Abstract
Traditionally, research on life-history traits has viewed the link between clutch size and offspring size as a straightforward linear trade-off; the product of these two components is taken as a measure of maternal reproductive output. Investing more per egg results in fewer but larger eggs and, hence, offspring. This simple size–number trade-off has proved attractive to modellers, but our experimental studies on keelback snakes (Tropidonophis mairii, Colubridae) reveal a more complex relationship between clutch size and offspring size. At constant water availability, the amount of water taken up by a snake egg depends upon the number of adjacent eggs. In turn, water uptake affects hatchling size, and therefore an increase in clutch size directly increases offspring size (and thus fitness under field conditions). This allometric advantage may influence the evolution of reproductive traits such as growth versus reproductive effort, optimal age at female maturation, the body-reserve threshold required to initiate reproduction and nest-site selection (e.g. communal oviposition). The published literature suggests that similar kinds of complex effects of clutch size on offspring viability are widespread in both vertebrates and invertebrates. Our results also challenge conventional experimental methodologies such as split-clutch designs for laboratory incubation studies: by separating an egg from its siblings, we may directly affect offspring size and thus viability.
Keywords: incubation dynamics, maternal investment, propagule size, size–number trade-off
1. Introduction
For many decades, biologists have attempted to understand the causal processes underlying life-history diversity. There have been many notable successes in that endeavour, with a resultant accumulation of sophisticated methodologies and extensive datasets (for recent examples, see Gillespie et al. 2008; Sprenger et al. 2008; Wheatley et al. 2008). Nonetheless, many gaps in our knowledge remain unfilled, and the empirical underpinnings for even the most basic concepts are often surprisingly weak. In this paper, we describe the results of some simple experimental studies designed to address a central issue in life-history theory: the relationship between clutch size and offspring size.
Living organisms display a remarkably wide range in both fecundity and offspring size, and attempts to explain interspecific and intraspecific diversity in these traits are a major focus of life-history theory. Indeed, the significance of clutch size and offspring size goes far beyond life-history questions: for example, rates of reproductive output are critical determinants of population viability, and thus are central concerns for biologists interested in topics such as conservation of endangered species (Dimond & Armstrong 2007; Fenner & Bull 2007; Burgess et al. 2008; Vincenzi et al. 2008) and sustainable levels of harvesting (Hailey 2000; Bardos et al. 2006). Accordingly, a massive empirical and theoretical literature has arisen to address these topics. The relationship between fecundity and offspring size has been a frequent focus of that work because, given finite resources, a reproducing animal must trade offspring size off against offspring number (reviewed in Stearns 1992; Roff 1992; Zehnder et al. 2007). In the common circumstance of energy limitation, for example, a female that allocates a certain amount of energy to her clutch or litter may thus be able to divide that allocation into X offspring of size Y, 2X offspring of size Y/2 or 4X offspring of size Y/4, and so forth. Although a female's ability to manipulate her expenditure in this way may be limited in actuality (e.g. high heritability in one component, such as egg size, may greatly reduce flexibility in allocation; Brown & Shine 2007), the trade-off concept has proven attractive to many theoreticians, and has played a pivotal role in the development of life-history theory (e.g. Smith & Fretwell 1974; Plaistow et al. 2007; Zehnder et al. 2007).
Many empirical studies have confirmed the existence of a trade-off between the size and number of offspring, at both interspecific and intraspecific levels (Stearns 1989; Roff 2002). The nature of the finite limit to overall resource allocation to reproduction remains contentious, however, with some authors identifying energy stores as the resource to be subdivided, whereas others stress physical limitations such as maternal abdominal volume (Du et al. 2005). Presumably, both are involved. For example, studies on reptiles suggest that total reproductive investment can be broadly predicted by maternal abdominal volume in interspecific comparisons (Vitt & Congdon 1978; Shine 1992) and that experimental reduction of available volume for the eggs induces female lizards to produce fewer eggs (Du et al. 2005). However, links between energy stores and reproductive output have also been well documented; for example, both total investment per clutch and the trade-off between egg size and number can shift among years within a single reptile population depending upon maternal feeding rates (Olsson & Shine 1997; Madsen & Shine 1999, 2000). On a directly mechanistic basis, ovarian follicle ablation redirects maternal yolk into fewer follicles and thus increases mean egg size (Sinervo & Huey 1990). Hence, despite continuing debate about the nature of upper limits to investment, the existence of a size–number trade-off is strongly supported by both models and empirical data.
In the present paper, we argue that the relationship between clutch size and offspring size may often be more complex than is captured in a simple size–number model. We suggest that it may often be the case that the size and/or fitness of progeny depend directly on clutch size, even at identical levels of maternal investment per progeny. Such a linkage has both methodological and theoretical implications; for example, it may impose selection on a wide range of related life-history traits such as ages at maturation, minimum energy threshold for reproduction, reproductive frequency, ontogenetic shifts in reproductive investment patterns and communal nesting behaviour. Because life-history models normally assume that clutch size does not affect offspring fitness except via a linear size–number trade-off, this extra complication has rarely been addressed in the previous literature.
2. Clutch size as a maternal effect
Although an extensive literature on the relationship between offspring size and clutch size posits a trade-off enforced by a finite supply of some resource, other kinds of causal relationships between these two variables have attracted little attention. Nonetheless, even a cursory survey reveals many cases wherein clutch size exerts a direct causal effect on either offspring size or offspring fitness, unrelated to the initial per-offspring provisioning ‘decision’ by parents. The most obvious and widespread such effect occurs in species that provision their young post-hatching, such as birds and mammals. Assuming some finite upper limit to the amount of parental care that can be provided, the size–number trade-off applies to this post-hatching phase as well. For example, both descriptive and experimental studies suggest that mean nutrient input per offspring may decrease if parents attempt to raise larger-than-usual clutches (e.g. artificially increasing clutch sizes in birds often reduces hatchling mass or viability; Lack 1968; but see Parejo & Danchin 2006). Such cases are only trivial extensions to the size–number trade-off; both kinds of examples rely on the mathematics of dividing a fixed amount of energy among a variable number of progeny.
The situation becomes more interesting when the resource in question is less directly linked to parental provisioning (table 1). Competition between developing siblings may be widespread, and can involve several mechanisms other than energy allocation. For example, a larger clutch may reduce oxygen availability for embryos (Lardies & Fernandez 2002; Green et al. 2006), imposing selection for the evolution of the jelly coat, and on clutch size per se (Lee & Strathmann 1998). Increased cues (visual, chemical, auditory, etc.) from a larger group of offspring (Taylor 1979; Friedlander 1985; McRae 1996; Kiesecker & Blaustein 1997; Hayes 2000) may render a larger egg clutch or litter easier for predators or pathogens to locate and destroy. Similar causal effects of clutch size on offspring fitness may occur via post-hatching mechanisms such as sibling competition after hatching (Godfray & Parker 1992); for example, clutch size affects offspring fitness in parasitic insects without post-ovipositional care (Le Masurier 1994; Fox et al. 1996; Desouhant et al. 2000).
Table 1.
