Abstract
Innate, inflammation-based immunity is the first line of vertebrate defence against micro-organisms. Inflammation relies on a number of cellular and molecular effectors that can strike invading pathogens very shortly after the encounter between inflammatory cells and the intruder, but in a non-specific way. Owing to this non-specific response, inflammation can generate substantial costs for the host if the inflammatory response, and the associated oxygen-based damage, get out of control. This imposes strong selection pressure that acts to optimize two key features of the inflammatory response: the timing of activation and resolution (the process of downregulation of the response). In this paper, we review the benefits and costs of inflammation-driven immunity. Our aim is to emphasize the importance of resolution of inflammation as a way of maintaining homeostasis against oxidative stress and to prevent the ‘horror autotoxicus’ of chronic inflammation. Nevertheless, host immune regulation also opens the way to pathogens to subvert host defences. Therefore, quantifying inflammatory costs requires assessing (i) short-term negative effects, (ii) delayed inflammation-driven diseases, and (iii) parasitic strategies to subvert inflammation.
Keywords: ageing, delayed costs, immune evasion, innate immunity, nitric oxide, reactive oxygen and nitrogen species
1. Introduction
Immune defences may be seen as one of the most sophisticated products of interspecific interactions. They are the result of frequent and long ‘arms races’ between hosts and parasites. As a result, hosts have evolved complex strategies to avoid the negative effects of parasites, and parasites have evolved many adaptive responses to counteract and evade the hosts' defences. Recognizing pathogenic organisms and clearing infection is the primary function of immunity (Medzhitov & Janeway 1997). This self-defence ability exists in unicellular organisms (that can produce microbicidal molecules), and reaches a high complexity in vertebrates (that possess a wide array of effectors that operate to cope with pathogenic invaders; Armstrong & Quikley 1999; Bulet et al. 2004).
Vertebrate immunity basically depends on two arms: innate and acquired immunity. The protective properties of innate immunity rely on constitutively produced receptors (pattern recognition receptors) that recognize distinct and conserved microbial molecular structures (pathogen-associated molecular patterns, PAMPs), which are absent from the host: once bound, these receptors directly activate the host's immune cells. The outcome of this activation is the initiation of the innate inflammatory response (Janeway & Medzhitov 2002; Akira et al. 2006). By contrast, acquired immunity is characterized by a vast repertoire of lymphocytes, bearing antigen-specific surface receptors that recognize specific antigenic configurations of pathogens and respond by triggering cellular (cytotoxic T-cells) and humoral (antibodies) effectors. In addition to specificity, acquired immunity differs from innate immunity in its ability to establish an immunological memory, which allows a more rapid and effective response upon re-exposure to the antigen (Cooper & Alder 2006). The dichotomy between innate and acquired immunity, while useful for a classification purpose, does not mean that these two branches work independent of one another. Acquired immunity largely depends upon the cells of the innate immune response to drive their functional maturation (Bayne 2003). In spite of the intimate connection between the innate and acquired immune responses and the major role played by the innate effectors in the process of parasite resistance, we still tend to focus on acquired immunity as the most important weapon against pathogens. This is evidenced by the biased representation of the acquired immune effector systems that have been studied in ecological immunity studies on vertebrates.
Inflammation is a non-specific process, elicited by trauma or infection, characterized by the delivery of fluids, molecules and cells from the blood into damaged or infected tissues, whose function is to fend off infectious agents. Although inflammation is commonly considered a vertebrate phenomenon, an inflammatory-like status has also been reported in invertebrates (Libert et al. 2006) where cells releasing toxic products and chemical signals are involved. The systemic effects of the vertebrate inflammatory response include fever and an increased number of leucocytes recruited for defence (Sell 2001). Several leucocyte families, e.g. granulocytes, monocytes (the precursors of macrophages) and lymphocytes, migrate to the focal area of the infected tissue and secrete metabolites, which have potent microbicidal properties that act through phagocytosis and exocytosis (Sell 2001). Upon encounter with an intruder, inflammatory cells produce peptides that play a key role in cellular communication. These peptides, called cytokines, can further activate and recruit other phagocytic cells, as well as drive their microbicidal activity. Phagocytes can kill engulfed pathogens mostly through the action of endogenously produced compounds with cytotoxic effects, such as enzymes, lytic peptides, as well as highly reactive oxygen and nitrogen species (ROS and RNS; Fang 2004; Swindle & Metcalfe 2007). In addition, and besides their cytotoxicity, at a low dose, ROS and especially nitric oxide (NO) also play a regulatory role as modulators of cellular communication and apoptosis (Matsuzawa et al. 2005; Swindle & Metcalfe 2007).
