Abstract
The environment that an offspring experiences during its development can have lifelong consequences for its morphology, anatomy, physiology and behaviour that are strong enough to span generations. One aspect of an offspring's environment that can have particularly pronounced and long-lasting effects is that provided by its parent(s) (maternal effects). Some disciplines in biology have been quicker to appreciate maternal effects than others, and some organisms provide better model systems for understanding the causes and consequences of the maternal environment for ecology and evolution than others. One field in which maternal effects has been poorly represented, and yet is likely to represent a particularly fruitful area for research, is the field of cooperative breeding (i.e. systems where offspring are reared by carers in addition to parent(s)). Here, we attempt to illustrate the scope of cooperative breeding systems for maternal effects research and, conversely, highlight the importance of maternal effects research for understanding cooperative breeding systems. To this end, we first outline why mothers will commonly benefit from affecting the phenotype of their offspring in cooperative breeding systems, present potential strategies that mothers could employ in order to do so and offer predictions regarding the circumstances under which different types of maternal effects might be expected. Second, we highlight why a neglect of maternal strategies and the effects that they have on their offspring could lead to miscalculations of helper/worker fitness gains and a misunderstanding of the factors selecting for the evolution and maintenance of cooperative breeding. Finally, we introduce the possibility that maternal effects could have significant consequences for our understanding of both the evolutionary origins of cooperative breeding and the rise of social complexity in cooperative systems.
Keywords: differential allocation, kin selection, load-lightening, offspring control, parental manipulation, social insects
1. Introduction
The tenet of evolution by natural selection is to provide a framework for understanding the origin and maintenance of form and function in the natural world (Darwin 1859). Historically, observed variation was thought to arise principally through mutation and subsequent selection, and/or drift (Fisher 1958; Falconer 1989). More recently, it has been emphasized that this sequence of events could also act in reverse, with phenotypic change being followed by genetic change (West-Eberhard 2003). For example, if an environment experienced during development causes offspring to adopt a certain form and the form adopted yields higher fitness returns given that environment, then selection will favour the mutations that fix the already existing association between environment and form. As a consequence, a number of recent publications have encouraged a greater appreciation of the potential role that the environment experienced by offspring during development, and the plasticity of their responses to this environment, has for influencing evolutionary processes (West-Eberhard 2003; Pigliucci 2007; Sultan 2007; Badyaev 2008; Uller 2008).
For the offspring of many organisms, the environment provided by their mother is among the most significant they experience during development. Maternal effects on offspring can be adaptive if the maternal environment provided has fitness consequences for the mother and/or her offspring (Mousseau & Fox 1998). If maternal fitness is maximized through increasing offspring fitness, then mothers will be selected to adjust offspring development to aid the survival and future reproductive potential of her offspring (termed anticipatory maternal effects, Marshall & Uller (2007)). Such an effect might be expected where offspring quality yields greater fitness to mothers than offspring quantity. By contrast, where maternal fitness is maximized more from increasing offspring quantity, mothers might be selected to adjust the offspring environment to the one that largely maximizes their ability to produce further offspring, rather than to the one that maximizes the fitness of individual offspring (termed selfish maternal effects, Marshall & Uller (2007)). Thus, an important point is that maternal strategies, and the effects that they have on their offspring, can be adaptive irrespective of whether they apparently help or hinder their own individual offspring.
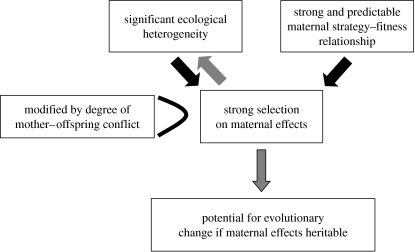
The environment provided by the mother, the effect that it has on offspring traits and its ramifications for ecological and evolutionary processes will depend on at least four factors (Trivers 1974; Stearns 1992; Godfray 1995; West & Sheldon 2002; Sockman et al. 2006; Marshall & Uller 2007; Rickard & Lummaa 2007; Badyaev 2008; Uller 2008). First, the degree to which ecology varies within and among mothers within a population, and the effect of that ecology on a mother's strategy, will govern the degree of variation in maternal strategies within that population. Second, the stronger and more predictable the relationship between a mother's strategy and her fitness, the more likely the strategy adopted will be under selection. Third, the degree of selection on maternal strategies and the effects that they have on offspring will be modified by the degree of mother–offspring conflict, with strong conflict increasing selection on maternal (and offspring) strategies. Finally, maternal effects might be expected to lead to evolutionary change if the maternal strategy is heritable in a given ecology (epigenetically or genetically) and is evolutionarily stable (see figure 1 for summary).
Figure 1.
Summary of the ecological causes and evolutionary consequences of maternal effects considered. Maternal effects can be passive or active (Badyaev 2005). Passive effects are likely to be common in all animal systems; here we concentrate on active maternal strategies and effects which we believe are likely to be particularly important in cooperative systems. The two most important factors selecting for active maternal effects will be ecological heterogeneity and the strength and predictability of the relationship between maternal strategy and fitness. If there is no ecological heterogeneity or a weak and/or unpredictable relationship between maternal strategy and fitness, active maternal effects will not be expected. The strength and extent of maternal effects will be further modified by the dynamics of mother–offspring conflict. Greater selection on maternal effects might be expected where maternal strategies and offspring strategies do not converge and where offspring have considerable scope for counteracting a given maternal strategy. Strong maternal effects will in turn further influence ecological heterogeneity, leading to positive feedback between each, again modified by the dynamics of maternal-offspring conflict. Finally, while within-generation maternal effects can be strong without inheritance, ultimately evolutionary change will become more likely when maternal effects are inherited, either epigenetically or genetically.
The ecological and evolutionary causes and consequences of maternal effects are thus best investigated in systems where within and between species: (i) ecology varies in its degree of heterogeneity, (ii) relationships among ecology, maternal investment strategy and maternal fitness vary in strength and predictability, and (iii) levels and outcomes of mother–offspring conflict are variable. This is because, in such systems, one can be armed with a set of predictions regarding the circumstances under which different types and extents of maternal strategies should be observed, and test these predictions using experimental approaches within species and comparative approaches between species. One system where all three sets of circumstances commonly occurs is in cooperative breeding systems. The specific aims of this paper are to: introduce cooperative breeding systems and highlight why they represent an appropriate model for investigating the causes and consequences of maternal effects (§2); introduce example maternal strategies that could be employed by mothers in behavioural time (§3); establish predictions regarding the circumstances under which different types of maternal strategies are expected in behavioural time and the stage of offspring development during which they are predicted to operate (§4); illustrate how maternal effects can confound measures of fitness in cooperative systems and lead to a misunderstanding of the pressures acting on such breeding systems (§5); provide important insights from research on maternal effects in humans, which have been largely neglected in animal research (§6); and discuss the potential role that maternal effects could have for the evolution of cooperative breeding and the rise of social complexity (§7).
2. Cooperative breeding systems: scope for maternal effects
Cooperative breeding is defined here as a system where individuals, in addition to genetic or putative parent(s), actively provide care to the offspring in a single location. Such behaviour poses a paradox for current evolutionary thinking, namely: how can a strategy evolve which not only reduces personal fitness but also increases the fitness of competitors? Far from being an esoteric exception, cooperative breeding is found in multiple animal classes (Insecta, Arachnida, Crustacea, Pisces, Aves, Mammalia, including humans), has had multiple evolutionary origins within classes and is the hallmark of some of the most successful animals on the planet (ants, termites, humans) (Wilson 1971; Hölldobler & Wilson 1990; Stacey & Koenig 1990; Taborsky 1994; Choe & Crespi 1997; Solomon & French 1997; Duffy et al. 2000; Whitehouse & Lubin 2005). As a consequence, an impressive amount of work has been conducted on cooperative breeding systems since the publication of Hamilton's seminal work on inclusive fitness, over four decades ago (Hamilton 1964). In this section, we introduce the definitions and terminologies that we will use throughout this paper, briefly summarize the main findings of relevance to maternal effects across cooperative systems and finally introduce the scope for studying maternal effects in cooperative breeders.
(a) Definitions and terminology
We define a maternal effect as the effect that a maternal investment strategy has on her offspring's phenotype, be it morphological, anatomical, physiological or behavioural. Primarily, we will be concerned with the effects of active maternal strategies that arise as a direct or indirect consequence of the numbers of other carers (or some strong correlate thereof). Terminology varies across the taxonomic groups of cooperative breeders. We use the term cooperative breeding irrespective of the taxonomic group and refer to the primary reproductive(s) as the queen only in invertebrates where significant and largely irreversible caste differences exist between the primary reproductive and her workforce. In such cases, the workforce will be referred to as workers; in all other cases, they will be referred to as helpers. It is important to note that in the vast majority of cooperative systems, multiple individuals can breed; the effects that a breeder has on its own offspring, irrespective of its status within the group, are maternal.
(b) Background to cooperative breeding
Four basic facts of cooperative breeding systems are relevant to the study of maternal effects. The key point in each is the fact that substantial variation exists within the same mother over time, among mothers within a population and between species (table 1). First, in the majority of species, cooperative groups consist of primary reproductive(s) and their offspring, which have foregone dispersal (Emlen 1995; Bourke 1997; Choe & Crespi 1997; Thorne 1997). However, the genetic structure of groups varies owing to immigration, co-breeding, breeder replacement, extra-group mating and/or group amalgamation (Stern & Foster 1997; Bourke 1999; Cockburn 2004; Russell 2004; Dierkes et al. 2005; Whitehouse & Lubin 2005; Bono & Crespi 2006; Ratnieks et al. 2006; Korb 2008). Second, to varying degrees, helpers/workers can be associated with a reduction in maternal contributions to offspring care, an increase in maternal productivity within seasons and survival between seasons, and/or an increase in the condition, survival and future reproductive capacity of offspring (Wilson 1971; Bourke 1997; Choe & Crespi 1997; Keller & Genoud 1997; Thorne 1997; Dickinson & Hatchwell 2004; Russell 2004; Russell et al. 2007a; Salomon & Lubin 2007). Thus, given that helpers/workers are usually kin of the breeder(s), and increase the reproductive success of those breeder(s), kin selection is likely to be an important force selecting for the incidence of cooperative breeding in most systems (Michod 1982; Bourke 1997; Emlen 1997; Griffin & West 2003). Moreover, because helpers/workers can have considerable effects on the fitness of breeding females, among the most important ecologies for a reproductive cooperative breeder is the size of workforce available to rear her young (e.g. Bourke 1997, 1999; Russell et al. 2002, 2003a; Roux & Korb 2004; Ridley 2007).
Table 1.
Summary of the variation found in life history and mating system across cooperative breeders. (Female skew indicates the proportion of other females breeding other than the primary in the group (high=less than 30% of group members, medium=30–60%, low=greater than 60%). Group size: minimum is one in invertebrates that found groups/colonies alone and is a pair in most birds, mammals and termites; maximum group sizes are the average maximum of a given species rounded to the nearest five (if less than 20), nearest 10 (if less than 100) and to the nearest order of magnitude (if more than 100) (see Bourke 1999). Castes involve significant and apparently irreversible differences between individuals within a group, which arise in order to perform a specific function (Q=queen morph, D=disperser morph, S=soldier morph; W=among-worker morphs). Fecundity represents the approximate maximum number of eggs laid (egg layers) or offspring delivered (viviparous species) per year by species in each category. Fecundity is highly variable and averages considerably less than these maxima. Tenure as alpha (days, months, years, decades) and an estimate of predictability (low, medium, high) with the most common first (e.g. med-low=medium to low predictability with medium being more likely that low). Helper effects refer to the effect of helper number on the reproductive output of the group: NR, no relationship; LR, linear; QR, quadratic; AR, accelerating relationship. (Sources: Wilson 1971; Taborsky 1984, 1994; Stacey & Koenig 1990; Duffy 1996; Crespi & Mound 1997; Crespi et al. 1997; Solomon & French 1997; Stern & Foster 1997; Avilés & Tufiño 1998; Bourke 1999; Duffy & Macdonald 1999; Hatchwell 1999; Russell 2004; Dierkes et al. 2005; Heg et al. 2005; Kranz 2005; Whitehouse & Lubin 2005; Bono & Crespi 2006; Lubin & Bilde 2007; Salomon & Lubin 2007; Korb 2008).)
| system/taxa | female skew | group size | castes | fecundity | tenure as alpha | helper effects |
|---|---|---|---|---|---|---|
| (a) avian type (common, female-biased immigration into groups) | ||||||
| birds | low-high | 2–15 | none | 10 | years×low-med | NR, LR, QR |
| mammals | med-high | 5–15 | humans only | 20 | years×low-med | NR, LR, QR |
| (b) mammalian type (common, male-biased immigration) | ||||||
| fishes | med-high | 5–30 | none | 1000 | months×low-med | LR, QR |
| birds | med-high | 3–15 | none | 15 | years×med-low | NR, LR, QR |
| mammals | low-high | 2–40 | none | 15 | years×med-low | LR, QR |
| (c) primitive insect-type systems (uncommon, male-biased immigration or no immigration) | ||||||
| nesting-thrips | med-high | 1–100 | yes (S) | 100 | months×med-low | LR |
| OP termites | high | 2–1000 | yes (Q, D, S) | 100 | years×high-med | NR, LR |
| non-gall aphids | low-med | 1–1000 | yes (D, S) | clonal | N/A clonal | unknown |
| prim. hymenop | low-high | 1–10 000 | yes (Q) | 1000 | months×med-high | LR, AR, QR |
| spiders | med-high | 1–1000 | none | 5000 | months×med-high | LR |
| crustaceans | high | 5–100 | yes (Q) | 50 | years×med | LR |
| mammals | med-high | 5–100 | yes (Q, D, W) | 30 | decades×med-high | unknown |
| (d) advanced insect-type systems (no immigration) | ||||||
| galling thrips | high | 1–1000 | yes (S, D) | 1000 | months×med-high | LR, AR |
| MP termites | high | 2–5 m | yes (Q, S, D, W) | 10 million | decades×high | AR |
| galling aphids | low-med | 1–10 000 | yes (S, D) | clonal | N/A clonal | AR |
| adv. hymenop | high | 1–10 m | yes (Q, S, D, W) | 5 million | months-decades×high | AR |
Third, offspring vary considerably in their scope for securing direct reproductive success within and among species (Wilson 1971; Emlen 1997; Clutton-Brock 1998; Beekman & Ratnieks 2003; Magrath et al. 2004; Russell 2004; Heg et al. 2006; Korb 2008; Smith et al. 2008; Hager & Jones in press). In some species, offspring might have a very high chance of dispersing successfully, co-breeding with the dominant or replacing it, while in others the chances are low and determined by phenotype (Choe & Crespi 1997; Bernasconi & Strassmann 1999; Bourke 1999; Clutton-Brock et al. 2006). Fourth, significant variation exists in the size and complexity of cooperative breeding units, again within and among species (Wilson 1971; Noirot 1991; Thorne 1997; Bourke 1999; Cockburn 2004; Miura 2004; Russell 2004; Kranz 2005; Whitehouse & Lubin 2005; Korb 2008; Hager & Jones in press). Among taxonomic groups, the number of female breeders varies from one to several, division of labour varies from absent to extreme and group sizes range from one to millions. Even within the same female, temporal variation in each can be considerable, because young groups tend to be smaller than older groups and because of stochasticity (table 1).
(c) Scope for maternal effects
The scope for maternal effects in cooperative breeders is therefore not only substantial, but also because of the degree of intra- and interspecific variations, cooperative breeders will provide a rich testing ground for ‘maternal effects theory’. There are four reasons for these suggestions. First, given that there is variation in the number of helpers/workers present, there is a high degree of ecological heterogeneity in cooperative systems. Second, given that there is variation in the relationships between helper/worker number and maternal fitness, variation exists in the strength and predictability of the relationships among ecology, maternal investment and fitness. Third, given that there is variation in the propensity and duration for offspring to remain at home and help their mother, there is potential for variable degrees of parent–offspring conflict and variable outcomes. Fourth, given that variation is intra- and interspecific in all cases, one can apply both within and comparative techniques to the study of maternal effects in cooperative breeders.
For example, consider the case of sex allocation in birds, itself a maternal effect. Within and across birds, consistent evidence for adaptive sex ratio skews is lacking with one exception—cooperative species (West & Sheldon 2002; Komdeur 2004). For example, in Seychelles warbler (Acrocephalus sechellensis), variation exists in territory quality and this variation dictates whether philopatric offspring can be accommodated (poor quality territories cannot accommodate more than a pair of birds) (Komdeur 1994). Helpers are females, and predictable variation exists between maternal breeding success and the numbers of female helpers present; on high-quality territories, the optimum number of helpers is two (Komdeur 1994). In accordance, females on low-quality territories lay male eggs (77% bias) while on high-quality territories they only lay male eggs in the presence of two helpers (85% bias); in the presence of no helpers, they lay female eggs (88% bias) (Komdeur et al. 1997). Experimental manipulations of helper number and territory quality confirm that females are capable of facultative adjustments (Komdeur et al. 1997). For example, reductions in helper numbers from two to one, on high-quality territories, lead females to switch from laying 85 per cent sons to laying 83 per cent daughters. Translocation of females from low- to high-quality territories leads to a switch from laying 90 per cent sons to 85 per cent daughters, while controls switched between high-quality territories produce 80 per cent daughters in both cases. A cross-fostering experiment in which sons or daughters are switched between nests confirms that maternal adjustments of sex ratio are adaptive (Komdeur 1998).
