Abstract
Maternal effects by which females provide their offspring with non-genetic factors such as hormones, nutrients and antibodies can have an important impact on offspring fitness. In vertebrates, maternal antibodies (matAb) are transferred from the mother, via the placenta, egg yolk or milk during lactation to offspring until they are 2 weeks (birds), 4–10 weeks (rodents) and 9 months (humans) old, respectively. matAb transfer can have direct effects on offspring growth rate in birds and rodents, probably by passively protecting the newborn from common pathogens before their endogenous immune system has matured. Indirect long-term effects of matAb transfer on the offspring's own immunity can be synergistic, if matAb act as antigen templates of the accumulated immunological experience of the mother and educate the newborn's immune system. However, it may also be suppressive if matAb reduce antigen presentation to the newborn resulting in antigen-specific blocking of offspring endogenous immunity. Our aim is to review the mechanisms and direct effects of matAb transfer in vertebrates with an emphasis on birds, outline a framework for research on the long-term effects of matAb on the endogenous immune system of the mature offspring and encourage ecological and evolutionary studies of matAb transfer in non-domesticated animals.
Keywords: maternal effects, maternal antibody transfer, trans-generational effects, immune system priming, offspring growth rate, epitope blocking
1. Introduction
When females provide their offspring with non-genetic benefits, they can have a dramatic impact on offspring fitness (Mousseau & Fox 1998), both during ontogeny as well as when that offspring becomes an adult (Agrawal et al. 1999; Tollrian & Harwell 1999; Grindstaff et al. 2003). In mammals, maternal factors can be transferred via the placenta, in the colostrum (the first milk transferred from the mother to her offspring just after birth) and in normal milk during lactation (Glezen 2003; Siegrist 2003; Lemke et al. 2004). In birds, reptiles and fishes, maternal factors are mainly transferred via the egg to the offspring (Bly et al. 1986; Schumacher et al. 1999; Grindstaff et al. 2003), hence the time period for the uptake of maternal factors is restricted to a period before, and shortly after birth.
Among the maternal factors that can be transferred by the mother to her offspring are hormones, antibodies and nutrients (macronutrients, mainly protein; micronutrients, e.g. carotenoids) (e.g. Blount et al. 2000, 2002; Rubolini et al. 2006; Biard et al. 2007). Previous research on trans-generational effects in mammals has focused on maternal antibody (matAb) transfer, in particular, in relation to vaccination in humans and mice. In birds, maternal transfer of antibodies via egg yolk to offspring has mainly been studied in poultry as a means of improving chick survival early in life, whereas research on wild birds has focused on maternal transfer of testosterone (e.g. Schwabl 1993; Schwabl et al. 1997; Gil et al. 1999) and to some extent carotenoids (Blount et al. 2002; Biard et al. 2007).
Parasites and other pathogens can have a decisive impact on the survival and performance of individuals hence protection against disease agents must be essential in natural populations (e.g. Loye & Zuk 1991; Lochmiller & Deerenberg 2000). The humoral, antibody-mediated immune system matures slowly in neonatal vertebrates, restricting them to fighting off infections and parasites with the innate immune system (Klasing & Leshchinsky1999; Grindstaff et al. 2003). However, maternally transferred antibodies may constitute an important addition to the neonate's ability to take care of antigens. The vulnerable window in young vertebrates is from birth to the age when the youngster starts to synthesize antibodies endogenously (Lawrence et al. 1981). The age at which the neonate starts to produce antibodies on its own differs markedly between species (Grindstaff et al. 2003). In some birds, increases in the levels of antibodies have been found 10–14 days post-hatch (Gasparini et al. 2006; Grindstaff et al. 2006; Pihlaja et al. 2006; Staszewski et al. 2007a), whereas it may take several weeks in poultry and rodents, and several months to years in humans (Apanius 1998; Grindstaff et al. 2003). The immune protection provided by maternally transferred antibodies is of rather short duration because they are catabolized. Most matAb may have disappeared from the offspring within 5–14 days in birds (Lozano & Ydenberg 2002; Grindstaff et al. 2003, 2006; Staszewski et al. 2007a), whereas in mammals lactation prolongs the period of passive protection to 5–10 weeks in rodents (Grindstaff et al. 2003; Kallio et al. 2006) and approximately 9 months in humans (Roitt et al. 1998). Hence, given that vertebrate neonates have a rudimentary immune defence early in life, the potential importance of matAb transfer to protect the neonate offspring from infection is considerable. Despite this, there are surprisingly few studies that have investigated how offspring are directly (in one- to two-week-old neonates) and indirectly (in particular long-term consequences in mature individuals and adults) affected by matAb transfer. Most studies conducted so far have focused on humans, mice and poultry, and only recently have mechanisms for matAb transfer and its consequences on offspring phenotype been investigated in non-domesticated vertebrates.
In this review, we concentrate on maternal transfer of antibodies in vertebrates with a special emphasis on birds. We begin with describing how the mother transfers antibodies to her offspring, to what extent it reflects the immunological history of the mother and the evidence for it being a passive process. Next we outline the direct effects of matAb as a passive immune protection for the neonate, and then examine the hypotheses proposed to explain the long-term effects of matAb on the endogenous immune system of the mature offspring. Finally, we highlight some aspects of matAb transfer that we rate as particularly interesting to focus on in future studies, with a special emphasis on evolutionary and ecological questions.
2. Trans-generational transfer of antibodies from the mother
Maternally transferred immunity factors potentially convey passive immunity to offspring primarily via IgG (sometimes referred to as IgY in birds). Available data suggest that the diversity and amount of antibodies transferred to offspring are a reflection of the local disease environment and mirror the circulating populations of antibodies in the female (Lemke & Lange 1999; Gasparini et al. 2001; Lemke et al. 2003). Thus, the offspring can get only those matAb against antigens that the mother herself has been exposed to during her lifetime (Heller et al. 1990), although in the case of allosuckling young, they may get an antibody repertoire from several females (Roulin & Heeb 1999). The maternal transfer of antibodies that the mother has accrued over a long time period is evident from some long-term immunization studies. Specific matAb were found to be transferred to offspring 1 year after maternal exposure in kittiwakes (Rissa tridactyla; Staszewski et al. 2007a) and seven to nine months after exposure in song sparrows (Melospiza melodia; Reid et al. 2006). Female pups of Wistar rats (Rattus norvegicus) were fed with antibodies that bind to a strain of Escherichia coli when 2 days old. In the next generation, the offspring of these females had higher levels of the specific antibodies than control offspring (Lundin et al. 1999). Thus, antibodies produced by the mother during an antigen challenge the previous season, as well as antibodies she has accrued from her grandmother, can be transferred from the mother to her offspring. Still, most studies of non-domesticated animals conducted so far have only examined the effect of an (experimentally induced) ongoing immune response in the mother on her immediate trans-generational transfer of antibodies to her offspring (e.g. Grindstaff et al. 2003).
