Abstract
Mothers in a range of taxa manipulate the phenotype of their offspring in response to environmental change in order to maximize their own fitness. Most studies have focused on changes in the mean phenotype of offspring. Focusing on mean offspring phenotypes is appropriate for species in which mothers are likely to successfully predict the environment their offspring will experience, but what happens when the offspring's environment is unpredictable? Theory suggests that when mothers face uncertainty regarding their offspring's environment, they should increase within-clutch variation in the offspring phenotype (i.e. they should bet hedge). While comparative analyses support the idea that mothers do bet hedge in response to environmental unpredictability, empirical tests are very rare and it remains unclear whether mothers adaptively adjust variance in offspring traits (a phenomenon we call dynamic bet hedging). As a first step towards examining dynamic bet hedging, we reanalysed data from five previously published studies. These studies were across a range of taxa, but all manipulated the maternal environment/phenotype and then examined changes in mean offspring size. We found some support for the theoretical predictions that mothers should increase within-clutch offspring size variation when faced with unpredictable environments. We predict that dynamic bet hedging is more common than previously anticipated and suggest that it has some interesting implications for the studies that focus on shifts in mean offspring traits alone. Hence, future studies should examine maternal effects on both the mean and the variance of offspring traits.
Keywords: egg size, maternal effect, variation, bet hedging, unpredictable environments, geometric mean
1. Introduction
Offspring phenotypes are remarkably variable at all levels—among species, populations within species, females within populations, clutches within mothers and individuals within clutches (e.g. offspring size, reviewed in Bernardo 1996; Marshall & Keough 2008). The role of maternal effects in generating this variability has long been the focus of research, and a range of factors have been identified that affect the mean phenotype of offspring produced by mothers (as demonstrated in this volume). While there has been a sustained and significant focus on the influence of maternal effects on the mean phenotype of offspring, the role of maternal effects on variability in offspring traits within clutches has received far less attention (Koops et al. 2003). This is surprising given the numerous conceptual and theoretical arguments that mothers should receive fitness benefits from producing offspring of variable phenotypes in unpredictable environments (e.g. Cohen 1966; Capinera 1979; Koops et al. 2003).
Generally, selection should favour mothers that produce offspring with a phenotype that maximizes maternal fitness (Mousseau & Fox 1998; Uller 2008). Importantly, the offspring phenotype that maximizes maternal fitness does not necessarily maximize offspring fitness and thus parents and offspring can be in conflict over what is the ‘best’ phenotype for both parties (Trivers 1974). Offspring size–number strategies best illustrate this conflict between mothers and offspring: mothers typically divide their resources among many offspring at the expense of each individual offspring's fitness (Smith & Fretwell 1974). The optimal offspring phenotype that mothers ‘should’ produce in order to maximize their fitness depends strongly on the conditions their offspring are likely to experience. Given most environments vary in space and time over the lifespan of mothers, mothers must often use cues from their own environment as predictors of the environment of their offspring. Numerous empirical studies demonstrate that mothers do indeed adjust the mean phenotype of their offspring according to changes in the maternal environment (reviewed in Mousseau & Fox 1998; Uller 2008). However, what if mothers cannot accurately anticipate the environment that their offspring experience?
When environments vary unpredictably, a single phenotype is unlikely to perform well in every circumstance. Therefore, if mothers cannot detect the likely environment of their offspring, or environmental cues in the maternal generation suggest that the offspring environment is likely to vary unpredictably, mothers should hedge their bets by producing a range of offspring phenotypes (Cohen 1966; Seger & Brockmann 1987; Philippi & Seger 1989). Bet hedging at reproduction has long been recognized as an adaptive strategy in unpredictable environments (e.g. Capinera 1979; Crump 1981; Koops et al. 2003; Simons & Johnston 2006). Increasing within-clutch variation in unpredictable environments can reduce the variation in reproductive success among generations, by ensuring that at least some offspring are likely to have the ‘right’ phenotype for the local environment (see box 1). This is because (over time) the cost of producing fewer offspring is larger than the benefit of producing more offspring (Gillespie 1977; Philippi & Seger 1989; Orr 2007). Therefore, in unpredictable environments, fitness across multiple generations may be maximized by increasing the variation in offspring phenotypes within generations, and thereby reducing the variation in offspring fitness across generations.
Box 1. Bet hedging: the basics revisited.
Reducing variation in reproductive success among generations carries an adaptive benefit because this reduction in variation increases the geometric mean fitness—the key component of any bet-hedging evolutionary strategy (Seger & Brockmann 1987; Philippi & Seger 1989). Geometric means—as opposed to arithmetic means—are calculated by multiplying values together and then raising this value to the power of 1/n where n=the number of values being considered. Because geometric means essentially estimate cumulative fecundity across generations, they give a far better estimate of relative population growth rates. Therefore, the mean fitness within a generation and across generations should be calculated as the arithmetic and geometric means, respectively (Gillespie 1977). For a detailed discussion of diversified bet hedging and maximizing geometric fitness, see Philippi & Seger (1989).
Mathematically, the geometric mean is related to the arithmetic mean by the equation
where μG is the geometric mean fitness; μA is the arithmetic mean fitness; and σ is the among-generation variation in fitness. Note that this equation applies to among-generation bet hedging only (see box 2) and only applies for fitness values greater than 0 (when fitness is zero for any generation, the geometric mean is always zero, i.e. the population goes extinct). As a simple rule of thumb, any strategy that has lower among-generation variance but the same arithmetic mean as another strategy will yield a higher geometric mean. However, a strategy with a very low arithmetic mean and low among-generation variance will not have a higher geometric mean than a strategy with a very high arithmetic mean and (relatively) higher among-generation variance (assuming that fitness in any one generation is always greater than 0). Thus, mothers are under selection to not only minimize among-generation variance in their reproductive success, but also to maximize their arithmetic mean fitness within generations (Gillespie 1977; Orr 2007), and this raises important trade-offs where the ability of mothers to predict the local offspring environment becomes crucial.