The effectors of inflammation endow organisms with efficient weapons to cope with the pervasiveness of infectious agents. The most compelling support to this view is the high sensitivity to infection, and the reduced survival prospect, of organisms showing deficiencies in the inflammatory process (Fang 2004). However, immune systems are not infallible, and the inflammatory response should be viewed as a double-edged sword that protects, but has the potential to harm, the host. Host tissue may be damaged when immune defences are misdirected, or overexpressed, leading to immunopathology. Although the distinction between immunopathologies and their origin is still debated (McGonagle & McDermott 2006), there is little doubt that chronic inflammation produces collateral undesirable effects on the host. In addition, it has been suggested that the cost of infection is more due to an over-reacting inflammatory response than a direct effect of the pathogen (Råberg et al. 1998; Mackintosh et al. 2004; Graham et al. 2005; Halliwell 2006 for reviews). To protect their tissues from inflammatory injury, hosts have evolved a regulatory network based on a class of cytokines (such as interleukin-10 or transforming growth factor-β) that control the resolution of inflammation once the pathogen has been cleared (Belkaid 2007).
Although the study of the molecular and cellular mechanisms of inflammation-based resistance is of prime importance from a biomedical perspective, understanding the selective forces and the constraints shaping the evolution of the diversity of host defences and parasite strategies to exploit the host requires an evolutionary approach. The aim of this paper is to provide a discussion of the evolutionary ecology of parasite-mediated inflammation. We will first briefly review the mechanisms, which are at the basis of the inflammatory response, with a special focus on the production of ROS and RNS. Then, we will discuss the selective forces and constraints that are likely to act on inflammation: the benefits of early and effective protection against intruders and the inevitable costs of non-specificity. Because parasite exposure and host response vary with ontogeny, we will address the question of age-related costs and benefits of inflammation. Finally, we will see that the pathogens can modify and manipulate host inflammation for their own survival and spread. This adds considerable complexity to the understanding of the evolutionary ecology of parasite-mediated inflammation.
2. The role of ROS and RNS in the inflammatory process
Phagocytic cells, such as macrophages and neutrophils (heterophils in birds), are at the core of the innate inflammatory response. Activation of phagocytic cells induces the production of antimicrobial compounds that, once transferred into the phagosome, play a primordial role in killing phagocytized pathogens.
ROS and RNS are probably the most important micromolecules that intervene during the inflammation-based control of invading pathogens. The most prominent feature of ROS and RNS is their generic cytotoxicity. These chemically reactive molecules cannot discriminate between the structure of host molecules, cells and tissues and infectious agents. This is in stark contrast to the fine-tuned capacity of the cells and molecules of the acquired immune system, which can recognize specific epitopes and surgically target the defence response. In §2a, we will first briefly describe the molecular nature of ROS and RNS, and their production and will then see how these reactive molecules achieve the goal of pathogen killing.
(a) ROS and RNS
Upon stimulation by a PAMP, neutrophils and macrophages respond by a marked increase in oxygen (O2) uptake. This increase in O2 consumption characterizes the so-called respiratory burst and is the consequence of the activation of an enzymatic system (NADPH oxidase or phox) that oxidizes NADPH into NADP+ plus superoxide anion () (Fang 2004). Superoxide, in addition to its own cytotoxic effect, participates in the generation of other, more unstable and damaging molecules, such as hydroxyl radicals (OH·), hydrogen peroxide (H2O2) and hypochlorous acid (HOCl). The half-life capacity to permeate cell membranes and the cytotoxicity vary greatly across different ROS. For instance, OH· has a half-life of a few nanoseconds whereas chloramines are stable for hours (Swindle & Metcalfe 2007).
RNS are the second class of micromolecules secreted by phagocytic cells during the inflammatory response. Nitric oxide (NO·) is the principal RNS produced by neutrophils and macrophages. NO· is generated enzymatically in a two-step reaction from l-arginine substrate (Fang 2004). This reaction is catalysed by a family of enzymes, the NO synthases (NOS). The inducible NOS (iNOS) is responsible for the burst in NO production following an inflammatory insult. Nitric oxide has a cytotoxic effect but it can also react with ROS to produce even more powerful oxidants, such as peroxynitrite (ONOO−) or dinitrogen trioxide (N2O3) (Halliwell 2006).
