Abstract
Sexual dimorphism in immune function is a common pattern in vertebrates and also in a number of invertebrates. Most often, females are more ‘immunocompetent’ than males. The underlying causes are explained by either the role of immunosuppressive substances, such as testosterone, or by fundamental differences in male and female life histories. Here, we investigate some of the main predictions of the immunocompetence handicap hypothesis (ICHH) in a comparative framework using mammals. We focus specifically on the prediction that measures of sexual competition across species explain the observed patterns of variation in sex-specific immunocompetence within species. Our results are not consistent with the ICHH, but we do find that female mammals tend to have higher white blood cell counts (WBC), with some further associations between cell counts and longevity in females. We also document positive covariance between sexual dimorphism in immunity, as measured by a subset of WBC, and dimorphism in the duration of effective breeding. This is consistent with the application of ‘Bateman's principle’ to immunity, with females maximizing fitness by lengthening lifespan through greater investment in immune defences. Moreover, we present a meta-analysis of insect immunity, as the lack of testosterone in insects provides a means to investigate Bateman's principle for immunity independently of the ICHH. Here, we also find a systematic female bias in the expression of one of the two components of insect immune function that we investigated (phenoloxidase). From these analyses, we conclude that the mechanistic explanations of the ICHH lack empirical support. Instead, fitness-related differences between the sexes are potentially sufficient to explain many natural patterns in immunocompetence.
Keywords: immunocompetence, handicap, Bateman's principle, insects, mammals, comparative analysis
1. Introduction
Sex differences in mortality and immunocompetence are well documented in humans. Women in most societies not only have longer lifespans, they also are more resilient against infectious and some non-infectious diseases such as cancer (Boyle & Ferlay 2004; WHO 2008, http://www.who.int/healthinfo/morttables/en/index.html). This, however, comes at the price of women being more susceptible to autoimmune diseases (Whitacre 2001). These effects are commonly attributed to sexual differences in endocrinology.
The phenomenon of higher female immunocompetence is not restricted to humans, but has been found in many vertebrates (Folstad & Karter 1992). In their landmark paper, Folstad & Karter developed the idea of hormones driving sexual dimorphism in vertebrates and applied this to Hamilton & Zuk's (1982) idea that sexually selected ornament traits are dependent on ‘health and vigour’ and therefore provide honest signals of genetic resistance.
Folstad & Karter's (1992) ‘immunocompetence handicap hypothesis’ (ICHH) assumes that testosterone suppresses immune function. In a nutshell, only males that are highly immunocompetent can handle the high testosterone titres that are needed to fully express their ornaments. This would result in a strong correlation between ornaments and immunocompetence (as a proxy for good genes) and hence male ornaments would be honest traits. This elegant concept provides an explanation for how parasite-mediated sexual selection sensu Hamilton & Zuk (1982) could work.
The ICHH hinges on a few important assumptions, most critically the assumption that testosterone is immunosuppressive (but see Wedekind & Folstad 1994 for a variant on this assumption). This assumption is debatable. While a great number of studies show a correlation between immunity and testosterone levels (Muehlenbein & Briebiscas 2005), the experimental evidence is scant (but see Yao et al. 2003). Moreover, a recent meta-analysis investigating the effects of testosterone on behaviour and immunity did not find consistent support for the assumption that testosterone suppresses immunity (Roberts et al. 2004). Lastly, many species of insect display female-biased sexual dimorphism in immunity (e.g. Kurtz et al. 2000; Joop et al. 2006), yet insects are devoid of sex-specific hormones. Given that many of these species also show a positive correlation between ornament traits and immunity (e.g. Ryder & Siva-Jothy 2000), this calls into question the necessity of invoking an ‘immunocompetence handicap’ for the expression of ornaments.
Consistent with the idea of higher female immunocompetence, many vertebrates (Poulin 1996; Zuk & McKean 1996; Moore & Wilson 2002) and some invertebrates (Sheridan et al. 2000) show higher incidence of parasitism in males than in females. However, immunocompetence is only one factor contributing to infection rates. Exposure to parasites due to differences in mating behaviour or foraging will also contribute to sexually dimorphic patterns of parasite infection (Skorping & Jensen 2004; Nunn & Altizer 2006). In general, male and female behaviour and life histories differ significantly in many species, and this is likely to be reflected in physiological traits such as immune function (Zuk & Stoehr 2002). Assuming that (i) females invest more into reproduction than males (Williams 1966) and (ii) males maximize fitness via mating rate and females via longevity (Bateman 1948), this would select for higher investment in immune function in females (Rolff 2002).
Here, we investigate specific predictions of the ICHH (Folstad & Karter 1992) and ‘Bateman's principle for immunity’ (Rolff 2002) using a comparative approach. We developed three refined predictions (see below) from the ICHH and Bateman's principle for immunity in an attempt to discriminate between the hypotheses within a single study (Lipton 2005). Finally, we conducted a meta-analysis of patterns of immunity in insects, which lack testosterone and thus serve as a useful comparison to the mammal analyses.
2. Testing the alternatives
Hamilton & Zuk (1982) predicted a positive correlation between ornaments and parasite resistance. The ICHH suggests a mechanism to ensure that such ornaments are honest signals, inasmuch as it focuses on the pleiotropic function of testosterone (or potentially other modulating molecules). The ICHH also predicts sexual dimorphism in immunity, but, as we will see, this prediction is not unique to the ICHH. While stimulating a great amount of evolutionary research, the main aim of the ICHH was to ‘provide a proximate model’ (Folstad & Karter 1992, p. 616).