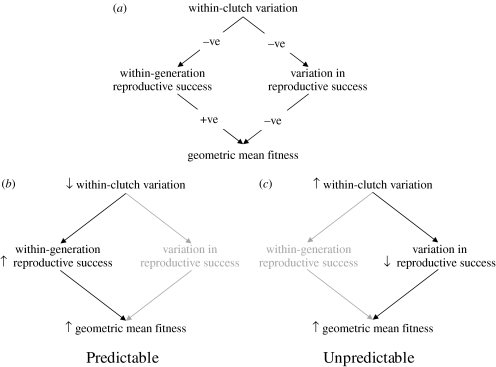
One of the best cited examples of maternal bet hedging concerns offspring size. Producing a range of offspring sizes within a single clutch inevitably means that mothers will produce some offspring that are not of an optimal size. So how do mothers face the dual challenge of maximizing the within-generation mean fitness and minimizing the among-generation variation in reproductive success? They achieve the former via plasticity in mean offspring size and the latter via within-clutch variation. Importantly, these two traits (i.e. the proportion of offspring close to the optimal size and variance in offspring size) trade off against each other: the more variance, the more offspring that are away from the optimal size (Marshall et al. 2008). Ultimately, the costs and benefits of the optimum variance trade-off will depend on the predictability of the environment and the scale at which the balancing selection pressures act. The ability of mothers to anticipate the environment that offspring will face determines the level of variation in offspring size that mothers will produce. When mothers have a good chance of predicting the environment their offspring will face, plasticity in mean offspring size (sometimes called ‘adaptive’ or ‘anticipatory’ maternal effects, Marshall & Uller 2007) will be favoured; when mothers have a poor chance of predicting the environment of their offspring, increasing variance in offspring size will be favoured (figure 1). The above argument indicates that there is significant uncertainty regarding the prevalence and importance of maternal bet hedging (including operational definitions of the term bet hedging itself: see box 2) and this uncertainty raises the important question: what empirical evidence is there for mothers employing bet-hedging strategies in unpredictable environments?
Figure 1.
(a) Schematic showing the relationships among within-clutch variability in offspring size, within-generation reproductive success, among-generation variability in reproductive success and geometric mean fitness. Increasing within-clutch variability decreases reproductive success within any one generation (assuming there is a single, optimal offspring size) but decreases variability among generations. (b) When environments are predictable, mothers should decrease within-clutch variability in offspring size so as to maximize reproductive success and thus geometric mean fitness. (c) However, when environments are unpredictable, mothers should increase within-clutch variability in offspring size, decreasing among-generation variation in reproductive success in order to increase geometric mean fitness.
Box 2. Bet hedging: the problems (aka the glass is half empty).
While bet hedging as a solution to environmental uncertainty has considerable intuitive appeal and some theoretical support, there are some significant doubts regarding its underlying adaptive value and applicability. Furthermore, there is significant ambiguity associated with the term ‘bet hedging’—the phrase covers a range of very different reproductive strategies that carry very different fitness pay-offs.
One of the often underappreciated issues associated when bet hedging is considered is that there are two distinct forms of bet hedging: (i) among generation and (ii) within generation. Hopper et al. (2003) provided an excellent and detailed overview of the important differences between these two forms of bet hedging, but we will provide a brief description of the issues associated with within- and among-generation bet hedging that are relevant to our discussion here. Among-generation bet hedging is essentially where mothers produce offspring with a range of different phenotypes that experience the same environment. Among-generation bet hedging is perhaps more common and more easily understood as the benefits of this form are straightforward: fitness benefits arise because at least some of the offspring will have the ‘right’ phenotype for the local environment. Within-generation bet hedging is more restricted and involves producing the same phenotype but spreading offspring across multiple environments (classically, this form of bet hedging is known as ‘not putting all one's eggs in the same basket’). Within-generation bet hedging is more complicated because population size strongly affects the benefits of this form of bet hedging: in large populations, the benefits of within-generation bet hedging are greatly reduced (Yasui 1998; Hopper et al. 2003; but see Proulx 2000). In this paper, we consider the benefits of producing variable offspring sizes as a bet-hedging strategy and in this regard, we are explicitly focused on among-generation bet hedging. Thus, we would expect that producing variable offspring sizes carries strong benefits in unpredictable environments. However, it is important to recognize that in all of the case studies we discuss below, offspring can disperse to different environments and so it could be argued that a limited amount of within-generation bet hedging also occurs. Regardless, our goal here is simply to highlight that caution should be exercised whenever one uses the term bet hedging in regard to maternal reproductive strategies.
A second issue associated with discussions of bet hedging is the distinction between diversified bet hedging and conservative bet hedging (Seger & Brockmann 1987). Diversified bet hedging is where mothers produce a range of phenotypes (as described above as among-generation bet hedging): conservative bet hedging is where mothers produce a single phenotype; but that phenotype is of such ‘quality’ that reproductive success is relatively constant from generation to generation. Specifically relating to offspring size, diversified bet hedging occurs when mothers produce a range of offspring sizes and conservative bet hedging occurs when mothers produce offspring that are consistently larger than a long-term optimal offspring size. Some models suggest that conservative bet hedging is more likely to be favoured than diversified bet hedging with regard to offspring size (Einum & Fleming 2004), but others find the converse (Marshall et al. 2008). Ultimately, the relative benefits of conservative and diversified bet hedging will depend on a number of factors including the offspring size–fitness function. Again, we simply want to highlight that it is important to recognize that there are multiple paths to reducing among-generation variation in reproductive success, and that the benefits of various bet-hedging strategies are not always clear.
Numerous correlative studies have cited bet hedging as an explanation for the observed within-clutch variation in offspring size. For example, Crump (1981) argued that some species of tree frogs exhibit within-clutch variability in the size of eggs as a bet-hedging strategy in response to environmental uncertainty. Species that breed in temporary ponds produce ‘flatter’ (i.e. more platykurtic) distributions of egg sizes than species that breed in permanent ponds (Crump 1981). Marshall et al. (2008) compared the offspring size variation across developmental modes in marine invertebrates, and found that within-clutch variability is the greatest in feeding, indirect developers that spend weeks to months in the plankton (where mothers are least able to predict offspring environment) and the smallest in species with direct development (where offspring hatch in the maternal environment). Within-species comparisons also suggest that bet hedging is likely. In the sea slug, Alderia modesta, mothers that produce feeding, highly dispersive offspring exhibit higher levels of within-clutch variation in offspring size than mothers of the same species that produce far less dispersive, non-feeding offspring (Krug 1998). Other studies examining within-species/intraspecific correlations between total offspring size variation and environmental predictability also suggest adaptive processes in egg size variability (Poulin & Hamilton 2000; Einum & Fleming 2002), although caution must be exercised as these studies did not distinguish between among- and within-clutch variations.
