Abstract
Species that depend on ephemeral habitat often evolve distinct dispersal strategies in which the propensity to disperse is closely integrated with a suite of morphological, behavioural and physiological traits that influence colonizing ability. These strategies are maintained by natural selection resulting from spatial and temporal variation in resource abundance and are particularly evident during range expansion. Yet the mechanisms that maintain close alignment of such strategies with resource availability, integrate suites of dispersal traits and generate variability in dispersal propensity are rarely known. Breeding females can influence offspring phenotype in response to changes in current environmental conditions, making maternal effects uniquely suited to bridge fluctuations in resource abundance in the maternal generation and variation in offspring dispersal ability. Western bluebirds' (Sialia mexicana) dependence on nest cavities—an ephemeral resource—has led to the evolution of two distinct dispersal phenotypes: aggressive males that disperse and non-aggressive males that remain philopatric and cooperate with their relatives. Over the last 40 years, western bluebirds rapidly expanded their geographical range, providing us with an opportunity to test, in newly established populations, the importance of maternal effects for generating variability in dispersal propensity. Here, I show that, under variable resource conditions, breeding females group offspring of different competitive ability in different positions in the egg-laying order and, consequently, produce aggressive males that are more likely to disperse when resources are low and non-aggressive philopatric males when resources are abundant. I then show experimentally that the association between resource availability and sex-specific birth order is robust across populations. Thus, this maternal effect enables close tracking of resource availability and may explain how variation in dispersal is generated in newly colonized populations. More generally, these results suggest that, as a key source of variation in colonizing phenotypes, maternal effects are of crucial importance for understanding the dynamics of range expansion.
Keywords: maternal effect, resource competition, adaptive plasticity, environmental heterogeneity, dispersal, aggression
1. Introduction
A major challenge for species undergoing range expansion is the colonization of a novel environment (Lewontin 1964; West-Eberhard 2003; Sol et al. 2005). Recently, maternal effects—the influence of parental phenotype on offspring phenotype—have been suggested to play a key role in this process (Cote et al. 2007; Badyaev 2008; Uller 2008). Maternal effects can enable breeding females to influence offspring phenotype in response to changes in current environmental conditions and may be particularly important in species that experience strong temporal or spatial environmental variation (e.g. Sutherland 1969; Donohue 1999; Galloway & Etterson 2007). In the case of range expansion, individuals colonizing areas at the range edge often encounter environmental conditions that differ markedly from those of their natal population. For example, newly colonized populations at the edge of the range will have a low density of conspecifics compared with the well-established populations interior to the range edge and may also vary in the community of competitors as well as abiotic conditions (Hoffman & Blows 1994; Case & Taper 2000; Huey et al. 2000; Holt 2003). Maternal effects have been shown to enhance survival in novel environmental conditions by either increasing phenotypic variance among offspring and thereby enabling at least some offspring to survive in the novel environment or through maternal response to a stressful environment that enables higher offspring survival (e.g. Räsänen et al. 2003; Badyaev 2005b). Finally, maternal effects may also facilitate range expansion when maternal adaptations to the historical environment pre-adapt species to colonizing new areas. Such pre-adaptations can occur when features of the novel environment are similar to the native environment and initiate an adaptive maternal response that enhances offspring survival and ultimately influences population growth.
A key trait that is crucial to the dynamics of range expansion is offspring dispersal ability, and an increasing number of studies across a wide range of taxa indicate that dispersal propensity depends on the conditions experienced by the mother (Sutherland 1969; Diss et al. 1996; Zera & Denno 1997; Donohue 1999; Massot & Clobert 2000). In many species, breeding females respond to changes in resource availability and population density by either affecting offspring development such that in times of low resource availability offspring develop phenotypes with higher dispersal and colonizing propensities (Sutherland 1969; Mousseau & Dingle 1991; Donohue 1999; Marshall 2008) or by overproducing the dispersing sex (West et al. 2002a,b; Dickinson 2004; Dubois et al. 2006; Silk & Brown 2008).
Distinct dispersal strategies within a species often evolve in response to dependence on ephemeral habitat, such that some individuals specialize at dispersing and colonizing new habitat, whereas other individuals remain in the natal population, do not develop dispersal-related traits and thus are able to avoid the costs of maintaining these traits (Harrison 1980; Roff 1984, 1994; Johnson & Gaines 1990; Hughes et al. 2003; Duckworth 2008). Yet the fitness costs and benefits of each strategy strongly depend on resource availability and local competition in the natal population—the colonizing phenotype is favoured when resource availability is diminishing and the philopatric phenotype is favoured when resources are abundant. Thus, maternal manipulation of offspring dispersal propensity will not only influence competition among offspring, but through the effects on local recruitment will also impact population dynamics and range expansion.
One common way that mothers mitigate within-family offspring competition over resources is by modifying the order in which they produce male and female offspring (Badyaev et al. 2008). In birds, breeding females lay one egg a day and changes in female behaviour and physiology during oogenesis can lead to a gradient in hormones, carotenoids and morphogens among sequentially laid eggs in a clutch (Schwabl 1993; Lipar et al. 1999; Reed & Vleck 2001; Badyaev et al. 2006, 2008), which in turn has the potential to affect oocyte sex determination (Rutkowska & Badyaev 2008) and exaggerate sex-specific dispersal strategies or morphologies. When such sex-biased ovulation order has consequences for resource allocation to offspring, as has frequently been shown (Dzus et al. 1996; Ryan & Vandenbergh 2002; Young & Badyaev 2004; Uller 2006), then these positional effects can link the environment experienced by the mother to offspring competitive abilities that may ultimately generate variance in dispersal strategies among offspring (Massot & Clobert 2000).
Here, I investigate the importance of maternal effects for determining variation in offspring dispersal ability and its potential to influence species range expansion. Western bluebirds (Sialia mexicana) provide a particularly suitable study system to address these problems because, not only is this species currently expanding its range in the northwestern United States, but it also experiences spatial and temporal variation in resource availability, has distinct phenotypic requirements for successful dispersal and it is possible to experimentally manipulate resource availability in the wild. I first review the history of western bluebirds' range expansion and what is currently known about dispersal evolution in this species. Then, I present new data showing that females can respond to the changes in resource abundance that occur during range expansion by adjusting the order in which males and females are produced, which in turn influences male colonization ability. I then discuss the implications of these findings for western bluebirds' range expansion and review examples of maternal effects influencing species colonization of novel environments to discuss the generality of these findings.
2. Role of dispersal strategies in species range expansion
(a) History of range expansion
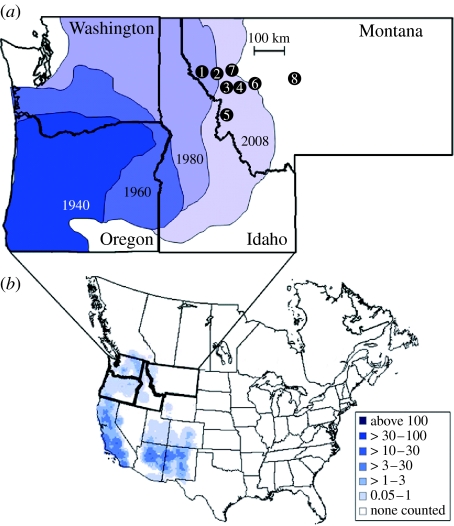
Western bluebirds' breeding range extends across a broad region of Western North America from southern British Columbia in the north to Central Veracruz, Mexico, in the south. The northern part of the range extends eastwards to Western and South-central Montana, whereas in the south it extends to extreme Western Texas (figure 1). Western bluebirds depend on tree cavities to breed (Guinan et al. 2000)—a resource that is often limited in the environment. In the early part of the twentieth century, implementation of fire suppression policy caused severe nest cavity limitation (Hejl 1994) and led to rapid declines in western bluebird populations. This nest cavity limitation was exacerbated when European starlings (Sturnus vulgaris) and house sparrows (Passer domesticus) reached the northwestern portion of the United States around the 1940s. The period between 1910 and 1950 corresponded to a decline of populations throughout the range and culminated with the extinction of this species from the northwestern part of its range. Starting in the 1960s, nest-box programmes, in conjunction with a reversal in the fire suppression policies, enabled western bluebirds to rapidly recolonize much of its former range (figure 1; Zeleny 1978; Duckworth & Badyaev 2007).