Examples of published reports describing the effects of increasing numbers of eggs (due either to larger clutch size or communal oviposition) on survivorship or phenotypes of offspring.
| species | effect of increasing egg number | reference |
|---|---|---|
| ladybird (Adalia bipunctata and Coccinella septempunctata) | decrease predation | Agarwala & Dixon (1993) |
| ladybird (Cycloneda sanguinea, Harmonia axyridis and Olla v-nigrum) | increased cannibalism | Michaud & Grant (2004) |
| leaf miner (Leucoptera sinuella) | decrease egg survival, larval survival and body size | Kagata & Ohgushi (2004) |
| weevil (Curculio elephas) | decrease larval mass | Desouhant et al. (2000) |
| seed beetle (Stator limbatus) | increased parasitism | Siemens & Johnson (1992) |
| seed beetle (Stator limbatus) | decrease survival, body size and development time | Fox et al. (1996) |
| butterfly (Chlosyne lacinia) | increase egg survival through desiccation resistance, decrease survival through cannibalism | Clark & Faeth (1998) |
| Hessian fly (Mayetiola destructor) | decrease survival and body size | Withers et al. (1997) |
| gall-making Diptera (Asteromyia carbonifera) | decrease parasitoid attack, increase sibling competition | Weis et al. (1983) |
| parasitic wasp (Hyssopus pallidus) | decrease body size | Zaviezo & Mills (2000) |
| mite (Iphiseius degenerans) | decrease predation of eggs | Faraji et al. (2002) |
| squid (Sepioteuthis australis) | decrease hatch success and developmental rate | Steer et al. (2003) and Steer & Moltschaniwskyj (2007) |
| gastropod (Melanochlamys diomedea) | decrease developmental rate | Chaffee & Strathmann (1984) |
| goby (Gymnogobius isaza) | increase fungal infection of eggs | Takahashi et al. (2004) |
| frog (Bufo boreas and Rana cascadae) | decrease egg survival through fungal infection | Kiesecker & Blaustein (1997) |
| frog (Rana sphenocephala) | decrease risk of pathogen infection | Ruthig (2008) |
| lizard (Lacerta schreiberi) | influence hatchling mass via water uptake | Marco et al. (2004) |
| lizard (Bassiana duperreyi) | increase hatchling mass via water uptake | Radder & Shine (2007) |
Positive feedback between clutch size and offspring viability may be less frequent, but can occur; for example, larger clutches may be able to satiate predators at hatching, thereby enhancing survival probability per offspring (McGinley 1989; Post 1998). In turtles deposited within deep nests, larger clutches may be better able to dig through compacted soil to escape the nest chamber (Nagle et al. 2004). Some larval insects are more effective feeding in groups (i.e. an Allee effect; Messina & Fox 2001). Metabolic heating due to embryo metabolism may accelerate embryogenesis in larger clutches of the eggs of sea turtles, thereby allowing earlier hatching and, perhaps, more viable phenotypes (Broderick et al. 2001; Ewert & Nelson 2003). Similarly, globular masses of amphibian eggs retain heat and accelerate larval development (Ryan 1978), and a larger total clutch mass in endothermic animals may confer advantages to each progeny in terms of thermal inertia (Gittleman 1985; Rhind 2003; Cooper et al. 2005; Rodel et al. 2008). Nest-attending male fishes may be less likely to predate upon their own eggs in larger clutches than in smaller ones (Klug & Bonsall 2007), or spend more time fanning the eggs (and thus increase egg-hatching success) in larger clutches (Karino & Arai 2006). Larger families may be socially dominant over smaller ones, thereby increasing offspring fitness in larger broods (in geese; Lepage et al. 1998). This diversity of potential causal links between clutch size and offspring size suggests that maternal effects relating to clutch size are not fully captured by the current generation of life-history models.
Recent research on reptiles suggests another type of relationship between clutch size and offspring size: one that is driven by hydric interactions between neighbouring eggs during the incubation period. Most squamate reptiles lay parchment-shelled eggs, which exchange water with the substrate during incubation (Mathies & Andrews 1996, 2000). Marco et al. (2004) reported that the amount of water taken up by a lacertid lizard egg (and hence the size of the hatchling that emerged from that egg) depended not only on the substrate water content, but also on the number of other eggs with which the focal egg was in contact. Eggs competed for water in a relatively dry nest, so that aggregated eggs took up less water and produced smaller hatchlings. In a study designed to clarify the fitness consequences of communal oviposition in scincid lizards, Radder & Shine (2007) reported a similar link between clutch size and water uptake, but the opposite effect on offspring size. Eggs incubated in larger groups took up less water during incubation, but produced larger hatchlings.
The present study is a direct outgrowth of Radder & Shine's (2007) work, and is designed to test the possibility that clutch size effects on water uptake by eggs (and thus on hatchling size) might be evident even within the range of clutch sizes observed within a single population (rather than the wider range of ‘incubation cluster’ sizes generated by communal oviposition). Keelback snakes (Tropidonophis mairii) provide an ideal model system for such a test; they exhibit a wide range in both clutch and hatchling body sizes within a single population, and the survival of hatchling snakes depends upon body size, in turn driven by rates of water uptake by the egg during incubation (Brown & Shine 2004). This system thus provides an ideal opportunity to establish direct links between clutch size, incubation dynamics and offspring fitness.
3. An experimental study of clutch size effects on incubation dynamics in snakes
(a) Study species and area
Keelbacks (T. mairii) are the only Australian species within the major cosmopolitan colubrid snake subfamily Natricinae, the phylogenetic lineage containing a diverse array of Asian taxa as well as European grass snakes and North American garter snakes (Malnate & Underwood 1988). They are small (up to 80 cm snout–vent length (SVL)) non-venomous snakes that are widely distributed throughout tropical and subtropical Australia, as well as much of New Guinea (O'Shea 1996; Cogger 2000). The keelbacks are found primarily in riparian habitats, and feed upon frogs (Shine 1991). In our study area, adult female keelbacks produce multiple clutches of eggs throughout the dry season (April to November; Brown & Shine 2002; Brown et al. 2002). Eggs are laid in relatively shallow nests (below 20 cm deep) on the edge of the flood plain (Brown & Shine 2002). The female keelbacks span an adult size range of 44.8–78.3 cm SVL, with most of that variation attributable to maternal age (for recaptured females that had been marked and released at hatching, and thus are of known age: age versus SVL, n=217, r=0.75, p<0.0001). Within our study population, clutch sizes range from 4 to 21, and are highly correlated with maternal body size (n=869, r=0.73, p<0.0001). Hatchlings range from 10.8 to 17.8 cm in SVL (1.1–3.8 g).
The Adelaide River flood plain, 60 km east of the city of Darwin in tropical Australia, lies within the wet–dry tropics (12°55′ S, 131°31′ E). Mean daily maximum air temperatures exceed 30°C in every month of the year, although minima fall much lower (15–24°C) mid-year than at other times. Rainfall is highly seasonal, with more than 75 per cent of the 1300 mm annual average rainfall occurring during a brief ‘wet season’ (December–March: Taylor & Tulloch 1985). Importantly, the seasonal timing of the onset and cessation of the monsoonal rains varies considerably from year to year as well as from place to place (Shine & Brown 2008). This seasonal and annual variation exposes natural nests of keelbacks to a wide range of hydric conditions over the prolonged nesting period.
(b) Methods
We conducted nightly surveys just after dusk, the main activity period for local keelbacks (Shine 1991). The observer walked and/or slowly drove a 1.3 km stretch of road along the top of Fogg Dam on the Adelaide River flood plain, using a spotlight to locate active animals. Any keelbacks seen were captured, and retained in captivity for processing (measuring, individual scale clipping, etc.) prior to release at their site of capture the following day. Gravid females were kept until they deposited eggs, generally less than 2 days after capture.