Furthermore, as we noted above (§2b), helpers also vary among cooperative species in the effect that they have on maternal fitness. Using this fact in birds and mammals, Griffin et al. (2005) conducted a meta-analysis to test whether sex ratio biases towards the helping sex increase with the strength of the relationship between helper number and maternal fitness. They found, as predicted, that the greatest sex ratio biases towards the helping sex were observed in those species where helpers had the greatest effect on maternal fitness. Thus, there is clear precedence, from both within-species and comparative approaches, for our suggestion that maternal effects will be strong in cooperative species and that their strength will be related to both ecological heterogeneity and predictability, and the strength and predictability of helper effects on maternal fitness.
3. Maternal strategies
Maternal effects can occur during four distinct phases: (i) the pre-reproductive phase, in which maternal effects can arise through influencing with whom the female mates and the timing of reproduction; (ii) the early reproductive phase, in which maternal effects can arise in egg-laying species through altering the number, size, sex and content of eggs laid and in viviparous species by altering the number, size, sex and quality of offspring delivered; (iii) the late reproductive phase, in which maternal effects can arise through differences in offspring care and provisioning post-hatching (egg layers) and post-birth (viviparous species); and (iv) the post-independence phase, in which maternal effects can arise through interactions with those offspring that remain at home. It is essential to note that the outcome of maternal strategies will not only depend on selection for the maternal strategy but also selection on offspring counter-strategies and genetic constraints on each (Parker et al. 2002; Beekman & Ratnieks 2003; Linksvayer 2006; Smiseth et al. 2008). Over evolutionary time, offspring could respond during any phase to a maternal strategy, but in behavioural time, responses are more restricted to the latter phases (e.g. Badyaev 2008). Our aim in this section is to outline the different strategies that could be employed by mothers in behavioural time to influence the outcome of offspring; we address the outcome of maternal and offspring strategies over evolutionary time below (§7). In particular, we are concerned here with a mother's ability to influence the options of her offspring, including the likelihood that they will show cooperative versus competitive tendencies. There is abundant evidence to show that mothers can influence the dispersive and competitive tendencies of their offspring (Marshall & Uller 2007; Groothuis & Schwabl 2008), suggesting that they could also influence their offspring's cooperative tendencies in cooperative breeders.
Evidence shows that offspring trajectories can be influenced profoundly by their early rearing environment, much of which is caused by levels of maternal investment. For example, early rearing conditions can have lifelong implications for individuals, influencing early growth and survival (Lindström 1999) as well as both health (Barker 1994) and reproductive capacity (Lummaa & Clutton-Brock 2002) in adulthood. Lifelong implications appear to arise in part because offspring are either incapable (Metcalfe & Monaghan 2001) or might be selected against (Gluckman et al. 2005) compensating for a bad start in life. Finally, early rearing conditions can have transgenerational phenotypic and fitness consequences for subsequent offspring (Lummaa & Clutton-Brock 2002; Lummaa 2003) that can be strong enough to affect population dynamics (Beckerman et al. 2002). The point is that in cooperative species, at least over behavioural time scales, differential maternal investment strategies could have profound and long-lasting effects on offspring (Alexander 1974; Michod 1982; Roisin 1994; Wade 2001; Linksvayer & Wade 2005; Strassmann & Queller 2008).
In §2c, we have illustrated, using the example of sex ratio biases, how mothers can benefit from producing offspring that are philopatric and helpful in some circumstances and, in others, are dispersive and potentially more competitive. Here, we provide four other ways in which mothers might be able to influence the cooperative nature of their offspring, one from each of the four life-history stages above (see table 2 for a more comprehensive list of possible strategies). Mothers could increase the helpfulness of offspring through providing suboptimal levels of resources per offspring, either by breeding during suboptimal times in the year or by breeding more rapidly than would be optimal for the offspring (Lummaa 2003; Lummaa & Tremblay 2003). If such offspring were unable to procure sufficient food to disperse or compete for reproduction with their mother, it is conceivable that they might settle for gaining fitness indirectly by helping their mother, thereby maximizing maternal fitness overall (Roisin 1994). However, offspring condition and contributions to helping can be positively related (e.g. Clutton-Brock et al. 2002); in such circumstances, maternal hormones employed during early reproductive phases could influence the sensitivity of offspring to the needs of subsequent offspring in their group. For example, in cooperative meerkats (Suricata suricatta), individuals in small groups can have higher levels of circulating cortisol (Young et al. 2006) and these levels in male helpers positively influence their contributions to care (Carlson et al. 2006). If mothers were also to have higher levels of circulating cortisol when in small groups, then such mothers could pass cortisol to their developing foetuses (Levine 1994) and produce helpful offspring. Other possibilities might be for mothers to malnourish offspring or terminate care early during the late reproductive phase, in order to limit the subsequent life-history options of offspring (Huck et al. 1987; Roisin 1994; Punzo & Alvarez 2002; Punzo & Ludwig 2002; Lummaa 2003). Finally, mothers could rear offspring to their full potential and attempt to suppress their chances for independent reproduction following their period of dependence. There is abundant evidence for maternal suppressive strategies of offspring reproduction during post-independent stages using both behavioural and physiological mechanisms (Bourke 1997; Clutton-Brock 1998; Beekman & Ratnieks 2003; Magrath et al. 2004; Russell 2004; Ratnieks et al. 2006; Hager & Jones in press).
Table 2.
Potential ways in which mothers could alter the outside options available to offspring during the four reproductive phases. (All examples are provided with respect to reducing outside options; the corollary might be expected to increase outside options. In all cases, the prediction is that mothers employ these different strategies to produce philopatric/helper offspring or dispersive/competitive offspring, although in many cases mothers might employ a given strategy to save resources without influencing options to offspring if helpers compensate for maternal reductions in investment.)
| maternal strategy | options | effect on offspring | rationale |
|---|---|---|---|
| (a) pre-reproductive phase | |||
| partner choicea | inbreeding/outbreeding, genetic compatibility, genetic quality | attractiveness or genetic viability | sexual selection—female partner choice influences offspring attractiveness/genetic quality |
| reproductive phenology | timing of reproduction, inter-birth intervals | size, condition, competitiveness | reproductive timing related to maternal condition and extrinsic food availability |
| investment phenology | incubation onset | competition among offspring | asynchronous offspring increases variance in form |
| (b) early reproductive phase | |||
| reproductive investment | alter brood/litter size | size, condition, competitiveness | number of offspring produced influences mean/variance offspring size/condition |
| alter sex ratio | sex | helpers are sex specific | |
| alter egg/foetus: nutrition, hormones, other micro-constituents | size, condition, propensity to cooperate/disperse | nutrition, hormones and other micro-constituents influence condition, growth and competitiveness and might influence sensitivity to begging offspring | |
| incubation schedules | condition | offspring growth and development in birds is related to the mean and variance in egg temperatures | |
| (c) late reproductive phase | |||
| provisioning level | as above regarding changes to body size and condition | ||
| level and duration of attention/care | neurodevelopment, psychology, personality | psychological research in humans shows diverse effects of maternal attention on offspring development/behaviour | |
| (d) post-reproductive phase | |||
| social/chemical interactions | alter offspring options/decisions | offspring can be coerced/informed into behaviour | |
While ambiguously leading to a maternal effect, it is certainly a maternal strategy that can have significant consequences on offspring outcomes regarding reproductive options and can be associated with maternal effects (Schwander et al. 2008).
4. Predictions and evidence for maternal effects
Our hypothesis so far is that maternal effects will be prevalent in cooperative species and mothers will have the capacity to employ both selfish and anticipatory strategies depending on their circumstance. However, the circumstances under which a given strategy is employed, the degree to which it is employed and when in the reproductive event it is so will be both numerous and complex. For example, ecology might influence the degree to which parent–offspring conflict exists and the outcome of any conflict (Beekman & Ratnieks 2003); selection on selfish strategies with long-term consequences for offspring will be seldom fruitful if outside options for offspring are commonly available (Emlen 1997; Magrath et al. 2004) or if offspring have little influence on breeder fitness through helping (Griffin & West 2003; Griffin et al. 2005). Ecology will also dictate maximum group size (Jarman 1974; Clutton-Brock & Harvey 1977) and hence set limits on the number of helpers that can be recruited (e.g. Komdeur 1994; Wcislo & Danforth 1997). Ecology in turn will interact with life history. For example, if maternal production exceeds optimal group size, then selection for anticipatory strategies will be greater than selfish ones. On the other hand, if the rate of maternal production is insufficient to permit all helpers to accrue fitness, then selfish maternal strategies employed to produce further helpers will also yield little fitness advantage. Finally, the costs and benefits of maternal strategies for the mother will depend upon the dispersal and mating system of a given species. For example, if the immigrating sex is female, then females will seldom produce the individuals with which they will compete, and hence might less often benefit from a selfish strategy.
Despite these complexities, we attempt here to provide some predictions regarding the circumstances under which different maternal strategies might be expected and the effects that they have on offspring, as well as provide evidence, where possible, which corroborates or counters our predictions. To this end, we divide all cooperative breeders into three broad classes according to their dispersal system (female biased, male biased, rare; table 1). These classes are denoted as follows: (i) avian-type systems wherein immigration into groups is relatively common and is female biased, (ii) mammalian-type systems wherein immigration into groups is relatively common and is primarily male biased, and (iii) insect-type systems wherein immigration into groups by either sex is rare or non-existent. Within each section, we predict the overall extent to which selfish versus anticipatory maternal strategies might be expected relative to the other systems, before being more specific about how a particular ecology and life history might influence the degree to which each type of maternal effect is found within each system.
Throughout, we use the terms selfish strategy (or effect) to denote when mothers appear to be investing towards their own optima and anticipatory strategy (or effect) when mothers appear to be investing towards the optima of their developing offspring (sensu Marshall & Uller 2007). It should be noted that maternal strategies will often have short-term influences on offspring, particularly, for instance, where helpers can compensate for any reductions in the maternal levels of care (e.g. Russell et al. 2007b, 2008; see §5). In addition, it will seldom be the case that the mothers benefit through a solely selfish strategy, and will often benefit from employing a mixed strategy where mothers influence the variance of offspring phenotype by employing both selfish and anticipatory strategies either simultaneously (i.e. within the same brood/litter) or sequentially (between different broods/litters). (For the species that produce a single offspring at a time, simultaneous strategies would be associated with a degree of alternation between selfish and anticipatory strategies, while a sequential strategy would be associated with a clear temporal divide.) Such strategies might often be sex specific. For example, anticipatory strategies might be more often employed for the dispersing sex, particularly if this sex never helps and its ability to secure success outside of the group is related to its phenotype.
Finally, two further points are of importance. First, a selfish maternal effect to developing offspring might be beneficial for helping offspring (see §7). Here, we are concerned only with one generation of offspring at a time (not multiple generations at the same time). Second, although we assign differences in offspring trajectory to maternal effects, it is likely that, to varying degrees, observed differences in offspring phenotype arise independently of the mother. For example, in eusocial aphids, the development of disperser morphs is suggested to be determined by the rate of contacts with conspecifics (Stern & Foster 1997), while in one-piece termites (i.e. those that live within their food), the development of offspring into dispersers or workers/soldiers is suggested to be determined by the amount of food left in the natal habitat (Korb & Katrantzis 2004). The degree to which offspring phenotype is governed by a maternally induced environment versus an external environment might be determined by the conflicts of interest between mother and offspring, as well as the amount of information available to mothers versus offspring concerning the maximally adaptive strategy (Beekman & Ratnieks 2003; Korb 2008). Notwithstanding, it is not possible to reject the importance of maternal effects unless they are measured.
(a) Avian-type systems
Avian-type systems are characterized here by relatively common and female-biased immigration into groups. Such systems are also largely characterized by facultative cooperative breeding as well as relatively low female fecundity and unpredictable tenures as dominant (table 1a). Avian-type systems include almost all birds and a few mammals (e.g. large canids, some callitrichids, humans; Greenwood 1980; Stacey & Koenig 1990; Solomon & French 1997; Cant & Johnstone 2008). Broadly, we predict that avian-type systems will be characterized by greater anticipatory maternal effects on developing offspring than is the case in either mammalian- or insect-type systems (see §§4b and 4c). There are three reasons to make this prediction. First, maternal longevity will be more unpredictable in avian- than insect-type systems (see §4c), because in all species mothers must forage for themselves and are susceptible to predation (Alexander et al. 1991; Thorne 1997). Second, maternal tenures as dominant will be more unpredictable in avian-type systems compared with either of the other two systems (see §§4b and 4c), since their tenure length is determined not only by their own survival but also by the survival of their partner. This is because in systems with female-biased immigration, partner death leads to a son assuming the dominant role, leading to mothers being evicted from their group (Hannon et al. 1985) or leaving voluntarily when partner death is likely (Hatchwell et al. 2000). Finally, in avian-type systems, mothers do not produce their own reproductive competitors (because their daughters disperse to breed) and so mother–offspring conflict will be reduced generally and mothers will benefit from producing competitive dispersers.
Overall anticipatory maternal effects can arise in three ways, depending on whether, in the presence of helpers, mothers increase, maintain or only partially reduce their investment in each offspring. In all these cases, the overall effects of maternal investment levels are beneficial for the offspring, although the degree to which they are so obviously varies. The available evidence is generally supportive of our prediction of largely anticipatory maternal effects operating in avian-type systems. First, while in the presence of helpers reductions in maternal investment and short inter-birth intervals are common in both birds (Hatchwell 1999; Dickinson & Hatchwell 2004) and avian-type mammals (Creel & Creel 2002; Sear & Mace 2008), these tend to occur when helper numbers are high and helpers can compensate for such effects (Hatchwell 1999; Sear et al. 2000; Russell 2004; Russell et al. 2008; Sear & Mace 2008). As a consequence, the maternal strategy in these cases appears more consistent with partially reducing the costs of reproduction or redirecting effort to other phases of reproduction (see §5), rather than attempting to produce offspring with cooperative tendencies. In addition, there is no evidence that offspring in avian-type systems are produced with reduced fertility and little evidence of physiological suppression of daughters by mothers generally (Russell 2004; Schoech et al. 2004, but see Creel & Creel 2002).
Although we predict (and evidence supports) that maternal strategies should be anticipatory, on average, we further predict that selfish strategies will occur in two scenarios. First, as indicated above, mothers can employ apparently selfish strategies during certain parts of the reproductive phase in order to save resources for future breeding. In these situations, the effects of these selfish strategies on offspring are expected to be short term and compensated by helpers (e.g. Hatchwell 1999). Nevertheless, simply measuring offspring outcome without due regard for maternal strategies will lead to a misrepresentation of the selective pressures acting on such cooperative breeding systems (see §§5 and 7). Second, we predict that mothers will attempt to produce some helpful offspring as part of a simultaneous mixed strategy, but whether or not this is the case has not been considered. Within and among females (and species), we expect that the latter scenario will arise when outside options for offspring are more limiting, group size is below optimal size, multiple unrelated males are present in the group and/or maternal longevity and tenure length are more closely linked and predictable. For example, reproductive skew theory predicts that outside options will determine the scope for maternal control of reproduction (Emlen 1997; Magrath et al. 2004), but no study to our knowledge has investigated how outside options modify maternal strategies during pre-, early or late reproductive phases (table 2). Evidence from sex ratio studies shows that the helping sex is often overproduced when group sizes are below optimum for the territory (Komdeur 2004), but whether or not the mother influences the cooperative tendencies of recruiting offspring is unknown. Female tenure length will be increased by having multiple unrelated males in the group (Creel & Creel 2002), and there is some evidence to suggest that the predictability of female tenure influences the maternal strategy. In superb fairy-wrens (Malurus cyaneus), in which the adult sex ratio is male biased, females live in single-female groups and female survival is tantamount to retention of dominance, mothers reduce investment in the caloric content of eggs (Russell et al. 2007b) as well as growing offspring (Russell et al. 2008), although such effects are compensated by helpers. By contrast, evidence from chestnut-crowned babblers (Pomatostomus ruficeps), in which adult sex ratios are more equal, females live in multi-female groups and where dominance from one year to the next is not guaranteed by survival, mothers increase investment in eggs in the presence of optimal numbers of helpers (A. F. Russell 2008, unpublished results), although their investment in provisioning young is currently unknown.
In conclusion, we predict that in avian-type systems maternal strategies will be anticipatory, on average, but that both within and among mothers (and species) mixed strategies will exist. We further predict that such mixed strategies will be more commonly simultaneous, rather than sequential, since maternal survival and retention of dominance between years is not necessarily predictable, and that selfish strategies (when they exist) will be restricted to subtle changes in offspring behaviour rather than significant changes to morphology, anatomy or physiology. There are two problems with the current approaches to studies in avian-type systems. First, maternal investment is seldom considered before the late reproductive stage, leading to a misrepresentation of a mother's overall investment strategy, and when it is considered, all the permeations of differential maternal investment are not considered. For example, the effects of maternal reproductive phenology and investment in the early reproductive phases on the cooperative versus competitive tendencies of offspring have not been considered for avian-type systems. Given that we are struggling to account for marked variation in individual contributions to cooperation within social groups of birds (Cockburn 1998), it seems sensible to explore the possibility that maternal effects during pre- and early reproductive phases have a significant effect on offspring development and, through this, influence their cooperative propensities. Second, given the relatively unpredictable nature of the relationship between female strategy and fitness (in avian-type systems relative to other cooperative breeding systems not relative to non-cooperative systems), we suggest that mothers will commonly employ a simultaneous mixed strategy, leading to offspring of varying phenotype within the same breeding attempt. The problem here is that mean outcomes in offspring traits are generally considered and not the variance, and when variance has been considered it is not considered a maternal effect (e.g. Reed & Walters 1996). We suggest a greater appreciation of the variance in offspring phenotype, the role that the mother plays in governing this variance and the potential consequences it has for maternal fitness (see also Crean & Marshall 2009).