(b) ROS and RNS are effective antimicrobial agents
The importance of ROS and RNS during the inflammatory response, and their role as effective antimicrobial agents, is provided by two lines of evidence: the identification of the molecular target of ROS and RNS in vitro and the fitness cost paid by individuals who cannot produce these molecules.
ROS and RNS have been shown to target several molecular structures of invading pathogens, in particular proteins, DNA and lipids. Depending on the chemical characteristics of each radical species (for instance, their capacity to cross lipid membranes), different molecular patterns of the pathogen can be targeted. For instance, Imlay & Linn (1986) showed that hydrogen peroxide (H2O2) can kill Escherichia coli in a dose-dependent manner, where low concentration mostly oxidized DNA, whereas higher concentration simultaneously oxidized several biomolecules. Nitric oxide can inhibit bacterial respiration and DNA replication (Fang 2004). Moreover, when in the presence of ROS, the NO-derived oxidative damage is greatly enhanced.
It should be noted, however, that for both ROS and RNS, the effectiveness of their antimicrobial activity also depends on the local redox environment. Reactive species can be scavenged by several detoxification systems (enzymatic and non-enzymatic). In most cases, therefore, the microbicidal effectiveness of the response is affected by the amount of ROS/RNS released and the availability of microbial antioxidant defences.
The second line of evidence on the importance of ROS and RNS as effective inflammatory effectors comes from studies that have either taken advantage of spontaneous mutations affecting the genes controlling the expression of phox, either using knockout mouse strains lacking phox or iNOS.
Chronic granulomatous disease (CGD) is a human pathology that occurs as a consequence of mutations in subunits of NADPH oxidase. Patients affected by CGD cannot produce superoxide anions () during the respiratory burst. The disease substantially reduces lifespan and is associated with persistent infection with a number of pathogenic micro-organisms, such as Staphylococcus aureus, Pseudomonas and Salmonella (Sell 2001).
Inducible NO synthase is the major enzymatic pathway responsible for the production of nitric oxide. Although there is no known human deficiency in the expression of iNOS, polymorphisms in the iNOS promoter region have been reported in several studies (Levesque et al. 1999; Kun et al. 2001). In most cases, increased iNOS expression is correlated with increased resistance to a number of different pathogens, including Plasmodium (Anstey et al. 1996; Hobbs et al. 2002). Similarly, human macrophages that express iNOS at high levels in vitro are better able to kill Leishmania and Mycobacterium parasites (Bogdan et al. 2000).
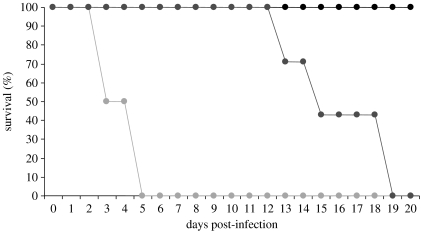
Studies on animal models have provided the best ultimate evidence for the importance of ROS and RNS in parasite resistance. Using knockouts lacking phox (gp91phox−/−), iNOS (iNOS−/−) and control wild-type mice infected with Salmonella typhimurium, Mastroeni et al. (2000) showed that the course of the infection was greatly affected by the absence of superoxide and NO (figure 1). Interestingly, inhibiting the NADPH synthase pathway (ROS production) or the iNOS pathway (NO production) resulted in a quite different pattern of infection-induced mortality, with phox−/− individuals already showing massive mortality at 3–4 days post-infection, whereas suppression of the iNOS pathway produced substantial mortality only approximately two weeks post-infection.
Figure 1.
Survival of mice infected with S. typhimurium. Wild-type (C57BL/6, black circles) mice show 100% survival at 20 days post-infection. On the contrary, KO mice that do not express phox (NADPH-based respiratory burst, light grey circles) or iNOS (NO production, dark grey circles) die during the course of the study. Interestingly, mortality was much more rapid in the phox−/− group (100% mortality at day 5 post-infection) than in the iNOS−/− group (100% mortality at day 19 post-infection). This suggests that respiratory burst precedes the NO response during the acute inflammatory response (adapted from Mastroeni et al. 2000).