Judging from a commonly observed positive relationship between the amount of specific antibodies circulating in mothers and the antibody levels in eggs or offspring, mothers seem to transfer antibodies into their eggs in proportion to the concentration in their circulation (Blount et al. 2002; Gasparini et al. 2002; Grindstaff et al. 2003; Staszewski et al. 2007a; Grindstaff 2008). That the relationship is due to maternal transfer of antibodies and not due to a common environment in general is reflected by the lack of such a relationship between antibody concentrations in fathers and the eggs of their mates (Gasparini et al. 2002; Reid et al. 2006). This passive way of depositing antibodies in the egg during yolk formation has several advantages. Repeated or recent exposures to diseases or parasites probably increase the circulating concentrations of specific antibodies in mothers, thus reflecting temporal and spatial variations in the risk of being exposed to a disease or parasite. In line with this, a study on kittiwakes showed that the prevalence of offspring with specific antibodies against Borrelia increased with an increase in the prevalence of ticks, the vector of Borrelia infections (Gasparini et al. 2001; Staszewski et al. 2007b). Furthermore, general yolk antibody concentrations have been found to be positively related to local breeding density (Müller et al. 2004), indicating a probable increase in the risk of being exposed to a pathogen.
If the maternal deposition of antibodies in the egg is connected to costs for the female, the concentration of antibodies in the yolk may be constrained by, for example, nutrient or energy limitations. Female hens (Gallus gallus) transfer approximately 10–20% of their steady-state level of antibodies into the eggs (Kowalczyk et al. 1985). To sustain this drainage of antibodies, egg-laying females increase their production of antibodies compared with non-laying hens (Klasing 1998). In line with this, yolk antibody levels have been reported to correlate positively with the condition of the mother (Hargitai et al. 2006). However, experimental addition of food to egg-laying females has produced mixed results as positive (Pihlaja et al. 2006), negative (Gasparini et al. 2007), and no effect (Grindstaff et al. 2005) have been found on the concentration of antibodies in the yolk. We believe that this variation in the outcome of feeding experiments reflects the highly variable effects of condition and food availability on the immune response in adults themselves (Hasselquist & Nilsson submitted). Thus, if antibody deposition in eggs is generally a reflection of the antibodies circulating in the mother, females that increase their immune response when food availability increases will also deposit higher concentrations in their eggs. In one of the food addition studies above, females did not respond to the added food with a variation in the circulating concentration of antibodies, and consequently, there was no effect on the concentration in the eggs (Grindstaff et al. 2005). Fed females transferring fewer antibodies to their eggs may seem counter-intuitive. However, if there are strong negative long-term costs to the offspring of matAb (see below), it could be adaptive for females in prime condition to transfer less matAb to their offspring and then compensate for the lack of short-term benefits of matAb by an increased parental effort (Gasparini et al. 2007).
If the cocktail of maternally transferred antibodies in the eggs is just a reflection of the diversity and concentration in the mother, we should not find any other patterns than the reflection of the local diversity of pathogens, in the deposition within or between clutches. However, such variation has been reported. Female barn swallows (Hirundo rustica) transfer more specific antibodies to their eggs if their mates were manipulated to advertise high quality (Saino et al. 2002a). Variation within a clutch has also been reported showing either increasing (Pihlaja et al. 2006), no trend (Grindstaff et al. 2005) or decreasing (Blount et al. 2002; Müller et al. 2004) antibody levels with laying sequence. Thus, these data may suggest that females, at least within certain limits, can actively adjust the concentration of matAb according to quality of breeding attempt or adaptive adjustments of the quality of eggs within a clutch.
In addition to matAb (i.e. a contribution from the acquired humoral immune system of the mother), lysozyme has been found to be transferred by the female to her young (Saino et al. 2002b). Lysozyme is part of the innate immune system and acts by digesting peptidoglycans that is a major component of the cell walls of bacteria (Roitt et al. 1998). This immune component is transferred via the albumen and, although conferring an antibacterial defence for the egg and enhancing hatching success (Saino et al. 2002b), a positive relationship between circulating levels of lysozyme in the female and in her offspring have also been found at the age of both 7 and 12 days (Saino et al. 2002b). Thus, these maternally transferred immune components may be taken up by the neonate and potentially boost the offspring's innate immune defence early in life.
3. Direct effects of maternal antibody transfer to offspring
The primary short-term benefit of matAb is believed to lie in pathogen resistance of neonates during the vulnerable period when their own immune system has not yet matured. This has been taken advantage of in the poultry industry where laying hens are vaccinated to provide passive immunity to their chicks (e.g. Goddard et al. 1994). Both the diversity and the amount of matAb being transferred to the neonate are of importance for the passive protection of the young. The time period when matAb are retained in the circulation of the neonate is dependent on the initial levels provided (Goddard et al. 1994; Nicoara et al. 1999). Below, we will review the benefits of the direct action of maternally transferred antibodies.
The most commonly observed short-term benefit of matAb is enhanced growth rate. This has been found as a result of both induced immune responses in the mothers (Gustafsson et al. 1994; Lozano & Ydenberg 2002) as well as enhanced growth of young raised in the same pathogen environment as their mothers (Heeb et al. 1998; Buechler et al. 2002; Kristan 2002; Grindstaff 2008). In the latter case, the experiments are mimicking the advantage of mothers conferring immunity against the local pathogen fauna. The hampered growth of young without specific matAb is believed to depend on the fact that these young have to rely on an innate immune response to clear the pathogens (Grindstaff 2008). The innate immune system is more expensive in terms of nutrients (Klasing & Leshchinsky 1999) and energy (Råberg et al. 2002) than the acquired (T- and B-cell mediated) immune system. Thus, matAb might reduce the need for invoking the innate system and thereby alleviate the growth reduction caused by a costly immune response (Grindstaff 2008). Nestlings of altricial mother birds that were exposed to fleas (Ceratophyllus gallinae), before egg laying grew better in an environment containing fleas than nestlings of unexposed mothers (Heeb et al. 1998; Buechler et al. 2002). However, this was not due to increased resistance to fleas, because flea survival and reproduction were unaffected by matAb (Heeb et al. 1998; Walker et al. 2003). Instead, the interpretation was that matAb take care of the antigen transmitted by the fleas when they bite, hindering a potentially much more energetically expensive immune response than the cost of the blood removed by the fleas (Nilsson 2003).
As an extension of enhanced growth rates, earlier maturation (Kallio et al. 2006) and earlier fledging (Heeb et al. 1998) are also potentially important fitness benefits of matAb transmission. Ultimately, matAb also enhance survival during the early part of life (Heller et al. 1990; Gustafsson et al. 1994; Pihlaja et al. 2006). Given the potentially important fitness benefits of transmitting antibodies to the next generation, it has been suggested that females should deliberately try to get infected by the local pathogens before egg formation (Heeb et al. 1998; Lozano & Ydenberg 2002). However, the costs to the females of such a strategy are probably larger than the benefits for the young. Costs of mounting an immune response include increased risk of immunopathology and increased rates of reactive oxygen species (ROS) formation (Hasselquist & Nilsson submitted). Especially running two ROS producing activities at the same time, viz. an immune response and a high work load in the form of egg formation, may drastically increase the risk of damage to important biomolecules such as DNA, lipids and proteins. Moreover, it is highly likely that the negative effects of the infection in the mother is carried over into the next breeding phase, i.e. the highly demanding period of nestling feeding (Drent & Daan 1980; Ilmonen et al. 2000), resulting in a diminished work capacity of the female and ultimately lowered reproductive success. Furthermore, carotenoid-fed female black-backed gulls (Larus fuscus) have been found to decrease their level of circulating antibodies compared with control-fed females, translating into a reduced transfer of matAb to eggs and hatchlings (Blount et al. 2002). One possible interpretation of these results is that females with a high availability of antioxidants (carotenoids) will clear any infection before egg laying starts, even though this will result in a reduced maternal transfer of immunity (Blount et al. 2002).