Figure 1.
Western bluebird's range expansion. (a) Changes in western bluebirds' breeding range in the northwestern United States. Black circles indicate eight study populations where aggressive behaviour of western and mountain bluebirds was measured. Population numbers indicate the order of population colonization by western bluebirds. (b) Breeding distribution of western bluebirds based on Breeding Bird Survey (BBS) data from 1994 to 2003. Contours are based on the mean number of birds counted on BBS survey routes (see Sauer et al. 2008 for details).
(b) Mechanisms underlying range expansion
New habitat at the edge of western bluebirds' range expansion differed from interior populations in the presence of an important interspecific competitor, mountain bluebirds (Sialia currucoides). These two congeners have similar habitat requirements and are typically segregated along an elevational gradient, with the two species overlapping at low to medium elevations and mountain bluebirds also breeding at high elevation (Pinkowski 1979; Power & Lombardo 1996; Guinan et al. 2000). When breeding sympatrically, they form interspecific territories; however, they are rarely observed to maintain stable sympatric populations over long periods of time (Duckworth & Badyaev 2007; Herlugson 1980). By the time that western bluebirds had expanded their range into Western Montana, the site of this study, mountain bluebirds had already colonized most of the available habitat; however, as western bluebirds colonized populations at the edge of the range, they rapidly displaced populations of mountain bluebirds (Duckworth & Badyaev 2007), providing strong evidence that the parapatric breeding distribution of these species is due to competitive displacement.
Competition for nest cavities among secondary cavity nesting species is intense and individuals have even been observed fighting until death (Gowaty 1984; Brawn & Balda 1988; Newton 1994; Merilä & Wiggins 1995). Not surprisingly, aggression is an important determinant of competitive ability, and in western bluebirds more aggressive males obtain larger territories with more nest cavities compared with non-aggressive males (Duckworth 2006a, 2008). Moreover, western bluebirds are more aggressive than mountain bluebirds and this enables them to rapidly displace mountain bluebirds at the edge of the range (Duckworth & Badyaev 2007). Yet, despite these benefits in competitive contexts, there is a trade-off between aggression and investment in parental care that results in a strong fitness cost of aggression (Duckworth 2006b, 2008).
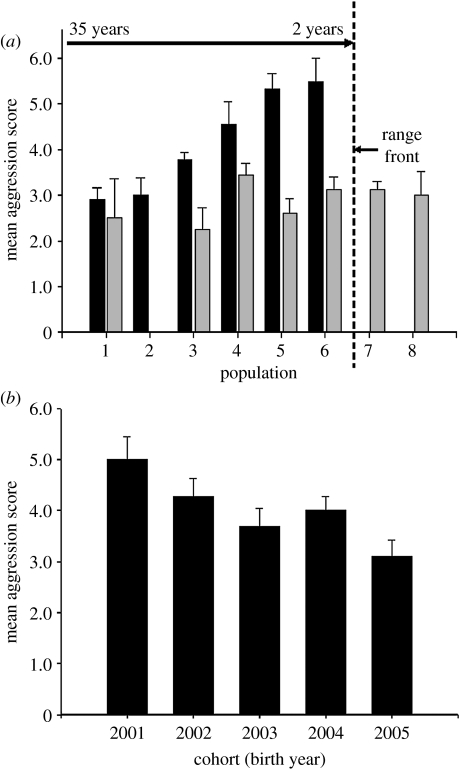
In concordance with these fitness costs and benefits, aggression of western bluebirds shifted rapidly across the range expansion. Newly colonized populations at the range front (where the two species overlapped) were highly aggressive, and older well-established populations (where mountain bluebirds had been displaced) were less aggressive (figure 2a). These shifts were due to the non-random settlement of new populations by highly aggressive individuals followed by rapid decreases within these populations to lower levels of aggressive behaviour (figure 2b). Changes in aggression within populations were not due to flexibility in the expression of aggression—aggression does not systematically decrease with age and is not influenced by the current competitive environment (Duckworth 2006a; Duckworth & Badyaev 2007). A key to understanding these patterns was the discovery that aggression and dispersal are both phenotypically and genetically correlated in this species such that aggressive males tend to be more dispersive than non-aggressive males (Duckworth & Badyaev 2007; Duckworth & Kruuk in press) and thus are more likely to colonize populations at the range edge (Duckworth 2008).
Figure 2.
Rapid cross-generational shifts in aggressive behaviour associated with western bluebirds' range expansion. (a) Aggression differed significantly among western bluebird (black bars) populations in Western Montana and was related to the time since colonization. Mountain bluebirds (grey bars) were generally less aggressive than western bluebirds. See figure 1 for population locations. (b) Aggression significantly decreased across cohorts. Data are from population 3. Shown are means and standard errors. Adapted from Duckworth & Badyaev (2007).
(c) Origin and evolution of distinct dispersal strategies
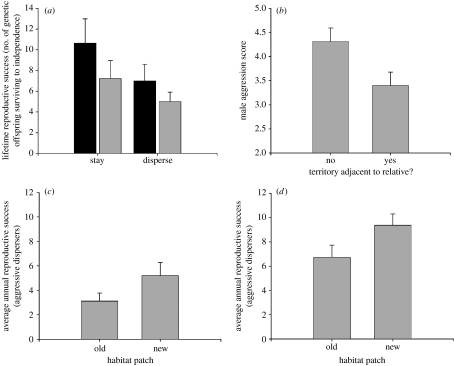
Integration of aggression and dispersal is adaptive—aggressive males have higher fitness when colonizing new populations, whereas non-aggressive males have the highest fitness when remaining in their natal population (figure 3; Duckworth 2008). The philopatric strategy is linked to facultative cooperative behaviour in this species—male offspring occasionally remain as a helper at their parents' nest and more frequently cooperate with their relatives in territorial defence (Dickinson & Akre 1998; Kraaijeveld & Dickinson 2001). Among males that remain in their natal population, non-aggressive males are more likely to acquire a territory next to their parents' territory, whereas aggressive males rarely settle near family members (figure 3b). These observations strongly suggest that non-aggressive males, which are poor competitors, acquire territories through cooperation with relatives whereas aggressive males must compete for territories on their own. When non-aggressive males are able to acquire a territory, they have higher lifetime fitness when compared with the aggressive males (figure 3a). Therefore, it is beneficial to the parents to produce non-aggressive males, but only if there are a sufficiently high number of nest cavities near their own nest cavity that would enable sons to benefit from familial interactions in territory acquisition. On the other hand, if resources are limited locally, then breeding females would benefit from producing aggressive males that can successfully compete for a territory on their own. In fact, aggressive males have the highest fitness when colonizing new areas where the density of conspecifics is lower than that in older populations enabling them to gain larger territories that may at least partially offset the trade-off between aggression and parental care (Duckworth 2008).
Figure 3.
Fitness consequences of integration between aggression and dispersal in western bluebirds. (a) Non-aggressive males that stay in their natal population to breed have higher fitness than both aggressive males and males that dispersed (black bars, non-aggressive; grey bars, aggressive). (b) Compared with aggressive males, non-aggressive males are more likely to acquire a territory by budding off their relatives' territory. In paired comparisons among old and newly created habitat patches at (c) population 1 and (d) population 3, aggressive males that colonized new habitat had higher fitness than aggressive males that colonized older habitat patches. See figure 1 for population locations. Shown are means and standard errors. Adapted from Duckworth (2008).
Historically, the distribution of nest cavities was patchy but occurred at high densities following forest fires. Forest fire creates suitable habitat for bluebirds by opening up understorey vegetation and creating patches of snags with a high abundance of nest cavities (Hutto 1995; Bagne et al. 2008; Hurteau et al. 2008). In these patches, the local availability of nest cavities is dynamic across years because new nest cavities are continually created by primary cavity nesters, old snags eventually decay and are no longer usable and there is a diverse community of both mammalian and avian cavity nesting species competing for prime cavities (Bagne et al. 2008). Mountain bluebirds are frequently among the earliest colonizers following forest fires (Hutto 1995), whereas western bluebirds, which as a species are less dispersive than mountain bluebirds, often show delayed patterns of colonization (Kotliar et al. 2007; Saab et al. 2007). Thus, the colonization of patches of post-fire habitat is similar to the colonization of populations at the edge of the range because in both contexts, colonization success depends on western bluebirds' ability to displace competitor species (Duckworth & Badyaev 2007; Kotliar et al. 2007; Saab et al. 2007). Indeed, the aggression of western bluebirds colonizing newly formed habitat patches in interior populations is similar to that of populations at the range front (figure 3c,d; Duckworth 2008). Thus, rapid shifts in aggression observed across the range expansion are not unique to the range front and occur whenever new areas are colonized, no matter where they are located.