Although correlative studies suggest that females may indeed be able to adjust variability in offspring size according to the anticipated environmental conditions, the strength of inference drawn from these studies is limited, and manipulative studies are required to produce more definitive conclusions. The particular problem with examining within-clutch variation in offspring size is that some offspring size variation is inevitable: mothers are unable to produce perfectly identical offspring and much of the observed variation in offspring size could be due simply to physiological constraints (Fox & Czesak 2000). Furthermore, variability in offspring size may increase in stressful or novel/rare conditions owing to developmental instability (de Jong 1995; Debat & David 2001; Olsson & Uller 2002). Thus, determining whether a particular level of variation in offspring size is adaptive or not for any one species is problematic, as several different selective regimes can lead to context-dependent variance in offspring size.
The issue of within-clutch variability is further complicated by the fact that selection can favour within-clutch variability in the absence of selection for bet-hedging strategies. Mothers may increase egg size variability in order to increase arithmetic mean fitness. For example, in stinkbugs, Kudo (2001) found subsocial species to lay smaller eggs around the periphery of the clutch in response to the positional risk of egg predation, whereas asocial species produce offspring of similar size (Kudo 2001, 2006). In this example, producing offspring of different sizes increases the number of surviving offspring in a constant and predictable environment. Furthermore, bet-hedging strategies may operate via different mechanisms. For example, many birds initiate incubation before their clutch is full to promote hatching asynchrony (Laaksonen 2004). Here, hatching asynchrony may be a method of maximizing the number of surviving chicks (i.e. arithmetic fitness) across a range of food availabilities, and is made possible as maternal provisioning continues after hatching and therefore clutch size adjustments can occur while parents feed their young. Hence, showing a difference in within-clutch variability is not sufficient evidence to support dynamic bet hedging as a maternal effect, and hypotheses and predictions must be clearly stated for each study system.
A more direct test of the adaptive nature of producing variable offspring sizes is to determine whether mothers dynamically adjust variation in the size of offspring in response to experimental manipulations of environmental unpredictability. Such an approach has been used to great effect in examining the selection pressures on mean offspring size (e.g. Fox et al. 1997) and we suggest that similar approaches could be used to determine whether mothers dynamically bet hedge. Unfortunately, we can find few studies that manipulate the maternal environment and directly measure within-clutch offspring size variation (but see Halpern 2005). As a first step, we have re-analysed some published studies that examine shifts in mean egg size across experimentally manipulated maternal environments to see whether there was a corresponding shift in the coefficient of variation (CV) within each clutch. These studies cover a variety of taxa and manipulations of maternal environment, both from our own work and that of colleagues who were generous enough to provide us with their raw data. Given the possibility that shifts in mean offspring size induce a shift in offspring size variation that is not adaptive, we have included two studies in which a change in offspring size variation would not be predicted, despite any shift in mean offspring size. However, these case studies represent datasets that we were able to access, and although we acknowledge that the list is far from exhaustive, they are purely designed to stimulate research and serve as a preliminary look at whether mothers may indeed control the within-clutch variance of their offspring. It is our hope that this paper encourages researchers to examine shifts in variance in addition to mean offspring traits in future studies.
2. Data compilation and analysis
We used the raw data from published studies to generate the estimates of within-clutch variation in offspring size for mothers experiencing different environmental treatments. Standard deviations and variances typically show strong relationships with means. Given that in most of our studies a shift in mean offspring size occurred, we used CVs as our estimates of offspring size variation. The CV describes the standard deviation as a percentage of the mean, and therefore CVs are less likely to increase as an artefact of increases in means (Quinn & Keough 2002). Furthermore, we have taken care to ensure that differences in the estimates of variation are unaffected by differences in sample sizes among the different treatment groups.
To analyse the data, we did our best to replicate the original studies' statistical analyses but, instead of analysing mean offspring size for each female as the unit of replication, we replaced it with the CV of offspring size for each female. When random factors were included, we used standard model simplification procedures to produce a reduced statistical model (Quinn & Keough 2002). In some instances, we could detect no effect of the experimental treatment and so we did power analyses to determine whether the lack of an effect represented a type II error. We arbitrarily set the effect size at a 50 per cent change in the CV because we had no a priori predictions of biologically significant effect sizes.
3. Case study 1
Plaistow et al. (2007) manipulated both the natal and the adult environment of the soil mite, Sancassania berlesei, and examined lifetime maternal provisioning strategies (egg size, egg number and maternal survival). Mites were reared from hatching in a low, medium or high ‘natal’ food environment; then at maturity, they were transferred to individual ‘adult’ environments (again manipulated as low-, medium- and high-feeding treatments) in a fully crossed, factorial design. Females increased their mean egg size as they aged, an effect that was strongest when adults were kept in high-food conditions. This result was interpreted as an adaptive shift in reproductive strategy with age—that is, the emphasis on egg number in young females shifted to an emphasis on egg size in older females, a response to likely increases in offspring competition.
With regard to within-clutch variation in offspring size, therefore, we suggest that older mothers are confronted with a more predictable environment, in that competition between offspring is much more likely when mothers are older (Plaistow et al. 2007). As such, we would predict that variation in offspring size should be lower in older mothers. Furthermore, we would predict that mothers that experience a constant environment with regard to food availability during rearing and reproduction would produce less variable offspring than mothers that experienced changes in their nutritional environment across their lifetime.