(b) Mammalian-type systems
Mammalian-type systems are defined here as those where immigration into groups is relatively common but is male biased. Such systems include almost all mammals and a few birds and cooperative fishes (Greenwood 1980; Clarke et al. 1997; Solomon & French 1997; Dierkes et al. 2005). Mammalian-type systems, in contrast to avian-type systems, are generally characterized by larger group sizes as well as greater degrees of obligatory cooperative breeding, female fecundity and predictability of tenures in the alpha position (Yaber & Rabenold 2002; Russell 2004; table 1b). These differences might stem, in part, from the opposing immigration system. First, in mammalian-type systems, mothers do not need to leave their group following the death of their partner, because partner death is followed by the immigration of an unrelated male into the group. As a consequence, maternal tenure length in mammalian-type systems will be more predictable than those in avian-type systems. Second, and more importantly, mothers have the potential to dictate their own level of reproductive competition within their group, for their reproductive competitors will not be immigrants but the daughters that they produce. Given that mothers will not benefit from being killed or usurped and will often lose fitness from sharing reproduction (Clutton-Brock 1998; Russell 2004), except under exceptional circumstances (Cant & Johnstone 1999), mothers should be under strong selection to reduce the competitive ability (and increase the helpfulness) of their female offspring. This, in turn, might be expected to lead to the larger group sizes and greater maternal fecundity observed in mammalian-type systems (see §7). We predict that in mammalian-type systems, mothers should be under greater selection than in avian-type systems to employ selfish strategies and that mixed simultaneous strategies will be common.
Whether or not mammalian-type systems are characterized by greater selfish maternal effects, on average, than in avian-type systems is unknown. However, the available evidence suggests that, at least prior to the post-reproductive phase, maternal effects are largely anticipatory, on average, in mammalian-type systems, and that selfish maternal effects, when they occur, are more consistent with reducing the costs of reproduction rather than influencing the cooperative tendencies of offspring (see also §4a). For example, if mothers were to attempt to reduce the quality of their offspring when in small groups in order to produce offspring with greater cooperative tendencies (and vice versa in large groups to produce competitive dispersers), we would predict that mothers in small groups (relative to those in large groups) should reproduce earlier and more frequently, should favour offspring quantity over quality and/or should contribute less to offspring rearing. On the contrary, in small groups, females are known to breed later (not earlier) in the reproductive season in cooperative meerkats (Russell et al. 2003a) and are known to breed less (not more) rapidly in a range of species (e.g. Solomon 1991; Powell & Fried 1992; Russell et al. 2003a). In addition, in the cichlid fish, Neolamprologus pulcher, females were found to lay smaller (not larger) eggs in groups of experimentally elevated size, and were not found to change clutch size (Taborsky et al. 2007). Similarly, in mammals, few studies have reported the expected negative relationship between helper number and litter size, although they have also failed to report a positive relationship (Solomon 1991; Powell & Fried 1992; Salo & French 1989; Russell et al. 2003a). Finally, increasing the numbers of helpers is generally associated with reductions (not increases) in the levels of maternal care during late reproductive phases in a range of species (Russell 2004).
There are at least five reasons for the apparent lack of corroborating evidence. First, one possibility is that few studies have attempted to test for selfish maternal effects by considering overall investment during all reproductive phases. For example, in meerkats, while maternal investment is consistent with an anticipatory strategy during some reproductive phases (Scantlebury et al. 2002; Clutton-Brock et al. 2004), overall, mothers wean light offspring in small groups (Russell et al. 2002, 2003), supporting the possibility that mothers employ selfish strategies to reduce outside options of offspring when help is most required. Second, mothers might be constrained from adopting selfish strategies owing to imprinting genes selecting for offspring to procure more resources (Haig 2000). However, we do not expect this to be likely in cooperative species where maternal death results in male dispersal to avoid inbreeding with daughters. Third, mothers might be further constrained from selfish effects if helpers compensate for such effects or groups are co-founded with unrelated conspecifics of the same sex, which produce competitive offspring. Fourth, despite the predictability of tenure as dominant, maternal survival might be insufficiently predictable to select for the production of offspring with reduced reproductive capacity. For example, in naked mole-rats (Heterocephalus glaber), one of the few insect-type mammals (see below), alpha females have unusually predictable (Alexander et al. 1991; Sherman & Jarvis 2002) reproductive tenures and monopolies of reproduction within their group (Faulkes & Bennett 2001). Finally, where offspring contributions to cooperation are condition dependent (e.g. Clutton-Brock et al. 2002; Russell et al. 2003b), mothers might gain few benefits from under-investing in offspring if this causes them to become poor helpers.
While evidence of selfish maternal effects prior to the post-independence period is generally lacking, maternal suppression of offspring reproduction post-independence is common. For example, in a range of mammal species where unrelated males are commonly present in the group, mothers suppress daughters physiologically (Solomon & French 1997; Russell 2004). In addition, in meerkats, evidence shows that mothers might be able to suppress their daughter's growth (Russell et al. 2004) and mothers are known to evict likely reproductive competitors from their group, causing them to abort if already pregnant or, if not, preventing them from conceiving through elevated stress (Young et al. 2006). Although such studies suggest that maternal strategies employed after their daughters reach maturity are partly effective in combating daughter reproduction (Clutton-Brock et al. 2001), it is possible that such apparent maternal control is most effective when mothers employ strategies earlier in the reproductive period, which improves suppressive strategies later, but this is yet to be determined.
In conclusion, we predicted that in mammalian-type systems, mothers might be selected to adopt a more mixed strategy, but the available evidence is currently unsupportive. This might be due to a lack of studies investigating both maternal investment strategies early in the reproductive phase and their outcomes after controlling for other sources of variation. Alternatively, owing to the constraints above, it might seldom benefit mothers to adopt a selfish strategy and, instead, as was the case with avian systems above, simultaneous mixed strategies might be more likely. If this is the case, then a greater appreciation of the role of mothers in creating within-brood/litter variance and its consequences is required. As yet, there is little evidence from cooperative mammals for adaptive sex ratio skews and therefore mothers appear not to attempt to overproduce daughters, possibly owing to the problems of increased competition in the group. It therefore seems more likely that mothers will attempt to influence the cooperative tendencies of their offspring through more subtle means, including hormone transfer. It is noteworthy that both individuals and litters can be highly repeatable in their contributions to cooperation over time (Clutton-Brock et al. 2002); the role of maternal effects in these patterns has yet to be determined.
(c) Insect-type systems
Insect-type systems are characterized by limited or no immigration into established groups by either sex. Such systems are commonly associated with obligate cooperative breeding and caste differentiation as well as extreme group sizes, female fecundity and the predictability of tenure length as queen (table 1c, d). Insect-type systems do not include any fishes or birds, but do include a few species of mammals (naked mole-rats: O'Riain et al. 1996; toothed whales: McAuliffe & Whitehead 2005) and a few species of Synalpheus crustaceans (Duffy et al. 2000), as well as all cooperative insects (thrips, aphids, termites, bees, wasps, ants, beetles) (Wilson 1971; Noirot 1991; Choe & Crespi 1997; Kranz 2005; Bono & Crespi 2006; Korb 2008) and spiders (Buskirk 1981; Whitehouse & Lubin 2005; Lubin & Bilde 2007). Unsurprisingly, given the diversity and size of taxonomic groups included here, there is an immense variation in group size and complexity, ranging from more mammalian-type systems to advanced eusociality with strongly defined queen–worker and among-worker castes (Wilson 1971; Choe & Crespi 1997; Bourke 1999; table 1c,d). As a consequence, insect-type systems allow an unrivalled assessment of the scope for maternal effects in cooperative breeders, the factors that affect the extent and types of maternal strategies employed and their consequences for ecological and evolutionary processes. Here, we split the insect-type systems into primitively cooperative species (table 1c) and more advanced cooperative species (table 1d). This split is largely arbitrary, and is motivated by the fact that a large number of insect-type systems differ little from mammalian-type systems described above wherein the studies of maternal effects prior to the post-independence phase are also largely lacking. We briefly summarize the scope for maternal effects in such systems, before describing in detail maternal effects in more advanced species.
Primitively cooperative species include (table 1c) the following: the cooperative bark and ambrosia beetles (Scolytinae; Kirkendall et al. 1997); one-piece termites (Korb 2008); non-gall forming thrips (Crespi & Mound 1997; Kranz 2005) and aphids (Stern & Foster 1997); Polistinae and Stenogastrinae wasps; Halictinae and Allodapini bees; Ponerinae ants (Bourke 1999; Ratnieks et al. 2006); all spiders (Whitehouse & Lubin 2005); Synalpheus crustaceans (Duffy et al. 2000); and among mammals, naked mole-rats (O'Riain et al. 1996, 2000) and some toothed whales (McAuliffe & Whitehead 2005). To our knowledge, maternal effects prior to the post-independence period have rarely been considered for any species included here. Instead, as is the case with cooperative systems outlined above, most studies have considered maternal control strategies during the post-independence phase. For example, during this phase, mothers appear to suppress offspring growth in ponerine ants (O'Donnell 1998) and naked mole-rats (O'Riain et al. 2000). In addition, mothers can evict competitors (allodapine bee Exoneura robusta, Bull et al. 1998), kill them (naked mole-rats, Sherman et al. 1991) or have them killed by workers (ponerine ants Dinoponera quadriceps, Monnin et al. 2002). That reproductive conflict can clearly exist in such species (Ratnieks et al. 2006) suggests that if maternal effects occur during early reproductive phases in such systems, they are largely inadequate to wholly control offspring phenotype. The obvious question is why do mothers not employ more extreme strategies earlier in offspring development to reduce competition with them later? This question is particularly valid, given that helpers are known to have significant influences on the reproductive capacity of offspring by various forms of mutilation soon after birth in insects (Liebig et al. 1999; Ratnieks et al. 2006). Potential explanations provided above for mammalian-type systems might offer an answer (see also §7).
In conclusion, studies of maternal investment strategies employed prior to offspring dependence are lacking in primitively cooperative insect-type systems. Broadly, we predict that mothers of such systems will adopt a mixed strategy with a greater propensity for a sequential element than in avian- or mammalian-type systems. This is because while groups start off small, they can reach hundreds of individuals in some species, with the production of competitors too early significantly reducing a group's capacity to grow and a female's capacity to maximize fitness. It is conceivable that individual differences in cooperative tendencies (O'Riain et al. 1996; Field et al. 2006) might arise, in part, from maternal effects, but these have not been considered. The species included here offer considerable opportunities for the study of maternal effects. First, they represent a continuum with more socially advanced species discussed below. Second, there is substantial variation in group size, complexity and caste differentiation. Third, ecology varies enormously, with differences in food proximity and predation risk. Fourth, offspring reproduction and investment in allocare is highly variable (e.g. in termites, workers provide little care (Korb 2008), while in spiders, mother and helpers allow themselves to be consumed by developing offspring (Salomon & Lubin 2007)). Fifth, some species can reproduce parthenogenically (aphids, Stern & Foster 1997), others are haplodiploid (hymenoptera and thrips, Crespi & Mound 1997), and the rest are diploid. Consequently, there is clearly substantial scope for research in such systems to test some of the ideas presented here and elsewhere regarding maternal effects (e.g. Linksvayer & Wade 2005; Marshall & Uller 2007; Badyaev 2008; Schwander et al. 2008).
Advanced insect-type systems include (table 1d) the following: multi-piece termites (those that forage outside their chamber; Korb 2008); gall forming thrips (Crespi et al. 1997) and aphids (Stern & Foster 1997); and all other cooperative hymenoptera (Bourke 1999; Ratnieks et al. 2006). These species are associated with eusociality and highly predictable female tenures as queen. Predictable tenures appear to result, in part, from life in the relative safety in various forms of confines and reduced threat of predation due to being emancipated from all forms of foraging, at least after the first worker is produced (Keller & Genoud 1997; Thorne 1997; Peeters & Ito 2001; Jemielity et al. 2005). Given both the lack of immigration and the predictability of female tenure as queen, in conjunction with significant and predictable temporal heterogeneity in the advantages of producing workers versus queens, we would expect that queens in eusocial-type systems, in contrast to the other three, will show a clear sequential mixed maternal strategy.
Indeed, the most remarkable aspect of advanced insect-type systems is the astonishing variation in form and function within and across species. In extreme cases, queens and workers might differ in size, longevity and fecundity by orders of magnitude and/or differ morphologically and anatomically in hundreds of characters (Wheeler 1986; Hölldobler & Wilson 1990; Crespi & Mound 1997; Stern & Foster 1997; Jemielity et al. 2005; Kranz 2005; Miura 2005). In addition, this variation appears to arise largely sequentially, with workers being produced before queens. The question is, how is this variation achieved? Early suggestions generally found favour with the idea that it was determined largely by differential provisioning of food by workers (Wilson 1971). However, more recently, it now appears that there is likely to be a significant genetic element in a broad range of species (Anderson et al. 2008; Smith et al. 2008). This is not to say that castes are necessarily determined genetically per se (although they can be in a few species), but more that castes are determined by gene×environment interaction. We propose that the primary environment in many cases will be maternal in origin, and less so a worker one as is traditionally suggested (Beekman & Ratnieks 2003; Ratnieks et al. 2006). We have three reasons for this suggestion.
First, it is usual for queens to control or signal to workers through aggression and/or pheromones (Wheeler 1986; Keller & Nonacs 1993). For example, while in honeybees (Apis mellifera) the workers provide the royal jelly that causes offspring to become queens, they do so largely on the current queen's signal (Wilson 1971). It seems to us more straightforward to invoke the ultimate controller/signaller rather than the simple messenger or implementer as the instigator of variation in offspring trajectory. Second, the workers that the queen controls/signals are generally her own offspring. At the extreme, it is conceivable that the ‘obedience’ of her working offspring is determined by the queen earlier during the worker's development and, at the least, the queen is influencing her own offspring post-independence, which itself is a maternal effect. Finally, there has been a general neglect of the role of pre- and early reproductive phase investment (i.e. when queens can act directly on developing offspring) in eusocial-type systems (Schwander et al. 2008); we believe that this stems, in part, from the belief that offspring development is governed almost wholly post-hatching through food and pheromones. While it is clear that the post-hatching environment is often important in determining offspring development, this does not mean that pre-hatching effects are non-existent or unimportant (Wheeler 1986; Schwander et al. 2008). Indeed, the realization that caste determination can often have a genetic element (Anderson et al. 2008; Smith et al. 2008) leads to a virtual inevitability that offspring trajectory will be initiated by the egg environment that is determined by the mother. Below, we consider the effect that queens have on their offspring directly as a direct maternal effect and the effects that workers have on offspring when acting with queen instructions as an indirect maternal effect.
Direct maternal effects occur during all reproductive stages in eusocial-type systems. For example, that queens tend to be the mother of workers and, through aggression and pheromones, alter their reproductive capabilities and behaviour, sometimes irreversibly (Wheeler 1986), is consistent with a selfish maternal effect during post-independence. Pheromones too can influence offspring development during the late reproductive phase. In termites, caste determination appears to be driven largely by the effect of queen pheromones on ecdysterone and juvenile hormone during larval development (Wilson 1971). Similarly, in both bumble-bees (Bombus terrestris) and some ants, queen pheromone can directly suppress the development of larvae into potential queens, particularly at certain times of the year (Wheeler 1986).
The most unambiguous maternal effects, however, are those that occur during the early and pre-reproductive phases, for these cannot be influenced by the direct effects of workers, at least in behavioural time. Although hormones are known to play an important role in influencing castes in higher termites (Nijhout & Wheeler 1982; Miura et al. 2003) and there is some evidence to suggest that caste outcome is influenced in the egg (Wheeler 1986; Roisin 2000), the primary evidence comes from ants. Queens and workers are known to come from differently provisioned eggs in the ant Formica polyctena. In early spring, queens of this species increase their production of potentially reproductive offspring by laying eggs that are larger, contain more RNA, have larger polar plasms and have nurse cells with larger nuclei (Wilson 1971; Wheeler 1986). Furthermore, it is known for the ant Pheidole pallidula that queens seal offspring outcome hormonally in the egg, in part, with additions of juvenile hormones, such that individuals hatch as either potential queens or workers (De Menten et al. 2005). Finally, in a recent experimental study on Pogonomyrmex harvester ants, Schwander et al. (2008) showed that eggs that give rise to queens have lower levels of the hormone ecdysterone (moulting hormone). To our knowledge, it is currently unknown to what degree differential provisioning of eggs causes caste differences through gene interactions versus simply representing differential provisioning of eggs that are already destined to become queens or workers genetically. Indeed, some egg substances could have causal influences on offspring outcome and others might have accessory functions that benefit the queen or the developing offspring depending on the caste developing. Either way, the evidence suggests that the scope for direct maternal effects in eusocial-type systems is significant and that they tend to be acting already in the early reproductive phase.