Taken together, these observations suggest that genetic integration of aggression and dispersal reflects an adaptive colonization strategy that evolved in response to the historical patchy and ephemeral distribution of nest cavities, and persistence of western bluebirds depends on their ability to continually assess resource availability and colonize new areas. As the density of bluebirds in newly colonized areas increases over time, resource availability decreases, leading to another cycle of dispersal behaviour and associated changes among populations in aggressive behaviour (Duckworth 2008). Yet there remains one unresolved question regarding these colonization dynamics: how is variation in aggression generated once new populations are colonized? Although there is an additive genetic variance for both aggression and dispersal and there is negative selection on both behaviours once populations are established (Duckworth 2008; Duckworth & Kruuk in press), previous work has shown that negative selection on these traits is not sufficient to produce the rapid changes in aggression observed within populations (Duckworth & Badyaev 2007) and that there is also little variation in aggression in newly colonized populations on which selection can act (Duckworth 2008). Moreover, there is significant unexplained residual variance in aggressive behaviour; this residual variance is not explained by behavioural flexibility because an individual's aggressive phenotype does not change substantially in adulthood and is largely determined early in life (Duckworth & Badyaev 2007; Duckworth & Kruuk in press). Here, I investigate the possibility that early ontogeny maternal effects are an important influence on the expression of aggression, and ultimately the key to understanding the rapid and adaptive shifts in aggression that are associated with the dynamic process of population colonization.
Based on knowledge of the functional significance of integration between aggression and dispersal, I predicted that because non-aggressive males gain territories by budding off their parents' territory, breeding females should produce non-aggressive sons when local resource availability is high. Moreover, because females are the more dispersive sex in this species, I also tested the prediction that mothers should produce more daughters when resources are low. Here, I first show that territory acquisition and the expression of aggression in adulthood are influenced by the order in which offspring are produced. I then combine the observations from a population where resource availability varies naturally and an experimental population where resource availability is manipulated to determine whether females adjust either offspring sex ratio or the order in which they produce male offspring within a clutch.
3. Material and methods
(a) Naturally varying population
To investigate the influence of natural variation in resource availability on brood sex ratios and offspring phenotype, I used data from a population of western bluebirds in Western Montana in which mountain bluebirds have been excluded for approximately 8 years (Duckworth 2006a). The habitat at this site is a mixed forest of conifers with open meadows interspersed throughout the site. In 2001, the study area was systematically searched for nest cavities suitable for bluebird nest sites. Only areas with less than 50 per cent tree cover were searched as bluebirds will not breed in areas with greater amounts of tree cover (Guinan et al. 2000). Most nest cavities were located in mature trees or dead snags which are conspicuous and easy to locate. Nest-boxes were placed over (whenever possible) or next to these natural cavities to mimic natural variation in the density of nest cavities across the population while standardizing for variation in nest cavity quality.
Western bluebirds are short-distance migrants (Guinan et al. 2000) and in Montana they arrive to the breeding grounds in mid-March. They begin territorial interactions almost immediately but do not start nest building until early April and first nests are initiated anytime from mid-April until the end of May. Data on territorial behaviour were collected during the 2002–2005 breeding seasons and each year resident adults were captured and marked with a unique colour band combination for individual recognition. From March to July, I visited territories twice weekly to monitor nests and record observations of male territorial behaviour. Most territorial interactions and foraging bouts of bluebirds occur within a 150 m radius of the nest-box (Pinkowski 1979; Duckworth 2006a) and therefore, to measure the differences in local resource abundance among territories, for each pair, I recorded the number of nest-boxes within this area. I also recorded, for each pair, the distance from their own nest-box to the next nearest nest-box (occupied or unoccupied), as well as the distance from their own box to the nearest box occupied by another bluebird. These measurements were used to determine whether females adjusted either the sex ratio of their clutches or the order in which they laid males and females in response to the variation in local resource density and availability.
To investigate whether hatching order influenced the expression of aggression in adulthood, during 2002–2007, I simulated territorial intrusions to measure aggression of offspring that had either returned to breed at the study site or had dispersed and were resighted off the study site. Dispersing offspring were located through extensive searches by the author as well as by monitoring of nest-box trails by members of Mountain Bluebird Trails throughout Western Montana (Duckworth & Kruuk in press). To simulate territorial intrusions, birds were presented with a common interspecific competitor, a live tree swallow (Tachycinetas bicolor). A heterospecific competitor was used to avoid infanticide that can occur when a conspecific male is presented at a nest site (R. A. Duckworth 2004, personal observation) and also to standardize measurements of aggressive response between males and females (Duckworth 2006b). The tree swallow was placed in a wire cage on the nest-box and the number of times an individual attacked, flew by, or hovered near the model during a 2 min period was recorded. These behaviours were summarized into an aggression score that varied from 1–6 with 1 indicating the least aggressive response and a 6 indicating the most aggressive response. Specifically, scores were assigned as follows: 1, no aggressive behaviour; 2, hovering or flying by one to five times and zero attacks; 3, hovering or flying by more than five times and zero attacks; 4, one to five attacks; 5, six to nine attacks; and 6, 10 or more attacks. Measurements of aggression using this method are repeatable within individuals (Duckworth 2006b).
To investigate whether offspring hatching order influenced a male's potential for cooperative behaviour, I compared hatching order of males that did and did not acquire a territory next to a first-order relative. Territories were considered adjacent to a relative if the focal male's nest-box was within 300 m from either their father's or brother's (nest-mates only) nest-box. Brothers were included because they are known to cooperate and for some individuals only a brother's territory separated them from their parents' territory. For focal individuals, only the first year of breeding was included in the analysis because territorial dominance is usually established during the first breeding attempt.
The majority of females in this population begin full incubation on the last day of egg laying; however, because females begin partial incubation before this time (R. A. Duckworth 2002–2007, unpublished data), the eggs hatch over a 24–48 hour period causing a size hierarchy between the nestlings. Because I acquired data on nestling size ranks for all nests, but laying and hatching order for only a subset of nests, I used nestling size rank as a proxy for laying and hatching order. To determine whether size rank is a good proxy, I monitored egg laying and/or hatching order for a subset of nests (n=17). Eggs were numbered sequentially on the day they were laid and nestlings were marked within 2–3 hours of hatching. Individual markings were renewed every second day until nestlings could be banded at 8–10 days of age. Five morphological traits (bill length, tarsus length, wing length, tail length and body mass) were measured for each nestling on days 5, 10 and 15. I used wing length as a primary criterion to determine nestling size ranks and used body mass as a secondary criterion in the cases where wing length was identical between two nestlings. Laying order, hatching order and nestling size ranks were highly correlated with one another (all r>0.70, p<0.001). Bluebirds grow sexually dimorphic plumage coloration as nestlings (Guinan et al. 2000) and therefore I determined sex of all nestlings in the population at approximately 15 days of age. For eggs or nestlings that did not survive to 15 days of age and for which we were able to obtain tissue samples, I determined the sex of nestlings molecularly. DNA was extracted using standard phenol–chloroform methods. We used PCR primers P8 and P2 (Griffiths et al. 1998) which anneal to conserved exonic regions and amplify across an intron in both CHD1-W and CHD1-Z genes. PCR on tissue-extracted DNA was carried out according to the protocol in Badyaev (2005b).