4. Indirect effects of maternal antibody transfer—mechanisms
There are two opposing scenarios for how trans-generational transfer of matAb affects the offspring's own (endogenous) humoral immune responses. One hypothesis states that matAb have long-lasting positive effects on the antigen-specific antibody responses of the mature offspring, whereas the alternative hypothesis states that matAb have a negative effect on the offspring's own production of antigen-specific antibodies.
(a) matAb enhance the humoral immunity of offspring
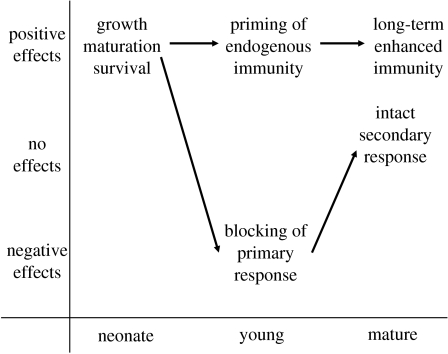
Studies of mice and humans have revealed that matAb transferred to the offspring do not only provide passive protection early in life, but may also have an instructive role during the development of the endogenous humoral (antibody-mediated) immune system of the offspring (figure 1; Anderson 1995; Lemke & Lange 2002; Lemke et al. 2004). This hypothesis builds on observations in mice that show (i) offspring that received antigen-specific matAb early in life produced higher (endogenous) antibody titres than controls when challenged with the same antigen when fully matured (e.g. Desowitz 1971; Lange et al. 2002; Lemke et al. 2003), (ii) the enhancement of offspring endogenous antibody responses was only through the maternal, and not the paternal, line (Cookson et al. 1992; for an example in birds see Reid et al. 2006), (iii) the positive effect of offspring antibody responses was not a result of matAb transferring the specific antigen to the neonate (Ono et al. 1974; Sasaki et al. 1977; Jarrett & Hall 1983; Roberts & Turner 1983, Lange et al. 2002), (iv) secondary Ab response levels can be reached in offspring after a first injection with an antigen to which their mother had been repeatedly exposed (Stern 1976; Okamoto et al. 1989), and (v) the enhanced effect could even be detected in the F2 generation, i.e. only when their grandmother had been injected with the antigen (Lemke et al. 1994; Ismail et al. 1995; Lange et al. 1999; Lundin et al. 1999).
Figure 1.
Positive, neutral and negative effects of matAb on the endogenous immune system and fitness of offspring in three different life stages. Neonate, from birth/hatching to the age when all the matAb are catabolized; young, from the start of the development of the offspring's endogenous immune system to a fully matured immune system; mature, the adult life stage. Arrows denote a schematic development of costs and benefits depending on the effect of matAb on the developing endogenous immune system of offspring.
Lemke & Lange (1999) proposed a mechanism for how matAb could play an instructive role in the development of the endogenous humoral immune system of the offspring. They suggested that immunoglobulins and antigen receptors of B-cells form an interacting network of idiotypes, which is functionally connected to the T-cell compartment. In this context, immunoglobulins function as an internal antigen in the network, and could thus directly influence the developing immune system of the neonate (Anderson 1995). The effect of matAb can either be specifically targeting its corresponding idiotype (or anti-idiotype) in the neonate (Victor et al. 1983; Lemke & Lange 1999), or if it is directed towards a highly connective idiotype, expressed by cross-reactive IgM antibodies, it may even alter the entire repertoire of the individual (Bernabe et al. 1981).
There seems to be a rather short time window early in life when matAb may function as internal antigens and have long-term consequences for the endogenous immune system of the offspring (Anderson 1995). In mice, matAb from breast milk can be transported from the neonate's gut to the bloodstream only during the first 12–16 days of life (Mackenzie & Keeler 1984). Moreover, long-lasting effects on a specific Ab response in offspring could be achieved only when specific syngeneic antibodies were administered at 2–11 days of age (Haba & Nisonoff 1995). This is in accordance with the finding that immunoglobulin-dependent selection of the T-cell repertoire operates only during the first three weeks of life in mice (Martinez et al. 1985). Additional support for the importance of exposure to matAb:antigen complexes early in life comes from studies on humans, where the incidence of hay fever in adults were lower in those living on a farm during the first year of life rather than years 1–5 and much lower than in those not living on a farm at all (Riedler et al. 2001). Hence, in the context of the ‘offspring immunity enhancement hypothesis’, matAb are proposed to function as ‘templates’ of antigens constituting the accumulated immunological experience of the mother, which could be passed on to the offspring and result in an education of the nascent immune system of the newborn.
Another advantage of matAb is that their epitope blocking may prevent the misrecognition of antigens as self (during the period early in life when the offspring develops its self-antigen tolerance through selective deletion of B-cells that produce antibodies that would attack self-antigens), thus avoiding the development of immunological tolerance to the specific antigen (Carlier & Truyens 1995).
(b) matAb suppress the humoral immunity of offspring
In studies of vaccination in infants, mainly in humans and mice, there is a common pattern that the presence of matAb results in a lower endogenous antigen-specific Ab response to the vaccine (Carlier & Truyens 1995; Siegrist 2003). Hence, at least part of the positive effect of matAb in protecting the offspring early in life (figure 1) would, under this scenario, be offset by the negative effect acting on the offspring's own antibody response to the antigen at an older age. A very important general finding in these studies is that infant B-cell, but not T-cell, responses are suppressed by matAb (Siegrist 2003). In essence, this means that the infant's endogenous primary antibody response to the specific antigen will be suppressed, whereas the T-cell mediated secondary antibody response to the antigen will be normal and robust (figure 1).
Several mechanisms have been proposed to explain the process of matAb inhibition of infant B-cell activation and hence antibody production. (i) matAb may rapidly neutralize the antigenic agent, preventing it from replicating and resulting in an antigen load that is too low to prime infant B- and T-cells to produce antigen-specific antibodies (Albrecht et al. 1977). (ii) The matAb:antigen immune complexes bind to infant B-cell receptors preventing B-cell activation and antibody production (Daeron 1997). (iii) matAb:antigen immune complexes are eliminated by Fc-dependent phagocytosis, resulting in reduced levels of antigen to be presented to infant B-cells. However, the antigen will be processed and peptide epitopes will be presented to infant T-cells allowing memory cell production. (iv) Epitope blocking—matAb bind to specific epitopes and hide them from infant B-cells (Heyman 2001). Only the two latter hypotheses are consistent with the key observation of a lack of B-cell activation but the presence of T-cell activation in infants, and other studies provide some additional support for epitope blocking rather than the Fc-dependent phagocytosis mechanism (Siegrist 2003). Hence, studies of human and mice emphasize that the negative effect of matAb on the infant antibody response is characterized by the importance of matAb:antigen concentration ratio (and hence the timing of antigen exposure), and of the suppression of primary but not secondary antibody response.
From an evolutionary point of view, a scenario of a negative effect of matAb on offspring endogenous humoral immune response in the mature offspring would imply costs in addition to the direct benefits (see above) of trans-generational transfer of matAb to offspring (figure 1). If, however, this negative effect is only transient, occurring the first time the antigen is exposed to the infant's mature immune system, the cost may be alleviated (Carlier & Truyens 1995). However, note that the first exposure to a pathogen, when the host is reliant on the less efficient and slower primary antibody response, is often associated with increased disease susceptibility and host mortality (e.g. Valkiūnas 2005; Zehtindjiev et al. 2008).
5. Empirical studies of how matAb affect the offspring's own immunity in birds
There are so far rather few studies of non-direct, long-term effects of matAb transfer on offspring immunity in animals. Besides studies on mice under laboratory conditions, only a few studies on wild birds have so far evaluated the effect of matAb on the development of the offspring's own immunity.