Indirect maternal effects require that queens control/signal worker behaviour and that worker behaviour influences caste determination. There is abundant evidence to suggest that queen–worker castes are influenced by post-embryonic food (quality, amount or type) provisioning, particularly at certain times of the year (see below). However, it is important to note that despite this evidence, none of it necessarily wholly counters the possibility that caste determination has a genetic element or that maternal effects are not already at work pre-hatching. Indeed, it is likely that if a queen would benefit from workers rearing a queen, she will already provision the egg accordingly, and simply require that the workers provision food to maintain offspring on the predetermined trajectory. In several species with queen-worker castes, pheromones from the queen determine when larvae should be reared into queens or workers. Such species include Monomorium pharaonis (Wilson 1971), as well as Myrmica rubra (Brian 1980), Plagiolepis pygmaea (Passera 1980), A. mellifera (Seeley 1985), Linepithema humile (Passera et al. 1988) and Solenopsis invicta (Vargo & Fletcher 1986). In species that live in smaller groups, pheromones seem able to diffuse around the colony, but in the species that live in large colonies or where queens are restricted to a certain area, other mechanisms of pheromone transfer are found. For example, in honeybees, messenger bees carry queen pheromones around the colony (Naumann et al. 1991), while in Camponotus floridanus ants, pheromones are carried around the colony in the form of hydrocarbon signatures on eggs (Endler et al. 2004). Thus, indirect maternal effects are consequently also of significant importance in eusocial-type systems and appear largely to function as signals to workers to differentially provision offspring (Wilson 1971; Keller & Nonacs 1993).
In conclusion, therefore, eusocial-type systems provide profound evidence for the potential role of maternal effects in influencing form and function in cooperative breeders. Through differential provisioning of eggs and offspring, queens can influence whether their offspring will function primarily as a helper or as a reproductive, and with it, a whole host of differing morphological, anatomical and physiological traits. Exciting avenues for future research will be to determine the following: the degree to which castes are determined by maternal effects across systems living in different ecological situations and with different numbers of queens; the roles that maternal effects play in influencing the outcome of maternal-offspring conflict and the sensitivity of offspring to queen signals; and whether maternal effects influence the altruistic versus selfish tendencies of offspring quantitatively as well as qualitatively. Nothwithstanding, it is clear that the earlier a queen acts during an offspring's development, the greater the potential control she will exercise (Wheeler 1986; Strassmann & Queller 2008). In addition, by exercising control early, a queen might increase the scope for more efficient division of labour among worker castes. Wheeler (1986) pointed out that the most successful ants are those with dimorphic and polymorphic worker castes, and suggested that the evolution of such castes will be more likely when offspring yield early to the fact that they will not become a reproductive.
5. Maternal effects as confounds of fitness estimates
In order to understand selection on the evolution and maintenance of cooperative breeding, it is essential to have an accurate estimate of helper fitness. Studies of avian-, mammalian- and primitive insect-type systems have been at the forefront of attempting to measure the fitness benefits of helping, mainly because the facultative nature of many of these systems allows one to measure breeder fitness with and without helpers (Bourke 1997; Dickinson & Hatchwell 2004; Russell 2004; Salomon & Lubin 2007). However, it should at least be possible to measure helper fitness in advanced insect-type systems that are annual, although doing so in perennial systems will be a challenge. As we have indicated above, maternal effects might be more prevalent in cooperative breeding systems than we have given credit for and that such effects might arise during pre- or early reproductive phases. Here, we provide an example from each phase as to why neglecting the possible role of maternal strategies during early phases can be problematic for our understanding of the adaptive significance of helping in cooperative breeding systems, even where maternal strategies do not permanently alter offspring phenotype.
(a) The case of inter-birth intervals
It is a common phenomenon across multi-brooded cooperative breeders that females have reduced inter-birth intervals in the presence of helpers/workers (Wilson 1971; Bourke 1999; Dickinson & Hatchwell 2004; Russell 2004). In insect-type systems, this can be extreme, given the fact that queens might produce 10 000 eggs per day (Wilson 1971) and will certainly be caused in part by worker presence (Roux & Korb 2004). However, in avian- and mammalian-type systems, the degree to which helpers cause reduced inter-birth intervals is less widely considered, and greater numbers of helpers might arise as a consequence rather than a cause of short inter-birth intervals. One of the problems concerns how to assign cause and effect in cooperative breeding systems. One option is to select females that have lost helpers, gained helpers and done neither, and compare the changes in breeding phenology (sensu Cockburn et al. (2008), but see Wright & Russell (2008) for discussion). Another option is to remove group members and compare the outcome (e.g. Brown et al. 1982; Solomon 1991; Powell & Fried 1992), but here one should be cautious about interpreting the results of such experiments because they change group size as well as helper number (Cockburn 1998; Russell et al. 2008).
A less appreciated problem is exemplified by the first helper removal experiment conducted in a cooperative vertebrate (Brown et al. 1982). Brown and colleagues removed helpers in cooperative grey-crowned babbler (Pomatostomus temporalis) and observed a reduction in reproductive success. The conclusion was that by allowing mothers to reduce investment in one breeding attempt, helpers caused mothers to save sufficient resources to breed again in the same season (i.e. to have reduced inter-birth intervals, see also Brown et al. (1978)). Given this conclusion, one can attribute differences in productivity in groups of different sizes to be caused by helpers, and calculate fitness benefits of help accordingly. Since this study, the idea of ‘load-lightening’ (Brown & Brown 1981) has been expanded to include the possibility that helpers cause mothers to save sufficient resources to improve survival prospects between reproductive seasons. Load-lightening has become one of the more commonly cited helper consequences for breeders in cooperative breeding systems, and has become deeply entrenched in our thinking (Crick 1992; Hatchwell 1999; Russell 2004; Quinlan & Quinlan 2008; Sear & Mace 2008). While helpers are clearly associated with reductions in breeder care of offspring, it does not automatically follow that all fitness consequences of this should be attributed to helpers.
For example, life-history theory (Williams 1966; Stearns 1992) and the differential allocation hypothesis (Burley 1986, 1988; see also its extension: Sheldon 2000; Harris & Uller 2009) predict that females should increase investment when the current reproductive value is higher than expected, on average, and reduce it when the reverse is true. Given that helpers provide females with a reliable estimate of the amount of food her brood will receive leads to a situation where females should increase investment when helper numbers are relatively high and decrease it when they are relatively low (see Wright (1998); Woxvold & Magrath (2005) for supporting evidence with clutch size). While observations of reductions in maternal contributions to brood provisioning (Hatchwell 1999; Russell 2004), for example, appear at odds with this idea, this is not necessarily the case. This is because a reduction in brood provisioning might be a consequence of high reproductive costs during a previous phase of reproduction (e.g. egg laying) or might be associated with high concurrent investment in a subsequent phase (e.g. mothers might be already mobilizing resources for the next brood). The point is that because maternal investment is seldom measured during all reproductive phases, it is seldom possible to evaluate whether load-lightening arises as a consequence of a helper strategy or a maternal strategy. One way to determine whether maternal investment is governed by helper or mother is to conduct a brood size manipulation experiment and measure the degree to which mothers versus helpers change their investment in the current attempt as well as the degree to which these changes influence maternal (inter-birth interval, clutch size and provisioning) versus helper (provisioning) investment in the subsequent attempt. A key prediction of load-lightening would be that for groups of increasing size, increases in brood size would cause reduced increases in investment by mothers compared with individual helpers, since mothers, in the presence of multiple helpers, benefit by saving resources for the future.
Another key difference between the two hypotheses concerns helper effects on maternal survival. Under load-lightening, a positive relationship should be observed between helper number and maternal survival, while under differential allocation relatively high numbers of helpers for a given territory should be associated with a reduction in maternal survival; no difference in maternal survival might be expected if both load-lightening and differential allocation hypotheses are operating. No study has tested the effects of helpers on maternal survival experimentally, and many observational studies are confounded by group size, maternal and habitat quality (Dickinson & Hatchwell 2004). However, distinguishing whether apparent load-lightening is a consequence of a helper versus maternal strategy can have significant implications for the calculations of helper fitness and our understanding of the complexity of cooperative societies. For example, under load-lightening, differences in fitness between groups of varying size should be apportioned to the helpers since it is their behavioural strategy that causes the effect in the mother. By contrast, under life-history-type hypotheses, the strategy of interest is maternal in origin and hence at least some of the fitness differences should be apportioned to her strategy. We will return to the issue of load-lightening versus differential allocation more generally below (§7), for we believe that it could have significant implications for who has control in cooperative societies as well as the rise of social complexity in cooperative systems.
(b) The case of cryptic investment
Egg layers have considerable advantages for the investigations of maternal effects, since all aspects of maternal investment during the early reproductive phase can be measured with precision. Evidence provided above (§4c) shows the potential for egg size and constituents to affect offspring outcomes in advanced insect-type systems. By contrast, there is little such evidence in other egg-laying species. This dearth is surprising, given the considerable evidence from non-cooperative birds, for example, showing significant among- and within-female variation in both overall energetic investment (Williams 1994; Bernardo 1996; Christians 2002; Wagner & Williams 2007) and investment in micro-constituents, particularly antioxidants (Royle et al. 2001; Blount 2004), immunoglobulins (Karell et al. 2008) and hormones (Schwabl 1993; Sockman et al. 2006; Groothuis & Schwabl 2008).
Outside of advanced insect-type systems (see above), we know of only one published study that has considered egg sizes in a cooperative fish (Taborsky et al. 2007) and one in a cooperative bird (Russell et al. 2007b). In the cichlid fish, N. pulcher, experimental increases in group size caused reductions in the size of eggs laid by the breeding female, but no change in clutch size. This reduction in egg size was suggested to arise because mothers were able to save resources without paying costs in the presence of many group members, for fry hatching from small eggs had lower predation in larger groups. In superb-fairy wrens, females breeding in the presence of helpers, again without changing clutch size, laid smaller eggs of lower nutritional content that gave rise to smaller chicks. Eggs were 5 per cent smaller and contained 13 per cent less protein and 12 per cent less lipid. Helpers compensated for such effects through increased nestling provisioning and mothers benefited through 30 per cent improved survival to the following year. Cross-fostering experiments showed that neglecting this reduction in maternal investment at the egg stage wholly obscures the helper provisioning effect on chick mass in this species, leading to an underestimation of the selective benefits of helping. In addition, we have recently found that maternal investment might be more complicated in the fairy-wrens than we previously supposed, for it appears that eggs laid in the presence of helpers, although of lower nutritional content, have 18 per cent higher concentrations of carotenoids (after controlling for differences in egg volume; A. F. Russell & L. B. Astheimer 2008, unpublished results). One explanation is that offspring that come from smaller eggs will incur greater oxidative stress during catch-up growth and require increased antioxidants to combat this (Blount 2004). Finally, we have found recently in chestnut-crowned babblers (Pomatostomus ruficeps) that females lay eggs that are 4 per cent larger in the presence of optimal helper numbers compared with no helpers, and that these eggs produce chicks that are significantly heavier at hatching and following cross-fostering are significantly heavier at fledging (A. F. Russell 2008, unpublished results).
Consequently, if investment in the early reproductive phase is neglected, one stands a significant chance of miscalculating the fitness benefits of helping (underestimating in the case of fairy-wrens and overestimating in the case of babblers; Russell et al. 2007b; A. F. Russell 2008, unpublished results). The babbler result is clearly consistent with the predictions of differential allocation (Cunningham & Russell 2000; Sheldon 2000): females live in multi-female groups and have no guarantee of being dominant between years, so should reproduce maximally in the presence of optimal numbers of helpers. By contrast, the egg size results in the fishes and fairy-wrens appear to be more consistent with load-lightening (Brown & Brown 1981). However, under load-lightening, maternal reductions are expected to be a flexible behavioural response to helper contributions (e.g. Hatchwell & Russell 1996; Russell et al. 2008). This is not the case for either the fish or wren results where females adjust egg investment prior to receiving any help, and in the case of fishes, helpers do not provide any care to developing offspring. An alternative is that they both represent a form of differential investment, where mothers reduce investment for future benefit without suffering current costs (Russell et al. 2007b; Taborsky et al. 2007), rather than the more usual case of increasing the current benefit and suffering the future cost (Sheldon 2000). Either way, more studies of egg packaging are clearly required to gauge the causes and consequences of maternal investment strategies during early reproductive phases, as well as the implications such maternal investment has for indications of power asymmetries and calculations of fitness.
6. Lessons from human studies
Humans are clearly a cooperative breeder, indeed one could argue that they are among the most advanced cooperatively breeding vertebrates: not only do they have pre-reproductive helpers (Sear & Mace 2008) but they are also one of the very few with a clear and irreversible worker caste in the form of sterile grandmothers (Foster & Ratnieks 2005). Here, we consider two aspects of maternal effects that are well studied in humans, but poorly represented in other species. These include the role of maternal signalling in governing offspring phenotype and the scope for maternal effects to span generations. Both are of interest: the role of signalling can elucidate the outcomes of mother–offspring conflict (Keller & Nonacs 1993) and the scope for transgenerational inheritance of maternal effects will influence the role of maternal effects in influencing evolutionary change (Uller 2008). Despite this, to our knowledge, no study has considered the ecological and evolutionary implications of maternal effects for cooperative systems outside of the eusocial insects (Wade 2001; Linksvayer & Wade 2005; Linksvayer 2006), and even here, the role of signalling is under-represented (Keller & Nonacs 1993). Thus, the results of human studies provide invaluable insights into the potential mechanisms of maternal effects and the potential role of maternal effects for influencing evolutionary change in a cooperative vertebrate.
(a) The role of signalling
In an influential paper, Keller & Nonacs (1993) pointed out that signalling via pheromones might more accurately reflect mother–offspring communication rather than maternal control in the eusocial insects. Correctly, in our mind, if adhering to information via pheromones were not adaptive for workers, they should be selected to employ counter-strategies that cause their indifference to pheromones. There is one potential caveat, however: if mothers were to use pheromones mostly as honest signals but sometimes as dishonest ones, the costs of workers attempting to distinguish between the two might be prohibitively high. This would also be the case of maternal signals in eggs and developing young.
Maternal signalling in humans is suggested to arise because of the benefits to offspring of setting their physiology early in foetal development to match the current conditions. In the foetal programming hypothesis, Lucas (1991) suggested that changes in nutrient and hormonal conditions during crucial periods of rapid cell division could alter foetal gene expression, leading to permanent changes to physiological processes. For example, offspring with impaired nutrition might ‘interpret’ their environment as being nutrient poor and, irreversibly, set themselves up for shorter stature, which could be adaptive in such conditions (Barker 1994; Bateson 2001). Evidence for this idea primarily comes from medical research which shows that where mismatches between early and later life conditions are pronounced, individuals are far more likely to succumb to health problems in adulthood (Hales & Barker 1992; Barker 1994). Thus, the idea is that if the outlook is poor, mothers would benefit by signalling this during gestation so that offspring can permanently develop ‘thrifty’ traits because they ‘expect’ the environment they will experience post-birth and beyond to be qualitatively similar (Gluckman et al. 2005). As such, a parsimonious explanation for induced thrifty traits in humans is an honest maternal strategy, by which mothers produce offspring that make metabolic demands that can be met (Wells 2007). Mothers that have a compromised ability to provide energy to a growing infant will do better to produce an infant with low requirements.
Recent debate on the existence and importance of such ‘predictive adaptive responses’ by offspring has centred on the inherently unpredictable nature of conditions throughout an animal's life and questioned the value of adopting irreversible trajectories early in development (Rickard & Lummaa 2007). However, given that a significant determinant of reproductive success in humans, and other cooperative species, is care by kin (Sear & Mace 2008), it is possible that the availability of such care will increase the adaptive value of maternal signalling and offspring responses. This is because, in cooperative species, environmental stochasticity will be partly buffered and the current and future conditions more predictable in the presence of carers.
Two aspects of this hypothesis are interesting with respect to cooperative species. First, the ideas presented here would predict that in the absence of helpers (i.e. rearing conditions relatively poor), mothers might benefit offspring by making them small, for large offspring might succumb more rapidly when conditions are unfavourable. At the same time, small offspring are known to have reduced probabilities of finding a partner and having reduced reproductive success (Phillips et al. 2001; Lummaa & Clutton-Brock 2002). Thus, because small offspring will have fewer outside options, they might benefit more from being cooperative rather than attempting to be competitive. In other words, even through honest signalling, mothers might be able to enhance the likelihood of rearing a helper, with both mother and offspring benefiting as a consequence. Second, however, the ideas also underscore the capacity for maternal control in cooperative systems: offspring that begin small, owing to reduced maternal investment, might not benefit from attempting to eat more to increase in size later. For example, a mother breeding in unusually favourable conditions might benefit by rearing a cooperator to help when conditions deteriorate. If mothers were able to undernourish her offspring, she could conceivably deceive it into perceiving poor conditions. Although this strategy would only be stable if employed sufficiently infrequently, the point is that the offspring's counter-strategy of catch-up growth following birth would appear to be unprofitable (Barker 1994; Bateson 2001; Metcalfe & Monaghan 2001; Gluckman et al. 2005).
(b) Maternal effects on multiple generations
Maternal effects might be expected to have greater evolutionary consequences if they are inherited through at least one generation. In eusocial hymenoptera, this is clear: workers can seldom develop fully functioning ovaries (Beekman & Oldroyd 2008) and cannot produce queens. In extreme situations, they do not have any reproductive capacity at all, and so no direct contribution to the gene pool (Ratnieks et al. 2006; Smith et al. 2008). However, the ‘inheritance’ of maternal effects is unknown for diploid cooperative breeders outside humans. In humans, the weight of a mother at birth as well as her growth and development during early life can have significant influences on the birth weight of her own offspring (Wells 2007). For example, foetuses that were exposed during the last trimester of their mother's pregnancy to the Dutch famine following World War II themselves produced offspring with lower birth weight (Lumey 1992). Mechanistic evidence from medical studies shows that maternal stress, blood pressure and general health influences placental function and, through this, influences offspring birth weight (Wells 2007).
