Abstract
When faced with changing environments, organisms rapidly mount physiological and behavioural responses, accommodating new environmental inputs in their functioning. The ubiquity of this process contrasts with our ignorance of its evolutionary significance: whereas within-generation accommodation of novel external inputs has clear fitness consequences, current evolutionary theory cannot easily link functional importance and inheritance of novel accommodations. One hundred and twelve years ago, J. M. Baldwin, H. F. Osborn and C. L. Morgan proposed a process (later termed the Baldwin effect) by which non-heritable developmental accommodation of novel inputs, which makes an organism fit in its current environment, can become internalized in a lineage and affect the course of evolution. The defining features of this process are initial overproduction of random (with respect to fitness) developmental variation, followed by within-generation accommodation of a subset of this variation by developmental or functional systems (‘organic selection’), ensuring the organism's fit and survival. Subsequent natural selection sorts among resultant developmental variants, which, if recurrent and consistently favoured, can be inherited when existing genetic variance includes developmental components of individual modifications or when the ability to accommodate novel inputs is itself heritable. Here, I show that this process is consistent with the origin of novel adaptations during colonization of North America by the house finch. The induction of developmental variation by novel environments of this species's expanding range was followed by homeostatic channelling, phenotypic accommodation and directional cross-generational transfer of a subset of induced developmental outcomes favoured by natural selection. These results emphasize three principal points. First, contemporary novel adaptations result mostly from reorganization of existing structures that shape newly expressed variation, giving natural selection an appearance of a creative force. Second, evolutionary innovations and maintenance of adaptations are different processes. Third, both the Baldwin and parental effects are probably a transient state in an evolutionary cycle connecting initial phenotypic retention of adaptive changes and their eventual genetic determination and, thus, the origin of adaptation and evolutionary change.
Keywords: Baldwin effect, evolution, developmental plasticity, hormones, inheritance, maternal effects
1. Introduction
Throughout the history of evolutionary biology, one general question remains most puzzling: ‘how and why do organisms produce a suitable adaptation where it is needed?’ (Weismann 1896; Gerhart & Kirschner 2007). The answer to this question hinges on understanding the evolution of organismal systems that enable continuing environmental input and homeostasis of already-present adaptive structures. Essentially, the main difficulty here is to envision the evolution of a system that reconciles variability and heredity—adaptation to changing environments requires generation of novel developmental variation, but heritability of such variation should, by definition, limit the range of future variability. In different reincarnations, the relationship between variability and heredity is expressed in the search for the ‘non-Lamarckian’ links between functional importance and heritability (Osborn 1896; Jablonka et al. 1995), between novelty and contingency in the evolutionary process (Cope 1887; Baldwin 1896; Müller & Wagner 1991; Müller & Newman 2003; West-Eberhard 2003), between adaptation and adaptability (Severtsov 1934; Mayr 1960; Kirschner & Gerhart 2005) and, more generally, the connection between adaptation and evolutionary change (Schmalhausen 1938; Lewontin 1970).
Observations that ‘the means of survival’ are distinct from the ‘fact of survival’—i.e. that accommodation of novel environmental inputs within a generation plays an important role in an organism's survival and functioning—fuel a search for the place of such accommodations in evolutionary theory and for the mechanisms that might link induction and retention of within-generation accommodations in evolutionary lineages (Dobzhansky 1937; Schmalhausen 1938; Mayr 1960, 1982; Simpson 1984; Gould 2002; West-Eberhard 2003). Four general observations aid in this search. First, a weakening or cessation of natural selection often coincides with evolutionary novelties and diversifications, such as in domestication, where shielding from natural selection leads to expression of novel developmental variation, or in ‘ecological release’ (Van Valen 1965) of developmental diversification in the wild that accompanies colonizations and invasions of competitor- and predator-free environments (Losos & Queiroz 1997; Reid 2007). Second, the expression of previously hidden or novel phenotypic variation—such as physiological or morphological plasticity—is delineated by existing organismal structures shaped by the accumulation of prior adaptations (Cope 1887; Baldwin 1902; Whyte 1965) and governed by either regulatory changes or rearrangements of pre-existing components (King & Wilson 1975; Stern 2000; West-Eberhard 2003; Carroll 2005). Third is the thesis that different inheritance systems include distinct levels of directionality and persistence in relation to generation span (e.g. Jablonka 2001). For example, maternal effects, the focus of this review, combine induction of novel and functionally important developmental variation in offspring with its directional inheritance over the span of at least two generations (Badyaev 2008). Fourth is the notion of an epigenetic-to-genetic continuum of regulatory changes in developmental evolution, where the most reliable and recurrent organism–environment associations (those with the greatest fit, i.e. fitness) become internalized and stabilized by progressively greater genetic determination (Chetverikov 1926; Newman & Müller 2000; Oyama 2000; Müller & Newman 2003; Badyaev 2005b, 2007). In turn, progressively stronger integration and genetic determination greatly amplify the spread of novel phenotypic variation both among organismal components and across individuals in a population (e.g. Simpson 1953; Newman & Müller 2000; Duckworth in press), thereby facilitating evolutionary change.
One hundred and twelve years ago, in a series of influential publications, J. M. Baldwin, H. F. Osborn and C. L. Morgan proposed a process by which within-generation developmental accommodation of induced environmental inputs, which makes an organism fit in its present environment (‘organic selection’ in Baldwin's writings), can become internalized in an evolutionary lineage and lead to evolutionary change without violating Weismann's rule of germ plasma continuity (Baldwin 1896; Morgan 1896; Osborn 1896). They proposed that organic selection is the process in which phenotypic accommodation of novel environmental inputs in ontogeny allows survival in changing environments, allowing time for subsequent natural selection to retain suitable adaptation. The key components of this evolutionary sequence (termed the Baldwin effect by Simpson 1953) are (i) initial overproduction of random (with respect to fitness) developmental variation, (ii) organismal complexity that assures channelling, directionality and initial retention of a subset of the induced developmental variation, and (iii) subsequent natural selection that both favours the ability to accommodate novel inputs when they increase an organism's fitness and sorts among the resultant developmental variants based on their survival value. When organismal complexity assures similar patterns of accommodation among individuals in a population and when natural selection consistently favours particular developmental outcomes of accommodation, the ability to accommodate the novel input can be inherited. Such inheritance is possible when either existing genetic variation includes developmental components of individual accommodations or the ability to accommodate novel inputs has genetic variation (Baldwin 1896; Simpson 1953; Ancel 1999; West-Eberhard 2005)—conditions likely to be met for a majority of modern organisms (Davidson 2006).
Demonstrating the Baldwin effect requires an integration of approaches from developmental biology, physiological ecology and evolutionary ecology and an empirical system in which one can observe organisms adapting to changing environments of variable recurrences. Here, I review the evidence that the origination of adaptive morphological modifications during ongoing colonization of North America by the house finch (Carpodacus mexicanus) is consistent with the Baldwin effect processes (figure 1). The house finch—a passerine bird native to southwestern North America—underwent an extensive expansion of historical range through both contemporary introductions and natural invasions. By 2008, just 70 years after the first introduction, house finches have occupied virtually all of continental USA, occurring at high densities at its coldest, hottest, wettest and driest locations and occupying the widest ecological range of any extant bird species. First, I briefly review the history of the house finch range expansion, concurrent phenotypic divergence among newly established populations and the rapid evolutionary change over 14 consecutive generations after the establishment of a population in the northern edge of the species range. Second, I show that exposure of breeding females to novel environments during range expansion induced behavioural and physiological responses that resulted in strong maternal effects on offspring developmental variation. Third, I review the evidence for integration of induced maternal effects and offspring sex determination, and suggest that this integration represents homeostatic channelling of induced developmental variation (i.e. phenotypic accommodation). Fourth, I document strong natural selection on both precision of accommodation in maternal generation and morphological outcomes of these accommodations in the offspring generation. I suggest that maternal effects may be a transient state in the evolutionary continuum of epigenetic-to-genetic inheritance systems and a powerful illustration of the Baldwin effect processes (figure 1). Furthermore, I suggest that the Baldwin effect process is a particularly likely pathway to the origin of novel adaptations in the complex of modern organisms because of the redundancy and integration of genetic determination of their development and homeostasis.
Figure 1.
Summary of the Baldwin effect processes in the origination of novel adaptations during ongoing range expansion of the house finch. Novel inputs (e.g. ambient temperature exceeding egg-viability limits in newly established populations in the northern and southern edges of the range and recurrent mite infestation in the native population) induce novel behavioural and physiological variation (e.g. modification of incubation onset, plasma hormone fluctuations; §3a,b). Shared involvement of the same hormonal mechanisms in regulating environmental assessment, incubation behaviour and oogenesis ensures channelling and accommodation of the induced variation (§3c), resulting in overproduction of novel, but functional, developmental variants (§3d). Subsequent natural selection acts on both reproductive homeostasis of females breeding under novel conditions and resulting developmental variants (§3d). Recurrent fitness benefits of developmental outcomes of induction might lead to their retention (§3e).