(b) Resource manipulation experiment
The experimental population consists of uniformly open ranchland habitat near St. Regis, MT, which is approximately 100 km from the naturally varying population. The uniform habitat of this population enabled isolation of the effects of nest-box density while controlling for differences among territories in habitat structure and quality. To manipulate local resource availability, in 2004, I established 44 territories by placing single nest-boxes approximately 150 m apart along a linear transect. These territories were established in early March before bluebirds arrived to the breeding grounds. Nest-boxes were monitored weekly and as soon as a pair had settled on a territory, as indicated by the initiation of nest building, I designated territories as either control (low resource abundance) or experimental (high resource abundance). On experimental territories (n=18), I placed a second box 10–15 m from the first box and on control territories (n=13), I visited them but did not add a second nest-box. Control and experimental territories were chosen so that they were spatially intermixed across the study area and so that the two groups did not differ in initiation date (t=0.85, p=0.40, n=30). By placing additional nest-boxes after bluebirds had already settled territories, it is possible to decouple aggression and territory quality and isolate the direct effect of changes in resource abundance from the indirect influence of territory acquisition ability on maternal effects. Thus, there was no difference in aggression among adults on the control and experimental territories in this experiment (Duckworth 2006a). Nest-boxes were visited once weekly to monitor the progress of nests and to band and measure nestlings. Nestlings were sexed using plumage differences at 15 days of age.
(c) Statistical analysis
To determine whether females adjusted offspring sex in relation to resource availability and offspring hatching order, I categorized offspring in the first three positions in the clutch as ‘early’ and offspring in positions four or greater as ‘late’ and then calculated a measure of sex-biased hatching order for each clutch as the number of males in early positions minus the number of males in late positions. Thus, a positive number meant that females produced more males early in the clutch and a negative number meant that females produced more males later in the clutch. This measure was used to avoid pseudo-replication of analysing each hatching position separately and three positions were included in each group because median clutch size was six. Only the first broods for which we had information on sex of offspring were included in the analysis. In the naturally varying population, some females bred in multiple years. Thus, I used a mixed model that initially included year and identity of the mother as a random factor, but retained only identity of the mother in the final model as year was not significant. Because we were not able to obtain DNA from all offspring that died before 15 days of age, I analysed the data for both the subset of clutches with complete data on hatching order and sex ratio as well as the entire dataset. Excluding incomplete broods did not alter the results, and therefore here I present results from the analyses of the entire dataset. For analyses of the influence of resource availability on sex-biased hatching order, I used one-tailed tests of significance as I had a priori predictions about the directionality of these relationships. I used two-tailed tests of significance for all other analyses. The number of extra nest-boxes on territories varied from zero to five with a median of two. For the naturally varying population, to facilitate a similar presentation of the results with the experimental population, I coded the number of nest-boxes on a territory as a dichotomous trait, with territories having up to two extra nest-boxes being coded as ‘low resource’ and territories having more than two boxes as ‘high resource’. To ensure that dichotomous coding did not change the results, I also carried out the analysis with the number of boxes coded as a continuous trait. Both analyses produced similar results and therefore I present only the results with nest-boxes as a dichotomous trait here. I calculated the coefficient of variation in aggression for brothers reared in the same nest to determine the influence of sex ratio on variance in aggression among siblings. A normal distribution of residuals was verified before analyses where appropriate.
4. Results
(a) Maternal effects on aggression and territory acquisition
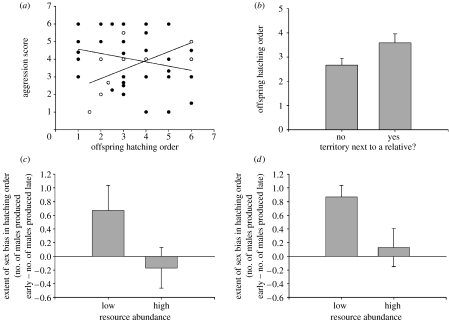
Both offspring sex and hatching order influenced the expression of aggression in adulthood (general linear model (GLM), interaction: F1,51=7.48, p<0.01) such that larger, earlier hatched male offspring were more aggressive than smaller, later hatched male offspring (r=−0.30, p=0.05, n=43; figure 4a) and larger, earlier hatched female offspring were less aggressive than smaller, later hatched female offspring (r=0.67, p<0.05, n=9; figure 4a). Consistent with previous findings, males were more aggressive than females (F1,51=8.43, p<0.01). Among brothers reared in the same nest, brood sex ratio was correlated with variance in their aggressive behaviour such that, in nests with a male-biased sex ratio, there was greater variance in aggression among brothers compared with brothers reared in nests with a female-biased sex ratio (r=0.84, p=0.02, n=7 nests). Hatching order was also related to territorial acquisition such that later hatched males were more likely than earlier hatched males to share a territorial boundary with a relative (t=−1.63, d.f. =53, p=0.05, figure 4b).
Figure 4.
Maternal effects on offspring aggressive behaviour, territory acquisition and in response to resource abundance. (a) The expression of aggression in adulthood is correlated with offspring position in the brood as measured by nestling size ranks. Earlier hatched males (filled circles) are more aggressive than later hatched males, whereas earlier hatched females (open circles) are less aggressive than later hatched females. (b) Offspring hatch order influenced territory settlement such that later hatched males were more likely to acquire a territory next to a relative compared with earlier hatched males. Shown are means and standard errors. In both (c) experimental and (d) naturally varying populations, females produced more males earlier when resources were low and more males later when resources were high. Shown are means and standard errors.
(b) Influence of resource abundance on maternal effects
Local resource abundance influenced sex bias in the hatching order in both the experimental and the naturally varying populations. In the experimental population, females on control territories produced more males earlier, whereas females on territories with an added nest-box produced more males later (t-test: t=−2.20, d.f.=19, pone-tailed=0.04; figure 4c). A similar pattern was found in the naturally varying population with females on territories with few extra nest cavities producing more males earlier and females on territories with many extra nest cavities producing more males later (mixed model: F1,53=2.52, pone-tailed<0.01; figure 4d). However, differences in resource abundance among territories did not affect brood sex ratios (effect of resource abundance in experimental, t=−0.06, d.f.=19, p=0.95, and naturally varying, F1,53=0.30, p=0.59, population).
In the naturally varying population, neither brood sex ratios nor sex bias in the laying order was related to distance to the nearest neighbour (sex-biased hatching order: F1,53=0.00, p=0.98; brood sex ratio: F1,53=0.32, p=0.57) or distance to the next nearest nest cavity (sex bias in offspring position: F1,53=0.12, p=0.73; brood sex ratio: F1,53=0.43, p=0.52).
5. Discussion
Maternal effects that enable breeding females to influence offspring dispersal phenotype in response to the changes in current environmental conditions may be particularly important during species range expansion as well as for species that depend on successional habitat (Sutherland 1969; Mousseau & Dingle 1991; Donohue 1999). During both range expansion and successional changes, the offspring generation is likely to develop in an environment with very different resource availability compared with the environment that their parents experienced. In the case of range expansion, newly colonized populations at the edge of the range will have a low density of conspecifics initially, but over time, density should increase. Similarly, species that depend on ephemeral resources will experience large fluctuations in resource abundance over time. In both of these situations, maternal effects that enable breeding females to influence offspring dispersal ability in response to changes in resource abundance should be highly beneficial. Once evolved, maternal effects on dispersal have a strong potential to influence population colonization, growth and density and ultimately population dynamics and species range expansion (Donohue 1999; Cote et al. 2007).
Breeding females can influence offspring access to resources by either determining the expression of dispersal-related traits (Sutherland 1969; Diss et al. 1996; Donohue 1999) or, in species with sex-biased dispersal, by manipulating offspring sex ratios (Hamilton 1967; West et al. 2002b; Silk & Brown 2008). In this study, I examined the ability of mothers to influence offspring phenotype in response to variation in resource abundance in both an experimental and a naturally varying population, and found near identical patterns of maternal effects across these populations. In both populations, mothers adjusted the order in which they produce males depending on the current availability of resources (figure 4c,d). When resource availability was low mothers produced more sons earlier and when resources availability was high mothers produced a higher proportion of sons later in the brood. Because the order in which mothers produced offspring had important consequences for the expression of aggression and offspring territory acquisition—sons produced later were less aggressive and more likely to acquire a territory near their parents (figure 4a,b)—such sex-biased maternal effects are concordant with the prediction that females should produce aggressive dispersers when local resource availability is low and non-aggressive philopatric males when local resource availability is high.