We expect the potential for helper-mediated maternal effects in humans under natural conditions to be high, for maternal exposure to both famines (Stanner & Yudkin 2001; Stein et al. 2004) and supplemental feeding programmes (Christiansen et al. 1980; Prentice et al. 1983; Ceesay et al. 1997) can affect the condition of their own offspring at birth. Evidence exists to suggest that both pre-reproductive helpers and post-reproductive grandmothers influence breeder reproductive success. For example, pre-reproductive helpers, through reducing the reproductive costs of the mother, can be associated with increased fecundity and/or offspring survival (Hill & Hurtado 1996; Bereczkei 1998; Draper & Hames 2000; Crognier et al. 2001; Sear et al. 2002). Furthermore, it is known that helping grandmothers can cause helper-mediated maternal effects. One of the primary ways in which post-menopausal females help is by reducing the workload of, and supplementing the resources to, their pregnant and lactating offspring (Sear & Mace 2008). In addition, it is known that offspring are in better condition when reared by a mother and grandmother (Sear et al. 2000, 2002). It is therefore likely that one way in which this effect arises is through improving maternal condition.
Finally, it is conceivable that selection for maternal effects can be strong enough to select for increased lifespan. Given that human offspring are born helpless, have a long period of dependence and benefit from the presence of help, it has been suggested that mothers that survive long after the birth of their final offspring would gain greater fitness through securing the survival and future reproductive success of those offspring (Williams 1957; Hawkes et al. 1998; Lee 2003). Whether or not this is the case is still contentious, but we have shown in pre-industrial Finns and Canadians that mothers gain two extra grandchildren for every 10 years they survive beyond 50 years (Lahdenperä et al. 2004). This effect arose because in the presence of mothers, offspring reproduced earlier, more rapidly and more successfully than those reproducing in the absence of their mother, either because she had died or because she lived in a different neighbourhood. Thus, evidence from humans not only shows that maternal effects acting during the early reproductive phase are strong enough to span generations, but also potentially indicate that maternal effects can be strong enough to cause increased longevity.
7. Role of maternal effects in cooperative evolution and directions for future research
In this paper, we have illustrated the potential scope for investigating maternal effects in cooperative breeding societies. In particular, we have provided rationale regarding why and when maternal effects would be expected to be operating and presented supporting evidence where possible. We have suggested that a neglect of maternal effects acting during certain reproductive phases might confound estimates of helper fitness and cloud our understanding of selection on cooperative breeding. While fewer studies have considered maternal effects in cooperative breeding systems than in other systems, the available evidence, particularly from eusocial insects and humans, suggests that maternal effects can have significant effects on a host of offspring traits and that these effects are sufficient to span generations. This latter point leads to the question, have maternal effects had a role in shaping the evolution and complexity of cooperative breeding systems? In §7a, we discuss the role of maternal effects in the evolutionary origin of cooperative breeding, its maintenance and the rise of complexity. In §7b, we provide some directions for future research.
(a) A role for maternal effects in the evolution of cooperative breeding
Scope for mother–offspring conflict clearly exists in cooperatively breeding systems (Bourke 1997; Emlen 1997; Ratnieks et al. 2006). The first question is, to what degree could mothers achieve the phenotype of offspring required to maximize their own fitness? A lack of outside options for offspring (Emlen 1982; Koenig et al. 1992; Lacey & Sherman 1997; Arnold & Owens 1998; Field et al. 1998) might lead to mothers gaining greater power (Alexander 1974; Beekman & Ratnieks 2003), with small changes in maternal strategy (table 2) potentially having large consequences in influencing outside options available to offspring. In addition, maternal strategies can be unusually varied and dynamic in cooperative breeders (table 2): while a mother might benefit from producing helpers in a certain group size (i.e. adopt a selfish maternal effects strategy), in others she might benefit from producing highly competitive dispersers (i.e. producing offspring close to their optimum, hence adopting an anticipatory strategy) (e.g. Komdeur 1994). The scope for offspring to evolve counter-strategies when mothers are selected to alter the variance, not the mean, in offspring phenotype is unclear, particularly bearing in mind that in cooperative breeders, offspring from the former investment strategy will be easily out-competed by offspring from the latter investment strategy when it comes to competition for breeding opportunities.
Nevertheless, all models of parent–offspring conflict in uni- and biparental care systems predict that offspring should evolve strategies to counteract attempts at maternal control of their fitness, should this differ from their own optimum (Trivers 1974; Godfray 1995; Parker et al. 2002; Smiseth et al. 2008). It is therefore inconceivable that offspring counter-strategies will not exist. For example, a simple counter-strategy for offspring would be to abstain from helping, although the success of this strategy will be limited if competition over breeding positions is intense and the only way of being assured fitness is by complimenting the maternal strategy and helping. A more extreme possibility would be for offspring to kill their younger siblings in order to improve personal condition and scope for direct fitness. We suggest below that cooperative breeding could either evolve from selection in maternal toleration of offspring philopatry or selection in offspring manipulation by mothers (see Linksvayer & Wade 2005). In the former, mothers will generally be in control and we predict that cooperative systems will commonly remain simple. In the second situation, perhaps counterintuitively, we believe that offspring will have substantial scope for dictating maternal strategy, leading to greater scope for the rise of social complexity in cooperative systems.
Cooperative breeding has multiple evolutionary origins and it is doubtful that the same factors are involved in all cases. Unsurprisingly, a large number of (non-mutually exclusive) models have been proposed to explain both the evolutionary origins and the rise of complexity, including kin selection (Hamilton 1964), ecological constraints (Emlen 1982; Koenig et al. 1992), life-history models (Rowley 1965), reproductive skew (Emlen 1997; Magrath et al. 2004; Hager & Jones in press), parental manipulation (Alexander 1974) and indirect genetic (maternal) effects (Wade 2001; Linksvayer & Wade 2005). The results of all such models are likely to have varying degrees of relevance, and our suggestions are not alternatives but compliments. In addition, our suggestions are necessarily speculative at this stage and serve primarily to illustrate how maternal effects could lead to the evolution of cooperative breeding and how social complexity is likely to be a consequence of offspring having a significant bearing on maternal strategy. An important point in this regard is that while conflict is likely to be significant in cooperative systems, ultimately, cooperative breeding will be most common, stable and complex where the conflicting interests of all parties are resolved close to each other's optima within the framework of the existing constraints.
Maternal effects are largely unconsidered in the evolutionary origins of cooperative breeding in vertebrates (Craig 1979; Jamieson 1989), but have been considered in insects (Linksvayer & Wade 2005; Linksvayer 2006). Bourke (1999) suggested that a precursor to complexity in cooperative systems is large group sizes while Hughes et al. (2008) inferred that monogamy might also be important. We noted above that numerous constraints exist on maximum group size; if constraints set group size to below a given threshold, social complexity is unlikely to evolve. In addition, we also noted that mothers will seldom benefit from producing helping offspring in avian-type systems for mothers do not produce their own competitors. As such, we expect that avian-type systems will be generally simple and division of labour will be uncommon. Here, we assume that (all else being equal) maternal fitness will be maximized by mothers using helpers to save resources for the future (i.e. load-lightening; §5) and that offspring (i.e. helpers) will benefit from mothers increasing their current allocation in their presence (i.e. differential allocation; §5). In the case of mothers, this is because maternal survival is enhanced by having helpful offspring and reproductive tenure length is one of the greatest contributors to maternal fitness in cooperative breeders (e.g. Bourke 1999; Clutton-Brock et al. 2006). In the case of offspring, this is because helpers are likely to benefit most through kin-selected benefits if mothers produce many, low-quality offspring in the current attempt: clearly if mothers produce few offspring or invest heavily in those offspring, not only are few kin selected benefits available to offspring through a helping strategy, but also the production of high-quality competitors or dispersers will be detrimental to the future indirect and direct fitness gains of current helpers (see the legend of figure 2 for more discussion).
Figure 2.
Hypothetical models for the evolution of cooperative breeding and the rise of complexity with (a) maternal control and (b) offspring control. In situation (a), consider offspring fitness is enhanced more by staying at home than maternal fitness, e.g. because of ecological constraints on breeding. If mothers never tolerate offspring or cannot resist offspring, offspring always leave or stay without helping, respectively. Cooperative breeding will evolve if maternal toleration is enhanced by offspring evolving cooperation. Mothers have four options in response to helpful offspring. If maternal tenure is predictable and maternal fitness is enhanced by producing high-quality offspring (as is likely in ecological constraints), mothers would benefit most from option (A) and/or (B) (bold lines). This could lead to a conflict of interest because helpers will often benefit from mothers producing many, low-quality offspring (i.e. option C) since such offspring will themselves have reduced outside options and so stay in the group, compete less for reproduction within the group and lighten the load of the helper. In addition, option C might allow helpers to maximize indirect fitness while waiting for an opportunity to breed (Gadakar 1990). However, mothers are in control and will evict offspring that counters the most adaptive maternal strategy. Thus, although cooperative breeding can evolve through this scenario, complexity is unlikely to arise, because there is little selection on mothers increasing current fecundity and hence group size, which is the precursor to increasing complexity (Bourke 1999). In scenario (b), mothers would benefit from having offspring that stay at home and help while offspring would benefit from dispersing to breed. Maternal effects are likely to be instrumental in achieving helpful offspring in the first place. In order to retain offspring, mothers can either offer them staying incentives (path 1) or ‘control’ them by reducing their outside options (path 2, table 2). Staying incentives can be either direct (1E) or indirect (1F). Direct staying incentives (in the form of shared reproduction) are unlikely to be stable because no individual can gain control and cooperative systems are thus likely to either break down or remain simple. Indirect staying incentives (in the form of indirect fitness benefits from helping kin) would lead to offspring gaining the upper hand because they would simply leave if mothers were not able to provide them with sufficient scope for gaining indirect fitness. All else being equal, sufficient scope is likely to arise in the form of increased current fecundity, which in turn could lead to selection for further helpers, further increases in group size and potentially an increase in complexity (obviously depending on constraints on group size and maternal fecundity). However, the evolvability of this route is unclear. Maternal control (path 2) of offspring will be best achieved by honest under-investment, since if under-investment is dishonest, offspring will be selected to procure withheld resources. Honest under-investment can arise if mothers increase current fecundity (owing to quality–quantity trade-offs). Under maternal control of offspring, mothers in the presence of helpers have the same four options as in (a) (here 2A–D), but this time mothers might already be committed to path 2C, since their ability to achieve helpful offspring in the first place is reliant upon having high fecundity. If mothers attempt any of the other three routes, offspring will be reared in good condition and will subsequently disperse (because offspring are ultimately in control).
Consider the situation where offspring are ecologically constrained from independent reproduction, and that offspring benefit (more than the mother does) from remaining at home (figure 2a). If mothers never tolerate offspring, or cannot resist them, then cooperative breeding will/need not evolve. Cooperative breeding might be expected if maternal toleration of offspring is enhanced by offspring evolving cooperative tendencies, which may or may not be facilitated by maternal effects. The point here is that mothers are in control and only helpful offspring are tolerated and, consequently, maternal strategies are likely to operate closer to the maternal optimum, which is unlikely to lead to social complexity because mothers will reduce effort and save resources for the future (i.e. they will not increase group size, Bourke 1999).
By contrast, consider the reverse situation, mothers benefit more from having cooperative offspring and offspring benefit more from dispersing to breed (figure 2b). In this situation, maternal effects are likely to be instrumental to the ‘evolution’ of helpful offspring. However, an evolutionary arms race between mother and offspring would ensue, with mothers attempting to keep offspring in the group and offspring attempting to gain control and either leaving the group or breeding in it. In such a scenario, offspring will have significant power and, consequently, through selection on offspring counter-strategies, maternal strategies need to evolve closer to the offspring optima (i.e. fast maternal reproduction). This is not only because fast maternal reproduction is likely to be closer to the offspring's optima, but also because fast reproduction might be selected by the mother in order to reduce the outside options of their offspring and maintain them in the group (table 2). Hence, the maternal strategy to acquire helpful offspring is the same strategy that offspring benefit from most. We therefore suggest that maternal effects are not only likely to be instrumental to the evolution of cooperative breeding (i.e. through producing helpful offspring) in this circumstance, but will also be influential for the rise of social complexity (i.e. through being flexible to adapt in behavioural time to evolved offspring counter-strategies).
In conclusion, the evolution of cooperative breeding and the rise of social complexity could evolve from either maternal responses to offspring strategies or offspring responses to maternal strategies. We suggest here that although cooperative breeding can evolve in either scenario, the rise of social complexity will be more probable when offspring have a substantial influence on the maternal strategy. An exciting prospect will be to attempt to elucidate the degree to which variation in form and function of cooperative societies can be explained by the outcome of conflict between mother and offspring and how these are constrained by phylogeny and modified by the current ecology and life history. Observations in combination with recent molecular advances and quantitative genetic models are revolutionizing our understanding of the dynamics of such conflict. That ponerine ants have largely lost queen–worker castes while mother harvester ants currently ensure the maintenance of their castes by producing pure versus hybrid offspring (Smith et al. 2008), with associating costs for colony growth (Schwander et al. 2006), suggests that cooperative systems offer an insight into ‘current’ evolutionary dynamics between mother and offspring. That castes are largely determined by genes or gene×environment interactions (Anderson et al. 2008; Smith et al. 2008) underscores the scope for selection to be acting on both maternal and offspring strategies. For example, recent discoveries of workers adopting largely reproductive rather than working strategies might represent examples of worker counter-strategies ‘in action’ (Neumann & Moritz 2002; Lopez-Vaamonde et al. 2004; Beekman & Oldroyd 2008). Finally, quantitative genetics is revealing the nature of genetic correlations that may enhance or constrain evolutionary responses to selection. For example, opposing selection on maternal and offspring strategies could lead to conflict without resolution because negative genetic correlations between strategies means that selection cannot lead to evolutionary responses (Linksvayer 2006).
(b) Directions for future research
Future research, across all cooperative systems, needs to have an increased awareness of the potential for maternal effects in understanding offspring morphology and behaviour, fitness calculations of helper behaviour and the evolution of cooperative breeding, as well as the rise of complexity. In eusocial hymenoptera, a number of recent publications have illustrated the significant potential of maternal effects, particularly employed early in the reproductive phase, for influencing the form and function of cooperative societies and the individuals within (Wade 2001; Linksvayer & Wade 2005; Linksvayer 2006; Schwander et al. 2006, 2008; Anderson et al. 2008; Smith et al. 2008). By stark contrast, extremely few studies have considered maternal effects prior to the late reproductive phase outside the advanced eusocial hymenoptera. Nevertheless, there is some evidence to suggest that, here too, maternal effects could play a significant role in influencing form and function, and at the very least confound estimates of helper fitness and our understanding of the forces selecting for cooperative breeding (Komdeur 1998; Griffin et al. 2005; Kranz 2005; Russell et al. 2007b; Taborsky et al. 2007). We suggest four further directions to future research.
First, in order to calculate helper fitness, we need to measure maternal (and paternal) contributions to offspring at all stages of reproduction and control for them. Of particular note is that mothers in cooperative breeding systems might commonly influence the variance of their offspring, and helpers might benefit from reducing this variance, maintaining it or increasing it. Either way, it is essential that helper fitness calculations are able to control for the maternal strategy and apportion fitness accurately to the helper strategy versus the maternal one. Currently, our assumption is generally that a heavy offspring is a good offspring, but this need not be the case. For example, while heavy offspring might contribute more to cooperation (Clutton-Brock et al. 2002) and might have greater lifetime breeding potential (Russell et al. 2007a), neither the fitness of the helper nor the mother might be maximized in all cases. If heavy offspring compete with group members for reproduction or disperse early, the lifetime fitness of related group members might be reduced (Russell 2004; Ratnieks et al. 2006). Thus, in addition to measuring the investment by mothers at all phases of reproduction, the lifetime consequences of rearing offspring in a different condition needs to be considered before fitness can be assessed and apportioned.
Second, maternal effects appear to be partly responsible for qualitative division of labour in eusocial insects (Schwander et al. 2008), but whether they affect quantitative differences in individual contributions to cooperation is unknown. Individual differences in individual contributions to cooperation are marked within all cooperatively breeding species, but we are struggling to account for much of the variance (Hölldobler & Wilson 1990; Clutton-Brock et al. 2002; Russell 2004; Arnold et al. 2005; Field et al. 2006). No study to our knowledge has investigated the potential role of maternal effects in influencing quantitative differences in individual contributions to cooperation.
Third, while evidence from eusocial insects and humans has confirmed that maternal effects can be transgenerational (see §6), no such study has considered the possibility in other systems. This is all the more remarkable given the fact that studies of cooperative birds, for example, represent some of the most impressive long-term datasets for any species. Future research aimed at quantifying the degree to which early conditions have lifelong and transgenerational ramifications, and determining the degree to which maternal versus helper strategies influence these, would elucidate the evolutionary dynamics of cooperative systems. Finally, although not discussed here, research pioneered by Bowlby (1951) and Harlow (1958) on humans and primates, respectively, has spawned an immense literature on the psychological effects of maternal investment, particularly the lack of it, on offspring behaviour (e.g. Flouri 2004). Few studies have done so on cooperative species outside humans, but even evidence from spiders has shown that the number of days of maternal investment can influence the neurophysiology and behaviour of offspring in adulthood (Punzo & Alvarez 2002; Punzo & Ludwig 2002). The effects that the duration of maternal care has on offspring behaviour are an untapped source of maternal effects in cooperative breeders outside of humans.