We incubated groups of eggs, in a single layer, in 1000 ml containers (70 mm×100 mm×145 mm) in a range of cluster sizes (n=4, 5, 8, 10, 15, 16, 25 and 27 eggs). The keelback eggs are elongate and were half buried in the incubation substrate with the long axis upwards. The eggs within clusters were in physical contact throughout incubation. To ensure equal moisture availability per egg for clutches of different sizes, we added a mass of vermiculite and water equal to the mass of the eggs within that cluster (e.g. 20 g eggs+20 g water+20 g vermiculite). Containers were weighed, covered with non-airtight lids and incubated in a styrofoam cooler at room temperature.
To deconfound maternal effects, we split clutches and combined eggs from four to five clutches within each treatment group (and for larger group sizes, within each cluster). Thus, the exact size of experimental clusters was determined by the availability of multiple females with relatively simultaneous oviposition. The clutches of five females were used to conduct the first set of incubation trials consisting of one replicate of each of four cluster size categories (n=4–5, 8–10, 15–16 and 25–27 eggs). The eggs from each clutch were distributed among each of the four cluster size categories. Approximately one month later, the clutches of four additional females were used to replicate the design. The nine different females contributed a total of 110 eggs (11–16 eggs each).
The eggs were individually marked, measured and weighed within 24 hours of oviposition. After intervals of 23, 33, 43 and 53 days post-laying, the eggs were reweighed and water was added to the incubation medium to replace moisture lost through evaporation. Immediately prior to hatching (approx. 60 days after laying), we transferred the eggs to individual 120 ml containers so that we could unambiguously assign each hatchling to its egg. Within 48 hours of hatching, each snake was sexed (by hemipene eversion in males), weighed and measured for SVL, head and tail length.
We assessed normality and variance homogeneity prior to analyses, and ln-transformed data when necessary to satisfy these assumptions for statistical testing. For analyses of treatment effects, we included maternal identity and cluster size as fixed factors in a repeated-measures ANOVA. We designated four cluster size categories representing small clutches (n=4 or 5 eggs), average clutches (n=8 or 10 eggs), large clutches (n=15 or 16 eggs) and very large (or composite, communally deposited) clutches (n=25 or 27 eggs).
(c) Results
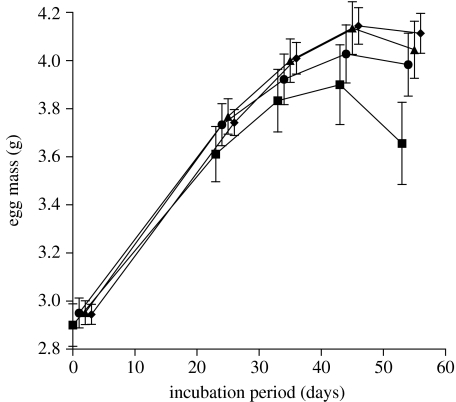
The mean initial (immediately post-laying) mass of eggs did not differ significantly among the four cluster size groups (F3,109=0.08, p=0.97). A repeated-measures ANOVA on changes in egg mass during incubation (with cluster size and maternal ID as independent factors) revealed that eggs increased in mass overall through the course of incubation (F4,296=775.9, p<0.0001), and that the treatment groups did not differ significantly in total mass increments (F3,74=2.23, p=0.09). The main effect for maternal (=clutch) ID was significant (F8,74=24.0, p<0.0001) but no higher order interaction terms containing clutch ID were significant (table 2). Notably, eggs in some treatments took up water (i.e. gained mass) over a different time course than did those in other treatments (i.e. a significant interaction between cluster size and the trajectory of increase in egg mass: F12,296=3.25, p=0.0002). Figure 1 reveals the nature of this interaction. Eggs in each cluster size took up water at similar rates until midway through incubation, but after this time the rate of water increase depended upon cluster size. Eggs incubated in small groups tended to lose mass, whereas eggs in large clusters continued to gain mass. Eggs in intermediate-sized clusters showed an intermediate pattern of changes in mass (figure 1), and the interaction between cluster size and time remains significant even if data from the smallest cluster size (n=4 or 5 eggs) are excluded from the analysis (table 2).
Table 2.
Results of statistical analyses of the effects of egg number (cluster size) on rates of water uptake of snake eggs (as measured by changes in egg mass through time during incubation). (The significance of the effects from each main factor in the repeated-measures ANOVA plus the interaction terms are shown. (a) The results based on the inclusion of all cluster size categories and (b) those based on a dataset excluding the smallest (and most divergent) cluster size (=four to five eggs). Cases where p<0.05 are shown in italics.)
| source | d.f. | F | p |
|---|---|---|---|
| (a) all cluster sizes included | |||
| maternal ID | 8,74 | F=3.04 | 0.005 |
| cluster size | 3,74 | F=2.23 | 0.092 |
| cluster size×ID | 24,74 | F=1.10 | 0.37 |
| time | 4,71 | F=61.9 | <0.0001 |
| time×cluster size | 12,188 | Wilk's lambda F=5.12 | <0.0001 |
| time×ID | 32,263 | Wilk's lambda F=1.16 | 0.26 |
| time×ID×cluster size | 96,284 | Wilk's lambda F=1.26 | 0.08 |
| (b) excluding the smallest (four to five egg) clusters | |||
| maternal ID | 8,74 | F=6.25 | 0.005 |
| cluster size | 2,74 | F=0.46 | 0.63 |
| cluster size×ID | 16,74 | F=0.92 | 0.55 |
| time | 4,71 | F=98.7 | <0.0001 |
| time×cluster size | 8,142 | Wilk's lambda F=2.45 | 0.016 |
| time×ID | 32,263 | Wilk's lambda F=1.91 | 0.003 |
| time×ID×cluster size | 64,280 | Wilk's lambda F=1.23 | 0.13 |
Figure 1.
Rate of mass increase (=water uptake from the substrate) by keelback eggs incubated in a range of cluster sizes. Eggs that were incubated in small groups lost mass in the latter part of incubation, whereas eggs in larger groups continued to increase in mass. Squares, 4–5 eggs; circles, 8–10 eggs; triangles, 15–16 eggs; diamonds, 25–27 eggs.
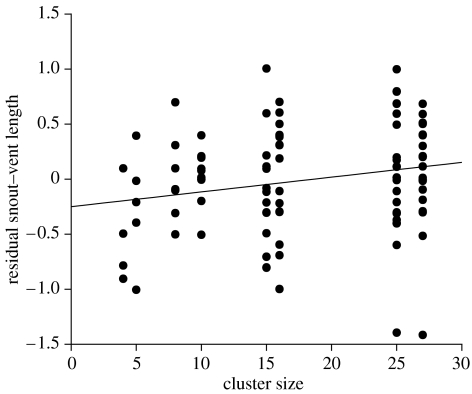
This effect of cluster size on water uptake rates resulted in significant effects on hatchling body sizes. We included initial (post-laying) egg mass as a covariate in these analyses, to ensure that any treatment effects were not due to minor differences in initial egg mass among groups. After correcting for initial egg mass, we found significant effects of cluster size on hatchling SVL (F1,106=5.23, p=0.024; figure 2), but not on body mass (F1,106=2.36, p=0.13). Sex ratios of the offspring were not affected by cluster size (Χ12=0.05, p=0.83).
Figure 2.