By contrast, investigating the role that sex-specific life histories play in shaping immunocompetence is more of an ultimate question; hence, the predictions by the ICHH and hypotheses based on sex-specific life histories are not mutually exclusive. The notion that life-history differences shape sexual dimorphism in immunity has at least two specific predictions (Rolff 2002). (i) Dimorphism in immunity should covary with the extent of dimorphism in reproductive behaviour and, given general differences between the sexes in the factors that influence lifetime reproductive success (Bateman 1948), should tend to be higher in females than males. For example, if males maximize their fitness early in adult life but females exhibit a strong correlation between fitness and longevity, this should select for greater investment in immunity by females than males. (ii) Males and females should differ in their quantitative genetic architecture for fitness and immune traits (partly investigated in Rolff et al. 2005).
Here, we investigated sexual dimorphism in white blood cell counts (WBC) and whether this dimorphism covaries negatively with sexual dimorphism in body mass across mammals, as expected if increased male-male competition leads to a reduction in male cell counts relative to female cell counts. Throughout, we assume that the number of circulating WBCs represents a reasonable measure of investment in immunity (Nunn et al. 2000, 2003; Nunn 2002; Semple et al. 2002; Anderson et al. 2004). We also assume that the extent of sexual size dimorphism in mammals reflects the strength of sexual selection (Andersson 1994) and is therefore used as a correlate for sexual selection.
Under the ICHH, we expect that measures of sexual selection should correlate with variation in cell counts calculated within a species. We argue that if (i) testosterone is immunosuppressive (but see discussion) and (ii) involved in the expression of sexually selected traits and male mating behaviour, that males of species with stronger sexual selection should not only exhibit higher average testosterone titres but also higher variation in testosterone titres, and thus variation in cell counts. This constitutes an indirect test of the assumption that testosterone is involved as a mediator of both immune function and the expression of ornament traits. We would like to highlight that this indirect test is based on one core assumption. We assume that not all additive genetic variance is removed, but partly captured by condition dependency of the traits under investigation (Rowe & Houle 1996).
If males and females invest differentially in immune system parameters based on Bateman's principle, we predict that mammalian species with greater longevity will have higher numbers of circulating WBCs. We further predict that sex differences in life expectancy and the duration of breeding covary positively with sex differences in WBC counts: species in which males have a shorter reproductive period than females should show lower cell counts in males than in females.
In our simultaneous approach we used a comparative mammal dataset to investigate four main predictions. (i) The intensity of sexual selection positively covaries with female-biased sexual dimorphism in immunity. This investigates the ICHH and more derived hypotheses on resource trade-offs. (ii) Variation in immunity increases with female-biased sexual dimorphism in immunity. This provides an indirect examination of the role of testosterone. (iii) Female-biased sexual dimorphism in longevity positively correlates with female-biased sexual dimorphism in immunity (a prediction from Rolff 2002). (iv) Female-biased sexual dimorphism in the duration of time that individuals are likely to breed successfully (the duration of effective breeding, or DEB) positively correlates with female-biased sexual dimorphism in immunity.
3. Material and methods
(a) Mammalian comparative data and analyses
We obtained data on WBC from the International Species Information System (2002). We focused on five major types of circulating white blood cells: lymphocytes; neutrophils; monocytes; eosinophils; and basophils. These counts therefore represent key cells involved in adaptive immunity (lymphocytes) and innate immunity (neutrophils and monocytes), and also cells involved in fighting macroparasites (eosinophils) and the production of pharmacologically active substances (basophils). While only a proxy for immunity WBC are routinely used to assess the immunocompetence of immune-deficient patients (e.g. Houwen 2001).
We obtained data on sex differences in life expectancy and DEB from Clutton-Brock & Isvaran (2007) and data on longevity from the Pantheria database (Jones et al. submitted). Data on body mass and body mass dimorphism were the same as used in Lindenfors et al. (2007) for all species except for Tursiops truncatus and Equus caballus (where data came from Dearolf et al. 2000 and Ruckstuhl & Neuhaus 2002, respectively). Life expectancy reflects age expected upon reaching adulthood in wild populations (Clutton-Brock & Isvaran 2007), while longevity represents maximum-recorded lifespan. In all cases, measures of dimorphism refer to male values divided by female values. All continuous characters were log-transformed prior to the analyses.
Analyses were conducted using species values and after controlling for phylogenetic relationships among mammalian species (Harvey & Pagel 1991; Nunn & Barton 2001). To control for phylogeny, we used the method of independent contrasts, as calculated in the PDAP module (Midford et al. 2005) of Mesquite (Maddison & Maddison 2007). Contrasts were calculated based on a recent composite estimate of mammalian phylogeny (Bininda-Emonds et al. 2007). We tested the assumptions of independent contrasts and found that using equal branch lengths best met the assumptions for tests across all mammals and for analyses of dimorphism in DEB, while log-transformed branch lengths best met the assumptions for analyses of dimorphism in life expectancy.
All of the hypotheses we investigated predicted either positive or negative associations. We used directed tests rather than one-tailed tests, if not specified otherwise, as these enable detection of patterns that are opposite to predictions while retaining much of the statistical power of one-tailed tests (Rice & Gaines 1994). We set γ/α to 0.8, giving values of γ=0.04 and δ=0.01. In analyses of variation across age and sex classes and for tests involving body mass, we used two-tailed tests, as no clear predictions were possible.
(b) Insect meta-analyses
We obtained data on sex differences in immunocompetence in insects from the literature. Certain criteria had to be met in selecting the studies for data collection to allow calculation of statistics (Cohen's d) for the meta-analysis. First, the mean values for male and female immune response had to be stated. Second, standard errors or standard deviations had to be included in the text or in figures. Third, separate sample sizes of males and females had to be accessible.
Data were collected using references from the literature and Google Scholar (sets of search terms as follows: sexual dimorphism immunity insect; sex difference immunity insect; gender difference immunity; haemocyte immunity insect; haemocyte sex difference, phenoloxidase immunity; phenoloxidase sex difference). After selecting all of the articles that measured male and female immunocompetence, studies that did not fit the criteria were omitted (see electronic supplementary material).