2. House finch establishment in novel environments in North America
(a) Brief history of the house finch range expansion
Prior to 1850, house finches occupied an area from southern Oregon, central Utah and southern Wyoming in the north and east, to Oaxaca, Mexico in the south (Hill 2002). In the 1850s, a small number of house finches from coastal California (sc CA; figure 2) were introduced to Oahu and, by 1901, they were abundant throughout the Hawaiian chain (Grinnell 1911). In the 1930s, 40–100 house finches, originally from Santa Barbara, California, were released from a pet store in New York City and, by the early 1950s, the city's population of house finches had increased to several hundred birds (Elliott & Arbib 1953; Mundinger & Hope 1982). From the 1960s to the 1990s, the New York population spread across all of the eastern USA and southeastern Canada. In the 1940s–1950s, house finches also began to expand their range in western North America (Badyaev & Hill 2000). The expansion proceeded along both the eastern and the western sides of the Rocky Mountains, reaching northwestern Montana, the focus of this review, from the south and west in the 1950s; house finches became a common breeding bird in Missoula, Montana by 1969 (P. L. Wright 1993, personal communication), although there are records of vagrant house finches in the area in the late 1940s. On the eastern side of the Rocky Mountains, house finches reached north-central Montana by 2002 and the expansion currently continues southwards along the Rocky Mountain Front. On the western side of the Rocky Mountains, house finches reached the continental divide from the west by 2005 and expansion now proceeds northwards. The first verified exchange of individuals between the western and eastern parts of the northern range expansion in Montana took place in the spring of 2002 (Duckworth et al. 2003).
Figure 2.
Rapid phenotypic divergence across selected house finch study populations. Shown is the difference between male and female size (in standard deviations of female trait). The bars from left to right are bill length, wing, tail, tarsus and body mass. Southwestern Arizona (sw AZ1 and sw AZ2), Mexico (Isla Guadalupe (IGU) and Guerrero (GUE)) and south-central California (sc CA) populations are within the species' historical range. The sc CA population is the source of introductions for the east coast populations (southeastern Michigan, se MI; southeastern Alabama, se AL; southern New York, s NY) and Hawaii (HI). The Montana populations on the western and southeastern fronts of the Rocky Mountains have been established during historical range expansion over the last 40 years (nw MT, sw MT and se MT), while the eastern MT populations have been derived from an expansion of the introduced range over the last 10 years (nc MT and ne MT). The numbers after abbreviations of the recently established population are the first breeding record. The nw MT population (Vigilante Ministorage Complex in Missoula, MT) is the main subject of the present review.
(b) Divergence among newly established populations
The expansion of the geographical range was accompanied by rapid allometric divergence and frequent reversals of sexual size dimorphism; new populations often differ by as much as two standard deviations of the mean (s.d.) in less than 10 generations in some traits (figure 2). The pattern of phenotypic divergence showed no clear historical or genetic constraints—the among-population covariance structure was distinct between the sexes and discordant with both within-population covariance patterns and the patterns expected from historical sequence of population settlement. Instead, the divergence reflected low phenotypic and genetic integration among traits during ontogeny; half of all examined ontogenetic allometries among seven morphological traits had significant genetic variance (Badyaev & Hill 2000; Badyaev & Martin 2000a).
In newly established populations at the northern and southern edges of the geographical range, the divergence in growth parameters was qualitatively concordant with within-population patterns of natural selection (see §2c; Badyaev et al. 2001b). However, neither evolved ontogenetic divergence nor mortality due to natural selection achieved the observed magnitude and speed of population differentiation. Instead, the divergence was produced by two interconnected phenomena: (i) population-specific changes in the frequency distribution of distinct ontogenies produced by biasing the birth sequence of male and female offspring, a maternal effect, and (ii) greater sensitivity of male offspring to environmental conditions during growth and, consequently, higher variance in male growth and morphology both within and among populations (Badyaev et al. 2001a, 2003a). Controlling for either of these phenomena experimentally and statistically erases the observed phenotypic differentiation among populations (Badyaev et al. 2002a,b).
(c) Rapid evolutionary change and natural selection following population establishment
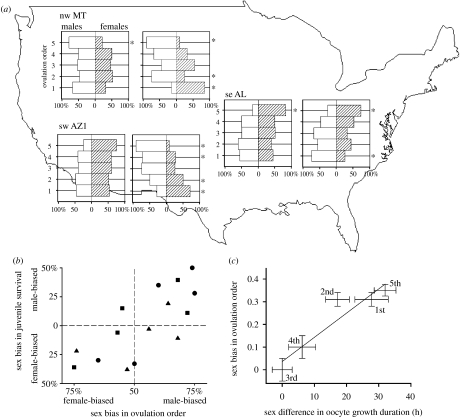
Low integration and high phenotypic and genetic variance during growth enabled rapid and extensive microevolution in morphology since the establishment of the northwestern Montana population (NWM hereafter; figures 3 and 4). Over 14 consecutive generations, the average between-generation change (generation is 1.7 years in house finches) exceeded 0.15 s.d. for bill dimensions and 0.1 s.d. for skeletal traits (figures 3 and 4). Peripheral location and recent origin of the NWM population, in addition to abundant developmental variation, might have contributed to high mortality caused by natural selection on morphology there; but in all examined populations, net natural selection was qualitatively concordant with observed morphologies in both sexes (Badyaev & Martin 2000b; Badyaev et al. 2000). Natural selection on morphology was especially strong on juveniles (figures 3 and 4), but in both age classes and sexes, selection fluctuated across years and seasons, often differed between the sexes, and was closely linked to local functional demands, such as exploitation of new food source in bill traits or a novel disease agent. Without the overproduction of morphologies that were favoured by survival selection and the underproduction of disfavoured morphologies (see §3b)—accomplished by phenotypic accommodation of environmentally induced organismal modifications over consecutive generations, components of the Baldwin effect (see §3a,c; figure 1)—such strong and fluctuating natural selection would have caused local population extinction and prevented the evolution of extensive and adaptive population divergence (figure 2; Badyaev et al. 2002a,b). Specifically, the initial population establishment and subsequent adaptation to climatic extremes of the expanding geographical range were enabled by population-specific sex bias in ovulation order (figure 5a). Below, I argue that this seemingly complex adaptation is a transient and likely passive maternal effect that is a novel by-product of natural selection on females' ability to overlap egg production (oogenesis) and incubation required by breeding under extreme environmental conditions in the north. Its central importance in contemporary phenotypic evolution of house finches is assured by strong natural selection on both the physiological modifications that enable finches to breed under novel conditions and resulting developmental variation in offspring morphology.
Figure 3.
Phenotypic evolution and natural selection on morphology of house finches across 14 consecutive generations following establishment in the northwestern Montana study population. (a,d,g) Cross-generational changes in means (±1 s.e.) of traits measured in fully grown juveniles born in the population in a given year. The 1970s is a sample of first-breeding founders of the Missoula population that were measured at the time of collection and held at the University of Montana's P. L. Wright Zoological Museum. Sample sizes of locally born offspring of locally born parents are as follows: 1995 (23 males, 24 females); 1996 (53, 42); 1997 (46, 52); 1998 (19, 22); 1999 (53, 44); 2000 (48, 26); 2001 (49, 55); 2002 (57, 66); 2003 (61, 67); 2004 (48, 36); 2005 (32, 25); 2006 (22, 24); 2007 (13, 20); and 2008 (25, 27). The right axis shows the mean between-generation change (in s.d./year) in morphological traits in males (black circles) and females (white circles). (b,e,h) Linear selection differentials of juvenile survival selection episode (from the end of the growth until the onset of post-juvenile moult). Sample sizes of local juveniles (before selection) are as follows: 1995 (82 males, 74 females); 1996 (131, 103); 1997 (132, 133); 1998 (154, 139); 1999 (170, 177); 2000 (313, 284); 2001 (346, 311); 2002 (416, 474); 2003 (534, 576); 2004 (262, 204); 2005 (156, 159); 2006 (63, 92); 2007 (65, 51); and 2008 (38, 43). (c,f,i) Linear selection differentials of subsequent adult survival selection episode (from post-juvenile moult to the first spring of breeding). Black bars, selection on males; white bars, selection on females. The horizontal lines show the mean strength of selection across 14 generations (solid line, males; dashed line, females). *p<0.05. (a–c) Beak length, (d–f) beak depth and (g–i) beak width.
Figure 4.
Phenotypic evolution and natural selection on morphology of house finches across 14 consecutive generations following establishment in the northwestern Montana study population. The description is the same as given in figure legend 3. (a–c) Wing length, (d–f) tarsus length, (g–i) tail length and (j–l) body mass.
Figure 5.