(a) matAb enhance the humoral immunity of offspring
Kittiwakes, a long-lived colonial seabird, are naturally exposed to the tick-borne parasite Borrelia burgdorferi and infected mothers transfer anti-Borrelia antibodies to their eggs and offspring (Gasparini et al. 2001, 2002). In this study system, kittiwake chicks were tested for either the presence or absence of anti-Borrelia antibodies at 5 days of age, the former groups were assumed to have obtained the antibodies from their exposed mothers whereas the latter groups were assumed to have uninfected mothers (Gasparini et al. 2006). The chicks were then tested at 10 and 20 days of age, i.e. when they had begun their own endogenous antibody production, and the test showed that chicks that had higher anti-Borrelia titres when 5 days old had significantly higher anti-Borrelia Ab titres also at 10 and 20 days of age. The interpretation of these results was that mothers previously exposed to Borrelia transferred specific anti-Borrelia matAb that enhanced the antigen-specific humoral response of their offspring (Gasparini et al. 2006). This study suggests that matAb transfer could enhance the endogenous immune system of the offspring in an antigen-specific way. Note, however, that the specific antibody response was not measured directly in the mothers before egg laying, but rather inferred from the chicks antibody titre at 5 days of age. This measure may hence be confounded by other factors, e.g. the antibody production of the chick itself (if the endogenous production of antibodies starts earlier than day 5 in gulls, something that is not known in detail).
However, further evidence for the enhancive effect of matAb on the endogenous immune system of the offspring comes from studies of wild passerine birds. In an experiment on wild pied flycatchers (Ficedula hypoleuca), females were injected with either an antigen (lipopolysaccharide, LPS) or saline (control) approximately one week before egg laying. Offspring of LPS-injected females had more circulating antibodies and tended to have more LPS-specific antibodies when 14 days old compared with offspring of saline-injected females (Grindstaff et al. 2006). The interpretation of these results was that maternally transferred antibodies can have positive effects on the endogenous humoral immune system of the offspring in general and not only being antigen specific. However, note that LPS was used as the antigen stimulating the mother's antibody production before egg laying, and that LPS is a very potent antigen that can stimulate a broad range of the immune system including B-cells in an unspecific way (Kuby 1992; Janeway & Travers 1996). Hence, we find it plausible that an LPS injection in the mother caused a general (unspecific) enhancement of offspring humoral immunity in this study. However, offspring could be sampled only during the first two weeks of life, so nothing is known about the long-term consequences of the matAb treatment in this study.
Long-term antigen-specific enhancement of offspring antibody responses has, however, been observed in a study on another wild passerine bird. In song sparrows at Mandarte Island, Canada, approximately 50 per cent of the adult population was injected with an antigen (keyhole limpet haemocyanin) in autumn (Reid et al. 2006). Approximately seven to nine months later, females laid their eggs and broods were raised until independence. Some two to four months after hatching, approximately 50 per cent of the offspring were caught, injected with the same antigen as their parents and primary antibody responses to the specific antigen were measured. The results of this study showed that fully grown offspring of mothers injected with the antigen a year before produced more antigen-specific antibodies than the offspring of non-injected mothers. Hence, these studies corroborate several of the ideas outlined in the ‘matAb-induced enhancement of offspring immunity’ hypothesis as they show that the mother's immunological memory of a previously encountered antigen can be transferred to the offspring, providing them with an endogenous humoral immune response that is specifically good at targeting these antigens (Reid et al. 2006).
(b) matAb suppress the humoral immunity of offspring
Negative non-direct effects of matAb transfer to offspring in animals come from a study of the kittiwake. Females were either injected with a Newcastle disease virus (NDV) vaccine or not injected at all in 1 year and the effect of this vaccination was studied in their nestlings the next year (Staszewski et al. 2007a). Chicks were cross-fostered, and when 1 day old they were injected with the NDV vaccine and then their blood was sampled until they were 25 days old. The results of this study showed that chicks of mothers, which had been exposed to NDV vaccination the year before had initially higher (when 5 days old and solely relying on the antibodies transferred from their mother), but when 20–25 days old significantly lower (when producing antibodies themselves) antigen-specific antibody titres (Staszewski et al. 2007a). This suggests that birds may face an antigen blocking effect of maternally transferred antibodies if exposed to the antigen early in life, as has been found in studies of humans and mice (reviewed in Carlier & Trouyens 1995; Siegrist 2003).
In conclusion, most studies of indirect effects of matAb transfer in non-domesticated animals have so far been carried out on birds during the first two to three weeks of their lives, i.e. during the early phase of the maturation of the endogenous immune system of the offspring. In these studies, matAb transfer seems to enhance offspring endogenous humoral immunity at the end of the nestling period (at an age of 14–20 days) when matAb should have been completely consumed. This enhancement of offspring humoral immunity was specific in the case of kittiwakes but possibly general in the pied flycatchers. Thus, at a time early in life when we expect primary immune responses against specific antigens, several studies report enhanced effects of matAb transfer on offspring immunity. Long-term effects of matAb transfer have only been conducted in one study of a non-domesticated bird, the song sparrows studied in the wild at Mandarte Island. In this study, transfer of matAb enhanced the offspring's antigen-specific humoral immune response when fully mature (Reid et al. 2006). In the kittiwake study that showed a blocking effect of the maternally transferred antibodies, the exposure to the antigen occurred very early in life (at day 1), which may be an important observation to reconcile the differing results of the studies of wild birds. Hence, so far there are very few studies of how matAb transfer can affect the endogenous immune system of the mature offspring, and this is particularly true for non-domesticated vertebrates. More studies are definitely needed before we can evaluate the long-term pros and cons of matAb transfer on the offspring's own immune system.
6. Discussion
Based on the available information from the studies of vertebrates, a general pattern seems to emerge where the effect of matAb transfer to offspring can be divided into three stages (figure 1). Early in life (lasting up to 2 weeks in birds, 4–10 weeks in rodents and 4–9 months in humans; Anderson 1995; Apanius 1998; Roitt et al. 1998; Grindstaff et al. 2003), during the period when there is a relatively high concentration of matAb in the blood of the neonate, matAb are beneficial through their directly protective effect against common pathogens. Then comes a period when the infant's own immune system is developing, which lasts roughly up to two to six months in chicken and six to seven weeks in mallards and macaws (Rose & Orlans 1981; Grindstaff et al. 2003), more than two weeks in rodents and up to 1–2 years in humans (Roitt et al. 1998; Grindstaff et al. 2003). During this maturation period, the matAb may have not only negative effects, e.g. through epitope blocking and inactivation of B- and T-cells (Carlier & Truyens 1995), but also positive, through an instructive priming effect (figure 1), on the development of the immune system of the offspring (Lemke & Lange 1999). In the third stage, when offspring immunity has matured, matAb transfer may, in most cases, have no or even positive effects on offspring endogenous immunity (Glezen 2003; Lemke et al. 2004; Reid et al. 2006).
In terms of immunology, there is still much to learn about the time frame of antibody transmission from the mother to the offspring in non-model animals. Is it the whole lifetime repertoire of the mother, which is transferred or is it mainly the antibodies from a recent or even ongoing response? In either case, a still untested prediction from matAb transfer theory is that rare pathogens would be in an advantageous position compared with common pathogens. Thus, in systems where maternal transfer of antibodies are important, we would predict pathogens to show cyclic population dynamics with the length of the cycles corresponding to the generation time of the most important hosts (cf. Hamilton & Zuk 1982).