Interestingly, the functional role of NO against invading parasites is not restricted to intracellular micro-organisms. Brugia malayi is an extracellular nematode that is responsible for lymphatic filariasis in humans. Rodents can also be infected with filarial parasites, which allow experimental tests of the effectors of immunity against such parasites. Using elegant experiments, Rajan et al. (1996) showed that iNOS is an important mediator of resistance to B. malayi. Mice were infected with B. malayi larvae and then were either injected with aminoguanidine (AG, an inhibitor of iNOS) or left untreated. A larger number of adult worms were yielded from AG-treated mice compared with controls, whatever the initial number of larvae inoculated. In a second complementary experiment, susceptible mice (SCID mice) were infected with Brugia larvae and then either injected with DEA/NO (a compound that releases NO) or left as control. In agreement with the results of the first experiment, the number of adult worms yielded from DEA/NO mice was much smaller compared with control, susceptible mice (Rajan et al. 1996).
(c) How does natural selection operate on the effectors of the inflammatory response?
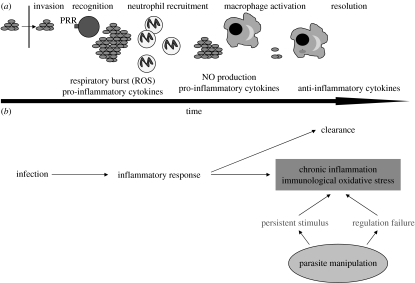
This brief survey shows that early inflammatory response is important for host resistance towards a range of parasitic organisms, since individuals lacking the ability to produce ROS and RNS are more susceptible to infection and suffer from an increased infection-induced mortality. At a first glance, one might be tempted to believe that natural selection operates on the effectors of inflammation to exhibit the highest toxicity. However, we should keep in mind the crucial feature of the innate inflammatory response: non-specificity. ROS and RNS do not discriminate between host and pathogen DNA, proteins or lipids. As such, ROS and RNS can be quite costly for the host. Therefore, one might wonder how natural selection has shaped the effectors of the inflammatory response as to maximize the protective effect while minimizing the risk of collateral damage. There are at least two solutions to this problem. The first is rapidity. The effectors of inflammation are usually activated within minutes (another major difference from adaptive immunity which needs days/weeks to be fully deployed). It appears that the chronological suite of events that are triggered by an immune insult is organized so as to progress from the most to the less costly response. Even within the inflammatory response, there is a chronological sequence suggesting that the more toxic ROS produced during the respiratory burst precedes the activation of the iNOS pathway and its milder NO delivery (Vazquez-Torres & Fang 2001). Costly inflammatory response is therefore rapidly activated but it should also be rapidly switched off to avoid the ‘horror autotoxicus’ of chronic inflammation (figure 2). Macrophages secrete a number of anti-inflammatory molecules, such as interleukin-10 (IL-10) and transforming growth factor β (TGF-β), which contribute to the resolution (the process of downregulation) of the inflammatory response. Once again, the dichotomy between the innate inflammatory response (antigen non-specific) and the acquired immune response (antigen specific) emphasizes the intrinsically costly nature of inflammation. This is because, at least in principle, antigen-specific immunity can be free of cost, in terms of autoimmunity, if T-lymphocytes with receptors that bind to autoantigens are negatively selected in the thymus or suppressed in peripheral organs. The non-specific nature of inflammatory effectors means, by definition, that the response generates costs whose magnitude depends on the intensity and duration of the response. In §3, we will review in more detail the costs of acute and especially chronic inflammation and how this has led to the evolution of regulatory mechanisms.
Figure 2.
(a) A schematic view of the time course of acute inflammation. Once a pathogen has entered the host, pattern recognition receptors (PRR) on cell membranes bind to some pathogen molecular signatures. This recognition phase is followed by the recruitment of neutrophils and their respiratory burst. Sustained production of pro-inflammatory cytokines activates macrophages. Macrophages with engulfed pathogens start the resolution process by secreting anti-inflammatory cytokines. (b) Two of the outcomes of infection-induced inflammation: (i) clearance and resolution; and (ii) chronic inflammation and immunological oxidative stress. Parasites, of course, can interfere with the normal outcome of the inflammatory response for their own survival.
3. Inflammatory diseases and oxidative stress
(a) Direct and indirect costs
There is now a large body of evidence showing that pathogen-induced inflammation is associated with an increase in ROS and RNS production. A number of correlative studies have reported associations between local or systemic infections and oxidative damage. For instance, humans infected with HIV suffer from an increased amount of serum hydroperoxides and malondialdehyde (the products of lipid peroxidation) and reduced availability of antioxidants (both enzymatic and non-enzymatic) (Pace & Leaf 1995). Septic shock, probably one of the most severe consequences of the inflammatory response (Annane et al. 2005) is accompanied by a substantial production of NO and peroxynitrite (Titheradge 1999; Sebbane et al. 2006).