To conclude, we suggest that maternal effects are likely to be particularly common in cooperative systems, having the potential to reflect the maternal investment strategy as well as having some bearing on the evolution of cooperative breeding and the rise of social complexity. It would appear critical to us that we begin to view parents as flexible participants in cooperative systems during all phases of the reproductive period, and measure their contributions to reproduction during each, to fully understand the dynamics and selective forces operating. An important issue in this regard is the fact that increasing variation in offspring might be a key aspect of the way in which mothers or helpers gain fitness, and it is important that variance in maternal effects are considered (see also Williams 2008). Finally, considering whether maternal effects might persist despite helper contributions, or be swamped or enhanced by them, will be important to gauging the degree to which parents and offspring differ in their current fitness optima and which party is gaining the greater fitness. We suggest that we would now benefit more from taking advantage of the unusual ability to test maternal effects in cooperative breeders, for it will yield a greater understanding and appreciation of the dynamics that underpin cooperative breeding systems, and the role of all carers to the maintenance and evolution of such systems. Through doing so, we expect studies of cooperative systems to elucidate hitherto unrealised aspects of the role of maternal effects to ecological and evolutionary processes.
Acknowledgments
We are extremely grateful to Tobias Uller for the invitation, encouragement, patience and guidance. We are also grateful to Jon Blount, Lucy Browning, Heikki Helanterä, Ani Kazem, Ian Rickard, Nick Royle, Tanja Schwander, Jon Wright and two anonymous referees for insightful and helpful criticism of various drafts, and to all those who sent reprints of various papers. Finally, we thank the Royal Society University Research Fellowship scheme for funding (A.F.R. and V.L.).
Footnotes
One contribution of 12 to a Theme Issue ‘Evolution of parental effects: conceptual issues and empirical patterns’.
References
- Alexander R.D. The evolution of social behaviour. Annu. Rev. Ecol. Syst. 1974;5:325–383. doi:10.1146/annurev.es.05.110174.001545 [Google Scholar]
- Alexander R.D., Noonan K., Crespi B.J. The evolution of eusociality. In: Sherman P.W., Jarvis J., Alexander R.D., editors. The biology of the naked mole rat. Princeton University Press; Princeton, NJ: 1991. pp. 3–44. [Google Scholar]
- Anderson K.E., Linksvayer T.A., Smith C.R. The causes and consequences of genetic caste determination in ants (Hymenoptera: Formicidae) Myrmecol. News. 2008;11:119–132. [Google Scholar]
- Arnold K.E., Owens I.P.F. Cooperative breeding in birds: a comparative analysis of the life history hypothesis. Proc. R. Soc. B. 1998;265:739–745. doi:10.1098/rspb.1998.0355 [Google Scholar]
- Arnold K.E., Owens I.P.F., Goldizen A.W. Division of labour within cooperatively breeding groups. Behaviour. 2005;142:1577–1590. doi:10.1163/156853905774831927 [Google Scholar]
- Avilés L., Tufiño P. Colony size and individual fitness in the social spider Anelosimus eximius. Am. Nat. 1998;152:403–418. doi: 10.1086/286178. doi:10.1086/286178 [DOI] [PubMed] [Google Scholar]
- Badyaev A.V. Maternal inheritance and rapid evolution of sexual size dimorphism: passive effects or active strategies? Am. Nat. 2005;166:S17–S30. doi: 10.1086/444601. doi:10.1086/444601 [DOI] [PubMed] [Google Scholar]
- Badyaev A.V. Maternal effects as generators of evolutionary change: a reassessment. Ann. N.Y. Acad. Sci. 2008;1133:151–161. doi: 10.1196/annals.1438.009. doi:10.1196/annals.1438.009 [DOI] [PubMed] [Google Scholar]
- Barker D.J.P. BMJ Publishing Group; London, UK: 1994. Mothers, babies and disease in later life. [Google Scholar]
- Bateson P. Fetal experience and good adult design. Int. J. Epidemiol. 2001;30:928–934. doi: 10.1093/ije/30.5.928. doi:10.1093/ije/30.5.928 [DOI] [PubMed] [Google Scholar]
- Beckerman A., Benton T.G., Ranta E., Kaitala V., Lundberg P. History making life-history: population dynamic consequences of delayed life-history effects. Trends Ecol. Evol. 2002;17:263–269. doi:10.1016/S0169-5347(02)02469-2 [Google Scholar]
- Beekman M., Oldroyd B.P. When workers disunite: intraspecific parasitism by eusocial bees. Annu. Rev. Entomol. 2008;53:19–37. doi: 10.1146/annurev.ento.53.103106.093515. doi:10.1146/annurev.ento.53.103106.093515 [DOI] [PubMed] [Google Scholar]
- Beekman M., Ratnieks F.L.W. Power over reproduction in social hymenoptera. Phil. Trans. R. Soc. B. 2003;358:1741–1753. doi: 10.1098/rstb.2002.1262. doi:10.1098/rstb.2002.1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereczkei T. Kinship network, direct childcare, and fertility among Hungarians and Gypsies. Evol. Hum. Behav. 1998;19:283–298. doi:10.1016/S1090-5138(98)00027-0 [Google Scholar]
- Bernardo J. Maternal effects in animal ecology. Am. Zool. 1996;36:83–105. doi:10.1093/icb/36.2.83 [Google Scholar]
- Bernasconi G., Strassmann J.E. Cooperation among unrelated individuals: the ant foundress case. Trends Ecol. Evol. 1999;14:477–482. doi: 10.1016/s0169-5347(99)01722-x. doi:10.1016/S0169-5347(99)01722-X [DOI] [PubMed] [Google Scholar]
- Blount J.D. Carotenoids and life-history evolution in animals. Arch. Biochem. Biophys. 2004;430:10–15. doi: 10.1016/j.abb.2004.03.039. doi:10.1016/j.abb.2004.03.039 [DOI] [PubMed] [Google Scholar]
- Bono J.M., Crespi B.J. Costs and benefits of joint colony founding in Australian Acacia thrips. Insectes Soc. 2006;53:489–495. doi:10.1007/s00040-005-0902-9 [Google Scholar]
- Bourke A.F.G. Sociality and kin selection in insects. In: Krebs J.R., Davies N.B., editors. Behavioural ecology: an evolutionary approach. Blackwell Science; Oxford, UK: 1997. pp. 203–227. [Google Scholar]
- Bourke A.F.G. Colony size, social complexity and reproductive conflict in social insects. J. Evol. Biol. 1999;12:245–257. doi:10.1046/j.1420-9101.1999.00028.x [Google Scholar]
- Bowlby J. Schocken; New York, NY: 1951. Maternal care and mental health. [Google Scholar]
- Brian M.V. Social factors affecting queen fecundity in the ant. Physiol. Entemol. 1980;14:381–389. doi:10.1111/j.1365-3032.1989.tb01106.x [Google Scholar]
- Brown J.L., Brown E.R. Kin selection and individual fitness in babblers. In: Alexander R.D., Tinkle D.W., editors. Natural selection and social behavior. Chiron Press; New York, NY: 1981. pp. 244–256. [Google Scholar]
- Brown J.L., Dow D.D., Brown E.R., Brown S.D. Effects of helpers on feeding of nestlings in grey-crowned babbler (Pomatostomus temporalis) Behav. Ecol. Sociobiol. 1978;4:43–59. doi:10.1007/BF00302560 [Google Scholar]
- Brown J.L., Brown E.R., Brown S.D., Dow D.D. Helpers: effects of experimental removal on reproductive success. Science. 1982;215:421–422. doi: 10.1126/science.215.4531.421. doi:10.1126/science.215.4531.421 [DOI] [PubMed] [Google Scholar]
- Bull N.J., Mibus A.C., Norimatsu Y., Jarmyn B.L., Schwartz M.P. Giving your daughters the edge: bequeathing reproductive dominance in a primitively social bee. Proc. R. Soc. B. 1998;265:1411–1415. doi:10.1098/rspb.1998.0450 [Google Scholar]
- Burley N. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 1986;127:415–445. doi:10.1086/284493 [Google Scholar]
- Burley N. The differential-allocation hypothesis: an experimental test. Am. Nat. 1988;132:611–628. doi:10.1086/284877 [Google Scholar]
- Buskirk, R. E. 1981 Sociality in the Arachnida. In Social insects, vol. II (ed. H. R. Herman), pp. 282–393. New York, NY: Academic Press.
- Cant M.A., Johnstone R.A. Costly young and reproductive skew in animal societies. Behav. Ecol. 1999;10:178–184. doi:10.1093/beheco/10.2.178 [Google Scholar]
- Cant M.A., Johnstone R.A. Reproductive conflict and the separation of reproductive generations in humans. Proc. Natl Acad. Sci. USA. 2008;105:5332–5336. doi: 10.1073/pnas.0711911105. doi:10.1073/pnas.0711911105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson A.A., Manser M.B., Young A.J., Russell A.F., Jordan N.R., McNeilly A.S., Clutton-Brock T.H. Cortisol levels are positively associated with pup-feeding rates in male meerkats. Proc. R. Soc. B. 2006;273:571–577. doi: 10.1098/rspb.2005.3087. doi:10.1098/rspb.2005.3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceesay S.M., Prentice A.M., Cole T.J., Foord F., Weaver L.T., Poskitt E.M., Whitehead R.G. Effects on birth weight and perinatal mortality of maternal dietary supplements in rural Gambia: 5 year randomised controlled trial. Br. Med. J. 1997;315:786–790. doi: 10.1136/bmj.315.7111.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J.C., Crespi B.J. Cambridge University Press; Cambridge, UK: 1997. The evolution of mating systems in insects and arachnids. [Google Scholar]
- Christians J.K. Avian egg size: variation within species and inflexibility within individuals. Biol. Rev. 2002;77:1–26. doi: 10.1017/s1464793101005784. [DOI] [PubMed] [Google Scholar]
- Christiansen N., Mora J.O., Navarro L., Herrera M.G. Effects of nutritional supplementation during pregnancy upon birth-weight—the influence of pre-supplementation diet. Nutr. Rep. Int. 1980;21:615–624. [Google Scholar]
- Clarke A.L., Saether B.-E., Roskaft E. Sex biases in avian dispersal: a reappraisal. Oikos. 1997;79:429–438. doi:10.2307/3546885 [Google Scholar]
- Clutton-Brock T.H. Reproductive skew, concessions and limited control. Trends Ecol. Evol. 1998;13:288–292. doi: 10.1016/s0169-5347(98)01402-5. doi:10.1016/S0169-5347(98)01402-5 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H., Harvey P.H. Primate ecology and social organization. J. Zool. 1977;183:1–39. [Google Scholar]
- Clutton-Brock T.H., et al. Cooperation, conflict, and concession in meerkat groups. Science. 2001;201:43–48. doi: 10.1126/science.291.5503.478. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H., Russell A.F., Sharpe L.L., Young A.J., Balmforth Z., McIlrath G.M. Evolution and development of sex differences in cooperative behavior in meerkats. Science. 2002;297:253–256. doi: 10.1126/science.1071412. doi:10.1126/science.1071412 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H., Russell A.F., Sharpe L.L. Behavioural tactics of breeders in cooperative meerkats. Anim. Behav. 2004;68:1029–1040. doi:10.1016/j.anbehav.2003.10.024 [Google Scholar]
- Clutton-Brock T.H., Hodge S.J., Spong G., Russell A.F., Jordan N.R., Bennett N.C., Sharpe L.L., Manser M.B. Intrasexual competition and sexual selection in cooperative mammals. Nature. 2006;444:1065–1068. doi: 10.1038/nature05386. doi:10.1038/nature05386 [DOI] [PubMed] [Google Scholar]
- Cockburn A. Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 1998;29:141–177. doi:10.1146/annurev.ecolsys.29.1.141 [Google Scholar]
- Cockburn A. Mating systems and sexual selections. In: Koenig W.D., Dickinson J.L., editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 81–101. [Google Scholar]
- Cockburn A., Sims R.A., Osmond H.L., Green D.J., Double M.C., Mulder R.A. Can we measure the benefits of help in cooperatively breeding birds: the case of superb fairy-wrens Malurus cyaneus? J. Anim. Ecol. 2008;77:430–438. doi: 10.1111/j.1365-2656.2007.01351.x. doi:10.1111/j.1365-2656.2007.01351.x [DOI] [PubMed] [Google Scholar]
- Craig R. Parental manipulation, kin selection, and the evolution of altruism. Evolution. 1979;33:319–334. doi: 10.1111/j.1558-5646.1979.tb04685.x. doi:10.2307/2407622 [DOI] [PubMed] [Google Scholar]
- Crean A.J., Marshall D.J. Coping with environmental uncertainty: dynamic bet hedging as a maternal effect. Phil. Trans. R. Soc. B. 2009;364:1087–1096. doi: 10.1098/rstb.2008.0237. doi:10.1098/rstb.2008.0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel S.R., Creel N.M. Princeton University Press; Princeton, NJ: 2002. The African wild dog: behavior, ecology, and conservation. [Google Scholar]
- Crespi B.J., Mound L.A. Ecology and evolution of social behaviour among Australian gall thrips and their allies. In: Choe J.C., Crespi B.J., editors. The evolution of mating systems in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 168–180. [Google Scholar]
- Crespi B.J., Carmean D.A., Chapman T.W. Ecology and evolution of galling thrips and their allies. Annu. Rev. Entomol. 1997;42:51–71. doi: 10.1146/annurev.ento.42.1.51. doi:10.1146/annurev.ento.42.1.51 [DOI] [PubMed] [Google Scholar]
- Crick H.Q.P. Load-lightening in cooperatively breeding birds and the cost of reproduction. Ibis. 1992;134:56–61. doi:10.1111/j.1474-919X.1992.tb07230.x [Google Scholar]
- Crognier É., Baali A., Hilali M.K. Do helpers at the nest increase their parents' reproductive success? Am. J. Hum. Biol. 2001;13:365–373. doi: 10.1002/ajhb.1060. doi:10.1002/ajhb.1060 [DOI] [PubMed] [Google Scholar]
- Cunningham E.J.A., Russell A.F. Egg investment is influenced by male attractiveness in mallards. Nature. 2000;404:74–77. doi: 10.1038/35003565. doi:10.1038/35003565 [DOI] [PubMed] [Google Scholar]
- Darwin C. 1st edn. John Murray; London, UK: 1859. The origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. [PMC free article] [PubMed] [Google Scholar]
- De Menten L., Fournier D., Brent C., Passera L., Vargo E.L., Aron S. Dual mechanism of queen influence over sex ratio in the ant Pheidole pallidula. Behav. Ecol. Sociobiol. 2005;58:527–533. doi:10.1007/s00265-005-0964-0 [Google Scholar]
- Dickinson J.L., Hatchwell B.J. Fitness consequences of helping. In: Koenig W.D., Dickinson J.L., editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 48–66. [Google Scholar]
- Dierkes P., Heg D., Taborsky M., Skubic E., Achmann R. Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol. Lett. 2005;8:968–975. doi: 10.1111/j.1461-0248.2005.00801.x. doi:10.1111/j.1461-0248.2005.00801.x [DOI] [PubMed] [Google Scholar]
- Draper P., Hames R. Birth order, sibling investment, and fertility among Ju'hoansi (!Kung) Hum. Nat. 2000;11:117–156. doi: 10.1007/s12110-000-1016-0. doi:10.1007/s12110-000-1016-0 [DOI] [PubMed] [Google Scholar]
- Duffy J.E. Eusociality in a coral-reef shrimp. Nature. 1996;381:512–514. doi:10.1038/381512a0 [Google Scholar]
- Duffy J.E., Macdonald K.S. Colony structure of the social snapping shrimp Synalpheus filidigitus in Belize. J. Crust. Biol. 1999;19:283–292. doi:10.2307/1549235 [Google Scholar]
- Duffy J.E., Morrison C.L., Ríos R. Multiple origins of eusociality among sponge-dwelling shrimps (Synalpheus) Evolution. 2000;54:503–516. doi: 10.1111/j.0014-3820.2000.tb00053.x. doi:10.1554/0014-3820(2000)054[0503:MOOEAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Emlen S.T. The evolution of helping. I: an ecological constraints model. Am. Nat. 1982;119:29–39. doi:10.1086/283888 [Google Scholar]
- Emlen S.T. An evolutionary theory of the family. Proc. Natl Acad. Sci. USA. 1995;92:8092–8099. doi: 10.1073/pnas.92.18.8092. doi:10.1073/pnas.92.18.8092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen S.T. Predicting family dynamics in social vertebrates. In: Krebs J.R., Davies N.B., editors. Behavioural ecology: an evolutionary approach. Blackwell Science; Oxford, UK: 1997. pp. 228–253. [Google Scholar]
- Endler A., Liebig J., Schmitt T., Parker J.E., Jones G.R., Schreier P., Hölldobler B. Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc. Natl Acad. Sci. USA. 2004;101:2945–2950. doi: 10.1073/pnas.0308447101. doi:10.1073/pnas.0308447101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D.S. 3rd edn. Wiley; Harlow, UK: 1989. Introduction to quantitative genetics. [Google Scholar]
- Faulkes C.G., Bennett N.C. Family values: group dynamics and social control of reproduction in African mole-rats. Trends. Ecol. Evol. 2001;16:184–190. doi: 10.1016/s0169-5347(01)02116-4. doi:10.1016/S0169-5347(01)02116-4 [DOI] [PubMed] [Google Scholar]
- Field J., Foster W., Shreeves G., Sumner S. Ecological constraints on independent nesting in facultatively eusocial hover wasps. Proc. R. Soc. B. 1998;265:973–977. doi:10.1098/rspb.1998.0386 [Google Scholar]
- Field J., Cronin A., Bridge C. Future fitness and helping in social queues. Nature. 2006;441:214–217. doi: 10.1038/nature04560. doi:10.1038/nature04560 [DOI] [PubMed] [Google Scholar]
- Fisher R.A. 2nd edn. Dover; New York, NY: 1958. The genetical theory of natural selection. [Google Scholar]
- Flouri E. Psychological outcomes in midadulthood associated with mother's child rearing attitudes in early childhood: evidence from the 1970 British birth cohort. Eur. Child Adolesc. Psychiatry. 2004;13:35–41. doi: 10.1007/s00787-004-0355-5. doi:10.1007/s00787-004-0355-5 [DOI] [PubMed] [Google Scholar]
- Foster K.R., Ratnieks F.L.W. A new eusocial vertebrate? Trends Ecol. Evol. 2005;20:363–364. doi: 10.1016/j.tree.2005.05.005. doi:10.1016/j.tree.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Gadagkar R. Evolution of eusociality: the advantage of assured fitness returns. Phil. Trans. R. Soc. B. 1990;329:17–25. doi:10.1098/rstb.1990.0146 [Google Scholar]
- Gluckman P.D., Hanson M.A., Spencer H.G. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. doi:10.1016/j.tree.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Godfray H.C.T. Evolutionary theory of parent–offspring conflict. Nature. 1995;376:133–138. doi: 10.1038/376133a0. doi:10.1038/376133a0 [DOI] [PubMed] [Google Scholar]
- Greenwood P.J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 1980;28:1140–1162. doi:10.1016/S0003-3472(80)80103-5 [Google Scholar]
- Griffin A.S., West S.A. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science. 2003;302:634–636. doi: 10.1126/science.1089402. doi:10.1126/science.1089402 [DOI] [PubMed] [Google Scholar]
- Griffin A.S., Sheldon B.C., West S.A. Cooperative breeders adjust offspring sex ratio to produce helpful helpers. Am. Nat. 2005;166:1–5. doi: 10.1086/491662. doi:10.1086/491662 [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G., Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Phil. Trans. R. Soc. B. 2008;363:1647–1661. doi: 10.1098/rstb.2007.0007. doi:10.1098/rstb.2007.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager, R. & Jones, C. B. In press. Reproductive skew in vertebrates. Proximate and ultimate causes Cambridge, UK: Cambridge University Press.