Residual SVLs (corrected for initial egg mass) of hatchling keelback snakes from eggs incubated in clusters of different sizes. Eggs that were tightly packed with many other eggs produced larger offspring than did eggs that were incubated in smaller groups.
(d) Discussion
In tropical snakes (as in alpine scincids (Radder & Shine 2007) but not in lacertids (Marco et al. 2004)), eggs that were incubated within a large group of other eggs produced larger hatchlings than did eggs that were incubated in smaller groups. Rates of water exchange with the substrate appear to be important in this process: larger clusters of keelback eggs exhibited higher rates of water uptake initially, and lower rates of water loss towards the end of incubation, and thus produced larger hatchlings. The mechanistic basis for shifts in hydric exchange remains obscure, but may involve less air flow around individual eggs within larger clusters (and hence lower rates of evaporative water loss from such eggs). Regardless of the exact mechanism, the existence of a link between egg aggregation and water uptake in very dissimilar reptile species suggests that the phenomenon may be widespread (e.g. Clark & Faeth 1998). Our data show that this effect can be seen even within the range of clutch sizes apparent within a single population of keelbacks, and occurs in a species where we have firm empirical evidence of a link between water uptake, hatchling size and hatchling fitness (Brown & Shine 2004).
This maternal effect has important implications, both for experimental methodologies and for our understanding of the selective forces operating on maternal reproductive tactics. First, we consider methodological implications, deferring discussion of selective forces until §4. In terms of methodology, it is difficult to avoid the conclusion that incubating eggs individually (as is done in most experimental studies, especially those that use split-clutch designs) may thereby modify the phenotype of the offspring. That is, the young reptile that emerges from that egg in the laboratory will differ—perhaps in important ways—from the offspring that would have emerged from the same egg in the field. This will be true even if the overall physical conditions (moisture, temperature, etc.) during incubation are identical. In the case of keelbacks, the difference in hatchling size induced by a cluster of surrounding eggs would be great enough to significantly affect conclusions about the fitness consequences of size variation. Based on a field study of 291 hatchlings recaptured from more than 5400 released, survivorship increased significantly with body size to a maximum rate of 11 per cent in the largest size class (G.P. Brown 2008, unpublished data). An increase of 0.6 cm in SVL at hatching (from 15.2 to 15.8 cm, as generated by a small versus large cluster of eggs) is likely to translate into a 12 per cent increase in the probability of survival throughout the first year of life (from 5.9 to 6.6%: G.P. Brown, unpublished data). In some cases—such as studies of fitness differentials, or in conservation-oriented programmes where the aim is to maximize offspring viability—such a difference might be very significant.
The other methodological issue involves artefacts introduced by individual incubation, and, especially, the problematic correlation between clutch size and offspring size introduced by incubation dynamics. Unfortunately, clutch size (and not, infrequently, egg size) also correlates with maternal body size—larger females tend to have more, and sometimes larger, offspring (Fitch 1970). Larger females also tend to be older (as is the case in our keelback population; see above). Thus, in practice, any hydrically driven link between clutch size and offspring size (as revealed by our study) is enmeshed within a series of tight correlations with other biologically important traits. For example, maternal reproductive success might well increase with maternal age simply because of the water uptake effect—an explanation very different from those usually offered for such correlations (Olsson & Shine 1996; Plaistow et al. 2007). Indeed, any attempt to quantify allometric trends in reproductive traits or life-history variables becomes far more difficult if these kinds of feedback effects between offspring size and number are incorporated, because the relationship between egg mass and offspring mass may vary non-randomly with clutch size, female body size and female age. Superficially simple concepts such as ‘propagule size’ become surprisingly ambiguous, and allometries depend upon the life-history stage at which one measures the trait in question (e.g. allometries for egg mass differ from those for hatchling mass).
An extensive literature suggests that the phenotypic traits of hatchling reptiles are exquisitely sensitive to even minor details of the incubation environment—such as the mean thermal and hydric regimes to which eggs are exposed, the degree of fluctuation in such regimes, and so forth (Shine et al. 1997; Qualls & Andrews 1999; Webb et al. 2001; Ji et al. 2002; Shine 2002; Deeming 2004). Our analysis identifies yet another component of the incubation environment that can affect hatchling phenotypes: the number of neighbouring eggs during incubation. Importantly, this additional factor is likely to be highly sensitive to the interaction between abiotic conditions in the nest (e.g. moisture levels) and biotic traits (e.g. egg mass and clutch size). That interactive effect will pose significant difficulties for empirical studies on offspring fitness from laboratory-incubated eggs. For example, presumably, the effect of a larger clutch size on water uptake rates and thus offspring size will be greater under some abiotic conditions (e.g. dry nests) than others (e.g. wet nests), and the exact degree of contact between adjacent eggs (itself a function of the number of other clutches nearby, and of the physical structure within egg-laying sites) may well influence fitness-relevant traits also. Such complexities may explain the contrasting results of incubation experiments with lacertid lizard eggs (clustering reduced offspring size; Marco et al. 2004) and scincid lizard and colubrid snake eggs (clustering increased offspring size; Radder & Shine 2007; current study). In Marco et al.'s (2004) study, the influence of egg clustering on hatchling mass disappeared at intermediate levels of substrate moisture.
4. Broader implications of clutch size effects for life-history theory
Our experiments clearly demonstrate a carry-over effect of clutch size on offspring fitness, i.e. clutch size can be a maternal effect above and beyond the direct trade-off between size and number at the timing of maternal investment. Such effects could potentially influence the evolution of reproductive effort and, perhaps, also other life-history traits and behaviours (e.g. nest-site choice). We explore these implications below, with emphasis on reptiles.
The kinds of size–number models generally used in life-history theory usefully could be expanded to explore the consequences of a more diverse range of relationships between clutch size and offspring size. The standard model assumes that the relationship is linear: increasing clutch size by 1 reduces mean offspring size by the same amount, divided equally among all offspring. If larger clutches directly increase offspring size via rates of water exchange through the eggshell (for example), the relationship not only could change slope but also could become nonlinear. Some of these maternal effects probably cut in at specific threshold clutch sizes, whereas others would apply incrementally as clutch sizes increase. Marco et al.'s (2004) results suggest that local environmental conditions could massively affect the slope of the clutch size versus offspring size trade-off, with minor spatial and temporal variation in soil moisture levels generating different size–number trade-offs for different females, or even for successive clutches produced by the same female. The assumption that any cost or benefit to offspring is divided equally among the clutch may also be violated; for example, eggs in the middle of a cluster may experience different costs or benefits than eggs on the periphery. Such complexities could easily be incorporated into mathematical models, an approach that might provide useful insights into the selective consequences of these maternal effects on size–number trade-offs.
Our literature review suggests that such complex maternal effects are widespread. In terms of the specific mechanism we have documented in keelbacks, similar allometric effects on water uptake might also occur in other species of oviparous terrestrial animals—but even within specific groups, substantial variation is likely. For example, the eggs of some lizards have calcareous rather than parchment-like shells, and hence have less hydric exchange with the incubation environment (Packard et al. 1982). Those same lizard lineages are typified by low and invariant clutch sizes, hinting that the evolution of these two traits (calcareous eggshells and low clutch sizes) might be linked. For example, any hydric advantages associated with large clutch size may disappear with the evolution of an eggshell morphology that greatly limits water exchange with the environment, and vice versa.