Sex-biased ovulation order in response to environmental stimulus in three house finch populations. (a) Left: lack of sex-ratio bias in most ovulation positions in the absence of environmental stimulus. Right: sex-ratio bias (shown by asterisks) in ovulation order associated with ambient temperature below egg-viability threshold (nw MT population), ambient temperatures above egg-viability threshold (se AL population) and nest mite infestation (native sw AZ1 population). (b) Evidence that selection on maternal strategies and offspring morphologies share the same proximate targets (squares, nw MT; triangles, se AL; circles, sw AZ1). Relationship between sex bias in ovulation order and sex bias in juvenile survival. Individuals in the most sex-biased positions within a clutch had the most sex-biased survival. Modified from Badyaev et al. (2002a). (c) The relationship between sex bias in ovulation order (in deviations from parity for each ovulation position) and sex differences (female minus male value) in duration of oocyte growth (h) in the nw MT population. Numbers are ovulation orders (1–5) and standard errors for sex bias were calculated from the among-year deviations during 1995–2003, for growth duration from resampling, for each ovulation position and for growth duration for all oocytes for both sexes. Modified from Badyaev et al. (2005).
3. Evidence for the Baldwin effect's processes
(a) Environmental induction of physiological variation in reproducing females
Three features of avian biology make environmentally induced cross-generational effects on the rate and duration of offspring development likely. First, avian embryos cannot develop without being incubated and breeding females determine the duration of development directly by modifying the onset of incubation in relation to egg-laying in response to their environment (such as predation risk, mate provisioning and ambient temperature; Conway & Martin 2000; Hébert 2002; Martin et al. 2007). Second, although several eggs develop in the ovary simultaneously, only one is laid per day, making egg-laying sequence, in combination with the onset of incubation, a ubiquitous and powerful way to modify offspring growth in relation to environmental variation (Clark & Wilson 1981; Ricklefs 1993; Stoleson & Beissinger 1995). Third, the same hormonal mechanisms are involved in the assessment of environmental conditions, regulation of female reproductive state and metabolism of resources for developing oocytes (Johnson 2000; Vleck 2002; Williams et al. 2005; Sockman et al. 2006). For example, incubation behaviour in many birds, including the house finch (figure 6e), is regulated by environmentally induced synthesis of prolactin, a pituitary hormone that is also involved in controlling proliferation of oocytes and the synthesis of follicular steroids (Etches et al. 1979; Tabibzadeh et al. 1995; Sockman & Schwabl 1999; Sockman et al. 2001).
Figure 6.
Physiological consequences of environmentally induced behaviour following population establishment. Onset of incubation (grey bars, left axis, mean percentage of full clutch ±s.e.) closely tracks ambient temperature (black line, right axis, 24 hours average ±s.e.) and overlaps with egg-laying in (a) first-breeding newly arriving females, but not in (b) previously bred local females. Modified from Badyaev et al. (2003a). (c) The relationship between the ambient temperature outside the limits of tolerance for unincubated eggs (below 4°C, critical temperature hereafter) during oogenesis and sex-biased ovulation sequence (number of biased positions) in the first-breeding attempt of newly arriving females (response proportional to ambient temperature, n=86 females, solid line and circles) and subsequent breeding attempts of these females in the same environment (threshold response, n=51, dashed spline and squares). Bubble radius is proportional to the number of overlapping data points. (d) Estimated number (+s.e.) of the critical temperature days during 10-day-long oogenesis needed to exert full population-specific response (three biases in ovulation sequence in the NWM population) across the lifetime of the 51 females in (c). Modified from Badyaev & Oh (2008). (e) Hormonal correlates of environmentally induced incubation. Circulating plasma prolactin (circles; n=82 females) and androgens (testosterone and 5α-dihydrotestosterone; triangles; n=28, mean±s.e.) in the first-breeding females that began full incubation with the laying of the first egg. The box shows the period of oogenesis in these females. Breeding stages are the ovulation of the first egg (E1-O), the ovulation of the second egg and laying of the first egg (E2-O) and the onset of full incubation (PI-1d). Modified from Badyaev et al. (2005). (f) Relationship between circulating prolactin and androgens during oogenesis (shaded box in (e)) in the continuously sampled first-breeding females that show an environmentally induced overlap of egg-laying and incubation.
A combination of these factors accounts for directionality of naive females' reaction to novel environmental conditions across recently established house finch populations and its consequences for offspring growth (see §3d). Specifically, shared hormonal regulation of female reproduction and sex-specific allocation of growth substances to developing offspring facilitates the emergence of sex-specific clustering of oocytes along the egg-laying sequence across house finch populations (figure 5a).
Environmental induction of sex-biased ovulation sequence is most evident in females newly arriving to breed in the NWM population (figure 6a). The onset of incubation in these females closely tracks ambient temperature in a pattern consistent with the maintenance of viability of newly laid eggs—full incubation starts at ambient temperatures below 4°C (prolonged exposure to such temperatures results in developmental failures; Webb 1987), and these females show a proportional linear relationship between ambient temperature during oogenesis and sex bias in ovulation (figure 6c). Females that previously bred in the NWM population require less environmental induction to produce sex-specific clustering of offspring and their incubation behaviour is less affected by ambient temperature (figure 6b,c); the ‘internalization’ of the inductive environmental stimulus in these females can be accomplished by a shared hormonal regulation of environmental assessment and reproduction (see §3d). Overall, ambient temperatures exceeding the range of viability for unincubated eggs are common across newly established house finch populations in the northern and southern parts of the range, producing strong gradients of growth duration and associated morphological variation in offspring produced in different positions in the egg-laying sequence (Badyaev et al. 2003a,b).
(b) Overproduction of novel developmental variation by environmentally induced maternal effects
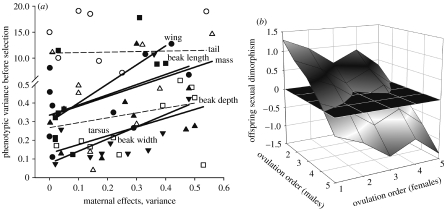
Environmentally induced maternal effects on offspring development result in greater phenotypic variation in most morphological traits (figure 7a). Because development of male offspring is more sensitive to environmental variation than is development of females in house finches (Badyaev et al. 2002b, 2003a, 2006b), environmentally induced variation in ovulation order of male oocytes produces a greater range of morphological variation than does variation in ovulation order of females (figure 7b), accounting for a greater contribution of male morphology to both within-population evolutionary change and among-population divergence (Badyaev et al. 2001a; Badyaev 2005a).
Figure 7.
Maternal effects widen developmental variation in offspring under novel environmental conditions. (a) Phenotypic variance in fully grown juvenile males in relation to maternal effects on the development of their traits (variance, ×102). Each point represents a generation from the 1995–2006 cohorts; the lines are least-squares regression. The dashed lines indicate non-significance. In female juveniles, phenotypic variance in tarsus is negatively associated with maternal effects and variances in bill length and width are unrelated to maternal effects. Variance in other traits is positively associated with maternal effects. Filled circles, beak length; filled up triangles, beak depth; filled down triangles, beak width; filled squares, wing; open squares, tarsus; open circles, tail; open triangles, mass. Modified from Badyaev (2005a). (b) Range of morphologies (approx. 10% of trait mean) at the end of the growth (shown as sexual dimorphism in male minus female tarsus lengths) that can be produced by variation in maternal adjustment of ovulation order of male and female oocytes. Values above the black plane represent male-biased dimorphism and values below the plane female-biased dimorphism. Modified from Badyaev et al. (2002b).
(c) Homeostatic channelling and organic selection of environmentally induced variation
An environmentally induced overlap between oogenesis and incubation imposes a trade-off between an increase in prolactin required for incubation and the synthesis and accumulation of steroids required for sex-specific allocation to growing oocytes (figure 6e,f). A resolution of this trade-off by clustering of oocytes of different sex along the ovulation sequence (figure 5a) favours integration of controls of sex determination and sex-specific allocation. Such integration might be facilitated by the involvement of the same environmentally sensitive hormonal mechanism in the regulation of maternal behaviours, oocyte growth and maturation, and syntheses of steroids linked to oocyte sex determination (Badyaev & Oh 2008; Rutkowska & Badyaev 2008).
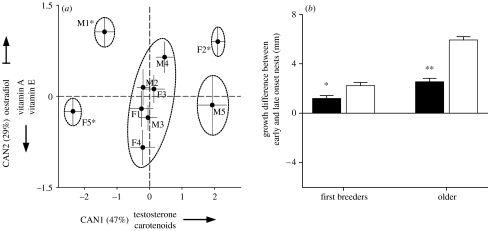
Several lines of evidence suggest that integration of sex determination and sex-specific resource allocation is an emergent outcome of phenotypic accommodation (e.g. organic selection in Baldwin's terminology) of novel environmental input by dynamics of oocyte growth. First, concentration of circulating hormones in female plasma changes consistently across oogenesis (figure 6e), and oocytes initiating growth at different points along this hormonal gradient are exposed to distinct hormonal mixtures and synthesize and accumulate different amounts of hormones as a result of such exposure (figure 8a, insert). Second, groups of oocytes recruited at the same time may form single-sex clusters, as evident from growth-inhibiting hormonal interactions between groups of spatially and temporally segregated oocytes of the same sex and age (figure 8c) and by sex-specific overlap in synthesis, accumulation and partitioning of resources (figure 8b,d). Third, the oocyte clustering is reversible and context dependent within a population, and can emerge rapidly in response to distinct environmental cues in different populations (ambient temperature in nw MT and se AL and nest mites in sw AZ1; figures 5a and 8e,f), as long as these cues are linked to a pronounced gradient of circulating steroids or prolactin in maternal plasma (Badyaev & Oh 2008). Fourth, epigenetic processes assuring random sex determination of avian meiosis can be modified to produce directional segregation distortion of sex chromosomes by factors linked to oocyte growth and steroid accumulation (Alonso-Alvarez 2006; Pike & Petrie 2006; Rutkowska & Badyaev 2008). Fifth, intra-ovarian dynamics of oocyte growth amplifies and maintains initial environmental induction—the onset of incubation has a particularly strong effect on sex determination of the first recruited oocytes, whereas sex determination of the subsequent oocytes is mostly affected by the sex of preceding eggs, possibly as a result of overlap in resource partitioning and inhibiting interactions (Badyaev et al. 2003b, 2006c). Finally, at least in two newly established populations, there are pronounced fitness consequences of integration of sex determination and sex-specific resource allocation—the misalignment of sex determination in ovulation order (e.g. when a male is produced in a female-biased ovulation position) is associated with hormonal accumulation that might be incompatible with normal sex-specific development (figure 9a), accounting for suboptimal growth and high mortality of such offspring at early developmental stages (figure 9b).