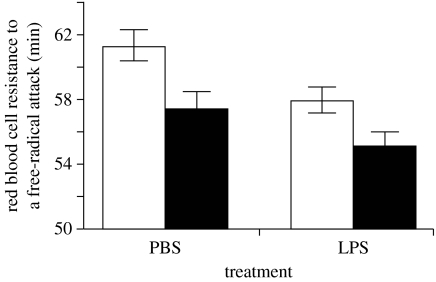
Experimental work has also shown that activation of the inflammatory response depletes antioxidants and exposes the host to increased risk of oxidative stress. Zebra finches (Taeniopygia guttata), whose innate immune response was activated by lipopolysaccharide (LPS) injection, showed a decrease in antioxidant status 24 hour post-injection compared with a control group (figure 3; Bertrand et al. 2006). Although one might wonder about the relevance of such an effect in terms of fitness, previous work has shown that a decrease in antioxidant status significantly predicts short-term survival prospects (Alonso-Alvarez et al. 2006). These results, therefore, suggest that the activation of the inflammatory response by PAMPs can incur fitness-relevant costs.
Figure 3.
Activation of the inflammatory response by the way of a LPS injection, which depletes antioxidant defences in male (white bars) and female (black bars) zebra finches (adapted from Bertrand et al. 2006).
The most compelling evidence that inflammatory response may alter host homeostasis comes from in vivo and in vitro experimental studies which controlled antioxidant availability, and/or ROS and RNS production through pharmacological treatments or using genetically modified organisms. For instance, experimental infection of mice with the retrovirus ts1 (a murine leukaemia virus) caused spongiform encephalopathy, thymus atrophy and other severe neuropathologies leading to death. In vitro experiments showed that the redox-sensitive transcription factor (Nrf2) was activated in cultured ts1-infected astrocytes. This factor upregulates the synthesis of glutathione (an antioxidant compound; Qiang et al. 2004). The astrocytes surviving to the infection were those producing the highest antioxidant defences, suggesting that ROS and RNS scavenging are crucial for cell survival and control of immunopathology (Qiang et al. 2006). In addition, treatment of astrocytes with an antioxidant (a refined monosodium α-luminol) reduced ROS accumulation and suppressed Nrf2 activation (Jiang et al. 2006). In short, this work shows that the degree of infection-induced immunopathology depends on the activation of antioxidant compounds that scavenge ROS and RNS produced during the inflammatory response.
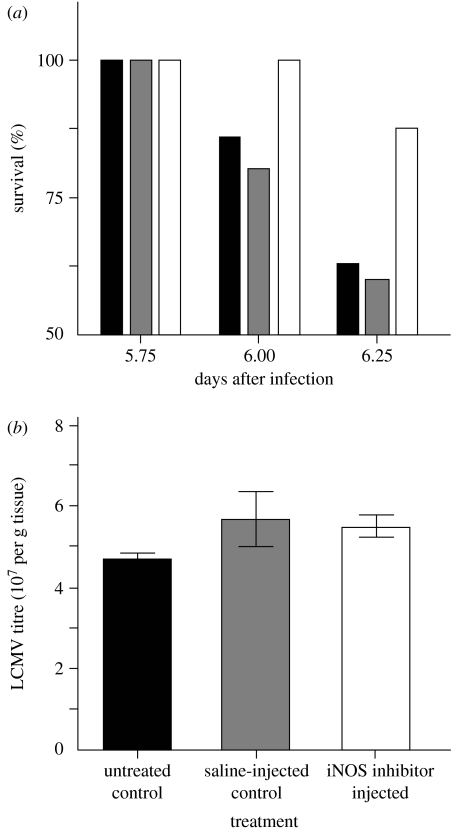
Blocking ROS or RNS production by means of specific inhibitors, or by the use of genetically modified organisms, has provided further evidence for the link between oxidative stress and inflammation. Humans infected with the Sin Nombre virus, the causative agent of cardiopulmonary syndrome, show an elevated expression of iNOS in the lungs, especially in areas with more severe pathology. Patients with the most severe symptoms were those with higher plasma NO-derived molecules NOx. A murine model of the disease (mice infected with lymphocyte choriomeningitis virus) showed, within a few days post-infection, identical pulmonary and cardiac symptoms associated with both elevated iNOS in the lungs and NOx in plasma. The periodic injection of a specific iNOS inhibitor in infected mice suppressed the cardiac symptoms and strongly improved short-term survival without changing virus titres (figure 4), suggesting that oxidative damage may outweigh the direct negative effects of viral replication (Davis et al. 2002).