Although such variation in relevant traits makes it difficult to frame generalities, that same variation provides a great opportunity for comparative analyses. There are a limited number of factors that affect offspring size and/or viability after the eggs are laid. Some of these occur during incubation, such as rates of physical exchange (of water, oxygen and waste products) with the nest environment and other eggs, and rates of attack by pathogens or predators. Other factors apply after hatching; for example, escape from the nest chamber or predator satiation (table 1; McGinley 1989; Espelta et al. 2008). Lastly, limited dispersal by offspring post-hatching may create circumstances where a larger clutch confers advantages (e.g. thermal inertia through clumping, or greater protection against predators via enhanced surveillance or cumulative defensive ability) or disadvantages (by attracting predators or creating greater competition for resources among siblings; e.g. Kagata & Ohgushi 2004; table 1). Even in cases where there is a consistent benefit to offspring size associated with clutch size variation, species may differ in the degree to which such a size benefit persists post-hatching (versus being eliminated by compensatory growth; Radder et al. 2007), and even if it persists, whether a size increment translates into a fitness increment (McGinley et al. 1987). Those kinds of costs and benefits should cut across phylogenetic lines, facilitating comparative analyses of putative links between such factors and the life-history variables predicted to evolve in response to maternal effects of this type. What form might these life-history modifications take? Possibilities include:
Nesting behaviour. Enhanced offspring size from larger clutches would favour tight clumping of eggs during oviposition, perhaps with adherent eggs to maintain the close physical contact, and active preference for laying eggs communally with those of other females (Radder & Shine 2007), perhaps even females of other species. In keeping with this prediction, keelback snakes do indeed oviposit communally rather than singly if given the opportunity (Brown & Shine 2005; figure 3), and thereby obtain the advantages of enhanced water uptake by eggs. Our laboratory studies suggest that the benefits of communal nesting might depend upon factors such as maternal fecundity and soil moisture levels, generating immense complexity.
Allometry of egg versus offspring size. Because eggs in larger clutches will be able to take up more water during incubation, we expect larger clutches to consist of smaller eggs (thus generating a similarity in offspring sizes). That is, a trade-off between egg size and number does not necessarily lead to a trade-off between offspring size and number. As predicted, mean egg mass declines with increasing clutch size in our keelback population (n=333 clutches, r=−0.14, p<0.01). Because we do not have data on neonatal masses from incubation in natural nests, we cannot assess the prediction concerning the relationship between clutch size and offspring mass.
Thresholds for initiating reproduction. We predict that female keelbacks should delay reproduction until they can produce a large-enough clutch to benefit their offspring in terms of optimal water uptake and, thus, hatchling size. All else being equal, we would expect this selective pressure to result in large females producing infrequent, but large, clutches of eggs. The same general prediction derives from the notion of fecundity-independent costs—that is, females should delay reproduction and produce infrequent large clutches if the act of reproduction involves a risk or energy cost whose magnitude is independent of the number of eggs produced (Bull & Shine 1979). In essence, these are two sides of the same coin: a life-history tactic based on producing occasional large clutches rather than numerous small clutches can be favoured because of fecundity-dependent advantages (as in the current study) or fecundity-independent costs (as in Bull & Shine's (1979) analysis). The two are entirely compatible, and both may well play a role.
Female body size. If hatchling viability increases with clutch size, we expect selection to favour traits such as higher maternal energy allocation to growth (because larger body size enhances clutch size), and delayed female maturation (because delaying reproduction allows a longer growth period and hence larger maternal body size at reproduction). The end result might affect the degree of sexual size dimorphism. Also, if larger clutch sizes enhance per-offspring fitness, larger females may be able to ‘afford’ smaller eggs, since these will produce relatively large offspring regardless of the smaller initial yolk investment. Such a scenario would offer a clear selective advantage to larger body size in females.
Reproductive modes. Although the three reptile species in which clutch size is known to affect offspring size are egg layers, approximately one-quarter of squamate species are viviparous (Blackburn 1982, 1985; Shine 1985). It is difficult to imagine hydric balance being affected by litter size in such cases, because the embryos are not in physical contact with each other, and all are maintained within a hydric environment under direct maternal control. If so, then the selective advantage of viviparity over oviparity may shift with fecundity. For a species producing small clutches, the shift to uterine incubation may enhance hydric balance of developing embryos more than would be the case for a species with higher fecundity (and, thus, in which large clutch sizes ensure more suitable hydric conditions inside the nest). If so, the selective advantages afforded by uterine retention of eggs may depend upon clutch sizes (and, hence, maternal mean adult body sizes).
Figure 3.
Two female keelbacks (T. mairii) ovipositing simultaneously in the same nest on the Fogg Dam wall. Subsequent excavation revealed that a third clutch was already present in the nest. Photograph by C. Beckmann.
5. Conclusions
In summary, clutch sizes may play a more complex role as maternal effects than has heretofore been appreciated. Not only can clutch size influence offspring size via a direct trade-off (given finite limits of energy or maternal abdominal volume), but also via a range of other pathways that can operate after the initial maternal allocation decision—either during egg incubation, at the time of hatching or after hatching. Such effects can occur in species without parental care, as well as in those that allocate additional resources to their progeny post-hatching. Increases in clutch size can increase progeny fitness in some circumstances, and decrease it in others—even within the same population, as a function of nest-site location. The mechanisms that generate causal effects of clutch size on offspring viability are diverse, ranging from competition among siblings for a limited resource (energy, oxygen and water) through to the advantages of larger numbers in predator satiation. Simple verbal models suggest that these effects not only need to be incorporated in our experimental designs for future work, but also should be incorporated in theoretical models to explore the consequences of this diversity in size–number trade-offs for the evolution of life-history tactics.
Acknowledgments
This work was authorized by the University of Sydney Animal Care and Ethics Committee.We thank T. Uller and E. Wapstra for inviting us to participate in the workshop, and the Northern Territory Land Corporation and Beatrice Hill Farm for accommodation and logistical support. Comments by the editors and reviewers substantially improved the manuscript. The work was funded by the Australian Research Council. The original impetus for studying these effects came from Raju Radder, who died while this current manuscript was under review. He will be sorely missed.
Footnotes
One contribution of 12 to a Theme Issue ‘Evolution of parental effects: conceptual issues and empirical patterns’.
