Report 25. Idaho Forest, Wildlife and Range Experiment Station; Moscow, ID: 1998. Assessing habitat quality using population fitness parameters: a remote-sensing-GIS based habitat explicit population model for sage grouse (Centrocercus urophasianus) [Google Scholar]
- Gaunt AS, Oring LW, Able KP, Anderson DW, Baptista LF, Barlow JC, Wingfield JC. Guidelines to the Use of Wild Birds in Research. Ornithological Council; Washington, DC: 1997. [Google Scholar]
- Goyman W. Noninvasive monitoring of hormones in bird droppings: physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann N Y Acad Sci. 2005;1046:35–53. doi: 10.1196/annals.1343.005. [DOI] [PubMed] [Google Scholar]
- Gregg MA, Dunbar MR, Crawford JA, Pope MD. Total plasma protein and renesting by greater sage-grouse. J Wildl Manag. 2006;70:472–478. [Google Scholar]
- Holloran MJ. PhD diss. University of Wyoming; Laramie: 2005. Greater Sage-Grouse (Centrocercus urophasianus) Population Response to Natural Gas Development in Western Wyoming. [Google Scholar]
- Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Kitaysky AS, Kitaiskaia EV, Wingfield JC, Piatt JF. Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J Comp Physiol B. 2001;171:701–709. doi: 10.1007/s003600100230. [DOI] [PubMed] [Google Scholar]
- Kitaysky AS, Romano MD, Piatt JF, Wingfield JC, Kikuchi M. The adrenocortical response of tufted puffin chicks to nutritional deficits. Horm Behav. 2005;47:606–619. doi: 10.1016/j.yhbeh.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Kotrschal K, Hirschenhauser K, Möstl E. The relationship between social stress and dominance is seasonal in greylag geese. Anim Behav. 1998;55:171–176. doi: 10.1006/anbe.1997.0597. [DOI] [PubMed] [Google Scholar]
- Laycock J, Wise P. Essential Endocrinology. 3. Oxford University Press; New York: 1996. [Google Scholar]
- Lyon AG, Anderson SH. Potential gas development impacts on sage-grouse nest initiation and movement. Wildl Soc Bull. 2003;31:486–491. [Google Scholar]
- MacArthur RH. Geographical Ecology: Patterns in the Distribution of Species. Harper & Row; New York: 1972. [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Millspaugh JJ, Washburn BE. Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol. 2004;138:189–199. doi: 10.1016/j.ygcen.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Millspaugh JJ, Washburn BE, Milanick MA, Slotow R, Van Dyk G. Effects of heat and chemical treatments on fecal glucocorticoid measurements: implications for sample transport. Wildl Soc Bull. 2003;31:399–406. [Google Scholar]
- Möstl E, Messmann S, Bagu E, Robia C, Palme R. Measurement of glucocorticoid metabolite concentrations in faeces of domestic livestock. Zentbl Vetmed Reihe A. 1999;46:621–631. doi: 10.1046/j.1439-0442.1999.00256.x. [DOI] [PubMed] [Google Scholar]
- Möstl E, Rettenbacher S, Palme R. Measurement of corticosterone metabolites in birds’ droppings: an analytical approach. Ann N Y Acad Sci. 2005;1046:1–18. doi: 10.1196/annals.1343.004. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE. Seasonal patterns of stress, disease, and sickness responses. Curr Dir Psychol Sci. 2004;13:198–201. [Google Scholar]
- Orrock JL, Danielson BJ, Brinkerhoff RJ. Rodent foraging is affected by indirect, but not direct, cues of predation risk. Behav Ecol. 2004;15:433–437. [Google Scholar]
- Palme R. Measuring fecal steroids: guidelines for practical application. Ann N Y Acad Sci. 2005;1046:1–6. doi: 10.1196/annals.1343.007. [DOI] [PubMed] [Google Scholar]
- Palme R, Rettenbacher S, Touma C, El-bahr SM, Möstl E. Stress hormones in mammals and birds: comparative aspects regarding metabolism, excretion, and non-invasive measurement in fecal samples. Ann N Y Acad Sci. 2005;1040:162–171. doi: 10.1196/annals.1327.021. [DOI] [PubMed] [Google Scholar]
- Remington TE. Why do grouse have ceca? a test of the fiber digestion theory. J Exp Zool. 1989;3(suppl):87–94. doi: 10.1002/jez.1402520515. [DOI] [PubMed] [Google Scholar]
- Romero ML, Reed JM. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp Biochem Physiol A. 2005;140:73–79. doi: 10.1016/j.cbpb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schroeder MA, Aldridge CL, Apa AD, Bohne JR, Braun CE, Bunnell SD, Connelly JW, et al. Distribution of sage-grouse in North America. Condor. 2004;106:363–376. [Google Scholar]
- Stevens L. Avian Biochemistry and Molecular Biology. Cambridge University Press; Cambridge: 1996. [Google Scholar]
- Stevenson RD, Tuberty SR, Defur PL, Wingfield JC. Ecophysiology and conservation: the contribution of endocrinology and immunology-introduction to the symposium. Integr Comp Biol. 2005;45:1–3. doi: 10.1093/icb/45.1.1. [DOI] [PubMed] [Google Scholar]
- Temple DJ, Gutiérrez RJ. Factors related to fecal corticosterone levels in California spotted owls: implications for assessing chronic stress. Conserv Biol. 2004;18:538–547. [Google Scholar]
- Tracy CR, Nussear KE, Esque TC, Dean-Bradley K, Tracy CR, Defalco LA, Castle KT, Zimmerman LC, Espinoza RE, Barber AM. The importance of physiological ecology in conservation biology. Integr Comp Biol. 2006;46:1191–1205. doi: 10.1093/icb/icl054. [DOI] [PubMed] [Google Scholar]
- Van Tienderen PH. Evolution of generalists and specialists in spatially heterogeneous environments. Evolution. 1991;45:1317–1331. doi: 10.1111/j.1558-5646.1991.tb02638.x. [DOI] [PubMed] [Google Scholar]
- Walker BG, Boersma PD, Wingfield JC. Field endocrinology and conservation biology. Integr Comp Biol. 2005;45:12–18. doi: 10.1093/icb/45.1.12. [DOI] [PubMed] [Google Scholar]
- Walker BL, Naugle DE, Doherty KE. Greater sage-grouse population response to energy development and habitat loss. J Wildl Manag. 2007;71:2644–2654. [Google Scholar]
- Washburn BE, Millspaugh JJ, Schulz JH, Jones SB, Mong T. Using fecal glucocorticoids for stress assessment in mourning doves. Condor. 2003;105:696–706. [Google Scholar]
- Wasser SK, Bevis K, King G, Hanson E. Noninvasive physiological measures of disturbance in the northern spotted owl. Conserv Biol. 1997;11:1019–1022. [Google Scholar]
- Wasser SK, Hunt KE. Noninvasive measures of reproductive function and disturbance in the barred owl, great horned owl, and northern spotted owl. Ann N Y Acad Sci. 2005;1046:109–137. doi: 10.1196/annals.1343.010. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol. 2000;120:260–275. doi: 10.1006/gcen.2000.7557. [DOI] [PubMed] [Google Scholar]
- Zablan MA, Braun CE, White GC. Estimation of greater sage-grouse survival in North Park, Colorado. J Wildl Manag. 2003;67:144–154. [Google Scholar]