Sex ratio theory predicts that when relatives compete over a limiting resource, parents should bias the sex ratio towards the more dispersive sex (Hamilton 1967; Silk 1984; West et al. 2002b); yet, in this study, we found no evidence for sex ratio adjustment in response to the variation in resource abundance. Instead, by manipulating offspring position in the brood, mothers influenced offspring aggressive behaviour. As a consequence, when resources were low, mothers produced males that were well suited to disperse and acquire territories on their own, whereas, when resources were abundant, mothers produced males that were most likely to remain philopatric and acquire a territory near their parents. A basic assumption of sex ratio theory is that the costs and benefits of producing a particular sex are uniform across all members of that sex. Yet, as is evident in western bluebirds as well as many other species, individuals within a sex often pursue distinct life-history strategies and vary extensively in morphology and behaviour (Gross 1996; Rhen & Crews 2002). Thus, maternal effects acting on offspring phenotype may alter the costs and benefits of producing a particular sex.
Such maternal adjustment of offspring phenotype based on an assessment of local resource abundance raises several important questions. First, what are the implications for understanding the process of colonization and population establishment in this species? Second, what are the proximate mechanisms linking maternal phenotype, offspring position in the brood and offspring aggressive behaviour? Third, do these maternal effects represent a passive consequence of a maternal response to local environmental conditions or active maternal adjustment of offspring phenotype? Finally, how does this enhance our understanding of the role of maternal effects in species range expansion?
(a) Implications for population colonization
In western bluebirds, the colonization of new populations is associated with rapid population-level decreases in aggression (Duckworth & Badyaev 2007; Duckworth 2008). While these changes are at least partially a response to natural selection, the extent and rapidity of the response cannot be fully explained by a selection on genetic variation alone (Duckworth & Badyaev 2007). However, the results of this study suggest that maternal effects may play a key role in this process by acting as a bridge that enables the parental generation of colonizers to produce offspring that are less aggressive and more likely to remain philopatric and take advantage of the surplus of resources acquired by their parents. At the same time, maternal effects can also enable offspring to escape a habitat patch that is overcrowded or declining in quality by producing aggressive males that are well prepared to leave their natal population and search for and colonize a new habitat patch. These results suggest that maternal effects on male aggressive phenotype, by enabling close tracking of resource availability, play a key role, not only in this species' recent range expansion, but also in this species' persistence.
(b) Proximate mechanisms underlying maternal effect
In birds, maternal clustering of offspring of different sex may be a common mechanism for manipulation of the offspring phenotype and provisioning of sex-specific resources (Krackow 1995; Blanco et al. 2002; Forstmeier et al. 2004; Badyaev & Oh 2008). Changing environmental conditions can influence fluctuations in hormone levels that occur when females are transitioning between oogenesis and egg laying and can lead to differential allocation of maternal resources to oocytes of different ovulation order and may also influence offspring sex (Schwabl 1993; Groothius & Schwabl 2002; Müller et al. 2002; Badyaev et al. 2006, 2008; Rutkowska & Badyaev 2008). This, in conjunction with a multitude of studies across a diverse group of taxa showing an early developmental effect of hormones and laying order on behaviour (Schwabl 1996; Forstmeier et al. 2004; Groothius et al. 2004; Amdan et al. 2006), strongly suggests that maternal adjustment of the order in which male and female offspring are produced may be a common mechanism for influencing the offspring phenotype. Thus, similar to other studies that have shown a link between aggression and maternal and egg yolk hormone profiles (Ros et al. 2002; Strasser & Schwabl 2004; Eising et al. 2006), the influence of offspring position on aggression of western bluebirds may be mediated by a hormonal gradient across the laying order. Alternatively, because the order of laying was tightly correlated with nestling size rank, the link between aggression and offspring position in the brood might reflect dominance interactions between nestlings of different sizes (e.g. Fargallo et al. 2003). Regardless of the proximate mechanism for the link between offspring position and aggression, this study provides evidence that mothers respond to the changes in resource availability by influencing offspring position in the clutch and that this in turn influences their aggression in adulthood.
How exactly female assessment of the changes in nest cavity availability is translated into an adjustment of offspring sex in relation to laying order is less clear. Western bluebirds prefer territories with multiple nest cavities and will actively defend all the cavities on their territory particularly during the early part of the breeding season when females are fertile (Plissner & Gowaty 1995; Duckworth 2006a). It could be that aggressive interactions over extra nest cavities alter female physiology in a way that causes sex-biased positioning of offspring. Several studies have shown that competition among females influences the androgen content of eggs (e.g. Müller et al. 2002; Navara et al. 2006). In tree swallows, Whittingham & Schwabl (2002) showed that females that experienced more aggressive interactions over nest cavities during their fertile period deposited higher levels of testosterone in their eggs. Thus, aggressive interactions during the fertile period can have important consequences for female physiology which in turn may influence offspring behaviour.
(c) Active and passive maternal effects: implications for range expansion
When are maternal effects most likely to influence species range expansion? To answer this question, it is useful to distinguish between ‘active’ and ‘passive’ maternal effects. An active maternal effect is an evolved maternal adaptation to a particular environment. On the other hand, passive maternal effects can occur in two ways. First, they occur when breeding females are exposed to novel environmental conditions to which they have not evolved the appropriate physiological, metabolic and behavioural adaptations necessary to maintain homeostasis, and this influences the offspring phenotype. Second, they occur when allocation trade-offs or physiological constraints prevent buffering of offspring from some aspects of the environmental variation, despite strong negative consequences on offspring fitness (Marshall & Uller 2007). For example, when food resources are scarce, a female may have to sacrifice allocation of resources to offspring in order to meet her own needs. This type of passive maternal effect is most frequently invoked in models linking maternal effects to population cycles where changes in the environmental conditions influence the mother's allocation of resources to offspring, producing a time lag in the reaction of populations to environmental changes (e.g. Rossiter 1994; Ginzburg 1998; Kendall et al. 2005).
Currently, there are few studies in animals which explicitly link maternal effects to the colonization of novel environments and range expansion, but two well-studied systems, the house finch (Carpodacus mexicana) and the seed beetle (Stator limbatus), provide instructive examples. The house finch, a species that is native to the southwestern deserts of the US, has recently expanded its range to encompass the entire continental US and parts of southern Canada. Badyaev (2005a) have shown that maternal effects are crucial to population persistence by enabling this passerine bird to survive under novel climactic conditions at the extremes of its range. In particular, breeding females modify the onset of incubation in each population differently depending on ambient temperatures during breeding, and, in turn, this produces sex biases in the laying order that ultimately act to increase variance in offspring morphology (Badyaev et al. 2003). In the absence of such a mechanism to increase offspring phenotypic variance, selection would drive this species to extinction in newly colonized areas (Badyaev et al. 2002). This provides an example of how colonization of a novel environment can disrupt homeostatic mechanisms resulting in increased offspring phenotypic variance that in turn can enable species persistence. Presumably, over evolutionary time, assuming there is genetic variability in maternal response to the environment, selection should fine-tune female responses leading to the possibility that this passive maternal effect will eventually become an active one.
In the seed beetle, maternally mediated plasticity in response to seeds of a native host allows larvae to successfully colonize a novel, non-native plant introduced to the southwestern US (Fox & Savalli 2000). The mechanism underlying this pattern is not entirely understood, but what is known is that females manipulate both egg content and egg size depending on which of the native host species that they experienced during egg maturation and this significantly impacts whether their offspring will survive on the non-native host plant (Carroll & Fox 2007). Thus, maternal adaptations to a native host have carry-over effects on the ability of offspring to survive on a novel non-native host. Because the precise mechanisms by which maternal experience during egg maturation influences offspring survival on the novel host are not known, it is difficult to say conclusively whether this is an active or passive maternal effect. It may, in fact, be a combination of both. Females adjust their investment in eggs when encountering a native host and this is probably an evolved adaptation to this particular host (i.e. an active maternal effect). By contrast, the fact that these changes in egg investment increase offspring survival on the novel host means that these changes are adaptive; however, it is still a passive maternal effect as it is not an adaptation to the novel host.