References
- Agarwala B.K., Dixon A.F.G. Why ladybirds lay eggs in clusters? Funct. Ecol. 1993;7:541–548. doi:10.2307/2390130 [Google Scholar]
- Bardos D.C., Day R.W., Lawson N.T., Linacre N.A. Dynamical response to fishing varies with compensatory mechanism: an abalone population model. Ecol. Model. 2006;192:523–542. doi:10.1016/j.ecolmodel.2005.07.015 [Google Scholar]
- Blackburn D.G. Evolutionary origins of viviparity in the Reptilia. I. Sauria. Amphib. Reptil. 1982;3:185–205. doi:10.1163/156853882X00419 [Google Scholar]
- Blackburn D.G. Evolutionary origins of viviparity in the Reptilia. II. Serpentes, Amphisbaenia, and Icthyosauria. Amphib. Reptil. 1985;6:259–291. doi:10.1163/156853885X00290 [Google Scholar]
- Broderick A.C., Godley B.J., Hays G.C. Metabolic heating and the prediction of sex ratios for green turtles (Chelonia mydas) Physiol. Biochem. Zool. 2001;74:161–170. doi: 10.1086/319661. doi:10.1086/319661 [DOI] [PubMed] [Google Scholar]
- Brown G.P., Shine R. Reproductive ecology of a tropical natricine snake, Tropidonophis mairii (Colubridae) J. Zool. (Lond.) 2002;258:63–72. doi:10.1017/S0952836902001218 [Google Scholar]
- Brown G.P., Shine R. Maternal nest-site choice and offspring fitness in a tropical snake (Tropidonophis mairii, Colubridae) Ecology. 2004;85:1627–1634. doi:10.1890/03-0107 [Google Scholar]
- Brown G.P., Shine R. Nesting snakes (Tropidonophis mairii, Colubridae) selectively oviposit in sites that provide evidence of previous successful hatching. Can. J. Zool. 2005;83:1134–1137. [Google Scholar]
- Brown G.P., Shine R. Repeatability and heritability of reproductive traits in free-ranging snakes. J. Evol. Biol. 2007;20:588–596. doi: 10.1111/j.1420-9101.2006.01256.x. doi:10.1111/j.1420-9101.2006.01256.x [DOI] [PubMed] [Google Scholar]
- Brown G.P., Shine R., Madsen T. Responses of three sympatric snake species to tropical seasonality in Northern Australia. J. Trop. Ecol. 2002;18:549–568. doi:10.1017/S0266467402002365 [Google Scholar]
- Bull J.J., Shine R. Iteroparous animals that skip opportunities for reproduction. Am. Nat. 1979;114:296–303. doi:10.1086/283476 [Google Scholar]
- Burgess K.S., Morgan M., Husband B.C. Interspecific seed discounting and the fertility cost of hybridization in an endangered species. New Phytol. 2008;177:276–283. doi: 10.1111/j.1469-8137.2007.02244.x. doi:10.1111/j.1469-8137.2007.02244.x [DOI] [PubMed] [Google Scholar]
- Chaffee C., Strathmann R.R. Constraints on egg masses. I. Retarded development within thick egg masses. J. Exp. Mar. Biol. Ecol. 1984;84:73–83. doi:10.1016/0022-0981(84)90231-4 [Google Scholar]
- Clark B.R., Faeth S.H. The evolution of egg clustering in butterflies: a test of the egg desiccation hypothesis. Evol. Ecol. 1998;12:543–552. doi:10.1023/A:1006504725592 [Google Scholar]
- Cogger H.G. 6th edn. Reed New Holland; Sydney, Australia: 2000. Reptiles and amphibians of Australia. [Google Scholar]
- Cooper C.B., Hochachka W.M., Butcher G., Dhondt A.A. Seasonal and latitudinal trends in clutch size: thermal constraints during laying and incubation. Ecology. 2005;86:2018–2031. doi:10.1890/03-8028 [Google Scholar]
- Deeming D.C. Post-hatching phenotypic effects of incubation on reptiles. In: Deeming D.C., editor. Reptilian incubation. Environment, evolution and behaviour. Nottingham University Press; Nottingham, UK: 2004. pp. 229–251. [Google Scholar]
- Desouhant E., Debouzie D., Ploye H., Menu F. Clutch size manipulations in the chestnut weevil, Curculio elephas: fitness of oviposition strategies. Oecologia. 2000;122:493–499. doi: 10.1007/s004420050971. doi:10.1007/s004420050971 [DOI] [PubMed] [Google Scholar]
- Dimond W.J., Armstrong D.P. Adaptive harvesting of source populations for translocation: a case study with New Zealand robins. Conserv. Biol. 2007;21:114–124. doi: 10.1111/j.1523-1739.2006.00537.x. doi:10.1111/j.1523-1739.2006.00537.x [DOI] [PubMed] [Google Scholar]
- Du W., Ji X., Shine R. Does body-volume constrain reproductive output in lizards? Biol. Lett. 2005;1:98–100. doi: 10.1098/rsbl.2004.0268. doi:10.1098/rsbl.2004.0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelta J.M., Cortes P., Molowny-Horas R., Sanchez-Humanes B., Retana J. Masting mediated by summer drought reduces accord predation in Mediterranean oak forests. Ecology. 2008;89:805–817. doi: 10.1890/07-0217.1. doi:10.1890/07-0217.1 [DOI] [PubMed] [Google Scholar]
- Ewert M.A., Nelson C.E. Metabolic heating of embryos and sex determination in the American alligator, Alligator mississippiensis. J. Therm. Biol. 2003;28:159–165. doi:10.1016/S0306-4565(02)00053-0 [Google Scholar]
- Faraji F., Janssen A., Sabelis M.W. The benefits of clustering eggs: the role of egg predation and larval cannibalism in a predatory mite. Oecologia. 2002;131:20–26. doi: 10.1007/s00442-001-0846-8. doi:10.1007/s00442-001-0846-8 [DOI] [PubMed] [Google Scholar]
- Fenner A.L., Bull C.M. Short-term impact of grassland fire on the endangered pygmy bluetongue lizard. J. Zool. (Lond.) 2007;272:444–450. doi:10.1111/j.1469-7998.2007.00287.x [Google Scholar]
- Fitch H.S. Reproductive cycles in lizards and snakes. Univ. Kansas Mus. Nat. Hist. Misc. Publ. 1970;52:1–247. [Google Scholar]
- Fox C.W., Martin J.D., Thakar M.S., Mousseau T.A. Clutch size manipulations in two seed beetles: consequences for progeny fitness. Oecologia. 1996;108:88–94. doi: 10.1007/BF00333219. doi:10.1007/BF00333219 [DOI] [PubMed] [Google Scholar]
- Friedlander T.P. Egg mass design relative to surface-parasitizing parasitoids, with notes on Asterocampa clyton (Lepidoptera: Nymphalidae) J. Res. Lepid. 1985;24:250–257. [Google Scholar]
- Gillespie D.O.S., Russell A.F., Lummaa V. When fecundity does not equal fitness: evidence of an offspring quantity versus quality trade-off in pre-industrial humans. Proc. R. Soc. B. 2008;275:713–722. doi: 10.1098/rspb.2007.1000. doi:10.1098/rspb.2007.1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittleman J.L. Communal care in mammals. In: Greenwood P.J., Harvey P.H., Slatkin M., editors. Evolution: essays in honour of John Maynard Smith. Cambridge University Press; Cambridge, UK: 1985. pp. 187–205. [Google Scholar]
- Godfray H.C.J., Parker G.A. Sibling competition, parent-offspring conflict and clutch size. Anim. Behav. 1992;43:473–490. doi:10.1016/S0003-3472(05)80106-X [Google Scholar]
- Green B.S., Anthony K.R.N., McCormick M.I. Position of egg within a clutch is linked to size at hatching in a demersal tropical fish. J. Exp. Mar. Biol. Ecol. 2006;329:144–152. doi:10.1016/j.jembe.2005.08.012 [Google Scholar]
- Hailey A. Implications of high intrinsic growth rate of a tortoise population for conservation. Anim. Conserv. 2000;3:185–189. doi:10.1111/j.1469-1795.2000.tb00102.x [Google Scholar]
- Hayes L.D. To nest communally or not to nest communally: a review of rodent communal nesting and nursing. Anim. Behav. 2000;59:677–688. doi: 10.1006/anbe.1999.1390. doi:10.1006/anbe.1999.1390 [DOI] [PubMed] [Google Scholar]
- Ji X., Qiu Q.B., Diong C.H. Influence of incubation temperature on hatching success, energy expenditure for embryonic development, and size and morphology of hatchlings in the oriental garden lizard, Calotes versicolor (Agamidae) J. Exp. Zool. 2002;292:649–659. doi: 10.1002/jez.10101. doi:10.1002/jez.10101 [DOI] [PubMed] [Google Scholar]