In terms of evolutionary and ecological consequences, it is essential to quantify the benefits and costs for neonates, maturing and fully grown offspring as well as mothers. Thus, we need to know under what circumstances there would be a positive or a negative effect of matAb on the development of the offspring's own immune system and to what extent this is carried over into adulthood. These measures would also reflect the potential benefits to the mother. However, equally important would be to estimate the costs for the mothers. This information would be essential for evaluating to what extent mothers can manipulate the concentration of matAb within and between clutches. Furthermore, knowledge about the nature and strength of short- and long-term costs and benefits of matAb transfer will make it possible to predict how differences between species are based on, for example, exposure to parasites (e.g. in relation to latitude and habitat distribution; Piersma 1997; Møller & Erritzøe 1998; Hasselquist 2007) and life-history strategies (e.g. slow versus fast pace of life; Ricklefs & Wikelski 2002; Hasselquist 2007). In this respect, several scenarios are possible.
One hypothesis is that fast pace-of-life species (typically short-lived species) are more likely to invest in an upregulation of the immune system to enhance matAb transfer to offspring if such an upregulation comes at a substantial cost in terms of the mother's survival or her future reproduction (Grindstaff et al. 2003). Alternatively, slow pace-of-life species (typically long-lived species) may be more likely to invest in an upregulation of the immune system to enhance matAb transfer to offspring, due to a higher potential pay-off from investments that improve offspring phenotype in species with a long life expectancy (Stearns 1992; Ricklefs & Wikelski 2002; Hasselquist 2007). This latter hypothesis relies on the assumption that upregulating the humoral immune response just before egg laying may have immediate, short-term costs but does not induce substantial long-term costs (that have impact on fitness) for the mother. There are some studies implying short-term fitness costs of humoral immune system activation on nestling feeding (Ilmonen et al. 2000; Råberg et al. 2000; Bonneaud et al. 2003), although no effect on egg laying has so far been found (Williams et al. 1999). Our knowledge about long-term costs of immune responses is very limited, although oxidative stress and immunopathology are candidates with the potential to reduce life expectancy (Hanssen et al. 2004; Hasselquist & Nilsson submitted). Furthermore, little is known about the possible synergistic or antagonistic effects between different maternal investments in the egg, e.g. matAb, hormones and carotenoids (Blount et al. 2002), which may affect the balance between costs and benefits of matAb transfer under different conditions.
In our view, this is an exciting area of research where the studies conducted so far have yielded exciting and unexpected results that challenge some of the traditional views of how the immune system works. From an evolutionary point of view, the trans-generational transmission of immunoglobulins, which have the potential to alter the phenotype of the offspring and hence may be seen as an epigenetic process (Lemke et al. 2004; Poulin & Thomas 2008), is interesting and challenging and deserves further study. For ecologists, studies of costs and benefits to offspring of matAb transfer are of considerable importance, given the high impact of parasites on population dynamics and life-history strategies in natural animal populations (Anderson & May 1982; Loye & Zuk 1991; Zuk & Stoehr 2002). A well-adapted trans-generational antibody protection mechanism would be particularly important for quality and survival of the neonate, although this may have to be traded off against potential long-term costs on offspring endogenous immunity. Hence, there may be room for different solutions and optima for matAb transfer in relation to for example population density, life-history strategy, parasite exposure, the condition of the female and the quality of her mate, which warrants further studies.
Acknowledgments
We thank Maria Sandell for many discussions of maternal effects in general and the transfer of antibodies to offspring in particular. The study was supported by grants from the Swedish Research Council (VR) (to D.H., J.-Å.N.), the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) and the Carl Trygger Foundation (to D.H.).
Footnotes
One contribution of 11 to a Theme Issue ‘Ecological immunology’.
References
- Agrawal A.A., Laforsch C., Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999;401:60–63. doi:10.1038/43425 [Google Scholar]
- Albrecht P., Ennis F.A., Saltzman E.J., Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J. Pediatr. 1977;91:715–718. doi: 10.1016/s0022-3476(77)81021-4. doi:10.1016/S0022-3476(77)81021-4 [DOI] [PubMed] [Google Scholar]
- Anderson R.W. On the maternal transmission of immunity: a ‘molecular attention’ hypothesis. BioSystems. 1995;34:87–105. doi: 10.1016/0303-2647(94)01444-c. doi:10.1016/0303-2647(94)01444-C [DOI] [PubMed] [Google Scholar]
- Anderson R.M., May R.M. Springer; Berlin, Germany: 1982. Population biology of infectious diseases. [Google Scholar]
- Apanius V. Ontogeny of immune function. In: Starck J.M., Ricklefs R.E., editors. Avian growth and development: evolution within the altrcial–precocial spectrum. Oxford University Press; Oxford, UK: 1998. pp. 203–222. [Google Scholar]
- Bernabe R.R., Couthino A., Cazenave P.A., Forni L. Suppression of a ‘recurrent’ idiotype results in profound alterations of the whole B-cell compartment. Proc. Natl Acad. Sci. USA. 1981;78:6416–6420. doi: 10.1073/pnas.78.10.6416. doi:10.1073/pnas.78.10.6416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biard C., Surai P.F., Møller A.P. Analysis of pre- and post-hatching maternal effects mediated by carotenoids in the blue tit. J. Evol. Biol. 2007;20:326–333. doi: 10.1111/j.1420-9101.2006.01194.x. doi:10.1111/j.1420-9101.2006.01194.x [DOI] [PubMed] [Google Scholar]
- Blount J.D., Houston D.C., Møller A.P. Why egg yolk is yellow. Trends Ecol. Evol. 2000;15:47–49. doi: 10.1016/s0169-5347(99)01774-7. doi:10.1016/S0169-5347(99)01774-7 [DOI] [PubMed] [Google Scholar]