In the case of western bluebirds, it seems likely that the maternal effect on offspring colonization ability is active, rather than passive, because the resource that induces the effect—changes in nest cavity availability—is the same that this species has depended on throughout its evolutionary history. Moreover, this maternal effect is probably adaptive because it enables females to produce sons with high colonizing ability when resources are scarce and to produce sons that are more likely acquire a territory locally when resources are abundant. Such active maternal effects may be most important during range expansions where species are expanding their range into areas that are similar to their native habitat, as is occurring in western bluebirds as well as species that are expanding their range in response to climate change (e.g. Thomas et al. 2001). On the other hand, when presented with a novel environment or resource, as is the case for house finches and seed beetles and many invasive species, passive maternal effects may be particularly important prior to the evolution of local adaptation. Thus, to understand the importance of maternal effects for range expansion, it is instructive to study these effects in both the native and recently expanded range and evaluate whether maternal effects are an evolved adaptation to similar environments or are a passive effect on offspring development that in the short term may increase offspring phenotypic variance and in the long term may lead to evolution of a new adaptive maternal effect.
Acknowledgments
The research conducted in this study was approved by the institutional animal care and use committee.I thank Tobias Uller for insightful comments and for inviting me to contribute to this issue. I am also grateful to two anonymous reviewers who provided comments that improved this manuscript. I thank the members of Mountain Bluebird Trails for donating nest-boxes and contributing information on resighted birds. Residents of the Hayes Creek neighbourhood kindly allowed me to observe bluebirds on their properties. This work was supported by the National Science Foundation (IRFP-0601751) and G.G. Simpson fellowship at the University of Arizona.
Footnotes
One contribution of 12 to a Theme Issue ‘Evolution of parental effects: conceptual issues and empirical patterns’.
References
- Amdan G.V., Csondes A., Fondrk M.K., Page R.E. Complex social behaviour derived from maternal reproductive traits. Nature. 2006;439:76–78. doi: 10.1038/nature04340. doi:10.1038/nature04340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V. Maternal inheritance and rapid evolution of sexual size dimorphism: passive effects or active strategies? Am. Nat. 2005a;166:S17–S30. doi: 10.1086/444601. doi:10.1086/444601 [DOI] [PubMed] [Google Scholar]
- Badyaev A.V. Stress-induced variation in evolution: from behavioral plasticity to genetic assimilation. Proc. R. Soc. B. 2005b;272:877–886. doi: 10.1098/rspb.2004.3045. doi:10.1098/rspb.2004.3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V. Maternal effects as generators of evolutionary change: a reassessment. In: Schlichting C.D., Mousseau T.A., editors. Year in evolutionary biology 2008. vol. 1133. Wiley-Blackwell; New York, NY: 2008. pp. 151–161. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Oh K.P. Environmental induction and phenotypic retention of adaptive maternal effects. BMC Evol. Biol. 2008;8:3. doi: 10.1186/1471-2148-8-3. doi:10.1186/1471-2148-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V., Hill G.E., Beck M.L., Dervan A.A., Duckworth R.A., McGraw K.J., Nolan P.M., Whittingham L.A. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science. 2002;295:316–318. doi: 10.1126/science.1066651. doi:10.1126/science.1066651 [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Hill G.E., Beck M.L. Interactions of maternal effects: onset of incubation and offspring sex in a passerine bird. Oecologia. 2003;135:386–390. doi: 10.1007/s00442-003-1203-x. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Acevedo Seaman D.A., Navara K.J., Hill G.E., Mendonça M.T. Evolution of sex-biased maternal effect in birds: III. Adjustment of ovulation order can enable sex-specific allocation of hormones, carotenoids, and vitamins. J. Evol. Biol. 2006;19:1044–1057. doi: 10.1111/j.1420-9101.2006.01106.x. doi:10.1111/j.1420-9101.2006.01106.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V., Young R.L., Hill G.E., Duckworth R.A. Evolution of sex-biased maternal effect in birds: IV. Intra-ovarian growth dynamics can link sex-determination and sex-specific acquisition of resources. J. Evol. Biol. 2008;21:449–460. doi: 10.1111/j.1420-9101.2007.01498.x. doi:10.1111/j.1420-9101.2007.01498.x [DOI] [PubMed] [Google Scholar]
- Bagne K.E., Purcell K.L., Rotenberry J.T. Prescribed fire, snag population dynamics, and avian nest site selection. For. Ecol. Manage. 2008;255:99–105. doi:10.1016/j.foreco.2007.08.024 [Google Scholar]
- Blanco G., Dávila J.A., López S., Rodríguez R., Martínez F. Sex-biased initial eggs favour sons in the slightly size-dimorphic Scops owl (Otus scops) Biol. J. Linn. Soc. 2002;76:1–7. doi:10.1111/j.1095-8312.2002.tb01709.x [Google Scholar]
- Brawn J.D., Balda R.P. Population biology of cavity nesters in northern Arizona: do nest sites limit breeding densities? Condor. 1988;90:61–71. doi:10.2307/1368434 [Google Scholar]
- Carroll S.P., Fox C.W. Dissecting the evolutionary impacts of plant invasions: bugs and beetles as native guides. Glob. Chang. Biol. 2007;13:1644–1657. doi:10.1111/j.1365-2486.2007.01403.x [Google Scholar]
- Case T.J., Taper M.L. Interspecific competition, environmental gradients, gene flow, and the coevolution of species' borders. Am. Nat. 2000;155:583–605. doi: 10.1086/303351. doi:10.1086/303351 [DOI] [PubMed] [Google Scholar]
- Cote J., Clobert J., Fitze P.S. Mother-offspring competition promotes colonization success. Proc. Natl Acad. Sci. USA. 2007;104:9703–9708. doi: 10.1073/pnas.0703601104. doi:10.1073/pnas.0703601104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson J.L. Facultative sex ratio adjustment by western bluebird mothers with stay-at-home helpers-at-the-nest. Anim. Behav. 2004;68:373–380. doi:10.1016/j.anbehav.2003.07.022 [Google Scholar]
- Dickinson J.L., Akre J.J. Extrapair paternity, inclusive fitness, and within-group benefits of helping in western bluebirds. Mol. Ecol. 1998;7:95–105. doi:10.1046/j.1365-294x.1998.00320.x [Google Scholar]
- Diss A.L., Kunkel J.G., Montgomery M.E., Leonard D.E. Effects of maternal nutrition and egg provisioning on parameters of larval hatch, survival and dispersal in the gypsy moth, Lymantria dispar. Oecologia. 1996;106:470–477. doi: 10.1007/BF00329704. doi:10.1007/BF00329704 [DOI] [PubMed] [Google Scholar]
- Donohue K. Seed dispersal as a maternally influenced character: mechanistic basis of maternal effects and selection on maternal characters in an annual plant. Am. Nat. 1999;154:674–689. doi: 10.1086/303273. doi:10.1086/303273 [DOI] [PubMed] [Google Scholar]
- Dubois N.S., Kennedy E.D., Getty T. Surplus nest boxes and the potential for polygyny affect clutch size and offspring sex ratios in house wrens. Proc. R. Soc. B. 2006;273:1751–1757. doi: 10.1098/rspb.2006.3509. doi:10.1098/rspb.2006.3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth R.A. Aggressive behavior affects selection on morphology by determining the environment of breeding in a passerine bird. Proc. R. Soc. B. 2006a;273:1789–1795. doi: 10.1098/rspb.2006.3517. doi:10.1098/rspb.2006.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth R.A. Behavioral correlations across reproductive contexts provide a mechanism for a cost of aggression. Behav. Ecol. 2006b;17:1011–1019. doi:10.1093/beheco/arl035 [Google Scholar]