- Haig D. The kinship theory of genomic imprinting. Annu. Rev. Ecol. Syst. 2000;31:9–32. doi:10.1146/annurev.ecolsys.31.1.9 [Google Scholar]
- Hales C.N., Barker D.J.P. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. doi:10.1007/BF00400248 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. doi:10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Hannon S.J., Mumme R.L., Koenig W.D., Pitelka F.A. Replacement of breeders and within-group conflict in the cooperatively breeding Acorn Woodpecker. Behav. Ecol. Sociobiol. 1985;17:303–312. doi:10.1007/BF00293208 [Google Scholar]
- Harlow H. The nature of love. Am. Psychol. 1958;13:573–685. doi:10.1037/h0047884 [Google Scholar]
- Harris W.E., Uller T. Reproductive investment when mate quality varies: differential allocation versus reproductive compensation. Phil. Trans. R. Soc. B. 2009;364:1039–1048. doi: 10.1098/rstb.2008.0299. doi:10.1098/rstb.2008.0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchwell B.J. Investment strategies of breeders in avian cooperative breeding systems. Am. Nat. 1999;154:205–219. doi: 10.1086/303227. doi:10.1086/303227 [DOI] [PubMed] [Google Scholar]
- Hatchwell B.J., Russell A.F. Provisioning rules in cooperatively breeding long-tailed tits Aegithalos caudatus: an experimental study. Proc. R. Soc. B. 1996;263:83–88. doi:10.1098/rspb.1996.0014 [Google Scholar]
- Hatchwell B.J., Russell A.F., Ross D.J., Fowlie M.K. Divorce in cooperatively breeding long-tailed tits: a consequence of inbreeding avoidance? Proc. R. Soc. B. 2000;267:813–819. doi: 10.1098/rspb.2000.1076. doi:10.1098/rspb.2000.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K., O'Connell J.F., Blurton Jones N.G., Alvarez H., Charnov E.L. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. doi:10.1073/pnas.95.3.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heg D., Brouwer L., Bachar Z., Taborsky M. Large group size yields group stability in the cooperatively breeding cichlid Neolamprologus pulcher. Behaviour. 2005;142:1615–1641. doi:10.1163/156853905774831891 [Google Scholar]
- Heg D., Bergmüller R., Bonfils D., Otti O., Bachar Z., Burri R., Heckel G., Taborsky M. Cichlids do not adjust reproductive skew to the availability of independent breeding options. Behav. Ecol. 2006;17:419–429. doi:10.1093/beheco/arj056 [Google Scholar]
- Hill K.R., Hurtado M.A. How much does grandmother help? In: Betzig L., editor. Human nature: a critical reader. Oxford University Press; New York, NY: 1996. pp. 140–146. [Google Scholar]
- Hölldobler B., Wilson E.O. Belknap Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Huck U.W., Labov J.D., Lisk R.D. Food-restricting first generation juvenile female hamsters (Mesocricetus auratus) affects sex ratio and growth of third generation offspring. Biol. Reprod. 1987;37:612–617. doi: 10.1095/biolreprod37.3.612. doi:10.1095/biolreprod37.3.612 [DOI] [PubMed] [Google Scholar]
- Hughes W.O., Oldroyd B.P., Beekman M., Ratnieks F.L.W. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science. 2008;320:1213–1216. doi: 10.1126/science.1156108. doi:10.1126/science.1156108 [DOI] [PubMed] [Google Scholar]
- Jamieson I.G. Behavioral heterochrony and the evolution of birds' helping at the nest: an unselected consequence of communal breeding? Nature. 1989;133:394–406. doi:10.1086/284925 [Google Scholar]
- Jarman P.J. The social organization of antelope according to their ecology. Behaviour. 1974;48:215–267. doi:10.1163/156853974X00345 [Google Scholar]
- Jemielity S., Chapuisat M., Parker J.D., Keller L. Long live the queen: studying aging in social insects. Age. 2005;27:241–248. doi: 10.1007/s11357-005-2916-z. doi:10.1007/s11357-005-2916-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karell P., Kontiainen P., Pietiainen H., Siitari H., Brommer J.E. Maternal effects on offspring IgGs and egg size in relation to natural and experimentally improved food supply. Funct. Ecol. 2008;22:682–690. doi:10.1111/j.1365-2435.2008.01425.x [Google Scholar]
- Keller L., Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. doi:10.1038/40130 [Google Scholar]
- Keller L., Nonacs P. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 1993;45:787–794. doi:10.1006/anbe.1993.1092 [Google Scholar]
- Kirkendall L.R., Kent D.S., Raffa K.A. Interactions among males, females and offspring in bark and ambrosia beetles: the significance of living in tunnels for the evolution of social behaviour. In: Choe J.C., Crespi B.J., editors. The evolution of mating systems in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 181–215. [Google Scholar]
- Koenig W.D., Pitelka F.A., Carmen W.J., Mumme R.L., Stanback M.T. The evolution of delayed dispersal in cooperative breeders. Q. Rev. Biol. 1992;67:111–150. doi: 10.1086/417552. doi:10.1086/417552 [DOI] [PubMed] [Google Scholar]
- Komdeur J. Experimental evidence for helping and hindering by previous offspring in the cooperative breeding Acrocephalus sechellensis. Behav. Ecol. Sociobiol. 1994;34:175–186. doi:10.1007/BF00167742 [Google Scholar]
- Komdeur J. Long-term fitness benefits of egg sex modification by the Seychelles Warbler. Ecol. Lett. 1998;1:56–62. doi:10.1046/j.1461-0248.1998.00009.x [Google Scholar]
- Komdeur J. Sex ratio manipulation. In: Koenig W.D., Dickinson J.L., editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 102–116. [Google Scholar]
- Komdeur J., Daan S., Tinbergen J., Mateman A.C. Extreme adaptive modification in sex ratio of Seychelles Warbler's eggs. Nature. 1997;385:522–525. doi:10.1038/385522a0 [Google Scholar]
- Korb, J. 2008 The ecology of social evolution in termites. In Ecology of social evolution (eds J. Korb & J. Heinze), pp. 151–174. Heidelberg, Germany: Springer.
- Korb J., Katrantzis S. Influence of environmental conditions on the expression of the sexual dispersal phenotype in a lower termite: implications for the evolution of workers in termites. Evol. Dev. 2004;6:342–352. doi: 10.1111/j.1525-142X.2004.04042.x. doi:10.1111/j.1525-142X.2004.04042.x [DOI] [PubMed] [Google Scholar]
- Kranz B.D. Egg size and reproductive allocation in eusocial thrips. Behav. Ecol. 2005;16:779–787. doi:10.1093/beheco/ari053 [Google Scholar]
- Lacey E.A., Sherman P.W. Cooperative breeding in naked-mole rats: implications for vertebrate and invertebrate sociality. In: Solomon N.G., French J.A., editors. Cooperative breeding in mammals. Cambridge University Press; Cambridge, UK: 1997. pp. 267–301. [Google Scholar]
- Lahdenperä M., Lummaa V., Helle S., Tremblay M., Russell A.F. Fitness benefits of prolonged post-reproductive lifespan in women. Nature. 2004;428:178–181. doi: 10.1038/nature02367. doi:10.1038/nature02367 [DOI] [PubMed] [Google Scholar]
- Lee R.D. Rethinking the evolutionary theory of aging: transfers, not births, shape senescence in social species. Proc. Natl Acad. Sci. USA. 2003;100:9637–9642. doi: 10.1073/pnas.1530303100. doi:10.1073/pnas.1530303100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the HPA axis: the influence of maternal factors. Ann. N. Y. Acad. Sci. 1994;746:275–288. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- Liebig J., Peeters C., Hölldobler B. Worker policing limits the number of reproductives in a ponerine ant. Proc. R. Soc. B. 1999;266:1865–1870. doi:10.1098/rspb.1999.0858 [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. doi:10.1016/S0169-5347(99)01639-0 [DOI] [PubMed] [Google Scholar]
- Linksvayer T.A. Direct, maternal, and sibsocial genetic effects on individual and colony traits in an ant. Evolution. 2006;60:2552–2561. doi: 10.1554/06-011.1. doi:10.1554/06-011.1 [DOI] [PubMed] [Google Scholar]
- Linksvayer T.A., Wade M.J. The evolutionary origin and elaboration of sociality in the aculeate hymenoptera: maternal effects, sib-social effects, and heterochrony. Q. Rev. Biol. 2005;80:317–336. doi: 10.1086/432266. doi:10.1086/432266 [DOI] [PubMed] [Google Scholar]
- Lopez-Vaamonde C., Koning J.W., Brown R.M., Jordan W.C., Bourke A.F.G. Social parasitism by male-producing reproductive workers in a eusocial insect. Nature. 2004;430:557–560. doi: 10.1038/nature02769. doi:10.1038/nature02769 [DOI] [PubMed] [Google Scholar]
- Lubin Y., Bilde T. The evolution of sociality in spiders. Adv. Study Behav. 2007;37:83–145. doi:10.1016/s0065-3454(07)37003-4 [Google Scholar]
- Lucas A. Programming by early nutrition in man. In: Bock G.R., Whelan J., editors. The childhood environment and adult disease. Wiley; Chichester, UK: 1991. pp. 38–55. [Google Scholar]
- Lumey L.H. Decreased birthweights in infants after maternal in utero exposure to the Dutch famine of 1944–1945. Paediatr. Perinat. Epidemiol. 1992;6:240–253. doi: 10.1111/j.1365-3016.1992.tb00764.x. doi:10.1111/j.1365-3016.1992.tb00764.x [DOI] [PubMed] [Google Scholar]
- Lummaa V. Early developmental conditions and reproductive success in humans: downstream effects of prenatal famine, birthweight, and timing of birth. Am. J. Hum. Biol. 2003;15:370–379. doi: 10.1002/ajhb.10155. doi:10.1002/ajhb.10155 [DOI] [PubMed] [Google Scholar]
- Lummaa V., Clutton-Brock T.H. Early development, survival and reproduction in humans. Trends Ecol. Evol. 2002;17:141–147. doi:10.1016/S0169-5347(01)02414-4 [Google Scholar]
- Lummaa V., Tremblay M. Month of birth predicted reproductive success and fitness in pre-modern Canadian women. Proc. R. Soc. B. 2003;270:2355–2361. doi: 10.1098/rspb.2003.2507. doi:10.1098/rspb.2003.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrath R.D., Johnstone R.A., Heinsohn R.G. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. Reproductive skew; pp. 157–176. [Google Scholar]
- Marshall D.J., Uller T. When is a maternal effect adaptive? Oikos. 2007;116:1957–1963. doi:10.1111/j.2007.0030-1299.16203.x [Google Scholar]
- McAuliffe K., Whitehead H. Eusociality, menopause and information in matrilineal whales. Trends Ecol. Evol. 2005;20:650. doi: 10.1016/j.tree.2005.09.003. doi:10.1016/j.tree.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Metcalfe N.B., Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. doi:10.1016/S0169-5347(01)02124-3 [DOI] [PubMed] [Google Scholar]
- Michod R.E. The theory of kin selection. Annu. Rev. Ecol. Syst. 1982;13:23–55. doi:10.1146/annurev.es.13.110182.000323 [Google Scholar]
- Miura T. Proximate mechanisms and evolution of caste polyphenism in social insects: from sociality to genes. Ecol. Res. 2004;19:141–148. doi:10.1111/j.1440-1703.2003.00618.x [Google Scholar]
- Miura T. Developmental regulation of caste-specific characters in social insect polyphenism. Evol. Dev. 2005;7:122–129. doi: 10.1111/j.1525-142X.2005.05014.x. doi:10.1111/j.1525-142X.2005.05014.x [DOI] [PubMed] [Google Scholar]
- Miura T., Shigeyuki K., Matsumoto T. Winged presoldiers induced by a juvenile hormone analog in Zootermopsis nevadensis: implications for plasticity and evolution of caste differentiation in termites. J. Morphol. 2003;257:22–32. doi: 10.1002/jmor.10100. doi:10.1002/jmor.10100 [DOI] [PubMed] [Google Scholar]
- Monnin T., Ratniek F.L.W., Jones G.R., Beard R. Pretender punishment induced by chemical signalling in a queenless ant. Nature. 2002;419:61–65. doi: 10.1038/nature00932. doi:10.1038/nature00932 [DOI] [PubMed] [Google Scholar]
- Mousseau T.A., Fox C.W. Oxford University Press; Oxford, UK: 1998. Maternal effects as adaptations. [Google Scholar]
- Naumann K., Winston M.L., Slessor K.N., Prestwich G.D., Webster F.X. Production and transmission of honey-bee queen (Apis mellifera L.) mandibular gland pheromone. Behav. Ecol. Sociobiol. 1991;29:321–332. doi:10.1007/BF00165956 [Google Scholar]
- Neumann P., Moritz R.F.A. The Cape honeybee phenomenon: the sympatric evolution of a social parasite in real time? Behav. Ecol. Sociobiol. 2002;52:271–281. doi:10.1007/s00265-002-0518-7 [Google Scholar]
- Nijhout H.F., Wheeler D.E. Juvenile hormone and the physiological basis of insect polymorphisms. Q. Rev. Biol. 1982;57:109–133. doi:10.1086/412671 [Google Scholar]
- Noirot C. Caste differentiation in Isoptera—basic features, role of pheromones. Ethol. Ecol. Evol. 1991;1:3–7. [Google Scholar]
- O'Donnell S. Reproductive caste determination in eusocial wasps (Hymenoptera: Vespidae) Annu. Rev. Entomol. 1998;43:323–346. doi: 10.1146/annurev.ento.43.1.323. doi:10.1146/annurev.ento.43.1.323 [DOI] [PubMed] [Google Scholar]
- O'Riain M.J., Jarvis J.U.M., Faulkes C.G. A dispersive morph in the naked mole-rat. Nature. 1996;380:619–621. doi: 10.1038/380619a0. doi:10.1038/380619a0 [DOI] [PubMed] [Google Scholar]
- O'Riain M.J., Jarvis J.U.M., Alexander R., Buffenstein R., Peeters C. Morphological castes in a vertebrate. Proc. Natl Acad. Sci. USA. 2000;97:13 194–13 197. doi: 10.1073/pnas.97.24.13194. doi:10.1073/pnas.97.24.13194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A., Royle N.J., Hartley I.R. Intrafamilial conflict and parental investment: a synthesis. Phil. Trans. R. Soc. B. 2002;357:295–307. doi: 10.1098/rstb.2001.0950. doi:10.1098/rstb.2001.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passera L. The inhibitory function of queens in the ant Plagiolepis pygmaea—the act of pheromones. Insectes Soc. 1980;27:212–225. doi:10.1007/BF02223665 [Google Scholar]
- Passera L., Keller L., Suzzoni J.-P. Queen replacement in dequeened colonies of the Argentine ant Iridomtrmex humilis. Psyche. 1988;95:59–66. doi:10.1155/1988/59259 [Google Scholar]
- Peeters C., Ito F. Colony dispersal and the evolution of queen morphology in social Hymenoptera. Annu. Rev. Entomol. 2001;46:601–630. doi: 10.1146/annurev.ento.46.1.601. doi:10.1146/annurev.ento.46.1.601 [DOI] [PubMed] [Google Scholar]
- Pigliucci M. Do we need an extended evolutionary synthesis? Evolution. 2007;61:2743–2749. doi: 10.1111/j.1558-5646.2007.00246.x. doi:10.1111/j.1558-5646.2007.00246.x [DOI] [PubMed] [Google Scholar]
- Phillips D.I.W., Handelsman D.J., Eriksson J.G., Forsen T., Osmond C., Barker D.J.P. Prenatal growth and subsequent marital status: a longitudinal study. Br. Med. J. 2001;322:771. doi: 10.1136/bmj.322.7289.771. doi:10.1136/bmj.322.7289.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell R.A., Fried J.J. Helping by juvenile pine voles (Microtus pinetorum), growth and survival of younger siblings, and the evolution of pine vole sociality. Behav. Ecol. 1992;3:325–333. doi:10.1093/beheco/3.4.325 [Google Scholar]
- Prentice A.M., Whitehead R.G., Watkinson M., Lamb W.H., Cole T.J. Prenatal dietary supplementation of African women and birth-weight. Lancet. 1983;1:489–492. doi: 10.1016/s0140-6736(83)92188-8. doi:10.1016/S0140-6736(83)92188-8 [DOI] [PubMed] [Google Scholar]
- Punzo F., Alvarez J. Effects of early contact with maternal parent on locomotor activity and exploratory behavior in spiderlings of Hogna carolinensis (Araneae: Lycosidae) J. Insect Behav. 2002;15:455–465. doi:10.1023/A:1016351531676 [Google Scholar]