Figure 8.
Channelling environmentally induced maternal effects into discrete and functional offspring phenotypes. (a) Exposure to androgens circulating in maternal plasma during oogenesis is expressed in the total concentration of yolk androgens in oocytes (solid line and triangles, males, n=33; dashed line and circles, females, n=36). Insert: bars are mean (±s.e.) daily exposures of male (black) and female (white) oocytes to androgens that had circulated in maternal plasma during the oocyte development. Modified from Badyaev et al. (2005). (b) Evidence that sex differences in timing of oocyte growth, allocation of hormones and sex determination are causally linked in this system. Bars and the left ordinate axis show mean acquisition (±s.e.) of androgens for male (black, solid lines) and female (white, dashed lines) oocytes. Lines are linear and quadratic regressions describing the within-sex patterns of acquisition in relation to ovulation order. Spline and the right ordinate axis show within-clutch gradient (least-squared means for each ovulation position) while statistically controlling for the effects of oocyte sex and biased sex ratio. Asterisks indicate sex-biased ovulation positions. Androgens are shown as an example; other steroids have similar patterns. Modified from Badyaev et al. (2006a). (c) Evidence for spatial sex-biased clustering of oocytes. Coefficient of variation (%,±s.e.) in yolk/albumen ratio (controlling for yolk size) as a measure of ovulation interval consistency in relation to the sex of the immediately preceding egg (black bars, preceding oocyte is male; white bars, preceding oocyte is female). *Significant differences between the effect of the sexes of preceding egg. Modified from Badyaev et al. (2006c). (d) Evidence for temporal sex-biased clustering of oocytes. The effect of the overlap among simultaneously growing oocytes on the accumulation of androgens depends on the sex of overlapping oocytes. Lines are partial regressions on residuals controlling for all other aspects of oocyte growth and substances. Circles and solid lines show overlap with same sex oocytes; triangles and dashed lines show overlap with oocytes of the opposite sex. Modified from Badyaev et al. (2008). (e) Clustering based on oocyte similarity in yolk acquisition (indicating spatial or temporal proximity) in the first-breeding females that experienced in the first-breeding females that experienced one or no critical temperature days during oogenesis (n=34 nests), and (f) the first-breeding females that experienced more than five critical temperature days during oogenesis (n=63 nests). Letters with numbers indicate sex (male or female) and ovulation order (1–5). Drawings show hypothetical arrangement of oocytes in the ovary that would correspond to (f) sex-specific clusters or (e) non-sex-specific hierarchical arrangements. Vertical bars indicate statistically distinct clusters. Modified from Badyaev & Oh (2008).
Figure 9.
Homeostatic integration of sex determination and sex-specific hormone allocation accommodates environmentally induced maternal effects to produce discrete and functional phenotypes. (a) Misalignment of sex determination and steroid acquisition results in likely non-survivable hormonal concentrations. Shown are the means (±bivariate s.e.) of the first two canonical discriminant axes and associated variance for oocyte concentration of steroids, carotenoids and vitamins. Letters with numbers indicate sex (male or female) and ovulation order (1–5). Ellipsoids enclose groups of oocytes that are not statistically different from each other. Asterisks indicate misaligned sex, e.g. males produced in strongly female-biased positions. Canonical axes are as follows: CAN1 (eigenvalue λ=0.40)=0.8testosterone−1.9oestradiol+0.5carotenoids+0.07vitamin A−0.31vitamin E; CAN2 (λ=0.25)=8.7oestradiol−0.5testosterone+0.3carotenoids+5.91vitamin A−0.89vitamin E. Modified from Badyaev et al. (2006a). (b) Coordination of incubation onset and sex-biased ovulation sequence in first-breeding versus older local females. Shown are the differences in growth gains (mm per 48 hours) in tarsus of offspring raised in the nests with the early versus late onset of incubation in relation to sex bias in ovulation order. Bars show greater variation in growth rates of males (white, sex avoided in the first ovulation position) compared with females (black, sex preferred in the first ovulation position) in nests with early incubation onset, and age differences in consequence of coordination of incubation onset and sex bias. *p<0.05, **p<0.01, significant differences by two-tailed t-test. Modified from Badyaev et al. (2003a).
Alternatively, the shared involvement of prolactin in both environmentally induced maternal behaviours and growth and, possibly, sex determination of oocytes might not be an emergent physiological response, but rather an evolved adaptive strategy. The link between the onset of incubation and sex bias in ovulation sequence could have evolved through natural selection for synchronization of offspring growth despite the asynchrony introduced by lengthy egg-laying periods, and capitalizing on greater sensitivity of one sex (typically males) to environmental conditions during growth. Indeed, the association between incubation onset and variable maternal allocations of resources, including in sex-specific pattern, in relation to ovulation order is frequently documented (e.g. Mead & Morton 1985; Bortolotti 1986; Blanco et al. 2002; Eising et al. 2003; Duckworth 2009).
(d) Natural selection on the resulting developmental variation
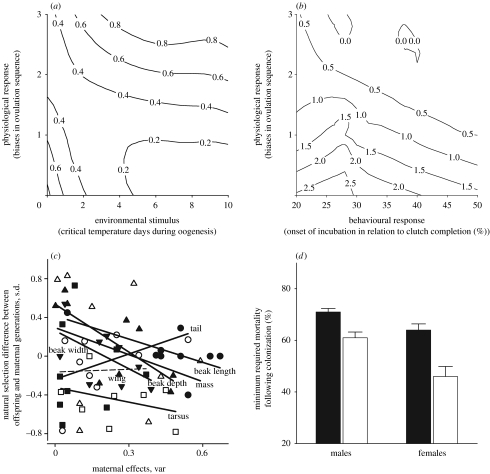
The developmental and physiological outcomes of phenotypic accommodation of environmental input by two generations—i.e. females' capacity to breed under novel environmental conditions and associated morphology of offspring—are subject to natural selection. In the maternal generation, females that respond to novel environmental stimulus with physiological adjustments (figure 10a), and have greater coordination of behavioural and physiological responses to environmental stimulus (figure 10b), have the highest fitness, even when the fitness consequences of their offspring morphology per se are statistically accounted (Badyaev et al. 2005). The proximate target of such selection might be the capacity of females' homeostasis to enable breeding under novel or fluctuating environments, as well as internal selection for alignment of sex determination and sex-specific allocation of oocytes recruited under pronounced environmentally induced hormonal gradients in female plasma. Such strong (figure 10a,b) natural selection on females' ability to breed under novel environments should facilitate phenotypic accommodation of novel environmental inputs even in the absence of any consequences for offspring morphology. However, there is evidence that natural selection on offspring in relation to their morphology and natural selection of female breeding under novel conditions share proximate targets (figure 5b,c), such that extensive initial divergence in morphology following population establishment (figure 2) is directly attributable to environmentally induced maternal effects (figure 5a).
Figure 10.
Natural selection on environmentally induced maternal behaviour and its consequences for offspring development. (a) Natural selection on the extent and precision of response (number of biases in ovulation sequence) to environmental stimulus (number of critical temperature days during oogenesis). Numbers are fledging success in relation to clutch size (n=128 nests). Modified from Badyaev & Oh (2008). (b) Natural selection on coordination of environmentally induced onset of incubation (figure 6) and physiological response to associated changes in hormonal profiles (figure 7). Numbers are offspring per female surviving to dispersal age (n=116 females). Modified from Badyaev et al. (2005). (c) Similarity in the strength of selection in offspring (t year) and maternal (t−1 year) generations as a function of maternal effects on trait development (variance, ×102, symbols are the same as given in figure 7). Ordinate values below zero indicate lesser mortality in offspring compared with maternal generations. Each point is a generation pair from the 1995–2005 cohorts; the lines are least-squared regressions and the non-significant slope is indicated by the dashed line. Only males are shown; female offspring show similar patterns. Modified from Badyaev (2005a). (d) Estimated mortality (‘minimum required mortality’ sensu Lande 1976) needed to produce the observed selection intensity on juvenile morphology in figure 4e, under the assumption of unweighted distribution of residual tarsus length (controlling for body mass) at fledging expected with no sex-biased ovulation order and under observed distribution of tarsus length for both sexes at fledging. Under the assumption of truncation selection, mortality was calculated from the mean selection intensities and a normal distribution. The difference in mortality was assessed with (white) and without (black) the sex-biased ovulation order. Modified from Badyaev et al. (2002a).