- Blount J.D., Surai P.F., Nager R.G., Houston D.C., Møller A.P., Trewby M.L., Kennedy M.W. Carotenoids and egg quality in the lesser black-backed gull Larus fuscus: a supplemental feeding study of maternal effects. Proc. R. Soc. B. 2002;269:29–36. doi: 10.1098/rspb.2001.1840. doi:10.1098/rspb.2001.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bly J.E., Grimm A.S., Morris I.G. Transfer of passive immunity from mother to young in a teleost fish: haemagglutinating activity in the serum and eggs of plaice, Pleuronectes platessa L. Comp. Biochem. Physiol. A. 1986;84:309–313. doi: 10.1016/0300-9629(86)90620-1. doi:10.1016/0300-9629(86)90620-1 [DOI] [PubMed] [Google Scholar]
- Bonneaud C., Mazuc J., Gonzalez G., Haussy C., Chastel O., Faivre B., Sorci G. Assessing the cost of mounting an immune response. Am. Nat. 2003;161:367–379. doi: 10.1086/346134. doi:10.1086/346134 [DOI] [PubMed] [Google Scholar]
- Buechler K., Fitze P.S., Gottstein B., Jacot A., Richner H. Parasite-induced maternal response in a natural bird population. J. Anim. Ecol. 2002;71:247–252. doi:10.1046/j.1365-2656.2002.00591.x [Google Scholar]
- Carlier Y., Truyens C. Influence of maternal infection on offspring resistance towards parasites. Parasitol. Today. 1995;11:94–99. doi: 10.1016/0169-4758(95)80165-0. doi:10.1016/0169-4758(95)80165-0 [DOI] [PubMed] [Google Scholar]
- Cookson W.O., et al. Maternal inheritance of atopic IgE responsiveness on chromosome 11q. Lancet. 1992;340:381–384. doi: 10.1016/0140-6736(92)91468-n. doi:10.1016/0140-6736(92)91468-N [DOI] [PubMed] [Google Scholar]
- Daeron M. Fc receptor biology. Annu. Rev. Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. doi:10.1146/annurev.immunol.15.1.203 [DOI] [PubMed] [Google Scholar]
- Desowitz R.S. Plasmodium berghei: enhanced protective immunity after vaccination of white rats of immune mothers. Science. 1971;172:1151–1152. doi: 10.1126/science.172.3988.1151. doi:10.1126/science.172.3988.1151 [DOI] [PubMed] [Google Scholar]
- Drent R.H., Daan S. The prudent parent—energetic adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Gasparini J., McCoy K.D., Haussy C., Tveraa T., Boulinier T. Induced maternal response to the Lyme disease spriochaete Borrelia burgdorferi sensu lato in a colonial seabird, the kittiwake Rissa tridactyla. Proc. R. Soc. B. 2001;268:647–650. doi: 10.1098/rspb.2000.1411. doi:10.1098/rspb.2000.1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini J., McCoy K.D., Tveraa T., Boulinier T. Related concentrations of specific immunoglobulins against the Lyme disease agent Borrelia burgdorferi sensu lato in eggs, young and adults of the kittiwake (Rissa tridactyla) Ecol. Lett. 2002;5:519–524. doi:10.1046/j.1461-0248.2002.00345.x [Google Scholar]
- Gasparini J., McCoy K.D., Staszewski V., Haussy C., Boulinier T. Dynamics of anti-Borrelia antibodies in black-legged kittiwake (Rissa tridactyla) chicks suggest a maternal educational effect. Can. J. Zool. 2006;84:623–627. doi:10.1139/Z06-024 [Google Scholar]
- Gasparini J., Boulinier T., Gill V.A., Gil D., Hatch S.A., Roulin A. Food availability affects the maternal transfer of androgens and antibodies into eggs of a colonial seabird. J. Evol. Biol. 2007;20:874–880. doi: 10.1111/j.1420-9101.2007.01315.x. doi:10.1111/j.1420-9101.2007.01315.x [DOI] [PubMed] [Google Scholar]
- Gil D., Graves J., Hazon N., Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. doi:10.1126/science.286.5437.126 [DOI] [PubMed] [Google Scholar]
- Glezen P. Effect of maternal antibodies on the infant immune response. Vaccine. 2003;21:3389–3392. doi: 10.1016/s0264-410x(03)00339-6. doi:10.1016/S0264-410X(03)00339-6 [DOI] [PubMed] [Google Scholar]
- Goddard R.D., Wyeth P.J., Varney W.C. Vaccination of commercial layer chicks against infectious bursal disease with maternally derived antibodies. Vet. Rec. 1994;135:273–274. doi: 10.1136/vr.135.12.273. [DOI] [PubMed] [Google Scholar]
- Grindstaff J.L. Maternal antibodies reduce costs of an immune response during development. J. Exp. Biol. 2008;211:654–660. doi: 10.1242/jeb.012344. doi:10.1242/jeb.012344 [DOI] [PubMed] [Google Scholar]
- Grindstaff J.L., Brodie E.D., III, Ketterson E.D. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transfer. Proc. R. Soc. B. 2003;270:2309–2319. doi: 10.1098/rspb.2003.2485. doi:10.1098/rspb.2003.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff J.L., Demas G.E., Ketterson E.D. Diet quality affects egg size and number but does not reduce maternal antibody transmission in Japanese quail Coturnix japonica. J. Anim. Ecol. 2005;74:1051–1058. doi:10.1111/j.1365-2656.2005.01002.x [Google Scholar]
- Grindstaff J., Hasselquist D., Nilsson J.-Å., Sandell M., Smith H.G., Stjernman M. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc. R. Soc. B. 2006;273:2551–2557. doi: 10.1098/rspb.2006.3608. doi:10.1098/rspb.2006.3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson E., Mattsson A., Holmdahl R., Mattsson R. Pregnancy in B-cell-deficient mice: postpartum transfer of immunoglobulins prevents neonatal runting and death. Biol. Reprod. 1994;51:1173–1180. doi: 10.1095/biolreprod51.6.1173. doi:10.1095/biolreprod51.6.1173 [DOI] [PubMed] [Google Scholar]
- Haba S., Nisonoff A. Prolongation of the responsiveness of newborn mice to syngeneic IgE by inhibition of IgE synthesis. Immunol. Lett. 1995;47:205–208. doi: 10.1016/0165-2478(95)00087-3. doi:10.1016/0165-2478(95)00087-3 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D., Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. doi:10.1126/science.7123238 [DOI] [PubMed] [Google Scholar]
- Hanssen S.A., Hasselquist D., Folstad I., Erikstad K.E. Cost of immunity: immune responsiveness reduces survival in a vertebrate. Proc. R. Soc. B. 2004;271:925–930. doi: 10.1098/rspb.2004.2678. doi:10.1098/rspb.2004.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargitai R., Prechl J., Török J. Maternal immunoglobulin concentration in collared flycatcher (Ficedula albicollis) eggs in relation to parental quality and laying order. Funct. Ecol. 2006;20:829–838. doi:10.1111/j.1365-2435.2006.01171.x [Google Scholar]
- Hasselquist D. Comparative immunoecology in birds: hypotheses and tests. J. Ornithol. 2007;148(Suppl. 2):S571–S582. doi:10.1007/s10336-007-0201-x [Google Scholar]
- Hasselquist, D. & Nilsson, J.-Å. Submitted. Cost of immune responses: what can we learn from studies of birds?