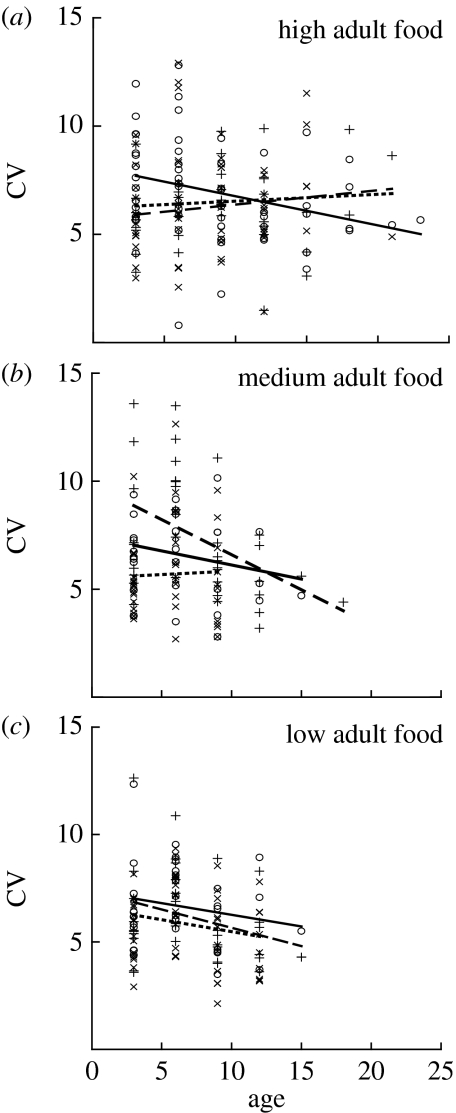
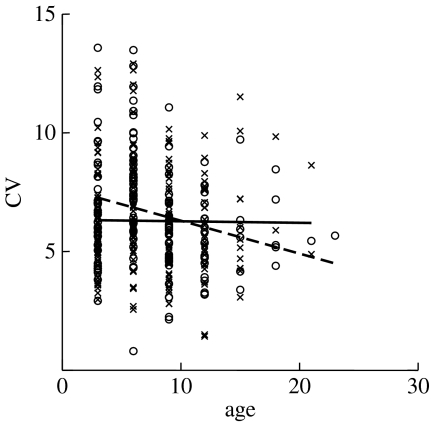
We found that the direction of change in the CV of egg size across maternal age depended on both the rearing and the adult food treatments (table 1; figure 2). As predicted, females generally showed a decrease in egg size variability with age (although the slope of this decrease depended on natal–adult feeding combinations, as shown by the significant interaction in table 1), but this was particularly the case when the natal environment matched the maternal environment. When mothers experienced a change in their nutritional environment from the natal stage to the adult stage, offspring size variability no longer decreased with age (table 2; figure 3). We suggest that this study represents an excellent example of mothers using their own experience of environmental predictability as an indicator of the predictability of the offspring environment, and modifying offspring size variability accordingly.
Table 1.
Effect of maternal age and food availability during juvenile and adult stages on within-clutch offspring size variation in the soil mite, S. berlesei. (Data from Plaistow et al. 2007.)
| source | d.f. | MS | F | p-value |
|---|---|---|---|---|
| rear food | 2 | 23.800 | 5.226 | 0.006 |
| adult food | 2 | 8.036 | 1.764 | 0.173 |
| age | 1 | 41.778 | 9.174 | 0.003 |
| rear×adult food | 4 | 11.640 | 2.556 | 0.039 |
| error | 378 | 4.554 |
Figure 2.
Effect of maternal age, natal diet and adult diet on offspring size variability in the soil mite, S. berlesei (data from Plaistow et al. 2007). Data are divided into adult food treatments ((a) high, (b) medium and (c) low), and divided within each adult treatment into rearing food treatments (circles and solid line, high; pluses and dashed line, medium; and crosses and dotted line, low).
Table 2.
Effect of maternal age and constancy of the maternal nutritional environment on within-clutch offspring size variation in the soil mite, S. berlesei. (Data from Plaistow et al. 2007.)
| source | d.f. | MS | F | p-value |
|---|---|---|---|---|
| change in food | 1 | 39.397 | 8.430 | 0.004 |
| age | 1 | 32.952 | 7.051 | 0.008 |
| change×age | 1 | 27.260 | 5.833 | 0.016 |
| error | 384 | 4.673 |
Figure 3.
Effect of maternal age and environmental constancy on offspring size variability in the soil mite, S. berlesei (data from Plaistow et al. 2007). Data are divided into a change in feeding treatments from rearing to adult food environment (crosses and solid line), and no change in feeding treatment (circles and dashed line).
4. Case study 2
The study by Allen et al. (2008) is similar to that of Plaistow et al. (2007), in that it also explored maternal responses to environmental variation in competition for resources. Allen et al. (2008) manipulated densities of the bryozoan, Bugula neritina (a sessile, filter feeding, colonial marine invertebrate), to examine whether the intensity of intraspecific competition influenced maternal provisioning. Colonies were reared from settlement in the field for five weeks in either low- or high-density treatments, then focal colonies were spawned in the laboratory and larval size measured. Mothers experiencing competition produced larger larvae than competition-free mothers, which was interpreted as an adaptive response to competition because larger larvae are better competitors. Allen et al. (2008) also included treatments in which mothers experienced either a constant or a variable level of competition. We predict that mothers in high-density environments would produce less variable offspring sizes, as offspring are highly likely to face competition, whereas it is unclear whether offspring from competition-free mothers will face competition. Furthermore, we would predict mothers that experience variable levels of competition to produce more variable offspring sizes than mothers that experience constant levels of competition.
Neither of our predictions was supported by our analyses, but in each instance, our power to detect a shift in within-clutch variation in offspring size was low. There was no effect of density on the level of variation in offspring size (density: F3,67=1.556, p=0.208), but our power to detect a 50 per cent difference in the CV of offspring size across competitive environments was approximately 0.67 (well below the desired value of 0.8). Similarly, there was no effect of ‘environmental constancy’ on the level of variation in offspring size (constancy: F1,58=0.05, p=0.824). Again, power analysis suggests that we did not have sufficient power to detect an effect (power to detect a 50% difference: approx. 0.61). We were surprised not to see an effect of environmental constancy in the maternal generation on within-clutch offspring size variation. Hence, it is unclear whether such effects are subtler and therefore harder to detect than expected, or simply do not occur in this species.