First, in several populations, natural selection on offspring morphology favours precise sex bias in ovulation order—such that sex-specific survival and sex-biased ovulation order are matched closely (figure 5b,c). Assuming that offspring morphology is at least partially affected by the egg's acquired hormones and other growth-affecting resources, such selection on offspring morphology acts to fine-tune the initial integration of sex determination and sex-specific allocation of resources along the environmentally induced hormonal gradient of breeding females, ultimately resulting in over-representation of some growth trajectories and under-representation of others—the main cause of population divergence. Second, when natural selection on morphology is similar between maternal and offspring generations, environmentally induced maternal effects on offspring morphology are concordant with patterns favoured by survival selection on offspring generation (figure 10c), the result expected when maternal effects and morphological variation share proximate mechanisms. Integration of complex internal mechanisms of sex determination with a contingent mechanism of sex-specific allocation of growth-affecting substances could enable not only retention and stabilization of environmentally induced effects (see §3e), but also facultative sex-specific adjustment of the effect of ovulation order on offspring morphology (e.g. Dijkstra et al. 1990; Cordero et al. 2001; Andersson et al. 2003).
Importantly, in the context of the house finch range expansion, when natural selection favours sexual size dimorphism (figures 3 and 4) and when the direction of dimorphism differs among populations (figure 2), clustering of offspring of different sex along the egg-laying sequence might lower offspring mortality by converting disruptive selection needed to produce sexual dimorphism in a newly established population to overall stabilizing selection on a bimodal distribution of morphologies produced by development (e.g. Kopp & Hermisson 2006). In fact, such conversion might have been the key to house finch establishment in the NWM population (figure 10d; Badyaev 2005a).
(e) The origins of heredity: initial retention and accommodation of induced environmental effects
Homeostatic channelling and phenotypic accommodation of environmentally induced variation assures similarity in individual responses to environmental change, improving the efficiency of natural selection in fine-tuning locally adaptive changes and facilitating their heritability without reducing developmental plasticity (Cope 1887; Baldwin 1902; Schmalhausen 1938; Jablonka & Lamb 1995; West-Eberhard 2003). When phenotypic accommodation includes elements of complex developmental and genetic pathways of existing structures or when the ability to accommodate novel inputs is itself heritable, recurrent natural selection can lead to retention and heritability of the induced changes (Baldwin 1902; Waddington 1953). However, natural selection accompanying the house finch establishment in new and diverse parts of the expanding range is unlikely to be recurrent over time, whereas the limits to expressed variability imposed by heritability might actually prevent population persistence when such persistence favours variability. Under these conditions, short-term cross-generational transfer of environmentally induced phenotypes (different in different populations) might be favoured by natural selection.
An important insight into heredity of environmentally induced changes is provided by within-generation changes in the processes of environmental induction (figure 6a,b) and phenotypic accommodation (figure 6c). In first-breeding females, newly arriving to breed in the NWM population from more southern locations, the link between maternal strategies and offspring development is mostly caused by strong environmental induction of incubation behaviour and corresponding, largely passive, propagation of this effect on offspring growth. However, in subsequent breeding attempts of these females, sex bias in ovulation order becomes the most important determinant of offspring morphology, independent of weaker inductive effects of ambient temperature on incubation behaviour—i.e. the incubation onset and sex bias in ovulation order become linked independently of mediating effects of ambient temperature (Badyaev et al. 2003a,b).
Facultative and precise sex bias in ovulation sequence can be an evolved strategy under recurrent natural selection, as demonstrated by house finches in the native southwestern Arizona population (figure 5). In this population, facultative sex bias in ovulation sequence is triggered by short-term infestation of nests by Pellonyssus reedi mites. The infestation occurs every year in all nests and the physiological response of females to mite infestation does not appear to be an induced strategy—naive females express sex-biased ovulation sequence in response to environmental stimulus similarly to experienced females (Badyaev & Oh 2008). More importantly, biased ovulation sequence is closely integrated with sex-specific adaptations in growth of male and female offspring (Badyaev et al. 2006b), a suite of coevolved mother–offspring adaptations triggered by mite-induced spikes in maternal plasma corticosterone, its transfer to egg yolk and subsequent corticosterone effect on Bmp-regulated ossification sequence (Badyaev, A. V., Young, R. L., Oh, K. P. & Landeen, E., personal observations).
The dependence of a physiological response on the environmental stimulus decreases rapidly across a female's lifetime, whereas the adaptive response itself is not only maintained, but also becomes more precise (figure 6c,d). The mechanisms that enable within-generation retention and fine-tuning of an induced adaptive response are of central importance to understanding the evolution of inheritance. Both within-generation retention of an induced effect (e.g. ovulation order of the first-breeding attempt) and within-generation fine-tuning of an induced effect (e.g. in relation to familiarity with mate, food fluctuations) are frequently documented (Cheng 1986; Yoo et al. 1986; Sockman et al. 2002; Pfaff et al. 2004) and commonly attributed to complexity and redundancy of endocrine reproductive systems where the same hormonal mechanisms are involved in the assessment of environmental variation and oocyte proliferation and ovulation (e.g. Ball & Balthazart 2008). Furthermore, the integration of mechanisms of sex determination and sex-specific allocation of resources should be under natural selection for sex-specific allocation of resources, because, in birds, multiple eggs develop at the same time but sex-specific allocation is often favoured and necessary for normal development (Carere & Balthazart 2007).
Integration of epigenetic mechanisms of sex determination and sex-specific accumulation of resources in relation to maternal hormonal state can facilitate the retention of maternal adaptations and eventual evolution of sex-specific maternal effects. The often documented precision in sex-ratio adjustment in relation to the context of breeding and incubation onset, including the reversal between subsequent breeding attempts, suggests a considerable degree of functional and genetic integration among these mechanisms in birds. I suggest that the environmentally induced interplay among maternal hormonal profiles, associated sex-specific acquisition of hormones by developing oocytes and developmental plasticity of offspring in the newly established population represent the initial stages of evolution of local adaptation and provide a link between environmental induction and genetic inheritance of novel adaptations.
4. Parental effects and the Baldwin effect as ‘ontogenetic accommodations of earlier generations’
The cornerstone of the Baldwin effect is the historical recurrence of an environmentally induced change that determines the speed of its accommodation in a lineage, such that the direction of evolution can be envisioned as ‘the direction of the ontogenetic accommodations of the earlier generations’ (Baldwin 1896). Three main prerequisites to this process are: the capacity of organismal homeostasis to accommodate and direct a novel input enabling survival in a novel environment; the ability to produce discrete and similar (i.e. directional) changes across many individuals simultaneously; and pre-existing heritable variation in elements of organismal modification (Baldwin 1902). Importantly, these features of homeostasis enable persistence of environmentally induced effects without their immediate fitness consequences per se—the spread of environmental induction is assured by a simple recurrence of inductive conditions and the complexity of existing organismal systems. The ability to produce rapid directional and facultative organismal modifications, shielded from natural selection, along with accommodation of distinct environmental conditions without limiting evolvability through genetic determination, puts the Baldwin effect processes at the forefront of evolutionary diversifications and innovations of modern organisms, especially in adaptations of homeostatic physiological systems.
The research described in this review suggests that the extensive phenotypic divergence among newly established house finch populations shown in figure 2 is an outcome of the Baldwin effect (figure 1)—the cross-generational changes in frequency distribution of environmentally induced phenotypes. More generally, house finches—the fastest dispersing bird species that over the last 70 years have occupied the widest ecological range of any extant bird—illustrate the evolutionary importance of emergent developmental processes even in organisms with pervasive, complex and redundant genetic regulation of homeostatic processes. Three phenomena are the keys to house finch adaptability during ongoing colonization of its current range. First, the capacity to persist and breed under exceptionally diverse novel environments enabled by behavioural and physiological modifications in response to novel conditions (see §3a). Directionality and consistency of such responses across individuals, as well as physiological amplification and propagation of initial environmental inputs, might be accomplished by functional integration of the reproductive system and by shared endocrinological controls of its multiple components (§3b; figure 1). Second, the ability to maintain precise phenotypic states while shielding abundant developmental variance in morphological traits from natural selection and, thus, retaining the capacity to change under novel environments of the expanding range (§2b). I suggest that this is accomplished by selection on integration of sex determination and sex-specific growth in offspring generation with environmentally induced hormonal gradients in the maternal generation (see §3d; figure 1). The developmental outcome of this selection is overproduction of some phenotypes and underproduction of others. Subsequent natural selection on morphology might further fine-tune the association between sex determination and growth, capitalizing on the link between growth and allocation of resources into eggs, eventually leading to consistent production of heritable locally advantageous morphologies.