- Duckworth R.A. Adaptive dispersal strategies and the dynamics of a range expansion. Am. Nat. 2008;172:S4–S17. doi: 10.1086/588289. doi:10.1086/588289 [DOI] [PubMed] [Google Scholar]
- Duckworth R.A., Badyaev A.V. Coupling of aggression and dispersal facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA. 2007;104:15017–15022. doi: 10.1073/pnas.0706174104. doi:10.1073/pnas.0706174104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth, R. A. & Kruuk, L. E. B. In press. Evolution of genetic integration between dispersal and colonization ability in a bird. Evolution (doi:10.1111/j.1558-5646.2009.00625.x) [DOI] [PubMed]
- Dzus E., Bortolotti G.R., Gerrard J.M. Does sex-biased hatching order in bald eagles vary with food resources? Ecosciences. 1996;3:252–258. [Google Scholar]
- Eising C.M., Müller W., Groothius T.G.G. Avian mothers create different phenotypes by hormones deposition in their eggs. Biol. Lett. 2006;2:20–22. doi: 10.1098/rsbl.2005.0391. doi:10.1098/rsbl.2005.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargallo J.A., Laaksonen T., Korpimäki E., Poyri V., Griffith S.C., Valkama J. Size-mediated dominance and begging behaviour in Eurasian kestrel broods. Evol. Ecol. Res. 2003;5:549–558. [Google Scholar]
- Forstmeier W., Coltman D.W., Birkhead T.R. Maternal effects influence the sexual behavior of sons and daughters in the zebra finch. Evolution. 2004;58:2574–2583. doi: 10.1111/j.0014-3820.2004.tb00885.x. doi:10.1554/04-325 [DOI] [PubMed] [Google Scholar]
- Fox C.W., Savalli U.M. Maternal effects mediate host expansion in a seed-feeding beetle. Ecology. 2000;81:3–7. doi:10.2307/177128 [Google Scholar]
- Galloway L.F., Etterson J.R. Transgenerational plasticity is adaptive in the wild. Science. 2007;318:1134–1136. doi: 10.1126/science.1148766. doi:10.1126/science.1148766 [DOI] [PubMed] [Google Scholar]
- Ginzburg L. Inertial growth: population dynamics based on maternal effects. In: Mousseau T.A., Fox C.W., editors. Maternal effects as adaptations. Oxford University Press; Oxford, UK: 1998. pp. 42–53. [Google Scholar]
- Gowaty P.A. House sparrows kill eastern bluebirds. J. Field Ornithol. 1984;55:378–380. [Google Scholar]
- Griffiths R., Daan S., Dijkstra C. Sex identification in birds using two CHD genes. Proc. R. Soc. B. 1998;263:1251–1256. doi: 10.1098/rspb.1996.0184. doi:10.1098/rspb.1996.0184 [DOI] [PubMed] [Google Scholar]
- Groothius T.G.G., Schwabl H. Determinants of within- and among-clutch variation in levels of maternal hormones in black-headed gull eggs. Funct. Ecol. 2002;16:281–289. doi:10.1046/j.1365-2435.2002.00623.x [Google Scholar]
- Groothius T.G.G., Müller W., von Engelhardt N., Carere C., Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2004;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. doi:10.1016/j.neubiorev.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Gross M.R. Alternative reproducitve strategies and tactics: diversity within sexes. Trends Ecol. Evol. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. doi:10.1016/0169-5347(96)81050-0 [DOI] [PubMed] [Google Scholar]
- Guinan J.A., Gowaty P.A., Eltzroth E.K., editors. Western bluebird (Sialia mexicana) The birds of North America, no. 510. Birds of North America, Inc; Philadelphia, PA: 2000. [Google Scholar]
- Hamilton W.D. Extraordinary sex ratios. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. doi:10.1126/science.156.3774.477 [DOI] [PubMed] [Google Scholar]
- Harrison R.G. Dispersal polymorphisms in insects. Annu. Rev. Ecol. Syst. 1980;11:95–118. doi:10.1146/annurev.es.11.110180.000523 [Google Scholar]
- Hejl S.J. Human-induced changes in bird populations in coniferous forests in western North America during the past 100 years. Studies Avian Biol. 1994;15:232–246. [Google Scholar]
- Herlugson, C. J. 1980 Biology of sypatric populations of western and mountain bluebirds. PhD thesis. Washington State University, Pullman, WA, USA, p. 134.
- Hoffman A.A., Blows M.W. Species borders: ecological and evolutionary perspectives. Trends Ecol. Evol. 1994;9:223–227. doi: 10.1016/0169-5347(94)90248-8. doi:10.1016/0169-5347(94)90248-8 [DOI] [PubMed] [Google Scholar]
- Holt R.D. On the evolutionary ecology of species' ranges. Evol. Ecol. Res. 2003;5:159–178. [Google Scholar]
- Huey R.B., Gilchrist G.W., Carlson M.L., Berrigan D., Serra L. Rapid evolution of a geographic cline in size in an introduced fly. Science. 2000;287:308. doi: 10.1126/science.287.5451.308. doi:10.1126/science.287.5451.308 [DOI] [PubMed] [Google Scholar]
- Hughes C.L., Hill J.K., Dytham C. Evolutionary trade-offs between reproduction and dispersal in populations at expanding range boundaries. Biol. Lett. 2003;270:S147–S150. doi: 10.1098/rsbl.2003.0049. doi:10.1098/rsbl.2003.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurteau S.R., Sisk T.D., Block W.M., Dickson B.G. Fuel-reduction treatment effects on avian community structure and diversity. J. Wildl. Manage. 2008;72:1168–1174. doi:10.2193/2007-351 [Google Scholar]
- Hutto R.L. Composition of bird communities following stand-replacement fires in northern rocky mountain (U.S.A.) conifer forests. Conserv. Biol. 1995;9:1041–1058. doi: 10.1046/j.1523-1739.1995.9051033.x-i1. doi:10.1046/j.1523-1739.1995.9051041.x [DOI] [PubMed] [Google Scholar]
- Johnson M.L., Gaines M.S. Evolution of dispersal: theoretical models and empirical tests using birds and mammals. Annu. Rev. Ecol. Syst. 1990;21:449–480. doi:10.1146/annurev.es.21.110190.002313 [Google Scholar]
- Kendall B.E., Ellner S.P., McCauley E., Wood S.N., Briggs C.J., Murdoch W.W., Turchin P. Population cycles in the pine looper moth: dynamical tests of mechanistic hypotheses. Ecol. Monogr. 2005;75:259–276. doi:10.1890/03-4056 [Google Scholar]
- Kotliar N.B., Kennedy P.L., Ferree K. Avifaunal responses to fire in southwestern montane forests along a burn severity gradient. Ecol. Appl. 2007;17:491–507. doi: 10.1890/06-0253. doi:10.1890/06-0253 [DOI] [PubMed] [Google Scholar]
- Kraaijeveld K., Dickinson J.L. Family-based winter territoriality in western bluebirds, Sialia mexicana: the structure and dynamics of winter groups. Anim. Behav. 2001;61:109–117. doi: 10.1006/anbe.2000.1591. doi:10.1006/anbe.2000.1591 [DOI] [PubMed] [Google Scholar]
- Krackow S. The developmental asynchrony hypothesis for sex ratio manipulations. J. Theor. Biol. 1995;176:273–280. doi: 10.1006/jtbi.1995.0197. doi:10.1006/jtbi.1995.0197 [DOI] [PubMed] [Google Scholar]
- Lewontin R.C. Selection for colonizing ability. In: Baker H.G., Stebbins G.L., editors. The genetics of colonizing species. Academic Press; New York, NY: 1964. pp. 77–94. [Google Scholar]
- Lipar J.L., Ketterson E.D., Val Nolan J. Intraclutch variation in testosterone content of red-winged blackbird eggs. Auk. 1999;116:231–235. [Google Scholar]
- Marshall D.J. Transgenerational plasticity in the sea: context-dependent maternal effects across the life history. Ecology. 2008;89:418–427. doi: 10.1890/07-0449.1. doi:10.1890/07-0449.1 [DOI] [PubMed] [Google Scholar]
- Marshall D.J., Uller T. When is a maternal effect adaptive? Oikos. 2007;116:1957–1963. doi:10.1111/j.2007.0030-1299.16203.x [Google Scholar]
- Massot M., Clobert J. Processes at the origin of similarities in dispersal behavior among siblings. J. Evol. Biol. 2000;13:707–719. doi:10.1046/j.1420-9101.2000.00202.x [Google Scholar]