5. Case study 3
Gagliano & McCormick (2007) manipulated maternal condition in the coral reef fish, Pomacentrus amboinensis, by applying a supplemented or non-supplemented feeding treatment to mothers placed on isolated patch reefs. They also examined unmanipulated mothers that were left on natural reefs. Overall, they found no difference in egg size among mothers subjected to different food treatments. We would predict that variation in offspring size should be lower on the two isolated patch reef treatments than on the natural reefs: mothers on isolated patch reefs will presumably experience greater environmental predictability, regardless of whether food was supplemented or not, than mothers experiencing natural competition and therefore variation in food availability on the main reef. We first tested whether there was a difference in offspring size variability between fed and unfed mothers, but there was none (F1,12=0.631, p=0.443). We therefore pooled these two groups and tested them against unmanipulated mothers. We found a significant difference between environmental treatments, whereby unmanipulated mothers on natural reefs produced offspring of more variable size than mothers exposed to constant experimental conditions (F1,12,=5.98, p=0.031). This study represents a case where no significant shift in the mean egg size occurred (presumably due to the dispersive larval phase obscuring a clear prediction of which direction to shift mean size), but a significant shift in egg size variability was still observed (possibly a bet-hedging response to increased environmental variability and therefore unpredictability, i.e. dynamic bet hedging).
6. Case study 4
Sprenger et al. (2008) manipulated the number of matings and the diversity of male partners to assess the effect of polyandry on maternal investment in the sea slug, Chelidonura sandrana. Each female was mated either singly, repeatedly mated with the same male or mated with four different males (polyandry). Polyandrous females produced larger egg capsules and larvae than both repeatedly and singly mated monandrous females. Here, while multiple mating can be interpreted as a genetic bet-hedging strategy (see Yasui 1998), we cannot envisage a difference in environmental predictability that the offspring of mothers in the different mating treatments would face. Consequently, we would predict that, despite a shift in mean egg size, there should be no change in the offspring size variability among the treatments. Our analyses support this prediction: we detected no difference in the CV of offspring size among mating treatments, despite having sufficient power to detect an effect (table 3; power to detect a 50% difference among treatment means: 0.87). However, it should be noted that it is currently unclear how genetic diversity affects the mother's perception of environmental predictability—as genetic diversity would increase variability in offspring performance even in a stable environment, it is possible that mothers could increase variability in offspring size to match the variability in offspring performance. This idea remains speculative, and warrants further investigation.
Table 3.
Effect of egg mass sequence, mating treatment (polyandry, multiple mating to a single male or single mating) and experimental run on within-clutch offspring size variation in the sea slug, C. sandrana. (Data from Sprenger et al. 2008.)
| source | d.f. | MS | F | p-value |
|---|---|---|---|---|
| egg mass | 1 | 0.000 | 0.028 | 0.868 |
| mating treatment | 2 | 0.005 | 1.750 | 0.177 |
| run | 3 | 0.008 | 2.597 | 0.054 |
| error | 176 | 0.003 |
7. Case study 5
Marshall & Keough (2004) simulated a predation event by halving the size of B. neritina colonies (same organism as case study 2), then examined its effect on subsequent offspring size. Colonies were reared from settlement in the field for six weeks, and then treatment colonies were halved in size and control colonies left intact. After one week, all colonies were harvested, induced to spawn and larval size measured. They found that halved colonies produced larvae that were on average 13 per cent smaller than unmanipulated colonies. This result was interpreted as mothers reducing the fitness of current offspring to increase their own long-term fitness (i.e. by diverting energy to their own growth). Under such a scenario, it is hard to imagine how the predictability of offspring environments would change among the two treatments and, thus, we would again predict no difference in the variability of offspring across maternal environments. However, the analyses in Marshall & Keough (2004) do not support our prediction: there was a strong effect of the predation treatment on offspring size variability, with halved colonies producing much more variable offspring (F1,10=28.38, p<0.001). It is possible that the change in larval size variability is a bet-hedging strategy, and we have misinterpreted how the females perceive the experimental manipulation and subsequent environmental predictability. However, we suspect that this effect is a physiological artefact (i.e. a stress response) of the experimental manipulation—the sudden redirection of resources back to asexual growth may inevitably result in an increase in offspring size variation within the colony. Thus we suggest that this case study provides a good example of a change in within-clutch offspring size variation that is not adaptive, but rather a product of physiological constraints. However, we acknowledge that this example highlights the problems associated with discriminating between adaptive and non-adaptive shifts in offspring size variation.
8. Conclusions
Our small sample of studies suggests that, at least in some cases, mothers appear to dynamically bet hedge in response to the changes in environmental predictability. Table 4 summarizes our findings and essentially shows that every combination of responses to maternal manipulation is possible. Overall, it appears that within-clutch variation in egg size may be a far more complex factor than previously acknowledged, and warrants further investigation. For some time, it has been recognized that environmental quality will affect the optimal size of offspring that mothers should produce, and that within-species offspring size variation is largely a product of mothers reacting to variation in the quality of environments that they face. However, we suggest that an additional source of offspring size variation—environmental predictability—be included in such considerations. Both among- and within-clutch offspring size variations contribute to total population-level variability, and we suggest that highly unpredictable environments are likely to induce mothers to produce a range of offspring sizes. More generally, we argue that variance in traits is often neglected in evolutionary ecology studies overall, and may have implications reaching beyond maternal effects.
Table 4.
Summary of results from case studies.