5. Are parental and the Baldwin effects distinct?
Both maternal (or more generally parental) effects and the Baldwin effect processes emphasize induction, functionality and directional inheritance (cross-generation changes in frequency distribution) of induced phenotypes (Baldwin 1902; Mousseau & Fox 1998; Pigliucci & Murren 2003; Badyaev 2005b, 2008). Are parental and the Baldwin ‘effects’ distinct? Both depend on emergent developmental processes and capitalize on homeostatic stability for accommodation and direction of either environmental input in the parental generation or parental input in the offspring generation. Both are produced by accumulation of prior adaptations or through adaptability of regulatory mechanisms, and both depend on the similarity of natural selection across generations (Mousseau & Dingle 1991; Uller 2008). Finally, both are defined by cross-generational changes in the distribution of phenotypes in relation to their functional importance.
I suggest that parental effects are a particularly clear illustration of the Baldwin effect processes (figure 1). First, the environmentally induced novel input that the parental phenotype delivers to developing offspring is an input that has been modified and prescreened by a functioning parental phenotype (Badyaev 2008). Such induction therefore not only limits detrimental or non-functional effects of environmental induction, but also is more efficient because it capitalizes on existing controls of developmental processes. By including elements of response to selection, such parental effects also modify selection experienced by offspring. Second, the developmental offset between environmental induction in the parental generation and subsequent parental induction in the offspring generation enables parental modification of earlier stages of offspring development—the environment of parental functioning becomes the environment of offspring development for offspring. Third, parental effect processes can enhance the evolutionary importance of environmentally induced changes by combining functional (in parental generation) and developmental (in offspring generation) accommodation of the same environmental input with directional inheritance and similar natural selection of its outcome. Importantly, both parental and the Baldwin effects include elements of genetic determination of existing organismal system that, under persistent natural selection, can genetically accommodate environmentally induced modifications.
6. Conclusions
Empirical investigations of parental and the Baldwin effect processes in the evolution of house finches emphasize three principal conclusions. First, in modern complex organisms, novel adaptations result mostly from reorganization of existing structures. Such pre-existing structures shape both novel or newly expressed variation on which natural selection can act (sometimes giving natural selection an appearance of a creative force). Second, both parental effects and the Baldwin effect emphasize that evolutionary diversifications and maintenance of adaptations are different processes (Lewontin 1974; Reid 1985; Müller & Newman 2005; Badyaev 2008), probably operating in alternation between emergence of novel developmental variation and stabilization of locally appropriate organism–environment associations by natural selection. Third, both processes probably represent a transient stage in an evolutionary cycle connecting phenotypic retention of adaptive changes and their genetic determination and, thus, origin of adaptation and evolutionary change.
Acknowledgments
I thank reviewers, Tobias Uller, Erik Wapstra, Massimo Pigliucci, James Cheverud, Renee Duckworth, Regis Ferriere, Gunter Wagner and participants of the symposium on the evolution of maternal effects for their critical comments and insightful discussions. I am grateful to many field and laboratory assistants who made this large-scale study possible. Funding for the research discussed herein was provided by the National Science Foundation (USA) and the David and Lucille Packard Fellowship.
Footnotes
One contribution of 12 to a Theme Issue ‘Evolution of parental effects: conceptual issues and empirical patterns’.
References
- Alonso-Alvarez C. Manipulation of primary sex-ratio: an updated review. Avian Poult. Biol. Rev. 2006;17:1–20. doi:10.3184/147020606783437930 [Google Scholar]
- Ancel L.W. A quantitative model of the Simpson–Baldwin effect. J. Theor. Biol. 1999;196:197–209. doi: 10.1006/jtbi.1998.0833. doi:10.1006/jtbi.1998.0833 [DOI] [PubMed] [Google Scholar]
- Andersson M., Wallander J., Oring L., Akst E., Reed J.M., Fleischer R.C. Adaptive seasonal trend in brood sex ratio: test in two sister species with contrasting breeding systems. J. Evol. Biol. 2003;16:510–515. doi: 10.1046/j.1420-9101.2003.00533.x. doi:10.1046/j.1420-9101.2003.00533.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V. Maternal inheritance and rapid evolution of sexual size dimorphism: passive effects or active strategies? Am. Nat. 2005a;166:S17–S30. doi: 10.1086/444601. doi:10.1086/444601 [DOI] [PubMed] [Google Scholar]
- Badyaev A.V. Stress-induced variation in evolution: from behavioral plasticity to genetic assimilation. Proc. R. Soc. B. 2005b;272:877–886. doi: 10.1098/rspb.2004.3045. doi:10.1098/rspb.2004.3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V. Evolvability and robustness in color displays: bridging the gap between theory and data. Evol. Biol. 2007;34:61–71. doi:10.1007/s11692-007-9004-5 [Google Scholar]
- Badyaev A.V. Maternal effects as generators of evolutionary change: a reassessment. In: Schlichting C.D., Mousseau T.A., editors. The year in evolutionary biology 2008. Wiley-Blackwell; New York, NY: 2008. pp. 151–161. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Hill G.E. The evolution of sexual dimorphism in the house finch. I. Population divergence in morphological covariance structure. Evolution. 2000;54:1784–1794. doi: 10.1111/j.0014-3820.2000.tb00722.x. doi:10.1111/j0014-3820.2000.tb00722.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Martin T.E. Individual variation in growth trajectories: phenotypic and genetic correlations in ontogeny of the house finch (Carpodacus mexicanus) J. Evol. Biol. 2000a;13:290–301. doi:10.1046/j.1420-9101.2000.00172.x [Google Scholar]
- Badyaev A.V., Martin T.E. Sexual dimorphism in relation to current selection in the house finch. Evolution. 2000b;54:987–997. doi: 10.1111/j.0014-3820.2000.tb00098.x. doi:10.1111/j.0014-3820.2000.tb0098.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Oh K.P. Environmental induction and phenotypic retention of adaptive maternal effects. BMC Evol. Biol. 2008;8:3. doi: 10.1186/1471-2148-8-3. doi:10.1186/1471-2148-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V., Hill G.E., Stoehr A.M., Nolan P.M., McGraw K.J. The evolution of sexual dimorphism in the house finch: II. Population divergence in relation to local selection. Evolution. 2000;54:2134–2144. doi: 10.1111/j.0014-3820.2000.tb01255.x. doi:10.1111/j.0014-3820.2000.tb01255.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Hill G.E., Whittingham L.A. The evolution of sexual size dimorphism in the house finch: IV. Population divergence in ontogeny of dimorphism. Evolution. 2001a;55:2534–2549. doi: 10.1111/j.0014-3820.2001.tb00767.x. doi:10.1111/j.0014-3820.2001.tb00767.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Whittingham L.A., Hill G.E. The evolution of sexual size dimorphism in the house finch: III. Developmental basis. Evolution. 2001b;55:176–189. doi: 10.1111/j.0014-3820.2001.tb01282.x. doi:10.1111/j.0014-3820.2001.tb01282.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Hill G.E., Beck M.L., Dervan A.A., Duckworth R.A., McGraw K.J., Nolan P.M., Whittingham L.A. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science. 2002a;295:316–318. doi: 10.1126/science.1066651. doi:10.1126/science.1066651 [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Hill G.E., Whittingham L.A. Population consequences of maternal effects: sex-biased hatching order produces divergence in sexual dimorphism between newly established bird populations. J. Evol. Biol. 2002b;15:997–1003. doi:10.1046/j.1420-9101.2002.00462.x [Google Scholar]