- Merilä J., Wiggins D.A. Interspecific competition for nest holes causes adult mortality in the collared flycatcher. Condor. 1995;97:445–450. doi:10.2307/1369030 [Google Scholar]
- Mousseau T.A., Dingle H. Maternal effects in insect life histories. Annu. Rev. Entomol. 1991;36:511–534. doi:10.1146/annurev.en.36.010191.002455 [Google Scholar]
- Müller W., Eising C.M., Dijkstra C., Groothius T.G.G. Sex differences in yolk hormones depend on maternal social status in Leghorn chickens (Gallus gallus domesticus) Proc. R. Soc. B. 2002;269:2249–2255. doi: 10.1098/rspb.2002.2159. doi:10.1098/rspb.2002.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navara K.J., Siefferman L.M., Hill G.E., Mendonça M.T. Yolk androgens vary inversely to maternal androgens in eastern bluebirds: an experimental study. Funct. Ecol. 2006;20:449–456. doi:10.1111/j.1365-2435.2006.01114.x [Google Scholar]
- Newton I. The role of nest sites in limiting the numbers of hole-nesting birds—a review. Biol. Conserv. 1994;70:265–276. doi:10.1016/0006-3207(94)90172-4 [Google Scholar]
- Pinkowski B.C. Foraging ecology and habitat utilization in the genus Sialia. In: Dickson J.G., Conner R.N., Fleet R.R., Kroll J.C., Jackson J.A., editors. The role of insectivorous birds in forest ecosystems. Academic Press; New York, NY: 1979. pp. 165–190. [Google Scholar]
- Plissner J.H., Gowaty P.A. Eastern bluebirds are attracted to two-box nest sites. Wilson Bull. 1995;107:289–295. [Google Scholar]
- Power H.W., Lombardo M.P. Mountain bluebird. In: Poole A., Gill F., editors. The birds of North America. vol. 222. Birds of North America, Inc; Philadelphia, PA: 1996. pp. 1–21. [Google Scholar]
- Räsänen K., Laurila A., Merilä J. Geographic variation in acid stress tolerance of the moor frog, Rana arvalis. II. Adaptive maternal effects. Evolution. 2003;57:363–371. doi:10.1554/0014-3820(2003)057[0363:GVIAST]2.0.CO;2 [PubMed] [Google Scholar]
- Reed W.L., Vleck C.M. Functional significance of variation in egg-yolk androgens in the American coot. Oecologia. 2001;128:164–171. doi: 10.1007/s004420100642. doi:10.1007/s004420100642 [DOI] [PubMed] [Google Scholar]
- Rhen T., Crews D. Variation in reproductive behaviour within a sex: neural systems and endocrine activation. J. Neuroendocrinol. 2002;14:517–531. doi: 10.1046/j.1365-2826.2002.00820.x. doi:10.1046/j.1365-2826.2002.00820.x [DOI] [PubMed] [Google Scholar]
- Roff D.A. The cost of being able to fly: a study of wing polymorphism in two species of crickets. Oecologia. 1984;63:30–37. doi: 10.1007/BF00379781. doi:10.1007/BF00379781 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Habitat persistence and the evolution of wing dimorphism in insects. Am. Nat. 1994;144:772–798. doi:10.1086/285706 [Google Scholar]
- Ros A.F.H., Dieleman S.J., Groothius T.G.G. Social stimuli, testosterone, and aggression in gull chicks: support for the challenge hypothesis. Horm. Behav. 2002;41:334–342. doi: 10.1006/hbeh.2002.1768. doi:10.1006/hbeh.2002.1768 [DOI] [PubMed] [Google Scholar]
- Rossiter M.C. Maternal effects hypothesis of herbivore outbreak. Bioscience. 1994;44:752–763. doi:10.2307/1312584 [Google Scholar]
- Rutkowska J., Badyaev A.V. Meiotic drive and sex determination: molecular and cytological mechanisms of sex ratio adjustment in birds. Phil. Trans. R. Soc. B. 2008;363:1675–1686. doi: 10.1098/rstb.2007.0006. doi:10.1098/rstb.2007.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan B.C., Vandenbergh J.G. Intrauterine position effects. Neurosci. Biobehav. Rev. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. doi:10.1016/S0149-7634(02)00038-6 [DOI] [PubMed] [Google Scholar]
- Saab V.A., Russell R.E., Dudley J.G. Nest densities of cavity-nesting birds in relation to postfire salvage logging and time since wildfire. Condor. 2007;109:97–108. doi:10.1650/0010-5422(2007)109[97:NDOCBI]2.0.CO;2 [Google Scholar]
- Sauer J.R., Hines J.E., Fallon J. USGS Patuxent Wildlife Research Center; Laurel, MD: 2008. The North American breeding bird survey, results and analysis 1966–2007. [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. doi:10.1073/pnas.90.24.11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Phys. C. 1996;114A:271–276. doi: 10.1016/0300-9629(96)00009-6. doi:10.1016/0300-9629(96)00009-6 [DOI] [PubMed] [Google Scholar]
- Silk J.B. Local resource competition and the evolution of male-biased sex ratios. J. Theor. Biol. 1984;108:203–213. doi: 10.1016/s0022-5193(84)80066-1. doi:10.1016/S0022-5193(84)80066-1 [DOI] [PubMed] [Google Scholar]
- Silk J.B., Brown G.R. Local resource competition and local resource enhancement shape primate birth sex ratios. Proc. R. Soc. B. 2008;275:1761–1765. doi: 10.1098/rspb.2008.0340. doi:10.1098/rspb.2008.0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D., Duncan R.P., Blackburn T.M., Cassey P., Lefebvre L. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA. 2005;102:5460–5465. doi: 10.1073/pnas.0408145102. doi:10.1073/pnas.0408145102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R., Schwabl H. Yolk testosterone organizes behavior and male plumage coloration in house sparrows. Behav. Ecol. 2004;56:491–497. doi:10.1007/s00265-004-0810-9 [Google Scholar]
- Sutherland O.R.W. The role of crowding in the production of winged forms by two strains of the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 1969;15:1385–1410. doi:10.1016/0022-1910(69)90199-1 [Google Scholar]
- Thomas C.D., Bodsworth E.J., Wilson R.J., Simmons A.D., Davies Z.G., Musche M., Conradt L. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. doi:10.1038/35079066 [DOI] [PubMed] [Google Scholar]
- Uller T. Sex-specific sibling interactions and offpsring fitness in vertebrates: patterns and implications for maternal sex ratios. Biol. Rev. 2006;81:207–217. doi: 10.1017/S1464793105006962. doi:10.1017/S1464793105006962 [DOI] [PubMed] [Google Scholar]
- Uller T. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 2008;23:432–438. doi: 10.1016/j.tree.2008.04.005. doi:10.1016/j.tree.2008.04.005 [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. Oxford University Press; New York, NY: 2003. Developmental plasticity and evolution. [Google Scholar]
- West S.A., Pen I., Griffin A.S. Cooperation and competition between relatives. Science. 2002a;296:72–75. doi: 10.1126/science.1065507. doi:10.1126/science.1065507 [DOI] [PubMed] [Google Scholar]
- West S.A., Reece S.E., Sheldon B.C. Sex ratios. Heredity. 2002b;88:117–124. doi: 10.1038/sj.hdy.6800018. doi:10.1038/sj.hdy.6800018 [DOI] [PubMed] [Google Scholar]
- Whittingham L.A., Schwabl H. Maternal testosterone in tree swallow eggs varies with female aggression. Anim. Behav. 2002;63:63–67. doi:10.1006/anbe.2001.1889 [Google Scholar]
- Young R.L., Badyaev A.V. Evolution of sex-biased maternal effects in birds. I. Sex-specific resource allocation among simultaneously growing oocytes. J. Evol. Biol. 2004;17:1355–1366. doi: 10.1111/j.1420-9101.2004.00762.x. doi:10.1111/j.1420-9101.2004.00762.x [DOI] [PubMed] [Google Scholar]
- Zeleny L. Nesting box programs for bluebirds and other passerines. In: Temple S.A., editor. Endangered birds: management techniques for preserving threatened species. University of Wisonsin Press; Madison, WI: 1978. pp. 55–60. [Google Scholar]
- Zera A.J., Denno R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997;42:207–230. doi: 10.1146/annurev.ento.42.1.207. doi:10.1146/annurev.ento.42.1.207 [DOI] [PubMed] [Google Scholar]