| treatment | species | difference in mean egg size | difference in CV egg size | power to detect a 50% change in CV | source |
|---|---|---|---|---|---|
| age and nutrition | Sancassania berlesei | yes | yes | — | Plaistow et al. (2007) |
| maternal density | Bugula neritina | yes | no | no | Allen et al. (2008) |
| maternal nutrition | Pomacentrus amboinensis | no | yes | — | Gagliano & McCormick (2007) |
| polyandry | Chelidonura sandrana | yes | no | yes | Sprenger et al. (2008) |
| simulated predation event | Bugula neritina | yes | yes | — | Marshall & Keough (2004) |
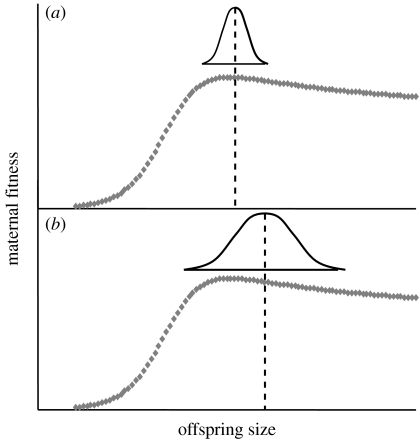
If mothers manipulate within-clutch variation in offspring size in response to changes in environmental predictability (as our case studies suggest they might), this may have implications for the interpretation of studies that focus solely on mean offspring size. If an offspring size fitness function is asymmetrical (as many seem to be, e.g. Einum & Fleming 2000; Marshall et al. 2006), then mothers should not produce any offspring below a certain minimum size threshold. Thus, if an experimental manipulation induces an increase in offspring size variation (because mothers perceive that manipulation as an indicator of environmental unpredictability), then it follows that mothers should also increase the mean size of their offspring simply to avoid producing those that ‘fall off’ the left-hand side of the fitness function (figure 4). It is therefore possible for an experiment to induce an adaptive shift in within-clutch offspring size variation where an increase in mean offspring size is simply a side effect of this shift, rather than an adaptive strategy in and of itself. This example remains purely speculative, but it does highlight the fact that mean and variance in offspring size are inextricably linked and should be considered simultaneously.
Figure 4.
Schematic showing the effect of increasing offspring size variability on the mean size of offspring that mothers produce. Panels show relationship between mean offspring size and maternal fitness (as indicated by the dotted curves) and distribution of offspring sizes that mothers produce (as indicated by the normal distributions). Relationship between mean offspring size and maternal fitness taken from Marshall et al. (2006). Predicted size distribution of eggs that mothers should produce in order to maximize their own fitness based on D. Marshall & L. Bussiere 2005, unpublished analyses. (a) Mothers produce only a small range of offspring sizes; hence, the mean offspring size they produce is close to both the minimum offspring size threshold for survival and the optimum offspring size for that environment (as indicated by the vertical dashed line). (b) Mothers produce a larger range of offspring sizes; hence, the mean offspring size they produce must be further from both the minimum size threshold for survival and the optimum offspring size.
The range of responses in egg size variation resulting from maternal manipulations demonstrates the potential difficulties associated with trying to discriminate between adaptive and non-adaptive shifts in offspring size variation. Similarly, it can be difficult to distinguish whether shifts in mean offspring traits result from adaptive or transmissive mechanisms in maternal effects studies in general. Within-clutch variation may be a non-adaptive physiological constraint, resulting from an inability of mothers to precisely allocate resources evenly between offspring (McGinley et al. 1987; Fox & Czesak 2000; Einum & Fleming 2004). Superficially, an increase in offspring variance in variable environments may appear to be evidence for adaptive plasticity. However, an increase in trait variation under stressful conditions may also be the result of increased non-adaptive developmental instability (Hoffman & Parsons 1991). It is sometimes argued that developmental instability itself may be a mechanism that produces adaptive variation if its expression changes across environments (Simons & Johnston 1997), although this idea remains contentious (Pigliucci 2001). Regardless, while we agree that part of the variance observed in offspring phenotypes is likely to be the result of physiological constraints, we believe our case studies imply that in certain cases, within-clutch variability in egg size may indeed be viewed as an adaptive maternal effect.
There are some important ecological implications of mothers manipulating within-clutch variation in offspring size. Within-species offspring size variation is traditionally underestimated (Turnbull et al. 2006). The fact that some mothers may be deliberately increasing size variation in their offspring suggests that offspring sizes are likely to be much more variable than previously anticipated. Any increases in offspring size variation within populations are likely to induce non-random mortality through size refuge effects and asymmetrical competitive interactions (Marshall et al. 2006). In some organisms, offspring size affects dispersal potential (Parciak 2002; Marshall & Keough 2003; Benard & McCauley 2008). If mothers produce a range of offspring sizes, then this could effectively spread her offspring throughout a range of habitats, further spreading her risk and minimizing among-generation variation in reproductive success (Strathmann 1974).
While we have focused specifically on offspring size, we expect that within-clutch variance in any offspring phenotype represents a trait that selection can shape. Some examples of other traits that exhibit a bet-hedging strategy include: high variance in the timing of seed germination (Simons & Johnston 2006); variation in sibling dispersal potential (Strathmann 1974; Krug & Zimmer 2004); and variation in settlement-cue requirements (Raimondi & Keough 1990; Krug 2001). Furthermore, a number of studies have begun to address adaptive changes in mean sperm traits from males facing varying levels of sperm competition (Snook 2005; Crean & Marshall 2008). We suggest that future studies also include the estimates of both mean and variance in sperm traits, given the substantial potential for males to hedge their bets with respect to sperm swimming longevity and competitive ability. This possibility, however, has been largely unexplored.
In summary, we propose that the emphasis of maternal effects studies should be modified from a focus on mean offspring traits in isolation to a focus on both the mean and the variance of offspring traits within a mother's clutch. At the very least, we encourage future studies to examine and report the variance of within-clutch traits. We predict that dynamic bet hedging in offspring size is more common than previously anticipated and we look forward to further studies that test this prediction.
Acknowledgments
We are extremely grateful to Stew Plaistow, Richard Allen, Monica Gagliano and Dennis Sprenger, who generously provided raw data files of egg size variation used in the case studies. We would also like to thank Tobias Uller and Erik Wapstra for organizing ‘Evolution of parental effects: conceptual issues and empirical patterns’, and our colleagues for many valuable discussions at this workshop. A.J.C. and D.J.M. were supported by an Australian Postgraduate Award and grants from the Australian Research Council during the preparation of this manuscript. Keyne Monro, Tobias Uller, Stew Plaistow, Tim Benton and two anonymous reviewers provided helpful comments that improved the manuscript.
Footnotes
One contribution of 12 to a Theme Issue ‘Evolution of parental effects: conceptual issues and empirical patterns’.