- Badyaev A.V., Beck M.L., Hill G.E., Whittingham L.A. The evolution of sexual size dimorphism in the house finch: V. Maternal effects. Evolution. 2003a;57:384–396. doi: 10.1111/j.0014-3820.2003.tb00272.x. doi:10.1111/j.0014-3820.2003.tb00272.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Hill G.E., Beck M.L. Interaction between maternal effects: onset of incubation is related to offspring sex in a passerine bird. Oecologia. 2003b;135:386–390. doi: 10.1007/s00442-003-1203-x. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Schwabl H., Young R.L., Duckworth R.A., Navara K., Parlow A.F. Adaptive sex differences in growth of pre-ovulation oocytes in a passerine bird. Proc. R. Soc. B. 2005;272:2165–2172. doi: 10.1098/rspb.2005.3194. doi:10.1098/rspb.2005.3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V., Acevedo Seaman D., Navara K.J., Hill G.E., Mendonça M.T. Evolution of sex-biased maternal effects in birds: III. Adjustment of ovulation order can enable sex-specific allocation of hormones, carotenoids, and vitamins. J. Evol. Biol. 2006a;19:1044–1057. doi: 10.1111/j.1420-9101.2006.01106.x. doi:10.1111/j.1420-9101.2006.01106.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Hamstra T.L., Oh K.P., Acevedo Seaman D. Sex-biased maternal effects reduce ectoparasite-induced mortality in a passerine bird. Proc. Natl Acad. Sci. USA. 2006b;103:14 406–14 411. doi: 10.1073/pnas.0602452103. doi:10.1073/pnas.0602452103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V., Oh K.P., Mui R. Evolution of sex-biased maternal effects in birds: II. Contrasting sex-specific oocyte competition in native and recently established populations. J. Evol. Biol. 2006c;19:909–921. doi: 10.1111/j.1420-9101.2005.01041.x. doi:10.1111/j.1420-9101.2005.01041.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Young R.L., Hill G.E., Duckworth R.A. Evolution of sex-biased maternal effects in birds: IV. Intra-ovarian growth dynamics can link sex-determination and sex-specific acquisition of resources. J. Evol. Biol. 2008;21:449–460. doi: 10.1111/j.1420-9101.2007.01498.x. doi:10.1111/j.1420-9101.2007.01498.x [DOI] [PubMed] [Google Scholar]
- Baldwin J.M. A new factor in evolution. Am. Nat. 1896;30:441–451. doi:10.1086/276408 [Google Scholar]
- Baldwin J.M. Macmillan; New York, NY: 1902. Development and evolution. [Google Scholar]
- Ball G.F., Balthazart J. Individual variation and the endocrine regulation of behavior and physiology in birds: a cellular/molecular perspective. Phil. Trans. R. Soc. B. 2008;363:1699–1710. doi: 10.1098/rstb.2007.0010. doi:10.1098/rstb.2007.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G., Dávila J.A., López Septiem J.A., Rodríguez R., Martínez F. Sex-biased initial eggs favour sons in the slightly size-dimorphic scops owl (Otus scops) Biol. J. Linn. Soc. 2002;76:1–7. doi:10.1111/j.1095-8312.2002.tb01709.x [Google Scholar]
- Bortolotti G.R. Influence of sibling competition on nestling sex ratios of sexually dimorphic birds. Am. Nat. 1986;127:495–507. doi:10.1086/284498 [Google Scholar]
- Carere C., Balthazart J. Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrinol. Metab. 2007;18:73–80. doi: 10.1016/j.tem.2007.01.003. doi:10.1016/j.tem.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Carroll S.B. Evolution at two levels: on genes and form. PLoS Biol. 2005;3:e245. doi: 10.1371/journal.pbio.0030245. doi:10.1371/journal.pbio.0030245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.-F. Female cooing promotes ovarian development in ring doves. Physiol. Behav. 1986;37:371–374. doi: 10.1016/0031-9384(86)90248-9. doi:10.1016/0031-9384(86)90248-9 [DOI] [PubMed] [Google Scholar]
- Chetverikov S.S. On certain aspects of the evolutionary process from the standpoint of modern genetics. J. Exp. Biol. Ser. A. 1926;2:1–40. [Google Scholar]
- Clark A.B., Wilson D.S. Avian breeding adaptations: hatching asynchrony, brood reduction, and nest failure. Q. Rev. Biol. 1981;56:253–277. doi:10.1086/412316 [Google Scholar]
- Conway C.J., Martin T.E. Evolution of passerine incubation behavior: influence of food, temperature, and nest predation. Evolution. 2000;54:670–685. doi: 10.1111/j.0014-3820.2000.tb00068.x. doi:10.1111/j.0014-3820.2000.tb00068.x [DOI] [PubMed] [Google Scholar]
- Cope E.D. Appleton; New York, NY: 1887. The origin of the fittest: essays on evolution. [Google Scholar]
- Cordero P.J., Vinuela J., Aparicio J.M., Veiga J.P. Seasonal variation in sex ratio and sexual egg dimorphism favouring daughters in first clutches of the spotless starling. J. Evol. Biol. 2001;14:829–834. doi:10.1046/j.1420-9101.2001.00320.x [Google Scholar]
- Davidson E.H. Academic Press; San Diego, CA: 2006. The regulatory genome: gene regulatory networks in development and evolution. [Google Scholar]
- Dijkstra C., Daan S., Buker J.B. Adaptive seasonal variation in the sex ratio of kestrel broods. Funct. Ecol. 1990;4:143–147. doi:10.2307/2389333 [Google Scholar]
- Dobzhansky T. Columbia University Press; New York: 1937. Genetics and the origin of species. [Google Scholar]
- Duckworth, R. A. In press. The role of behavior in evolution: a search for mechanism. Evol. Ecol.22 (doi:10.1007/s10682-008-9252-6)
- Duckworth R.A. Maternal effects and range expansion: a key factor in a dynamic process? Phil. Trans. R. Soc. B. 2009;364:1075–1086. doi: 10.1098/rstb.2008.0294. doi:10.1098/rstb.2008.0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth R.A., Badyaev A.V., Farmer K.L., Hill G.E., Roberts S.R. First case of mycoplasmosis in the native range of the house finch (Carpodacus mexicanus) Auk. 2003;120:528–530. doi:10.1642/0004-8038(2003)120[0528:FCOMGI]2.0.CO;2 [Google Scholar]
- Eising C.M., Muller W., Dijkstra C., Groothuis G.G. Maternal androgens in egg yolks: relation with sex, incubation time and embryonic growth. Gen. Comp. Endocrinol. 2003;132:241–247. doi: 10.1016/s0016-6480(03)00090-x. doi:10.1016/S0016-6480(03)00090-X [DOI] [PubMed] [Google Scholar]
- Elliott J.J., Arbib R.S. Origin and status of the house finch in the eastern United States. Auk. 1953;70:31–37. [Google Scholar]
- Etches R.J., Gargbutt A., Middleton A.L. Plasma concentrations of prolactin during egg laying and incubation in the ruffed grouse (Bonasa umbellus) Can. J. Zool. 1979;57:1624–1627. doi:10.1139/z79-213 [Google Scholar]
- Gerhart J., Kirschner M. The theory of facilitated variation. Proc. Natl Acad. Sci. USA. 2007;104:8582–8589. doi: 10.1073/pnas.0701035104. doi:10.1073/pnas.0701035104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S.J. Belknap Press, Harvard University Press; Cambridge, MA: 2002. The structure of evolutionary theory. [Google Scholar]
- Grinnell J. The linnet of the Hawaiian Islands: a problem in speciation. Univ. Calif. Publ. Zool. 1911;7:79–95. [Google Scholar]
- Hébert P.N. Ecological factors affecting initiation of incubation behaviour. In: Deeming D.C., editor. Avian incubation: behaviour, environment, and evolution. Oxford University Press; New York, NY: 2002. pp. 271–279. [Google Scholar]
- Hill G.E. Oxford University Press; New York, NY: 2002. A red bird in a brown bag: the function and evolution of colorful plumage in the house finch. [Google Scholar]
- Jablonka E. The systems of inheritance. In: Oyama S., Griffiths P.E., Gray R.D., editors. Cycles of contingency: developmental systems and evolution. MIT Press; Cambridge, MA: 2001. pp. 99–116. [Google Scholar]
- Jablonka E., Lamb M.J. Oxford University Press; Oxford, UK: 1995. Epigenetic inheritance and evolution: the Lamarckian dimension. [Google Scholar]
- Jablonka E., Oborny B., Molnar I., Kisdi E., Hofbauer J., Czaran T. The adaptive advantage of phenotypic memory in changing environments. Phil. Trans. R. Soc. B. 1995;350:133–141. doi: 10.1098/rstb.1995.0147. doi:10.1098/rstb.1995.0147 [DOI] [PubMed] [Google Scholar]
- Johnson A.L. Reproduction in the female. In: Whittow G.C., editor. Sturkie's avian physiology. Academic Press; San Diego, CA: 2000. pp. 569–596. [Google Scholar]
- King M.C., Wilson A.C. Evolution at two levels in humans and chimnanzees. Science. 1975;188:107–116. doi: 10.1126/science.1090005. doi:10.1126/science.1090005 [DOI] [PubMed] [Google Scholar]
- Kirschner M., Gerhart J.C. Yale University Press; New Haven, CT: 2005. The plausibility of life: resolving Darwin's Dilemma. [Google Scholar]
- Kopp M., Hermisson J. The evolution of genetic architecture under frequency-dependent disruptive selection. Evolution. 2006;60:1537–1550. doi:10.1554/06-220.1 [PubMed] [Google Scholar]
- Lande R. Natural selection and random genetic drift in phenotypic evolution. Evolution. 1976;30:314–334. doi: 10.1111/j.1558-5646.1976.tb00911.x. doi:10.2307/2407703 [DOI] [PubMed] [Google Scholar]
- Lewontin R.C. The units of evolution. Annu. Rev. Ecol. 1970;1:1–23. doi:10.1146/annurev.es.01.110170.000245 [Google Scholar]