- Punzo F., Ludwig L. Contact with maternal parent and siblings affects hunting behavior, learning, and central nervous system development in spiderlings of Hogna carolinensis (Araeneae: Lycosidae) Anim. Cogn. 2002;5:63–70. doi: 10.1007/s10071-002-0128-9. doi:10.1007/s10071-002-0128-9 [DOI] [PubMed] [Google Scholar]
- Quinlan R.J., Quinlan M.B. Human lactation, pairbonds, and alloparents: a cross-cultural analysis. Hum. Nat. 2008;19:87–102. doi: 10.1007/s12110-007-9026-9. doi:10.1007/s12110-007-9026-9 [DOI] [PubMed] [Google Scholar]
- Ratnieks F.L.W., Foster K.R., Wenseleers T. Conflict resolution in insect societies. Annu. Rev. Entomol. 2006;51:581–608. doi: 10.1146/annurev.ento.51.110104.151003. doi:10.1146/annurev.ento.51.110104.151003 [DOI] [PubMed] [Google Scholar]
- Reed M.J., Walters J.R. Helper effects on variance components of fitness in the cooperatively breeding red-cockaded woodpecker. Auk. 1996;113:608–616. [Google Scholar]
- Rickard I.J., Lummaa V. The predictive adaptive response and metabolic syndrome: challenges for the hypothesis. Trends Endocrinol. Metab. 2007;18:94–99. doi: 10.1016/j.tem.2007.02.004. doi:10.1016/j.tem.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Ridley A.R. Factors affecting offspring survival and development in a cooperative bird: social, maternal and environmental effects. J. Anim. Ecol. 2007;76:750–760. doi: 10.1111/j.1365-2656.2007.01248.x. doi:10.1111/j.1365-2656.2007.01248.x [DOI] [PubMed] [Google Scholar]
- Roisin Y. Intragroup conflicts and the evolution of sterile castes in termites. Am. Nat. 1994;143:751–765. doi:10.1086/285631 [Google Scholar]
- Roisin Y. Diversity and evolution of caste patterns. In: Abe T., Bignell D.E., Higashi M., editors. Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2000. pp. 95–119. [Google Scholar]
- Roux E.A., Korb J. Evolution of eusociality and the soldier caste in termites: a validation of the intrinsic benefit hypothesis. J. Evol. Biol. 2004;17:869–875. doi: 10.1111/j.1420-9101.2004.00727.x. doi:10.1111/j.1420-9101.2004.00727.x [DOI] [PubMed] [Google Scholar]
- Rowley I.C.R. The life history of the superb blue wren. Emu. 1965;64:251–297. [Google Scholar]
- Royle N.J., Surai P.F., Hartley I.R. Maternally derived androgens and antioxidants in bird eggs: complementary but opposing effects? Behav. Ecol. 2001;12:381–385. doi:10.1093/beheco/12.4.381 [Google Scholar]
- Russell A.F. Mammals: comparisons and contrasts. In: Koenig W.D., Dickinson J.L., editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 210–227. [Google Scholar]
- Russell A.F., Clutton-Brock T.H., Brotherton P.N.M., Sharpe L.L., McIlrath G.M., Dalerum F.D., Cameron E.Z., Barnard J.A. Factors affecting pup growth and survival in co-operatively breeding meerkats Suricata suricatta. J. Anim. Ecol. 2002;71:700–709. doi:10.1046/j.1365-2656.2002.00636.x [Google Scholar]
- Russell A.F., Brotherton P.N.M., McIlrath G.M., Sharpe L.L., Clutton-Brock T.H. Breeding success in cooperative meerkats: effects of helper number and maternal state. Behav. Ecol. 2003a;14:486–492. doi:10.1093/beheco/arg022 [Google Scholar]
- Russell A.F., Sharpe L.L., Brotherton P.N.M., Clutton-Brock T.H. Cost minimization by helpers in cooperative vertebrates. Proc. Natl Acad. Sci. USA. 2003b;100:3333–3338. doi: 10.1073/pnas.0636503100. doi:10.1073/pnas.0636503100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A.F., Carlson A.A., McIlrath G.M., Jordan N.R., Clutton-Brock T. Adaptive size modification by dominant female meerkats. Evolution. 2004;58:1600–1607. doi: 10.1111/j.0014-3820.2004.tb01739.x. doi:10.1554/03-480 [DOI] [PubMed] [Google Scholar]
- Russell A.F., Young A.J., Spong G., Jordan N.R., Clutton-Brock T.H. Helpers increase the reproductive potential of offspring in cooperative meerkats. Proc. R. Soc. B. 2007a;274:513–520. doi: 10.1098/rspb.2006.3698. doi:10.1098/rspb.2006.3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A.F., Langmore N.E., Cockburn A., Astheimer L.B., Kilner R.M. Reduced egg investment can conceal helper effects in cooperatively breeding birds. Science. 2007b;317:941–944. doi: 10.1126/science.1146037. doi:10.1126/science.1146037 [DOI] [PubMed] [Google Scholar]
- Russell A.F., Langmore N.E., Gardner J.L., Kilner R.M. Maternal investment tactics in superb fairy-wrens. Proc. R. Soc. B. 2008;275:29–36. doi: 10.1098/rspb.2007.0821. doi:10.1098/rspb.2007.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo A.L., French J.A. Early experience, reproductive success and development of parental behaviour in Mongolian gerbils. Anim. Behav. 1989;38:693–702. doi:10.1016/S0003-3472(89)80015-6 [Google Scholar]
- Salomon M., Lubin Y. Cooperative breeding increases reproductive success in the social spider Stegodyphus dumicola (Araneae Eresidae) Behav. Ecol. Sociobiol. 2007;61:1743–1750. doi:10.1007/s00265-007-0406-2 [Google Scholar]
- Scantlebury M., Russell A.F., McIlrath G.M., Speakman J.R., Clutton-Brock T.H. The energetics of lactation in cooperatively breeding meerkats Suricata suricatta. Proc. R. Soc. B. 2002;269:2147–2153. doi: 10.1098/rspb.2002.2108. doi:10.1098/rspb.2002.2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoech S.J., Reynolds S.J., Boughton R.K. Endocrinology. In: Koenig W.D., Dickinson J.L., editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 128–141. [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11 446–11 450. doi: 10.1073/pnas.90.24.11446. doi:10.1073/pnas.90.24.11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander T., Helms Cahan S., Keller L. Genetic caste determination in Pogonomyrmex harvester ants imposes costs during colony founding. J. Evol. Biol. 2006;19:402–409. doi: 10.1111/j.1420-9101.2005.01023.x. doi:10.1111/j.1420-9101.2005.01023.x [DOI] [PubMed] [Google Scholar]
- Schwander T., Humbert J.Y., Brent C.S., Cahan S.H., Chapuis L., Renai E., Keller L. Maternal effect on female caste determination in a social insect. Curr. Biol. 2008;18:265–269. doi: 10.1016/j.cub.2008.01.024. doi:10.1016/j.cub.2008.01.024 [DOI] [PubMed] [Google Scholar]
- Sear R., Mace R. Who keeps children alive? A review of the effects of kin on child mortality. Evol. Hum. Behav. 2008;29:1–18. doi:10.1016/j.evolhumbehav.2007.10.001 [Google Scholar]
- Sear R., Mace R., McGregor I.A. Maternal grandmothers improve the nutritional status and survival of children in rural Gambia. Proc. R. Soc. B. 2000;267:1641–1647. doi: 10.1098/rspb.2000.1190. doi:10.1098/rspb.2000.1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sear R., Steele F., McGregor I.A., Mace R. The effects of kin on child mortality in rural Gambia. Demography. 2002;39:43–63. doi: 10.1353/dem.2002.0010. doi:10.1353/dem.2002.0010 [DOI] [PubMed] [Google Scholar]
- Seeley T.D. Princeton University Press; Princeton, NJ: 1985. Honey bee ecology. [Google Scholar]
- Sheldon B.C. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 2000;15:397–402. doi: 10.1016/s0169-5347(00)01953-4. doi:10.1016/S0169-5347(00)01953-4 [DOI] [PubMed] [Google Scholar]
- Sherman P.W., Jarvis J.U.M. Extraordinary life spans of naked mole-rats (Heterocephalus glaber) J. Zool. 2002;258:307–311. doi:10.1017/S0952836902001437 [Google Scholar]
- Sherman P.W., Jarvis J., Alexander R.D., editors. The biology of the naked mole rat. Princeton University Press; Princeton, NJ: 1991. [Google Scholar]
- Smiseth P.T., Wright J., Kölliker M. Parent-offspring conflict and co-adaptation: behavioural ecology meets quantitative genetics. Proc. R. Soc. B. 2008;275:1823–1830. doi: 10.1098/rspb.2008.0199. doi:10.1098/rspb.2008.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.R., Toth A.L., Suarez A.V., Robinson G.E. Genetic and genomic analyses of the division of labour in insect societies. Nat. Rev. Genet. 2008;9:735–748. doi: 10.1038/nrg2429. doi:10.1038/nrg2429 [DOI] [PubMed] [Google Scholar]
- Sockman K.W., Sharp P.J., Schwabl H. Orchestration of avian reproductive effort: an integration of the ultimate and proximate bases for flexibility in clutch size, incubation behaviour, and yolk androgen deposition. Biol. Rev. 2006;81:629–666. doi: 10.1017/S1464793106007147. doi:10.1017/S1464793106007147 [DOI] [PubMed] [Google Scholar]
- Solomon N.G. Current indirect fitness benefits associated with philopatry in juvenile prairie voles. Behav. Ecol. Sociobiol. 1991;29:277–282. doi:10.1007/BF00163985 [Google Scholar]
- Solomon N.G., French J.A. Cambridge University Press; Cambridge, UK: 1997. Cooperative breeding in mammals. [Google Scholar]
- Stacey P.B., Koenig W.D. Cambridge University Press; Cambridge, UK: 1990. Cooperative breeding in birds: long-term studies of ecology and behavior. [Google Scholar]
- Stanner S.A., Yudkin J.S. Fetal programming and the Leningrad Siege study. Twin Res. 2001;4:287–292. doi: 10.1375/1369052012498. doi:10.1375/1369052012498 [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Stein A.D., Zybert P.A., van de Bor M., Lumey L.H. Intrauterine famine exposure and body proportions at birth: the Dutch Hunger Winter. Int. J. Epidemiol. 2004;33:831–836. doi: 10.1093/ije/dyh083. doi:10.1093/ije/dyh083 [DOI] [PubMed] [Google Scholar]
- Stern D.L., Foster W.A. The evolution of sociality in aphids: a clone's eye view. In: Choe J.C., Crespi B.J., editors. The evolution of mating systems in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 150–165. [Google Scholar]
- Strassmann J.E., Queller D.C. Social evolution: ant eggs lacking totipotency. Curr. Biol. 2008;18:R299–R301. doi: 10.1016/j.cub.2008.02.032. doi:10.1016/j.cub.2008.02.032 [DOI] [PubMed] [Google Scholar]
- Sultan S.E. Development in context: the timely emergence of eco-devo. Trends Ecol. Evol. 2007;22:575–582. doi: 10.1016/j.tree.2007.06.014. doi:10.1016/j.tree.2007.06.014 [DOI] [PubMed] [Google Scholar]
- Taborsky M. Broodcare helpers in the cichlid fish Lamprologus brichardi—their costs and benefits. Anim. Behav. 1984;32:1236–1252. doi:10.1016/S0003-3472(84)80241-9 [Google Scholar]
- Taborsky M. Sneakers, satellites, and helpers: parasitic and cooperative behavior in fish reproduction. Adv. Study Behav. 1994;23:1–100. doi:10.1016/S0065-3454(08)60351-4 [Google Scholar]
- Taborsky B., Skubic E., Bruintjes R. Mothers adjust egg size to helper number in a cooperatively breeding cichlid. Behav. Ecol. 2007;18:652–657. doi:10.1093/beheco/arm026 [Google Scholar]
- Thorne B.L. Evolution of eusociality in termites. Annu. Rev. Ecol. Syst. 1997;28:27–54. doi:10.1146/annurev.ecolsys.28.1.27 [Google Scholar]
- Trivers R.L. Parent–offspring conflict. Am. Zool. 1974;14:249–264. doi:10.1093/icb/14.1.249 [Google Scholar]
- Uller T. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 2008;23:432–438. doi: 10.1016/j.tree.2008.04.005. doi:10.1016/j.tree.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Vargo E.L., Fletcher J.D.C. Evidence of pheromonal queen control over the production of male and female sexuals in the fire ant, Solenopsis invicta. J. Comp. Physiol. 1986;159:741–749. doi:10.1007/BF00603727 [Google Scholar]
- Wade M.J. Maternal effect genes and the evolution of sociality in haplo-diploid organisms. Evolution. 2001;55:453–458. doi: 10.1554/0014-3820(2001)055[0453:megate]2.0.co;2. doi:10.1554/0014-3820(2001)055[0453:MEGATE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wagner E.C., Williams T.D. Experimental (antiestrogen-mediated) reduction in egg size negatively affects offspring growth and survival. Physiol. Biochem. Zool. 2007;80:293–305. doi: 10.1086/512586. doi:10.1086/512586 [DOI] [PubMed] [Google Scholar]
- Wcislo W.T., Danforth B.N. Secondarily solitary: the evolutionary loss of social behaviour. Trends Ecol. Evol. 1997;12:468–474. doi: 10.1016/s0169-5347(97)01198-1. doi:10.1016/S0169-5347(97)01198-1 [DOI] [PubMed] [Google Scholar]
- Wells J.C.K. The thrifty phenotype as an adaptive maternal effect. Biol. Rev. 2007;82:143–172. doi: 10.1111/j.1469-185X.2006.00007.x. doi:10.1111/j.1469-185X.2006.00007.x [DOI] [PubMed] [Google Scholar]
- West S.A., Sheldon B.C. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. doi:10.1126/science.1069043 [DOI] [PubMed] [Google Scholar]
- West-Eberhard M.-J. Oxford University Press; Oxford, UK: 2003. Developmental plasticity and evolution. [Google Scholar]
- Wheeler D.E. Developmental and physiological determinants of caste in social hymenoptera: evolutionary implications. Am. Nat. 1986;128:13–34. doi:10.1086/284536 [Google Scholar]
- Whitehouse M.E.A., Lubin Y. The functions of societies and the evolution of group living: spider societies as a test case. Biol. Rev. 2005;80:1–15. doi: 10.1017/s1464793104006694. doi:10.1017/S1464793104006694 [DOI] [PubMed] [Google Scholar]
- Williams G.C. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. doi:10.2307/2406060 [Google Scholar]
- Williams G.C. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 1966;100:687–692. doi:10.1086/282461 [Google Scholar]
- Williams T.D. Intraspecific variation in egg size and egg composition in birds—effects on offspring fitness. Biol. Rev. Camb. Philos. Soc. 1994;69:35–39. doi: 10.1111/j.1469-185x.1994.tb01485.x. doi:10.1111/j.1469-185X.1994.tb01485.x [DOI] [PubMed] [Google Scholar]
- Williams T.D. Individual variation in endocrine systems: moving beyond the ‘tyranny of the Golden Mean’. Phil. Trans. R. Soc. B. 2008;363:1687–1698. doi: 10.1098/rstb.2007.0003. doi:10.1098/rstb.2007.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E.O. Harvard University Press; Cambridge, MA: 1971. The insect societies. [Google Scholar]
- Woxvold I.A., Magrath M.J.L. Helping enhances multiple components of reproductive success in the cooperatively breeding apostlebird. J. Anim. Ecol. 2005;74:1039–1050. doi:10.1111/j.1365-2656.2005.01001.x [Google Scholar]
- Wright J. Helping-at-the-nest and group size in the Arabian babbler, Turdoides squamiceps. J. Avian Biol. 1998;29:105–112. doi:10.2307/3677187 [Google Scholar]
- Wright J., Russell A.F. How helpers help: disentangling ecological confounds from the benefits of cooperative breeding. J. Anim. Ecol. 2008;77:427–429. doi: 10.1111/j.1365-2656.2008.01391.x. doi:10.1111/j.1365-2656.2008.01391.x [DOI] [PubMed] [Google Scholar]
- Yaber M.C., Rabenold K.N. Effects of sociality on short-distance, female-biased dispersal in tropical wrens. J. Anim. Ecol. 2002;71:1042–1055. doi:10.1046/j.1365-2656.2002.00667.x [Google Scholar]
- Young A.J., Carlson A.A., Montfort S.L., Russell A.F., Bennett N.C., Clutton-Brock T.H. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA. 2006;103:12 005–12 010. doi: 10.1073/pnas.0510038103. doi:10.1073/pnas.0510038103 [DOI] [PMC free article] [PubMed] [Google Scholar]