References
- Allen R.M., Buckley Y.M., Marshall D.J. Offspring size plasticity in response to intraspecific competition: an adaptive maternal effect across life-history stages. Am. Nat. 2008;171:225–237. doi: 10.1086/524952. doi:10.1086/524952 [DOI] [PubMed] [Google Scholar]
- Benard M.F., McCauley S.J. Integrating across life-history stages: consequences of natal habitat effects on dispersal. Am. Nat. 2008;171:553–567. doi: 10.1086/587072. doi:10.1086/587072 [DOI] [PubMed] [Google Scholar]
- Bernardo J. The particular maternal effect of propagule size, especially egg size: patterns, models, quality of evidence and interpretations. Am. Zool. 1996;36:216–236. doi:10.1093/icb/36.2.216 [Google Scholar]
- Capinera J.L. Qualitative variation in plants and insects: effect of propagule size on ecological plasticity. Am. Nat. 1979;114:350–361. doi:10.1086/283484 [Google Scholar]
- Cohen D. Optimizing reproduction in a randomly varying environment. J. Theor. Biol. 1966;12:119–129. doi: 10.1016/0022-5193(66)90188-3. doi:10.1016/0022-5193(66)90188-3 [DOI] [PubMed] [Google Scholar]
- Crean A.J., Marshall D.J. Gamete plasticity in a broadcast spawning marine invertebrate. Proc. Natl Acad. Sci. USA. 2008;105:13 508–13 513. doi: 10.1073/pnas.0806590105. doi:10.1073/pnas.0806590105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump M.L. Variation in propagule size as a function of environmental uncertainty for tree frogs. Am. Nat. 1981;117:724–737. doi:10.1086/283755 [Google Scholar]
- Debat V., David P. Mapping phenotypes: canalization, plasticity and developmental stability. Trends Ecol. Evol. 2001;16:555–561. doi:10.1016/S0169-5347(01)02266-2 [Google Scholar]
- de Jong G. Phenotypic plasticity as a product of selection in a variable environment. Am. Nat. 1995;145:493–512. doi: 10.1086/285542. doi:10.1086/285752 [DOI] [PubMed] [Google Scholar]
- Einum S., Fleming I.A. Highly fecund mothers sacrifice offspring survival to maximize fitness. Nature. 2000;405:565–567. doi: 10.1038/35014600. doi:10.1038/35014600 [DOI] [PubMed] [Google Scholar]
- Einum S., Fleming I.A. Does within-population variation in fish egg size reflect maternal influences on optimal values? Am. Nat. 2002;160:756–765. doi: 10.1086/343876. doi:10.1086/343876 [DOI] [PubMed] [Google Scholar]
- Einum S., Fleming I.A. Environmental unpredictability and offspring size: conservative versus diversified bet-hedging. Evol. Ecol. Res. 2004;6:443–455. [Google Scholar]
- Fox C.W., Czesak M.E. Evolutionary ecology of progeny size in arthropods. Annu. Rev. Entomol. 2000;45:341–369. doi: 10.1146/annurev.ento.45.1.341. doi:10.1146/annurev.ento.45.1.341 [DOI] [PubMed] [Google Scholar]
- Fox C.W., Thakar M.S., Mousseau T.A. Egg size plasticity in a seed beetle: an adaptive maternal effect. Am. Nat. 1997;149:149–163. doi: 10.1111/j.1558-5646.1999.tb03790.x. doi:10.1086/285983 [DOI] [PubMed] [Google Scholar]
- Gagliano M., McCormick M.I. Maternal condition influences phenotypic selection on offspring. J. Anim. Ecol. 2007;76:174–182. doi: 10.1111/j.1365-2656.2006.01187.x. doi:10.1111/j.1365-2656.2006.01187.x [DOI] [PubMed] [Google Scholar]
- Gillespie J.H. Natural selection for variances in offspring numbers: a new evolutionary principle. Am. Nat. 1977;111:1010–1014. doi:10.1086/283230 [Google Scholar]
- Halpern S.L. Sources and consequences of seed size variation in Lupinus perennis (Fabaceae): adaptive and non-adaptive hypotheses. Am. J. Bot. 2005;92:205–213. doi: 10.3732/ajb.92.2.205. doi:10.3732/ajb.92.2.205 [DOI] [PubMed] [Google Scholar]
- Hoffman A.A., Parsons P.A. Oxford University Press; Oxford, UK: 1991. Evolutionary genetics and environmental stress. [Google Scholar]
- Hopper K.R., Rosenheim J.A., Prout T., Oppenheim S.J. Within-generation bet hedging: a seductive explanation? Oikos. 2003;101:219–222. doi:10.1034/j.1600-0706.2003.12051.x [Google Scholar]
- Koops M.A., Hutchings J.A., Adams B.K. Environmental predictability and the cost of imperfect information: influences on offspring size variability. Evol. Ecol. Res. 2003;5:29–42. [Google Scholar]
- Krug P.J. Poecilogony in an estuarine opisthobranch: planktotrophy, lecithotrophy, and mixed clutches in a population of the ascoglossan Alderia modesta. Mar. Biol. 1998;132:483–494. doi:10.1007/s002270050414 [Google Scholar]
- Krug P.J. Bet-hedging dispersal strategy of a specialist marine herbivore: a settlement dimorphism among sibling larvae of Alderia modesta. Mar. Ecol. Prog. Ser. 2001;213:177–192. doi:10.3354/meps213177 [Google Scholar]
- Krug P.J., Zimmer R.K. Developmental dimorphism: consequences for larval behavior and dispersal potential in a marine gastropod. Biol. Bull. 2004;207:233–246. doi: 10.2307/1543212. doi:10.2307/1543212 [DOI] [PubMed] [Google Scholar]
- Kudo S.I. Intraclutch egg-size variation in acanthosomatid bugs: adaptive allocation of maternal investment? Oikos. 2001;92:208–214. doi:10.1034/j.1600-0706.2001.920202.x [Google Scholar]
- Kudo S.I. Within-clutch egg-size variation in a subsocial bug: the positional effect hypothesis. Can. J. Zool. Rev. Can. Zool. 2006;84:1540–1544. doi:10.1139/Z06-163 [Google Scholar]
- Laaksonen T. Hatching asynchrony as a bet-hedging strategy—an offspring diversity hypothesis. Oikos. 2004;104:616–620. doi:10.1111/j.0030-1299.2004.12858.x [Google Scholar]