- Lewontin R.C. Columbia University Press; New York, NY: 1974. The genetic basis of evolutionary change. [Google Scholar]
- Losos J.B., Queiroz K.D. Evolutionary consequences of ecological release in Caribbean Anolis lizards. Biol. J. Linn. Soc. 1997;61:459–483. doi:10.1111/j.1095-8312.1997.tb01802.x [Google Scholar]
- Martin T.E., Auer S.K., Bassar R.D., Niklison A.M., Lloyd P. Geographic variation in avian incubation periods and parental influences on embryonic temperature. Evolution. 2007;61:2558–2569. doi: 10.1111/j.1558-5646.2007.00204.x. doi:10.1111/j.1558-5646.2007.00204.x [DOI] [PubMed] [Google Scholar]
- Mayr, E. 1960 The emergence of evolutionary novelties. In Evolution after Darwin, vol. 1 (ed. S. Tax), pp. 349–380. Chicago, IL: The University of Chicago
- Mayr E. Belknap Press, Harvard University Press; Cambridge, MA: 1982. The growth of biological thought. [Google Scholar]
- Mead P.S., Morton M.L. Hatching asynchrony in the mountain white-crowned sparrow (Zonotrichia leucophyris oriantha): a selected or incidental trait? Auk. 1985;102:781–792. [Google Scholar]
- Morgan C.L. Arnold; London, UK: 1896. Habit and instinct. [Google Scholar]
- Mousseau T.A., Dingle H. Maternal effects in insects: examples, constraints, and geographical variation. In: Dudley E.C., editor. The unity of evolutionary biology. Dioscorides Press; Portland, OR: 1991. pp. 745–761. [Google Scholar]
- Mousseau T.A., Fox C.W., editors. Maternal effects as adaptations. Oxford University Press; Oxford, UK: 1998. [Google Scholar]
- Müller G.B., Newman S. MIT Press; Cambridge, MA: 2003. Origination of organismal form: beyond the gene in developmental and evolutionary biology. [Google Scholar]
- Müller G.B., Newman S.A. The innovation triad: an EvoDevo agenda. J. Exp. Zool. B (Mol. Dev. Evol.) 2005;304:487–503. doi: 10.1002/jez.b.21081. doi:10.1002/jez.b.21081 [DOI] [PubMed] [Google Scholar]
- Müller G.B., Wagner G.P. Novelty in evolution: restructuring the concept. Annu. Rev. Ecol. Syst. 1991;22:229–256. doi:10.1146/annurev.es.22.110191.001305 [Google Scholar]
- Mundinger P.C., Hope S. Expansion of the winter range of the house finch: 1947–1979. Am. Birds. 1982;36:347–353. [Google Scholar]
- Newman S.A., Müller G.B. Epigenetic mechanisms of character origination. J. Exp. Zool. 2000;288:304–314. doi: 10.1002/1097-010X(20001215)288:4<304::AID-JEZ3>3.0.CO;2-G. doi:10.1002/1097-010X(20001215)288:4<304::AID-JEZ3>3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- Osborn H.F. A mode of evolution requiring neither natural selection nor the inheritance of acquired characteristics. Trans. NY Acad. Sci. 1896;15:141–142. [Google Scholar]
- Oyama S. Science and cultural theory. Duke University Press; Durham, NC: 2000. The ontogeny of information: developmental systems and evolution. [Google Scholar]
- Pfaff D.W., Phillips M.I., Rubun R.T. Elsevier; Amsterdam, The Netherlands: 2004. Principles of hormone/behavior relations. [Google Scholar]
- Pigliucci M., Murren C.J. Perspective: genetic assimilation and a possible evolutionary paradox: can macroevolution sometimes be so fast as to pass us by? Evolution. 2003;57:1455–1464. doi: 10.1111/j.0014-3820.2003.tb00354.x. doi:10.1111/j.0014-3820.2003.tb00354.x [DOI] [PubMed] [Google Scholar]
- Pike T.W., Petrie M. Experimental evidence that corticosterone affects offspring sex ratios in quail. Proc. R. Soc. B. 2006;273:1093–1098. doi: 10.1098/rspb.2005.3422. doi:10.1098/rspb.2005.3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R.G.B. Crom Helm; Beckenham, UK: 1985. Evolutionary theory: the unfinished synthesis. [Google Scholar]
- Reid R.G.B. Vienna Series in Theoretical Biology. MIT Press; Cambridge, MA: 2007. Biological emergences: evolution by natural experiment. [Google Scholar]
- Ricklefs R.E. Sibling competition, hatching asynchrony, incubation period, and lifespan in altricial birds. Curr. Ornithol. 1993;11:199–276. [Google Scholar]
- Rutkowska J., Badyaev A.V. Meiotic drive and sex determination: molecular mechanisms of sex ratio adjustment in birds. Phil. Trans. R. Soc. B. 2008;363:1675–1686. doi: 10.1098/rstb.2007.0006. doi:10.1098/rstb.2007.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalhausen I.I. Academy of Sciences, USSR; Leningrad, Russia: 1938. Organism as a whole in individual development and history. [Google Scholar]
- Severtsov A.H. Biomedgiz; Leningrad, Russia: 1934. Principal directions of the evolutionary process. [Google Scholar]
- Simpson G.G. The Baldwin effect. Evolution. 1953;7:110–117. doi:10.2307/2405746 [Google Scholar]
- Simpson G.G. A Columbia classic in evolution. Columbia University Press; New York, NY: 1984. Tempo and mode in evolution. [Google Scholar]
- Sockman K.W., Schwabl H. Daily estradiol and progesterone levels relative to laying and onset of incubation in canaries. Gen. Comp. Endocrinol. 1999;114:257–268. doi: 10.1006/gcen.1999.7252. doi:10.1006/gcen.1999.7252 [DOI] [PubMed] [Google Scholar]
- Sockman K.W., Schwabl H., Sharp P.J. Regulation of yolk-androgen concentrations by plasma prolactin in the American Kestrel. Horm. Behav. 2001;40:462–471. doi: 10.1006/hbeh.2001.1715. doi:10.1006/hbeh.2001.1715 [DOI] [PubMed] [Google Scholar]
- Sockman K.W., Gentner T.Q., Ball G.F. Recent experience modulates forebrain gene-expression in response to mate choice cues in European starlings. Proc. R. Soc. B. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. doi:10.1098/rspb.2002.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman K.W., Sharp P.J., Schwabl H. Orchestration of avian reproductive effort: an integration of the ultimate and proximate bases for flexibility of clutch size, incubation behavior, and yolk-androgen deposition. Biol. Rev. 2006;81:629–666. doi: 10.1017/S1464793106007147. doi:10.1017/S1464793106007147 [DOI] [PubMed] [Google Scholar]
- Stern D.L. Evolutionary developmental biology and the problem of variation. Evolution. 2000;54:1079–1091. doi: 10.1111/j.0014-3820.2000.tb00544.x. doi:10.1111/j.0014-3820.2000.tb00544.x [DOI] [PubMed] [Google Scholar]
- Stoleson S.H., Beissinger S.R. Hatching asynchrony and the onset of incubation in birds, revisited: when is the crucial period? Curr. Ornithol. 1995;12:191–270. [Google Scholar]
- Tabibzadeh C., Rozenboim I., Silsby J.L., Pitts G.R., Foster D.N., El Halawani M.E. Modulation of ovarian cytochrome P 450 17a-hydroxylase and cytochrome aromatase messenger ribonucleic acid by prolacin in the domestic turkey. Biol. Reprod. 1995;52:600–608. doi: 10.1095/biolreprod52.3.600. doi:10.1095/biolreprod52.3.600 [DOI] [PubMed] [Google Scholar]
- Uller T. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 2008;23:432–438. doi: 10.1016/j.tree.2008.04.005. doi:10.1016/j.tree.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Van Valen L. Morphological variation and width of the ecological niche. Am. Nat. 1965;94:377–390. doi:10.1086/282379 [Google Scholar]
- Vleck C.M. Hormonal control of incubation behaviour. In: Deeming D.C., editor. Avian incubation: behaviour, environment, and evolution. Oxford University Press; New York, NY: 2002. pp. 54–62. [Google Scholar]
- Waddington C.H. Genetic assimilation of an acquired character. Evolution. 1953;7:119–127. doi:10.2307/2405747 [Google Scholar]
- Webb D.R. Thermal tolerance of avian embryos; a review. Condor. 1987;89:874–898. doi:10.2307/1368537 [Google Scholar]
- Weismann A. Gustav Fischer; Jena, Germany: 1896. Uber Germinal Selektion: Eine Quelle bestimmt gerichterer Variation. [Google Scholar]
- West-Eberhard M.J. Oxford University Press; Oxford, UK: 2003. Developmental plasticity and evolution. [Google Scholar]
- West-Eberhard M.J. Phenotypic accommodation: adaptive innovation due to developmental plasticity. J. Exp. Zool. B (Mol. Dev. Evol.) 2005;304:610–618. doi: 10.1002/jez.b.21071. doi:10.1002/jez.b.21071 [DOI] [PubMed] [Google Scholar]
- Whyte L.L. George Braziller; New York, NY: 1965. Internal factors in evolution. [Google Scholar]
- Williams T.D., Ames C.E., Kiparissis Y., Wynne-Edwards K.E. Laying-sequence-specific variation in yolk oestrogen levels, and relationship to plasma oestrogen in female zebra finches (Taeniopygia guttata) Proc. R. Soc. B. 2005;272:173–177. doi: 10.1098/rspb.2004.2935. doi:10.1098/rspb.2004.2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo B.H., Sheldon B.L., Podger R.N. Analyses of oviposition times and intervals in a wide range of layer flocks under normal and continuous lighting regimes. Br. Poult. Sci. 1986;27:267–288. doi: 10.1080/00071668608416880. doi:10.1080/00071668608416880 [DOI] [PubMed] [Google Scholar]