Following this process, a total of 43 studies were included in the meta-analysis. We intended to carry out separate analyses of sexual dimorphism in immune function for each immune trait. However, the only traits for which there were sufficient sample sizes to do this were phenoloxidase activity (11 species, highly clustered in very few families) and haemocyte counts (11 species). Phenoloxidase activity is part of the humoral immune response in insects, and has been identified as a key component of innate immunity against a range of pathogens (Cerenius & Söderhall 2004; Eleftherianos et al. 2007). The phenoloxidase enzyme is activated following pathogen recognition by the insect, and the result is a cascade of immunological defence mechanisms. These mechanisms serve to physically shield the host body from invading micro-organisms and damaged self-tissue, and to release compounds to fight infection (Cerenius & Söderhall 2004). Haemocytes are also crucial components of innate immunity and are important measures of cellular immune responses. A higher number of circulating haemocytes has been used to indicate a superior immune response (Kraaijeveld et al. 2001). Haemocytes perform a number of roles in insect immune defences; specifically they aggregate to encapsulate larger foreign material, they demonstrate nodule formation in response to large numbers of pathogens and they clear small microbes via phagocytosis (Lavine & Strand 2002).
As most of the data were clustered in a limited number of taxonomic groups (crickets and damselflies), we did not carry out a formal comparative analysis. Instead, we conducted a meta-analysis, where each data point for the meta-analysis represented a comparison of the male and female immune response for a single immune trait. The effect size of each measure of sexual dimorphism in immune function was calculated using the sample sizes, means and standard errors or standard deviations stated in the original data (Cohen 1988). An effect size of 0 indicated no sex difference in immunocompetence, a positive value indicated that females exhibited a superior immune response, and a negative value suggested a higher level of immune function in males. Funnel plots were used to investigate the effects of selective reporting of significant results (Palmer 2000).
4. Result I: comparative analyses in mammals
(a) Comparative analyses
(i) Background
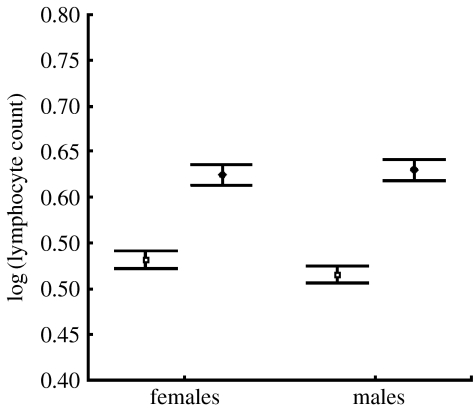
Before testing the specific predictions outlined above, we examined variation in WBC in all mammals combined and in the three best-represented groups of mammals in the database (carnivores, primates and artiodactyls). Data were identified as being from adults or immatures. We found significant differences in cell counts between adult and immature animals for all cell types except for basophils, either at the level of all mammals or within one of these three groups. The strongest results were obtained for lymphocytes, where immatures had higher levels than adults for females (t186=−12.80; p<0.001, two-tailed) and males (t186=−12.25; p<0.0001, two-tailed) (figure 1).
Figure 1.
Juveniles (diamonds) have significantly more lymphocytes than adults (squares) in mammals. Whiskers indicate standard error.
Based on these results, we restricted all subsequent tests to values of cell counts taken from adults. We found that cell counts were significantly higher in females than in males for two of the five white blood cell types that we investigated: eosinophils (t193=2.154, p=0.032), and lymphocytes (t193=4.196, p<0.001, all tests two-tailed, see figure 1). In addition, sex differences in monocyte counts approached significance (t193=1.909, p=0.058). These results held in the primates. Among the artiodactyls, we found sex differences for eosinophils and lymphocytes. In carnivores, however, only one cell type produced a significant difference between the sexes, and it was opposite to the pattern found in the other analyses: neutrophil counts were higher in males than in females (t40=−3.251, p=0.002, two-tailed).
We also investigated whether body mass covaries with WBC using independent contrasts. Across mammals and in both sexes, the number of neutrophils (t135=1.877, p=0.063 and t135=3.118, p<0.002 for females and males, respectively) and monocytes (t135=2.560, p=0.012 and t135=3.556, p<0.001 for females and males, respectively) increased with body mass in adults, while the number of lymphocytes instead decreased (t135=−2.317, p=0.022 and t135=1.992, p=0.048 for females and males, respectively, all tests two-tailed). Similarly, in analyses of life-history traits, we found that body mass covaried with longevity across mammals (female body mass b=0.147, t127=7.476, p<0.001; male body mass b=0.139, t127=7.478, p<0.001) and with measures of body mass dimorphism in both males (b=0.041, t135=4.649, p<0.001) and females (b=0.024, t135=2.411, p<0.001). Based on these findings, we included body mass as a covariate in most tests reported below.
(ii) Sexual selection and immunity
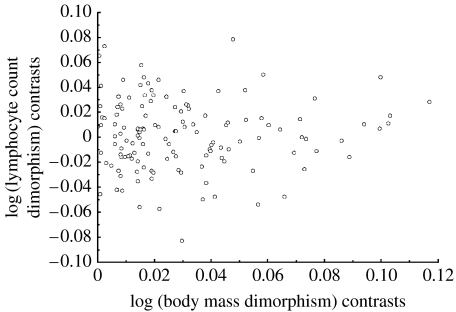
We first investigated a general prediction of immune system investment in relation to sexual selection: sexual dimorphism in body mass should covary negatively with sexual dimorphism in immune cell counts. We found no statistical support for this prediction using independent contrasts or in analyses of species values (table 1; figure 2). We also ran these tests controlling for body mass in a multiple regression, and this similarly produced no significant results.
Table 1.
Dimorphism in body mass and dimorphism in immune cell counts across mammals. (Note: t-statistics are given with sign indicating whether slopes were positive or negative, and p-values are from directed tests. Results are based on independent contrasts analyses (n=136 independent contrasts))
| cell type | t-statistic | p-value |
|---|---|---|
| neutrophils | 0.833 | 0.99 |
| monocytes | −0.022 | 0.61 |
| lymphocytes | 0.467 | 0.85 |
| eosinophils | −0.026 | 0.61 |
| basophils | 0.470 | 0.85 |
Figure 2.