- Marshall D.J., Keough M.J. Variation in the dispersal potential of non-feeding invertebrate larvae: the desperate larva hypothesis and larval size. Mar. Ecol. Prog. Ser. 2003;255:145–153. doi:10.3354/meps255145 [Google Scholar]
- Marshall D.J., Keough M.J. When the going gets rough: effect of maternal size manipulation on larval quality. Mar. Ecol. Prog. Ser. 2004;272:301–305. doi:10.3354/meps272301 [Google Scholar]
- Marshall D.J., Keough M.J. The evolutionary ecology of offspring size in marine invertebrates. Adv. Mar. Biol. 2008;53:1–60. doi: 10.1016/S0065-2881(07)53001-4. doi:10.1016/S0065-2881(07)53001-4 [DOI] [PubMed] [Google Scholar]
- Marshall D.J., Uller T. When is a maternal effect adaptive? Oikos. 2007;116:1957–1963. doi:10.1111/j.2007.0030-1299.16203.x [Google Scholar]
- Marshall D.J., Cook C.N., Emlet R.B. Offspring size effects mediate competitive interactions in a colonial marine invertebrate. Ecology. 2006;87:214–225. doi: 10.1890/05-0350. doi:10.1890/05-0350 [DOI] [PubMed] [Google Scholar]
- Marshall D.J., Bonduriansky R., Bussiere L.F. Offspring size variation within broods as a bet-hedging strategy in unpredictable environments. Ecology. 2008;89:2506–2517. doi: 10.1890/07-0267.1. doi:10.1890/07-0267.1 [DOI] [PubMed] [Google Scholar]
- McGinley M.A., Temme D.H., Geber M.A. Parental investment in offspring in variable environments—theoretical and empirical considerations. Am. Nat. 1987;130:370–398. doi:10.1086/284716 [Google Scholar]
- Mousseau T.A., Fox C.W. Oxford University Press; Oxford, UK: 1998. Maternal effects as adaptations. [Google Scholar]
- Olsson M., Uller T. Developmental stability and genetic architecture: a comparison within and across thermal regimes in tadpoles. J. Evol. Biol. 2002;15:625–633. doi:10.1046/j.1420-9101.2002.00417.x [Google Scholar]
- Orr H.A. Absolute fitness, relative fitness, and utility. Evolution. 2007;61:2997–3000. doi: 10.1111/j.1558-5646.2007.00237.x. doi:10.1111/j.1558-5646.2007.00237.x [DOI] [PubMed] [Google Scholar]
- Parciak W. Environmental variation in seed number, size, and dispersal of a fleshy-fruited plant. Ecology. 2002;83:780–793. doi:10.2307/3071881 [Google Scholar]
- Philippi T., Seger J. Hedging one's evolutionary bets, revisited. Trends Ecol. Evol. 1989;4:41–44. doi: 10.1016/0169-5347(89)90138-9. doi:10.1016/0169-5347(89)90138-9 [DOI] [PubMed] [Google Scholar]
- Pigliucci M. John Hopkins University Press; Baltimore, MD: 2001. Phenotypic plasticity: beyond nature and nurture. [Google Scholar]
- Plaistow S.J., St Clair J.J.H., Grant J., Benton T.G. How to put all your eggs in one basket: empirical patterns of offspring provisioning throughout a mother's lifetime. Am. Nat. 2007;170:520–529. doi: 10.1086/521238. doi:10.1086/521238 [DOI] [PubMed] [Google Scholar]
- Poulin R., Hamilton W.J. Egg size variation as a function of environmental variability in parasitic trematodes. Can. J. Zool. Rev. Can. Zool. 2000;78:564–569. doi:10.1139/cjz-78-4-564 [Google Scholar]
- Proulx S.R. The ESS under spatial variation with applications to sex allocation. Theor. Popul. Biol. 2000;58:33–47. doi: 10.1006/tpbi.2000.1474. doi:10.1006/tpbi.2000.1474 [DOI] [PubMed] [Google Scholar]
- Quinn G.P., Keough M.J. Cambridge University Press; Cambridge, UK: 2002. Experimental design and data analysis for biologists. [Google Scholar]
- Raimondi P.T., Keough M.J. Behavioral variability in marine larvae. Aust. J. Ecol. 1990;15:427–437. doi:10.1111/j.1442-9993.1990.tb01468.x [Google Scholar]
- Seger J., Brockmann H.J. What is bet-hedging? Oxf. Surv. Evol. Biol. 1987;4:182–211. [Google Scholar]
- Simons A.M., Johnston M.O. Developmental instability as a bet-hedging strategy. Oikos. 1997;80:401–406. doi:10.2307/3546608 [Google Scholar]
- Simons A.M., Johnston M.O. Environmental and genetic sources of diversification in the timing of seed germination: implications for the evolution of bet hedging. Evolution. 2006;60:2280–2292. doi:10.1554/05-396.1 [PubMed] [Google Scholar]
- Smith C.C., Fretwell S.D. Optimal balance between size and number of offspring. Am. Nat. 1974;108:499–506. doi:10.1086/282929 [Google Scholar]
- Snook R.R. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 2005;20:46–53. doi: 10.1016/j.tree.2004.10.011. doi:10.1016/j.tree.2004.10.011 [DOI] [PubMed] [Google Scholar]
- Sprenger D., Anthes N., Michiels N.K. Multiple mating affects offspring size in the opisthobranch Chelidonura sandrana. Mar. Biol. 2008;153:891–897. doi:10.1007/s00227-007-0861-3 [Google Scholar]
- Strathmann R.R. Spread of sibling larvae of sedentary marine invertebrates. Am. Nat. 1974;108:29–44. doi:10.1086/282883 [Google Scholar]
- Trivers R.L. Parent–offspring conflict. Am. Nat. 1974;14:249–264. [Google Scholar]
- Turnbull L.A., Santamaria L., Martorell T., Rallo J., Hector A. Seed size variability: from carob to carats. Biol. Lett. 2006;2:397–400. doi: 10.1098/rsbl.2006.0476. doi:10.1098/rsbl.2006.0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uller T. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 2008;23:432–438. doi: 10.1016/j.tree.2008.04.005. doi:10.1016/j.tree.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Yasui Y. The ‘genetic benefits’ of female multiple mating reconsidered. Trends Ecol. Evol. 1998;13:246–250. doi: 10.1016/s0169-5347(98)01383-4. doi:10.1016/S0169-5347(98)01383-4 [DOI] [PubMed] [Google Scholar]