- Heeb P., Werner I., Kölliker M., Richner H. Benefits of induced host responses against an ectoparasite. Proc. R. Soc. B. 1998;265:51–56. doi:10.1098/rspb.1998.0263 [Google Scholar]
- Heller E.D., Leitner G., Drabkin N., Melamed D. Passive immunisation of chicks against Escherichia coli. Avian Pathol. 1990;19:345–354. doi: 10.1080/03079459008418685. doi:10.1080/03079459008418685 [DOI] [PubMed] [Google Scholar]
- Heyman B. Functions of antibodies in the regulation of B cell responses in vivo. Springer Semin. Immunopathol. 2001;23:421–432. doi: 10.1007/s281-001-8168-4. doi:10.1007/s281-001-8168-4 [DOI] [PubMed] [Google Scholar]
- Ilmonen P., Taarna T., Hasselquist D. Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc. R. Soc. B. 2000;267:665–670. doi: 10.1098/rspb.2000.1053. doi:10.1098/rspb.2000.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail J., Brait M., Leo O., Urbain J. Assessment of a functional role of auto-anti-idiotypes in idiotypic dominance. Eur. J. Immunol. 1995;25:830–837. doi: 10.1002/eji.1830250330. doi:10.1002/eji.1830250330 [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Travers P. Current Biology, Ltd; London, UK: 1996. Immunobiology: the immune system in health and disease. [Google Scholar]
- Jarrett E.E., Hall E. IgE suppression by maternal IgG. Immunology. 1983;48:49–58. [PMC free article] [PubMed] [Google Scholar]
- Kallio E.R., Poikonen A., Vaheri A., Vapalahti O., Henttonen H., Koskela E., Mappes T. Maternal antibodies postpone hantavirus infection and enhance individual breeding success. Proc. R. Soc. B. 2006;273:2771–2776. doi: 10.1098/rspb.2006.3645. doi:10.1098/rspb.2006.3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K.C. Nutritional modulation of resistance to infectious diseases. Poult. Sci. 1998;77:1119–1125. doi: 10.1093/ps/77.8.1119. [DOI] [PubMed] [Google Scholar]
- Klasing K.C., Leshchinsky T.V. Functions, costs, and benefits of the immune system during development and growth. In: Adams N.J., Slotow R.H., editors. Proc. 22 Int. Ornithological Cong. BirdLife South Africa; Durban, South Africa: 1999. pp. 2817–2835. [Google Scholar]
- Kowalczyk K., Daiss J., Halpern J., Roth T.F. Quantitation of maternal–fetal IgG transport in the chicken. Immunology. 1985;54:755–762. [PMC free article] [PubMed] [Google Scholar]
- Kristan D.M. Maternal and direct effects of the intestinal nematode Heligmosomoides polygyrus on offspring growth and susceptibility to infection. J. Exp. Biol. 2002;205:3967–3977. doi: 10.1242/jeb.205.24.3967. doi:10.1242/jeb.012344 [DOI] [PubMed] [Google Scholar]
- Kuby J. Freeman; New York, NY: 1992. Immunology. [Google Scholar]
- Lange H., Kobarg J., Yazynin S., Solterbeck M., Henningsen M., Hansen H., Lemke H. Genetic analysis of the maternally induced affinity enhancement in the non-Ox1 idiotypic antibody repertoire of the primary immune response to 2-phenyloxazolone. Scand. J. Immunol. 1999;49:55–66. doi: 10.1046/j.1365-3083.1999.00472.x. doi:10.1046/j.1365-3083.1999.00472.x [DOI] [PubMed] [Google Scholar]
- Lange H., Kiesch B., Linden I., Otto M., Thierse H.-J., Shaw L., Maehnss K., Hansen H., Lemke H. Reversal of the adult IgE high responder phenotype in mice by maternally transferred allergen-specific monoclonal IgG antibodies during a sensitive period in early ontogeny. Eur. J. Immunol. 2002;32:3133–3141. doi: 10.1002/1521-4141(200211)32:11<3133::AID-IMMU3133>3.0.CO;2-0. doi:10.1002/1521-4141(200211)32:11<3133::AID-IMMU3133>3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- Lawrence E.C., Arnaud-Battandier F., Grayson J., Koski I.R., Dooley N.J., Muchmore A.V., Blaese R.M. Ontogeny of humoral immune function in normal chickens: a comparison of immunoglobulin-secreting cells in bone marrow, spleen, lungs and intestine. Clin. Exp. Immunol. 1981;43:450–457. [PMC free article] [PubMed] [Google Scholar]
- Lemke H., Lange H. Is there a maternally induced immunological imprinting phase á la Konrad Lorenz? Scand. J. Immunol. 1999;50:348–354. doi: 10.1046/j.1365-3083.1999.00620.x. doi:10.1046/j.1365-3083.1999.00620.x [DOI] [PubMed] [Google Scholar]
- Lemke H., Lange H. Generalization of single immunological experiences by idiotypically mediated clonal connections. Adv. Immunol. 2002;80:203–241. doi: 10.1016/s0065-2776(02)80016-5. doi:10.1016/S0065-2776(02)80016-5 [DOI] [PubMed] [Google Scholar]
- Lemke H., Lange H., Berek C. Maternal immunization modulates the primary immune response to 2-phenyl-oxazolone in BLAB/c mice. Eur. J. Immunol. 1994;24:3025–3030. doi: 10.1002/eji.1830241216. doi:10.1002/eji.1830241216 [DOI] [PubMed] [Google Scholar]
- Lemke H., Hansen H., Lange H. Non-genetic inheritable potential of maternal antibodies. Vaccine. 2003;21:3428–3431. doi: 10.1016/s0264-410x(03)00394-3. doi:10.1016/S0264-410X(03)00394-3 [DOI] [PubMed] [Google Scholar]
- Lemke H., Coutinho A., Lange H. Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends Immunol. 2004;25:180–186. doi: 10.1016/j.it.2004.02.007. doi:10.1016/j.it.2004.02.007 [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L., Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. doi:10.1034/j.1600-0706.2000.880110.x [Google Scholar]
- Loye J.E., Zuk M. Oxford University Press; Oxford, UK: 1991. Bird—parasite interactions. [Google Scholar]
- Lozano G.A., Ydenberg R.C. Transgenerational effects of maternal immune challenge in tree swallows (Tachycineta bicolour) Can. J. Zool. 2002;80:918–925. doi:10.1139/z02-063 [Google Scholar]
- Lundin B.S., Dahlman-Höglund A., Pettersson I., Dahlgren U.I.H., Hansson L.Å., Telemo E. Antibodies given orally in the neonatal period can affect the immune response for two generations: evidence for active maternal influence on the newborn's immune system. Scand. J. Immunol. 1999;50:651–656. doi: 10.1046/j.1365-3083.1999.00651.x. doi:10.1046/j.1365-3083.1999.00651.x [DOI] [PubMed] [Google Scholar]
- Mackenzie N.M., Keeler K.D. A flow microfluorimetric analysis of the binding of immunoglobulins to Fcg receptors of the neonatal mouse jejunal epithelium. Immunology. 1984;51:529–533. [PMC free article] [PubMed] [Google Scholar]
- Martinez C., Bernabe R.R., de la Hera A., Pereira P., Cazenave P.A., Coutinho A. Establishment of idiotypic helper T-cell repertoires early in life. Nature. 1985;317:721–723. doi: 10.1038/317721a0. doi:10.1038/317721a0 [DOI] [PubMed] [Google Scholar]
- Møller A.P., Erritzøe J. Host immune defence and migration in birds. Evol. Ecol. 1998;12:945–953. doi:10.1023/A:1006516222343 [Google Scholar]
- Mousseau T.A., Fox C.W. Oxford University Press; Oxford, UK: 1998. Maternal effects as adaptations. [Google Scholar]
- Müller W., Groothius T.G.G., Dijkstra C., Siitari H., Alatalo R. Maternal antibody transmission and breeding densities in the black-headed gull Larus ridibundus. Funct. Ecol. 2004;18:719–724. doi:10.1111/j.0269-8463.2004.00902.x [Google Scholar]
- Nicoara C., Zäch K., Trachsel D., Germann D., Matter L. Decay of passively acquired maternal antibodies against measles, mumps, and rubella viruses. Clin. Diag. Lab. Immunol. 1999;6:868–871. doi: 10.1128/cdli.6.6.868-871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J.-Å. Ectoparasitism in marsh tits: costs and functional explanations. Behav. Ecol. 2003;14:175–181. doi:10.1093/beheco/14.2.175 [Google Scholar]