There is no significant relationship between dimorphism in body mass and dimorphism in lymphocyte counts in mammals.
We tested the ICHH using comparative methods. First, we investigated whether body mass dimorphism covaries positively with variation in WBC counts among adult males, as would be expected if sexual selection results in greater variation in immune system parameters within the sex that typically experiences the most intra-sexual competition. Variation in WBC was measured as the coefficient of variation (CV). However, no significant results were found in examination of each of the five white blood cell types (table 2). In a second prediction of the ICHH, we investigated whether sexual dimorphism in immune system cell variation covaries with body mass dimorphism, again using the CV. We found no support for this prediction (table 2).
Table 2.
Comparative tests of the ICHH. (Notes: table presents t-statistics from analyses of independent contrasts, with sign of the t-statistic indicating the slope of the relationship from linear regression forced through the origin. None of the analyses produced statistically significant results (n=136 independent contrasts). The leftmost two columns depict the relationship between body mass dimorphism and leucocyte CV in males, while the rightmost two columns depict the relationship between body mass dimorphism and dimorphism in leucocyte CV.)
| leucocyte coefficient of variation (CV) | sexual dimorphism in leucocyte CV | |||
|---|---|---|---|---|
| t-statistic | p-value | t-statistic | p-value | |
| neutrophils | −2.050 | 0.11 | 0.085 | 0.58 |
| monocytes | 0.001 | 0.62 | −0.074 | 0.66 |
| lymphocytes | −1.373 | 0.43 | −0.692 | 0.94 |
| eosinophils | 0.244 | 0.50 | −0.189 | 0.72 |
| basophils | 0.746 | 0.29 | 0.483 | 0.39 |
Under the first of two predictions for Bateman's principle, we tested for an association between longevity and immune system parameters while also controlling for the effects of body mass. In phylogenetic tests, we found that evolutionary increases in longevity in females were correlated with increases in the number of monocytes (b=0.069, t126=2.328, p=0.013) and eosinophils (b=0.136, t126=2.794, p=0.004), but that the same did not apply to males (monocytes: b=0.026, t126=0.751, p=0.28; eosinophils: b=0.016, t126=0.382, p=0.44). All other results were not significant. In non-phylogenetic tests that controlled for body mass, however, longevity was a significant (positive) predictor of lymphocytes (females: b=0.204, t148=3.571, p<0.001; males: b=0.189, t147=3.528, p<0.001), monocytes (females: b=0.147, t148=4.842, p<0.001; males: b=0.152, t147=4.852, p<0.001) and neutrophils (females: b=0.164, t148=2.285, p=0.015; males: b=0.184, t147=2.623, p=0.006, directed tests in all cases). Thus, we found some support for the first prediction of Bateman's principle.
It is possible that our measures of longevity are too indirect for testing the hypothesis, as the critical issue concerns the DEB. In addition, it is the differences between the sexes in life-history traits that are relevant, rather than absolute estimates, and we know from previous work that other factors can influence overall numbers of white blood cells (Nunn et al. 2000, 2003; Nunn 2002; Semple et al. 2002; Anderson et al. 2004).
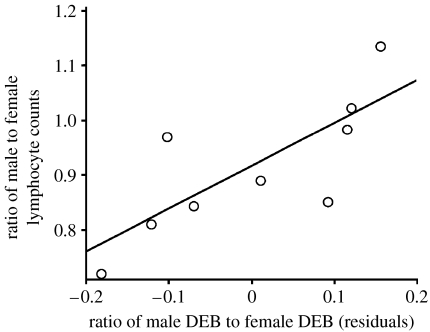
In a second set of tests, we therefore investigated how sex differences in the DEB and adult longevity (Clutton-Brock & Isvaran 2007) covary with sex differences in adult WBC. In non-phylogenetic tests involving the DEB and female body mass, we found that dimorphism in basophil and lymphocyte counts covaries positively with dimorphism in the DEB (table 3). These effects remained statistically significant in phylogenetic tests that used independent contrasts (table 3; figure 3). Results were similar in tests involving sexual dimorphism in life expectancy. In non-phylogenetic tests, dimorphism in immune cells was significantly associated with dimorphism in life expectancy for three types of leucocytes: basophils; lymphocytes; and eosinophils (table 4). Similar results were obtained when using independent contrasts (table 4), although results for basophils and eosinophils became marginally non-significant (p<0.07 in both cases).
Table 3.
Dimorphism in duration of effective breeding. (Note: body mass was significantly associated with dimorphism in effective breeding duration in non-phylogenetic tests (t7=−3.20, p=0.015, two-tailed) and in tests based on independent contrasts (t7=−3.74, p=0.007, two-tailed). Thus, analyses were conducted with a statistical model that included log female body mass and dimorphism in breeding duration as covariates. The t-statistics are given with sign indicating whether slopes were positive or negative, and p-values are based on directed tests (predicting a positive association between m : f dimorphism in effective breeding duration and m : f dimorphism in each of the cell types). Analyses based on nine species (eight independent contrasts).)
| non-phylogenetic test | independent contrasts | |||
|---|---|---|---|---|
| dimorphism in: | t-statistic | p-value | t-statistic | p-value |
| neutrophils | 0.11 | 0.58 | −0.14 | 0.69 |
| monocytes | −2.38 | 0.14 | −2.60 | 0.10 |
| lymphocytes | 3.14 | 0.013 | 2.77 | 0.02 |
| eosinophils | 0.49 | 0.40 | 0.75 | 0.30 |
| basophils | 2.56 | 0.027 | 3.59 | 0.007 |
Figure 3.
Sex differences in breeding duration and lymphocyte counts. Sex differences in DEB covary positively with sex differences in lymphocyte counts after controlling for body mass effects. The x-axis represents residuals from the regression of the DEB ratio on body mass.