- Kagata H., Ohgushi T. Conflict between optimal clutch size for mothers and offspring in the leaf miner, Leucoptera sinuella. Ecol. Entomol. 2004;29:429–436. doi:10.1111/j.0307-6946.2004.00623.x [Google Scholar]
- Karino K., Arai R. Effect of clutch size on male egg-fanning behavior and hatching success in the goby, Eviota prasina (Klunzinger) J. Exp. Mar. Biol. Ecol. 2006;334:43–50. doi:10.1016/j.jembe.2006.01.018 [Google Scholar]
- Kiesecker J.M., Blaustein A.R. Influences of egg laying behavior on pathogenic infection of amphibian eggs. Conserv. Biol. 1997;11:214–220. doi:10.1046/j.1523-1739.1997.95509.x [Google Scholar]
- Klug H., Bonsall M.B. When to care for, abandon, or eat your offspring: the evolution of parental care and filial cannibalism. Am. Nat. 2007;170:886–901. doi: 10.1086/522936. doi:10.1086/522936 [DOI] [PubMed] [Google Scholar]
- Lack D. Chapman and Hall; London, UK: 1968. Ecological adaptations for breeding in birds. [Google Scholar]
- Lardies M.A., Fernandez M. Effect of oxygen availability in determining clutch size in Acanthina monodon. Mar. Ecol. Prog. Ser. 2002;239:139–146. doi:10.3354/meps239139 [Google Scholar]
- Lee C.E., Strathmann R.R. Scaling of gelatinous clutches: effects of siblings' competition for oxygen on clutch size and parental investment per offspring. Am. Nat. 1998;151:293–310. doi: 10.1086/286120. doi:10.1086/286120 [DOI] [PubMed] [Google Scholar]
- Le Masurier A.D. Costs and benefits of egg clustering in Pieris brassicae. J. Anim. Ecol. 1994;63:677–685. doi:10.2307/5233 [Google Scholar]
- Lepage D., Gauthier G., Desrochers A. Larger clutch size increases fledging success and offspring quality in a precocial species. J. Anim. Ecol. 1998;67:210–216. doi:10.1046/j.1365-2656.1998.00182.x [Google Scholar]
- Madsen T., Shine R. The adjustment of reproductive threshold to prey abundance in a capital breeder. J. Anim. Ecol. 1999;68:571–580. doi:10.1046/j.1365-2656.1999.00306.x [Google Scholar]
- Madsen T., Shine R. Rain, fish and snakes: climatically-driven population dynamics of Arafura filesnakes in tropical Australia. Oecologia. 2000;124:208–215. doi: 10.1007/s004420050008. doi:10.1007/s004420050008 [DOI] [PubMed] [Google Scholar]
- Malnate E.V., Underwood G. Australasian natricine snakes of the genus Tropidonophis. Proc. Acad. Nat. Sci. Phila. 1988;140:59–201. [Google Scholar]
- Marco A., Diaz-Paniagua C., Hidalgo-Vila J. Influence of egg aggregation and soil moisture on incubation of flexible-shelled lacertid lizard eggs. Can. J. Zool. 2004;82:60–65. doi:10.1139/z03-209 [Google Scholar]
- Mathies T., Andrews R.M. Extended egg retention and its influence on embryonic development and egg water balance: implications for the evolution of viviparity. Physiol. Zool. 1996;69:1021–1035. [Google Scholar]
- Mathies T., Andrews R.M. Does reduction of the eggshell occur concurrently with or subsequent to the evolution of viviparity in phrynosomatid lizards? Biol. J. Linn. Soc. 2000;71:719–736. doi:10.1111/j.1095-8312.2000.tb01287.x [Google Scholar]
- McGinley M.A. The influence of a positive correlation between clutch size and offspring fitness on the optimal offspring size. Evol. Ecol. 1989;3:150–156. doi:10.1007/BF02270917 [Google Scholar]
- McGinley M.A., Temme D.H., Geber M.A. Parental investment in offspring in variable environments: theoretical and empirical considerations. Am. Nat. 1987;130:370–398. doi:10.1086/284716 [Google Scholar]
- McRae S.B. Family values: costs and benefits of communal nesting in the moorhen. Anim. Behav. 1996;52:225–245. doi:10.1006/anbe.1996.0169 [Google Scholar]
- Messina F.J., Fox C.W. Offspring size and number. In: Fox C.W., Roff D.A., Fairbairn D.J., editors. Evolutionary ecology: concepts and case studies. Oxford University Press; New York, NY: 2001. pp. 113–127. [Google Scholar]
- Michaud J.P., Grant A.K. Adaptive significance of sibling egg cannibalism in Coccinellidae: comparative evidence from three species. Ann. Entomol. Soc. Am. 2004;97:710–719. doi:10.1603/0013-8746(2004)097[0710:ASOSEC]2.0.CO;2 [Google Scholar]
- Nagle R.D., Lutz C.L., Pyle A.L. Overwintering in the nest by hatchling map turtles (Graptemys geographica) Can. J. Zool. 2004;82:1211–1218. doi:10.1139/z04-096 [Google Scholar]
- Olsson M., Shine R. How and why does reproductive success increase with age? A case study using sand lizards (Lacerta agilis) Oecologia. 1996;105:175–178. doi: 10.1007/BF00328543. [DOI] [PubMed] [Google Scholar]
- Olsson M., Shine R. The limits to reproductive output: offspring size versus number in the sand lizard (Lacerta agilis) Am. Nat. 1997;149:179–188. doi:10.1086/285985 [Google Scholar]
- O'Shea M. Independent Publishing; Port Moresby, Papua New Guinea: 1996. A guide to the snakes of Papua New Guinea. [Google Scholar]
- Packard M.J., Packard G.C., Boardman T.J. Structure of eggshells and water relations of reptilian eggs. Herpetologica. 1982;38:136–155. [Google Scholar]
- Parejo D., Danchin E. Brood size manipulation affects frequency of second clutches in the blue tit. Behav. Ecol. Sociobiol. 2006;60:184–194. doi:10.1007/s00265-005-0155-z [Google Scholar]
- Plaistow S.J., St Clair J.J.H., Grant J., Benton T.G. How to put all your eggs in one basket: empirical patterns of offspring provisioning throughout a mother's lifetime. Am. Nat. 2007;170:520–529. doi: 10.1086/521238. doi:10.1086/521238 [DOI] [PubMed] [Google Scholar]
- Post W. Advantages of coloniality in female Boat-tailed Grackles. Wilson Bull. 1998;110:489–496. [Google Scholar]
- Qualls C.P., Andrews R.M. Cold climates and the evolution of viviparity in reptiles: cold incubation temperatures produce poor-quality offspring in the lizard, Sceloporus virgatus. Biol. J. Linn. Soc. 1999;67:353–376. doi:10.1006/bijl.1998.0307 [Google Scholar]
- Radder R., Shine R. Why do female lizards lay their eggs in communal nests? J. Anim. Ecol. 2007;76:881–887. doi: 10.1111/j.1365-2656.2007.01279.x. doi:10.1111/j.1365-2656.2007.01279.x [DOI] [PubMed] [Google Scholar]
- Radder R., Warner D.A., Shine R. Compensating for a bad start: catch-up growth in juvenile lizards (Amphibolurus muricatus, Agamidae) J. Exp. Zool. A. 2007;307:500–508. doi: 10.1002/jez.403. doi:10.1002/jez.403 [DOI] [PubMed] [Google Scholar]
- Rhind S.G. Communal nesting in the usually solitary marsupial, Phascogale tapoatafa. J. Zool. (Lond.) 2003;261:345–351. doi:10.1017/S0952836903004308 [Google Scholar]
- Rodel H.G., Hudson R., von Holst D. Optimal litter size for individual growth of European rabbit pups depends on their thermal environment. Oecologia. 2008;155:677–689. doi: 10.1007/s00442-008-0958-5. doi:10.1007/s00442-008-0958-5 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Chapman and Hall; London, UK: 1992. The evolution of life histories: theory and analysis. [Google Scholar]
- Roff D.A. Sinauer Associates; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Ruthig G.R. The influence of temperature and spatial distribution on the susceptibility of southern leopard frog eggs to disease. Oecologia. 2008;156:895–903. doi: 10.1007/s00442-008-1026-x. doi:10.1007/s00442-008-1026-x [DOI] [PubMed] [Google Scholar]
- Ryan M.J. A thermal property of the Rana catesbeiana (Amphibia, Anura, Ranidae) egg mass. J. Herpetol. 1978;12:247–248. doi:10.2307/1563415 [Google Scholar]
- Shine, R. 1985 The evolution of viviparity in reptiles: an ecological analysis. In Biology of the Reptilia, vol. 15 (eds C. Gans & F. Billett), pp. 605–694. New York, NY: Wiley.