- Okamoto Y., Tsutsumi H., Kumar N.S., Ogra P.L. Effect of breast feeding on the development of anti-idiotype antibody response to F glycoprotein of respiratory syncytial virus in infant mice after post-partum maternal immunization. J. Immunol. 1989;142:2507–2512. [PubMed] [Google Scholar]
- Ono Y., Sasaki T., Ishida N. Regulation of clonal development of immune responding cells by antibody of maternal origin. Nature. 1974;250:593–594. doi: 10.1038/250593a0. doi:10.1038/250593a0 [DOI] [PubMed] [Google Scholar]
- Piersma T. Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos. 1997;80:623–631. doi:10.2307/3546640 [Google Scholar]
- Pihlaja M., Siitari H., Alatalo R.V. Maternal antibodies in a wild altricial bird: effects on offspring immunity, growth and survival. J. Anim. Ecol. 2006;75:1154–1164. doi: 10.1111/j.1365-2656.2006.01136.x. doi:10.1111/j.1365-2656.2006.01136.x [DOI] [PubMed] [Google Scholar]
- Poulin R., Thomas F. Epigenetic effects of infection on the phenotype of host offspring: parasites reaching across host generations. Oikos. 2008;117:331–335. doi:10.1111/j.2007.0030-1299.16435.x [Google Scholar]
- Råberg L., Nilsson J.-Å., Ilmonen P., Stjernman M., Hasselquist D. The cost of an antibody response: vaccination reduces parental effort. Ecol. Lett. 2000;3:382–386. doi:10.1046/j.1461-0248.2000.00154.x [Google Scholar]
- Råberg L., Vestberg M., Hasselquist D., Holmdahl R., Svensson E., Nilsson J.-Å. Basal metabolic rate and the evolution of the adaptive immune system. Proc. R. Soc. B. 2002;269:817–821. doi: 10.1098/rspb.2001.1953. doi:10.1098/rspb.2001.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J.M., Arcese P., Keller L.F., Hasselquist D. Long-term maternal effect on offspring immune response in song sparrows Melospiza melodia. Biol. Lett. 2006;2:573–576. doi: 10.1098/rsbl.2006.0544. doi:10.1098/rsbl.2006.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R.E., Wikelski M. The physiology/life-history nexus. Trends Ecol. Evol. 2002;17:462–468. doi:10.1016/S0169-5347(02)02578-8 [Google Scholar]
- Riedler J., et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. doi:10.1016/S0140-6736(01)06252-3 [DOI] [PubMed] [Google Scholar]
- Roberts S.A., Turner M.W. Specific suppression of rat IgE responses with milk from immunized females and with feeds of serum antibody. Immunology. 1983;48:195–199. [PMC free article] [PubMed] [Google Scholar]
- Roitt I., Brostoff J., Male D.K. 5th edn. Mosby; London, UK: 1998. Immunology. [Google Scholar]
- Rose M.E., Orlans E. Immunoglobulins in the egg, embryo, and young chick. Dev. Comp. Immunol. 1981;5:15–20. doi: 10.1016/s0145-305x(81)80003-1. doi:10.1016/S0145-305X(81)80003-1 [DOI] [PubMed] [Google Scholar]
- Roulin A., Heeb P. The immunological function of allosuckling. Ecol. Lett. 1999;2:319–324. doi: 10.1046/j.1461-0248.1999.00091.x. doi:10.1046/j.1461-0248.1999.00091.x [DOI] [PubMed] [Google Scholar]
- Rubolini D., Romano M., Bonisoli Alquati A., Saino N. Early maternal, genetic and environmental components of antioxidant protection, morphology and immunity of yellow-legged gull (Larus michahellis) chicks. J. Evol. Biol. 2006;19:1571–1584. doi: 10.1111/j.1420-9101.2006.01121.x. doi:10.1111/j.1420-9101.2006.01121.x [DOI] [PubMed] [Google Scholar]
- Saino N., Ferrari R.P., Martinelli R., Romano M., Rubolini D., Møller A.P. Early maternal effects mediated by immunity depend on sexual ornamentation of the male partner. Proc. R. Soc. B. 2002a;269:1005–1009. doi: 10.1098/rspb.2002.1992. doi:10.1098/rspb.2002.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino N., Dall'ara P., Martinelli R., Møller A.P. Early maternal effects and antibacterial immune factors in the eggs, nestlings and adults of the barn swallow. J. Evol. Biol. 2002b;15:735–743. doi:10.1046/j.1420-9101.2002.00448.x [Google Scholar]
- Sasaki T., Ono Y., Ishida N. Modification of fetal immune system by maternal anti-DNA antibody. I. Enhanced immuneresponse to DNA in the mice exposed to anti-DNA antibody early in life. J. Immunol. 1977;119:26–30. [PubMed] [Google Scholar]
- Schumacher I.M., Rostal D.C., Yates R.A., Brown D.R., Jacobson E.R., Klein P.A. Persistence of maternal antibodies against Mycoplasma agassizii in desert tortoise hatchlings. Am. J. Vet. Res. 1999;60:826–831. [PubMed] [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11 446–11 450. doi: 10.1073/pnas.90.24.11446. doi:10.1073/pnas.90.24.11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H., Mock D.W., Gieg J.A. A hormonal mechanism for parental favouritism. Nature. 1997;386:231. doi:10.1038/386231a0 [Google Scholar]
- Siegrist C.-A. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine. 2003;21:3406–3412. doi: 10.1016/s0264-410x(03)00342-6. doi:10.1016/S0264-410X(03)00342-6 [DOI] [PubMed] [Google Scholar]
- Staszewski V., Gasparini J., McCoy K.D., Tveraa T., Boulinier T. Evidence of an interannual effect of maternal immunization on the immune response of juveniles in a long-lived colonial bird. J. Anim. Ecol. 2007a;76:1215–1223. doi: 10.1111/j.1365-2656.2007.01293.x. doi:10.1111/j.1365-2656.2007.01293.x [DOI] [PubMed] [Google Scholar]
- Staszewski V., McCoy K.D., Tveraa T., Boulinier T. Interannual dynamics of antibody levels in naturally infected long-lived colonial birds. Ecology. 2007b;88:3183–3191. doi: 10.1890/07-0098.1. doi:10.1890/07-0098.1 [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Stern C.M. The materno-foetal transfer of carrier protein sensitivity in the mouse. Immunology. 1976;30:443–448. [PMC free article] [PubMed] [Google Scholar]
- Tollrian R., Harwell C.D. Princeton University Press; Princeton, NJ: 1999. The ecology and evolution of inducible defenses. [Google Scholar]
- Valkiūnas G. CRC Press; Boca Raton, FL: 2005. Avian malaria parasites and other Haemosporidia. [Google Scholar]
- Victor C., Bona C., Pernis B. Idiotypes on B lymphocytes: association with immunoglobulins. J. Immunol. 1983;130:1819–1825. [PubMed] [Google Scholar]
- Walker M., Steiner S., Brinkhof M.W.G., Richner H. Induced responses of nestling great tits reduce hen flea reproduction. Oikos. 2003;102:67–74. doi:10.1034/j.1600-0706.2003.12208.x [Google Scholar]
- Williams T.D., Christians J.K., Aiken J.J., Evanson M. Enhanced immune function does not depress reproductive output. Proc. R. Soc. B. 1999;266:753–757. doi:10.1098/rspb.1999.0701 [Google Scholar]
- Zehtindjiev P., Ilieva M., Westerdahl H., Hansson B., Valkiūnas G., Bensch S. Dynamics of parasitemia of malaria parasites in a naturally and experimentally infected migratory songbird, the great reed warbler Acrocephalus arundinaceus. Exp. Parasitol. 2008;119:99–110. doi: 10.1016/j.exppara.2007.12.018. doi:10.1016/j.exppara.2007.12.018 [DOI] [PubMed] [Google Scholar]
- Zuk M., Stoehr A.M. Immune defense and host life history. Am. Nat. 2002;160:S9–S22. doi: 10.1086/342131. doi:10.1086/342131 [DOI] [PubMed] [Google Scholar]