Table 4.
Dimorphism in life expectancy. (Notes: body mass was not significantly associated with dimorphism in life expectancy in non-phylogenetic tests (t12=−1.27, p=0.23, two-tailed) and in tests based on independent contrasts (t12=−0.94, p=0.36, two-tailed). Thus, bivariate results are presented in the table, with dimorphism in life expectancy as the independent variable. The t-statistics are given with sign indicating whether slopes were positive or negative, and p-values are based on directed tests (predicting a positive association between m : f dimorphism in life expectancy and m : f dimorphism in each of the cell types). Analyses based on 14 species (13 independent contrasts))
| non-phylogenetic test | independent contrasts | |||
|---|---|---|---|---|
| dimorphism in: | t-statistic | p-value | t-statistic | p-value |
| neutrophils | 0.47 | 0.40 | 0.17 | 0.54 |
| monocytes | 0.29 | 0.49 | −0.10 | 0.67 |
| lymphocytes | 2.00 | 0.043 | 2.00 | 0.043 |
| eosinophils | 2.14 | 0.033 | 1.90 | 0.051 |
| basophils | 2.45 | 0.019 | 1.73 | 0.068 |
5. Result II: meta-analysis in insects
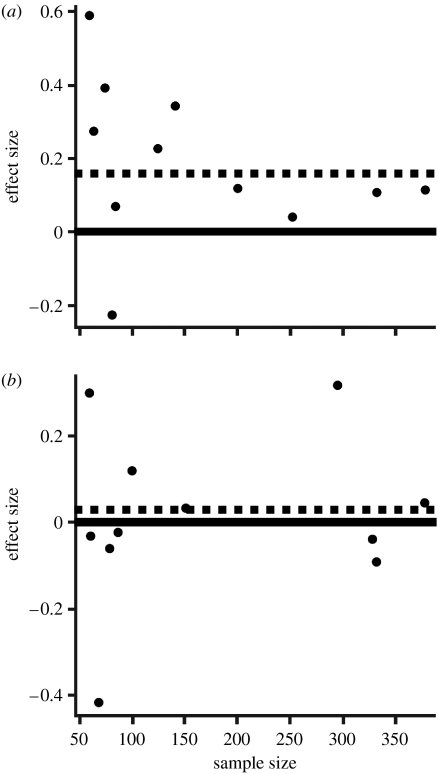
The mean effect size for phenoloxidase is 0.187. All but one of the studies showed a female bias in PO activity (see figure 4). For haemocytes, the mean effect size is 0.0136, suggesting a weak sexual dimorphism in haemocyte counts in the direction of female superiority (figure 4). The funnel plots did not provide any evidence of selective reporting, as there are no apparent gaps in the data (Palmer 2000).
Figure 4.
(a) Effect size of male and female phenoloxidase activity as a function of sample size. The solid line indicates zero effect size, i.e. no sexual dimorphism in immune function. Data points above this line represent studies in which females were found to have higher phenoloxidase activity. The dashed line denotes the mean effect size, which was 0.187. (b) Effect of male and female haemocyte counts as a function of sample size. The solid and dashed lines represent zero and mean effect size, respectively. The mean effect size was 0.013. The data points above the zero line indicate higher female haemocyte counts; those below the line suggest higher counts in males. The mean effect size is slightly above the zero line, suggesting a very weak sexual dimorphism in haemocyte counts in the direction of higher values in females.
6. Discussion
Here, using a comparative approach, we tested critical predictions of the ICHH and Bateman's principle for immunity. Overall, the analyses failed to support predictions from the ICHH. Instead, we found support for sexual dimorphism driven by life-history differences (i.e. Bateman's principle applied to immunity, Rolff 2002). In mammals, sex differences in WBCs were not associated with sexual dimorphism in body mass, although adult females tended to show higher cell counts than adult males. The insect dataset provided an inconclusive message, with a consistent sex difference in immunity in one out of two traits.
Roberts et al. (2004) provided a meta-analysis of the ICHH using studies that experimentally manipulated testosterone titres. They found an effect of testosterone application on immune function, but this effect failed to reach significance when controlling for multiple studies of the same species. The sample size in the meta-analysis by Roberts et al. (2004) was limited (36 species across mammals, reptiles and birds) and they combined a number of different ways to assess immunocompetence: white blood cells counts, measurement of PHA (a debated in vitro assay applied in vivo in the cited studies, see Siva-Jothy & Ryder 2001) and antibody titres.
Here, we took this analysis a step further by using a comparative approach and refining the predictions. This allowed us to use a larger sample size and hence should provide greater statistical power. We assumed that testosterone has a pleiotropic effect on immune function, the expression of sexually selected traits and male mating behaviour, and that the strength of sexual selection should result from higher average testosterone titres and should also result in higher variation in testosterone titres. If the latter holds true, then we expected to find a positive covariance between sexual dimorphism in size (as a measure for strength of sexual selection) and variation in WBC. However, we did not find any support for this prediction, despite having high statistical power to detect an effect (power=0.95 in a post hoc two-tailed test with medium effect size, n=136 and α=0.05, using the program G*Power 3, Faul et al. 2007). One possible explanation is that the presumed immunosuppressive effect of testosterone is not present or sufficiently strong for the ICHH to work. Studies specifically testing the effect of defined testosterone applications on subsets of white blood cells, such as the ones used in our comparative study, are limited. Yao et al. (2003) measured the response in WBCs for the five classes of leucocytes used here against three different concentrations of testosterone in rats. Only monocytes showed a significant decrease with increasing testosterone concentrations. The message with lymphocytes was mixed, but the proliferation of lymphocytes seems to be slightly hampered when high doses of testosterone were administered.
The focus on testosterone, or any other modulating substance, still leaves the question of ‘what is driving the observed differences in immunocompetence?’. Is the advent of testosterone imposing evolutionary constraints, which prevent males from exhibiting stronger immunocompetence? As testosterone is probably as old as the vertebrates, it is surprising that selection has not modified the pleiotropic effects.