- Shine R. Strangers in a strange land: ecology of the Australian colubrid snakes. Copeia. 1991;1991:120–131. doi:10.2307/1446254 [Google Scholar]
- Shine R. Relative clutch mass and body shape in lizards and snakes: is reproductive investment constrained or optimised? Evolution. 1992;46:828–833. doi: 10.1111/j.1558-5646.1992.tb02088.x. doi:10.2307/2409650 [DOI] [PubMed] [Google Scholar]
- Shine R. Eggs in autumn: responses to declining incubation temperatures by the eggs of montane lizards. Biol. J. Linn. Soc. 2002;76:71–77. doi:10.1111/j.1095-8312.2002.tb01715.x [Google Scholar]
- Shine R., Brown G.P. Adapting to the unpredictable: reproductive biology of vertebrates in the Australian wet–dry tropics. Invited review. Phil. Trans. R. Soc. B. 2008;363:363–373. doi: 10.1098/rstb.2007.2144. doi:10.1098/rstb.2007.2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine R., Elphick M.J., Harlow P.S. The influence of natural incubation environments on the phenotypic traits of hatchling lizards. Ecology. 1997;78:2559–2568. doi:10.2307/2265914 [Google Scholar]
- Siemens D.H., Johnson C.D. Density dependent egg parasitism as a determinant of clutch size in bruchid beetles (Coleoptera: Bruchidae) Environ. Entomol. 1992;21:610–619. [Google Scholar]
- Sinervo B., Huey R.B. Allometric engineering: an experimental test of the causes of interpopulational differences in performance. Science. 1990;248:1106–1109. doi: 10.1126/science.248.4959.1106. doi:10.1126/science.248.4959.1106 [DOI] [PubMed] [Google Scholar]
- Smith C.C., Fretwell S.D. The optimal balance between size and number of offspring. Am. Nat. 1974;108:499–506. doi:10.1086/282929 [Google Scholar]
- Sprenger D., Anthes N., Michiels N.K. Multiple mating affects offspring size in the opisthobranch Chelidonura sandrana. Mar. Biol. 2008;153:891–897. doi:10.1007/s00227-007-0861-3 [Google Scholar]
- Stearns S.C. Trade-offs in life-history evolution. Funct. Ecol. 1989;3:259–268. doi:10.2307/2389364 [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Steer M.A., Moltschaniwskyj N.A. The effects of egg position, egg mass size, substrate and biofouling on embryo mortality in the squid Sepioteuthis australis. Rev. Fish Biol. Fish. 2007;17:173–182. doi:10.1007/s11160-006-9023-9 [Google Scholar]
- Steer M., Moltschaniwskyj N., Jordan A. Embryonic development of southern calamari (Sepioteuthis australis) within the constraints of an aggregated egg mass. Mar. Freshw. Res. 2003;54:217–226. doi:10.1071/MF02107 [Google Scholar]
- Takahashi D., Asada H., Takeyama T., Takahata M., Katoh R., Awata S., Kohda M. Why egg-caring males of Isaza (Gymnogobius isaza, Gobiidae) refuse additional females: preliminary field observations. J. Ethol. 2004;22:153–159. doi:10.1007/s10164-004-0116-4 [Google Scholar]
- Taylor R.J. The value of clumping to prey when detectability increases with group size. Am. Nat. 1979;113:299–301. doi:10.1086/283387 [Google Scholar]
- Taylor J.A., Tulloch D. Rainfall in the wet-dry tropics: extreme events at Darwin and similarities between years during the period 1870–1983. Aust. J. Ecol. 1985;10:281–295. doi:10.1111/j.1442-9993.1985.tb00890.x [Google Scholar]
- Vincenzi S., Crivelli A.J., Jesensek D., Rubin J.F., Poizat G., De Leo G.A. Potential factors controlling the population viability of newly introduced endangered marble trout populations. Biol. Conserv. 2008;141:198–210. doi:10.1016/j.biocon.2007.09.013 [Google Scholar]
- Vitt L.J., Congdon J.D. Body shape, reproductive effort, and relative clutch mass in lizards: resolution of a paradox. Am. Nat. 1978;112:595–608. doi:10.1086/283300 [Google Scholar]
- Webb J.K., Brown G.P., Shine R. Body size, locomotor speed and antipredator behaviour in a tropical snake (Tropidonophis mairii, Colubridae): the influence of incubation environments and genetic factors. Funct. Ecol. 2001;15:561–568. doi:10.1046/j.0269-8463.2001.00570.x [Google Scholar]
- Weis A.E., Price P.W., Lynch M. Selective pressures on clutch size in the gall maker Asteromyia carbonifera. Ecology. 1983;64:688–695. doi:10.2307/1937190 [Google Scholar]
- Wheatley K.E., Bradshaw C.J.A., Harcourt R.G., Hindell M.A. Feast or famine: evidence for mixed capital-income breeding strategies in Weddell seals. Oecologia. 2008;155:11–20. doi: 10.1007/s00442-007-0888-7. doi:10.1007/s00442-007-0888-7 [DOI] [PubMed] [Google Scholar]
- Withers T.M., Madie C., Harris M.O. The influence of clutch size on survival and reproductive potential of Hessian fly. Entomol. Exp. Appl. 1997;83:205–212. doi:10.1023/A:1002963722041 [Google Scholar]
- Zaviezo T., Mills N. Factors influencing the evolution of clutch size in a gregarious insect parasitoid. J. Anim. Ecol. 2000;69:1047–1057. doi:10.1046/j.1365-2656.2000.00460.x [Google Scholar]
- Zehnder C.B., Parris M.A., Hunter M.D. Effects of maternal age and environment on offspring vital rates in the Oleander aphid (Hemiptera: Aphididae) Environ. Entomol. 2007;36:910–917. doi: 10.1603/0046-225x(2007)36[910:eomaae]2.0.co;2. doi:10.1603/0046-225X(2007)36[910:EOMAAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]