Longevity has long been speculated to be a driver of the evolution of the vertebrates' acquired immune system in the immunological literature. However, evidence to back this assertion remains scant (see Hedrick 2004). Only a handful of theoretical studies (e.g. Miller et al. 2007) support the notion that longevity selects for immunity. Here we investigated the intuitive prediction that longevity correlates positively with investment in immune function, and found some support for this prediction, yet longevity itself might be a poor predictor of fitness. One explanation for this finding is that selection is weaker after the termination of reproduction (Kirkwood 2005). We therefore examined the DEB (Clutton-Brock & Isvaran 2007), which revealed that the extent of sexual dimorphism in lymphocytes and basophils covaries positively with the dimorphism in the DEB. Some analyses of dimorphism in expected longevity also produced significant effects. These findings are consistent with the predictions made by Rolff (2002): sex differences in life histories select for sex differences in immunity.
We acknowledge that it might be possible to derive a prediction from the ICHH that males should be shorter lived than females owing to their lower immunocompetence. If accepted at face value, it would be impossible to distinguish between the different hypotheses. We would like to argue from first principles, however, that the prediction derived from ‘Bateman's principle’ is more parsimonious and theoretically sound. Williams (1966) stated that females show greater sacrifice for reproduction per unit offspring. If this were true, it predicts that females need to spend on average more time to get the same number of offspring than males. In other words, owing to their comparatively low costs, males can have more offspring in a shorter period of time. Moreover, the limited experimental support for the ICHH (Roberts et al. 2004) limits the value of deriving another post hoc prediction. On theoretical grounds, a posteriori hypotheses are weaker (Lipton 2005).
It is important to point out that we interpret the findings involving WBCs cautiously for three reasons. First, high-quality data on sex differences in the DEB (Clutton-Brock & Isvaran 2007) were available for only a small subset of mammalian species. Second, we assumed that WBC counts are a good proxy for investment in immune function. The relevance of peripheral cell counts as a measure of immunity is supported by the medical practice of using WBC to investigate the health status and immunocompetence of individual patients. One of the white blood cell types for which we found an effect involved lymphocytes. While they represent approximately 31.8 per cent of the leucocytes in the mammals in our dataset, they are part of the adaptive immune system and hence subject to fast proliferation. Lastly, we assumed that cell counts in captive animals are reflective of variation in the wild. We acknowledge that conditions of captivity might reduce variation in cell counts, including through reduced exposure to infectious agents. Moreover, because veterinarians select healthy animals for inclusion in the ISIS dataset, variation in cell counts could be further reduced.
We note that Moore & Wilson (2002) reported positive covariation between sexual size dimorphism in mammals and dimorphism in parasitism in comparative analyses. Males in species with larger male biased size dimorphism exhibited higher parasite prevalence. Our results are not consistent with the findings by Moore & Wilson (2002), as we did not find a negative correlation between size dimorphism and dimorphism in WBC counts. This might be explained by the fact that parasitism only partially reflects immunocompetence (see Skorping & Jensen 2004).
Even though insects are popular subjects in studies of ecological immunology (Rolff & Siva-Jothy 2003; Schmid-Hempel 2005), the available data for the study envisaged here were surprisingly limited. We can conclude that we find a consistent sex difference only in phenoloxidase activity, but not in haemocytes. A great variety of different immune responses exist in insects that have not been discussed here (Siva-Jothy et al. 2005). Taking the lack of sexual dimorphism in haemocyte counts at face value, we have three possible explanations: (i) there is no sex effect, (ii) the sample size is insufficient (even though there is no indication of publication bias in the funnel plots), and (iii) the ideas about life histories shaping the investment in immune function are correct, but we presently lack sufficient data from the wild to study this. At the moment, we are not in the position to make an informed decision, but the variety in life histories in insects might be higher than in mammals and thus could offer a means for more convincing tests of the role of life-history traits on immune defences. Task partitioning in social insects is one example where the correlation between task-specific measures of longevity and immunity could be investigated (e.g. Gerloff et al. 2003; Baer et al. 2005).
7. Conclusions
Overall we did not find support for the ICHH using a comparative approach, but we did find support for the idea that sex-specific life histories could select for investment in immune function. Despite the highly active research in ‘ecological immunology’ (Sheldon & Verhulst 1996), we are not yet in a position to fully explore the role of life history on immune defences. In addition, remarkably little data support the intuitive belief that investment in immune function is beneficial for long-lived species. This question is not only important for understanding sex differences in immunity, but potentially also for understanding the evolution of the acquired immune system in vertebrates (Hedrick 2004; Cooper & Alder 2006; Rolff 2007). It would be desirable to have data on insects, or any other groups that lack sex-specific hormones, to evaluate the relation between DEB and immune function. Moreover, insects would provide useful study organisms to unravel the longevity/immunity nexus using experimental evolution approaches.
Acknowledgments
We would like to thank Sophie Armitage for valuable and rapid comments on the manuscript. Two anonymous reviewers provided very helpful comments. C.N. was supported by the Max Planck Society, and E.R.P. was supported by a BBSRC studentship.
Footnotes
One contribution of 11 to a Theme Issue ‘Ecological immunology’.
Supplementary Material
Meta analysis data & sources
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Anderson M.J., Hessel J.K., Dixson A.F. Primate mating systems and the evolution of immune responses. J. Reprod. Immunol. 2004;61:31–38. doi: 10.1016/j.jri.2003.11.001. doi:10.1016/j.jri.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Bateman A. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. doi:10.1038/hdy.1948.21 [DOI] [PubMed] [Google Scholar]
- Baer B., Krug A., Boomsma J., Hughes W. Examination of the immune reponses of males and workers of the leaf-cutting ant Acromyrmex echinatior and the effect of infection. Insect. Soc. 2005;52:298–303. doi:10.1007/s00040-005-0809-x [Google Scholar]
- Binida-Emonds O.R.P., et al. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. doi:10.1038/nature05634 [DOI] [PubMed] [Google Scholar]
- Boyle P., Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann. Oncol. 2004;16:481–488. doi: 10.1093/annonc/mdi098. doi:10.1093/annonc/mdi098 [DOI] [PubMed] [Google Scholar]
- Cerenius L., Söderhall K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. doi:10.1111/j.0105-2896.2004.00116.x [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T., Isvaran K. Sex differences in ageing in natural populations of vertebrates. Proc. R. Soc. B. 2007;274:3097–3104. doi: 10.1098/rspb.2007.1138. doi:10.1098/rspb.2007.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Lawrence Erlbaum Associates; Mahwah, NJ: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Cooper M., Alder M. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. doi:10.1016/j.cell.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Dearolf J.L., McLellan W.A., Dillaman R.M., Frierson D., Pabst D.A. Precocial development of axial locomotor muscle in bottlenose dolphins (Tursiops truncatus) J. Morphol. 2000;244:203–215. doi: 10.1002/(SICI)1097-4687(200006)244:3<203::AID-JMOR5>3.0.CO;2-V. doi:10.1002/(SICI)1097-4687(200006)244:3<203::AID-JMOR5>3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- Eleftherianos I., et al. An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidas inhibition. Proc. Natl Acad. Sci. USA. 2007;104:2419–2424. doi: 10.1073/pnas.0610525104. doi:10.1073/pnas.0610525104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Folstad I., Karter J. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992;139:603–622. doi:10.1086/285346 [Google Scholar]
- Gerloff G., Ottmer B., Schmid-Hempel P. Effects of inbreeding on immune response and body size in a social insect, Bombus terrestris. Funct. Ecol. 2003;17:582–589. doi:10.1046/j.1365-2435.2003.00769.x [Google Scholar]
- Hamilton W., Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. doi:10.1126/science.7123238 [DOI] [PubMed] [Google Scholar]
- Harvey P.H., Pagel M.D. Oxford Series in Ecology and Evolution. Oxford University Press; Oxford, UK: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Hedrick S.M. The acquired immune system: a vantage from beneath. Immunity. 2004;21:617–625. doi: 10.1016/j.immuni.2004.08.020. doi:10.1016/j.immuni.2004.08.020 [DOI] [PubMed] [Google Scholar]
- Houwen B. The differential cell count. Lab. Hematol. 2001;7:89–100. [Google Scholar]
- International Species Information System. Minnesota Zoological Garden; Apple Valley, MN: 2002. Physiological reference values CD-ROM: international species information system. [Google Scholar]
- Jones, K. E. et al Submitted. PanTHERIA: a species-level database of life-history, ecology and geography of extant and recently extinct mammals.
- Joop G., Mitschke A., Rolff J., Siva-Jothy M. Immune function and parasites resistance in male and polymorphic female Coenagrion puella. BMC Evol. Biol. 2006;6:19. doi: 10.1186/1471-2148-6-19. doi:10.1186/1471-2148-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood T. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. doi:10.1016/j.cell.2005.01.027 [DOI] [PubMed] [Google Scholar]
- Kraaijeveld A.R., Limentani E., Godfray H.C.J. Basis of the trade-off between parasitoid resistance and larval competitve ability in Drosophila melanogaster. Proc. R. Soc. B. 2001;268:259–261. doi: 10.1098/rspb.2000.1354. doi:10.1098/rspb.2000.1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J., Wiesner A., Götz P., Sauer K. Gender differences and individual variation in the immune system of the scorpionfly Panorpa vulgaris (Insecta: Mecoptera) Dev. Comp. Immunol. 2000;24:1–12. doi: 10.1016/s0145-305x(99)00057-9. doi:10.1016/S0145-305X(99)00057-9 [DOI] [PubMed] [Google Scholar]
- Lavine M.D., Stranf M.R. Insect haemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002;32:1295–1309. doi: 10.1016/s0965-1748(02)00092-9. doi:10.1016/S0965-1748(02)00092-9 [DOI] [PubMed] [Google Scholar]
- Lindenfors P., Gittleman J.L., Jones K.E. Sexual size dimorphism in mammals. In: Fairbairn D.J., Blanckenhorn W.U., Szekely T., editors. Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford University Press; Oxford, UK: 2007. pp. 19–26. [Google Scholar]
- Lipton P. Testing hypotheses: prediction and prejudice. Science. 2005;307:219–221. doi: 10.1126/science.1103024. doi:10.1126/science.1103024 [DOI] [PubMed] [Google Scholar]
- Maddison, W. P. & Maddison, D. R. 2007 Mesquite: a modular system for evolutionary analysis, v. 2.01. See http://mesquiteproject.org
- Midford, P. E., Garland Jr, T. & Maddison, W. P. 2005 PDAP package of Mesquite See http://mesquiteproject.org/pdap_mesquite
- Miller M., White A., Boots M. Host life span and the evolution of resistance characteristics. Evolution. 2007;61:2–14. doi: 10.1111/j.1558-5646.2007.00001.x. doi:10.1111/j.1558-5646.2007.00001.x [DOI] [PubMed] [Google Scholar]
- Moore S.L., Wilson K. Parasites as a viability cost of sexual selection in natural populations of mammals. Science. 2002;297:2015–2018. doi: 10.1126/science.1074196. doi:10.1126/science.1074196 [DOI] [PubMed] [Google Scholar]
- Muehlenbein M., Bribiescas R. Testosterone-mediated immune functions and male life histories. Am. J. Hum. Biol. 2005;17:527–558. doi: 10.1002/ajhb.20419. doi:10.1002/ajhb.20419 [DOI] [PubMed] [Google Scholar]
- Nunn C.L. A comparative study of leukocyte counts and disease risk in primates. Evolution. 2002;56:177–190. doi: 10.1111/j.0014-3820.2002.tb00859.x. doi:10.1111/j.0014-3820.2002.tb00859.x [DOI] [PubMed] [Google Scholar]
- Nunn C., Altizer S. Oxford University Press; Oxford, UK: 2006. Infectious diseases in primates: behaviour, ecology, evolution. [Google Scholar]
- Nunn C.L., Barton R.A. Comparative methods for studying primate adaptation and allometry. Evol. Anthropol. 2001;10:81–98. doi:10.1002/evan.1019 [Google Scholar]
- Nunn C.L., Gittleman J.L., Antonovics J. Promiscuity and the primate immune system. Science. 2000;290:1168–1170. doi: 10.1126/science.290.5494.1168. doi:10.1126/science.290.5494.1168 [DOI] [PubMed] [Google Scholar]
- Nunn C.L., Gittleman J.L., Antonovics J. A comparative study of white blood cell counts and disease risk in carnivores. Proc. R. Soc. B. 2003;270:347–356. doi: 10.1098/rspb.2002.2249. doi:10.1098/rspb.2002.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A.R. Quasireplication and the contract of error: lessons from sex ratios, heritabilities and fluctuating asymmetry. Annu. Rev. Ecol. Syst. 2000;31:441–480. doi:10.1146/annurev.ecolsys.31.1.441 [Google Scholar]
- Poulin R. Sexual inequalities in helminth infections: a cost of being a male? Am. Nat. 1996;147:287–295. doi:10.1086/285851 [Google Scholar]
- Rice W.R., Gaines S.D. Heads I win, tails you lose—testing directional alternative hypotheses in ecological and evolutionary research. Trends Ecol. Evol. 1994;9:235–237. doi: 10.1016/0169-5347(94)90258-5. doi:10.1016/0169-5347(94)90258-5 [DOI] [PubMed] [Google Scholar]
- Roberts M., Buchanan K., Evans M. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 2004;68:227–239. doi:10.1016/j.anbehav.2004.05.001 [Google Scholar]
- Rolff J. Bateman's principle and immunity. Proc. R. Soc. B. 2002;269:867–872. doi: 10.1098/rspb.2002.1959. doi:10.1098/rspb.2002.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolff J. Why did the acquired immune system of vertebrates evolve? Dev. Comp. Immunol. 2007;31:476–482. doi: 10.1016/j.dci.2006.08.009. doi:10.1016/j.dci.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Rolff J., Siva-Jothy M. Invertebrate ecological immunology. Science. 2003;301:472–475. doi: 10.1126/science.1080623. doi:10.1126/science.1080623 [DOI] [PubMed] [Google Scholar]
- Rolff J., Armitage S., Coltman D. Genetic constraints and sexual dimorphism in immunity. Evolution. 2005;59:1844–1850. doi:10.1554/04-747.1 [PubMed] [Google Scholar]
- Rowe L., Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. B. 1996;263:1415–1421. doi:10.1098/rspb.1996.0207 [Google Scholar]
- Ruckstuhl K.E., Neuhaus P. Sexual segregation in ungulates: a comparative test of three hypotheses. Biol. Rev. 2002;77:77–96. doi: 10.1017/s1464793101005814. [DOI] [PubMed] [Google Scholar]
- Ryder J., Siva-Jothy M. Male calling song provides a reliable signal of immune function in a cricket. Proc. R. Soc. B. 2000;263:1171–1175. doi: 10.1098/rspb.2000.1125. doi:10.1098/rspb.2000.1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005;50:529–551. doi: 10.1146/annurev.ento.50.071803.130420. doi:10.1146/annurev.ento.50.071803.130420 [DOI] [PubMed] [Google Scholar]
- Semple S., Cowlishaw G., Bennett P.M. Immune system evolution among anthropoid primates: parasites, injuries and predators. Proc. R. Soc. B. 2002;269:1031–1037. doi: 10.1098/rspb.2001.1950. doi:10.1098/rspb.2001.1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon B., Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. doi:10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Sheridan L., Poulin R., Ward D., Zuk M. Sex differences in parasitic infections among arthropod hosts: is there a male bias? Oikos. 2000;88:327–334. doi:10.1034/j.1600-0706.2000.880211.x [Google Scholar]
- Siva-Jothy M., Ryder J. Assaying PHA-induced mitosis: out of control? Funct. Ecol. 2001;15:813–814. doi:10.1046/j.0269-8463.2001.00573.x [Google Scholar]
- Siva-Jothy M., Moret Y., Rolff J. Insect immunity: an evolutionary ecology perspective. Adv. Insect Physiol. 2005;32:1–48. doi:10.1016/S0065-2806(05)32001-7 [Google Scholar]
- Skorping A., Jensen K. Disease dynamics: all caused by males? Trends Ecol. Evol. 2004;19:219–220. doi: 10.1016/j.tree.2004.02.006. doi:10.1016/j.tree.2004.02.006 [DOI] [PubMed] [Google Scholar]
- Wedekind C., Folstad I. Adaptive or nonadaptive immunosuppression by sex hormones? Am. Nat. 1994;143:936–938. doi:10.1086/285641 [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1966. Adaptation and natural selection. [Google Scholar]
- Whitacre C. Sex differences in autoimmune disease. Nat. Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. doi:10.1038/ni0901-777 [DOI] [PubMed] [Google Scholar]
- Yao G., Liang J., Han X., Hou Y. In vivo modulation of the circulating lymphocyte subsets and monocytes by androgen. Int. Immunopharmacol. 2003;3:1853–1860. doi: 10.1016/j.intimp.2003.09.002. doi:10.1016/j.intimp.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Zuk M., McKean K. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 1996;26:1009–1024. doi:10.1016/S0020-7519(96)00086-0 [PubMed] [Google Scholar]
- Zuk M., Stoehr A. Immune defense and host life history. Am. Nat. 2002;160:S9–S22. doi: 10.1086/342131. doi:10.1086/342131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Meta analysis data & sources