Figure 4.
(a) Survival of mice infected with lymphocytic choriomeningitis virus. Black bars refer to control, untreated control mice; grey bars refer to control, saline-injected control mice; white bars refer to mice injected with 1400 W (an iNOS inhibitor). Whereas the survival of control mice dramatically drops after day 6 post-infection (approx. 60%), 1400 W-treated mice still have a 90% survival. (b) These differences in survival prospects are not due to viral titres, since they are similar in the three groups. These results, therefore, suggest that survival improvement of 1400 W-treated mice is due to the suppression of an immunopathological response (adapted from Davis et al. 2002).
(b) Immediate and delayed costs
Inflammation incurs costs. Assessing such costs is essential if we want to appraise the outcome of selective forces operating on the inflammatory response. Having a complete picture, however, requires considering not only the immediate, short-term, costs of inflammation but also those expressed once the infection has been cleared. Chronic inflammation may lead to delayed negative outcomes. Probably the most important delayed disabling consequence of chronic inflammation is cancer. There is now a large body of evidence showing that many cancers are associated with pathogens, and epidemiologic studies attribute to 15–20% of worldwide malignancies to infection (Coussens & Werb 2002). A wide array of pathogens may contribute to cancer occurrence through the inflammatory response of the host, and particularly the release of ROS and RNS (Ames et al. 1995). Under chronic inflammation, cells damaged by cytotoxic products are permanently replaced, but sustained ROS and RNS production can result in genomic alteration (De Marzo et al. 2008). Cultured mouse macrophages continuously exposed to interferon-γ (an effector of the inflammatory response) show a much higher mutation rate compared with control cells after 14 and 23 days of exposure, and this effect was drastically reduced by the addition of a NOS inhibitor (Zhuang et al. 1998). Epidemiological and experimental findings support the view that other disabling diseases, such as age-related macular degeneration (Hollyfield et al. 2008), may be seen as the delayed outcomes of infection and oxidative damage. Interestingly, epidemiological and demographic studies have also suggested that exposure to inflammatory insults early in life (during infancy) may contribute to age-related diseases (Finch & Crimmins 2004; Crimmins & Finch 2006). One might thus tentatively speculate that the reduction in early life infection risks in wealthy countries (with the associated reduction in inflammation) has contributed to the spectacular extension in longevity experienced by humans in the last decades.
Overall, the evidence of delayed occurrence of the disabling consequences of inflammation underlines that time is a crucial dimension that should be considered when weighing the benefits and costs associated with this component of the immune response. Importantly, these delayed effects also make the evolutionary analyses more complex because the time elapsed between the initiation of inflammatory damage and the occurrence of detectable costs may allow organisms to achieve crucial fitness-related functions, such as reproduction. This also underlines that many immunopathologies, and particularly those with an inflammatory determinism, are age related. This point leads us to address the link between inflammation and an important life-history trait: ageing.
(c) Inflammation, oxidative stress and ageing
From both a proximate and ultimate point of view, inflammation plays a central role in the ageing process, especially owing to the link between inflammation and oxidative stress. At the proximate level, the free-radical theory of ageing argues that ageing is the cumulative result of oxidative damage to cells and tissues that arises as a result of aerobic metabolism and the effect of external factors (Harman 1956; Finkel & Holbrook 2000). It provides a plausible, and currently accepted, global mechanism to explain ageing (Sarkar & Fisher 2006). At the ultimate level, antagonistic pleiotropy postulates that genes with late deleterious effects can be maintained and selected for, owing to their beneficial effects in early life (Williams 1957). Innate immune genes might fall within this category: inflammation might be essential for parasite resistance in early life but exert costs later on.
The importance of inflammation during ageing arises through two non-exclusive pathways. First, there is an increasing incidence of inflammatory diseases with age, at least in humans and animal models, and some of the most common age-related diseases have an inflammatory component triggered by past infections (Licastro et al. 2005; Sarkar & Fisher 2006). Second, ageing is characterized by a low grade, chronic and systemic pro-inflammatory status (the upregulation of inflammatory effectors) resulting from the imbalance between pro- and anti-inflammatory cytokines (Giunta 2006). This upregulation of inflammation with age seems evolutionarily conserved because a pro-inflammatory status has also been observed in aged fruitflies (Drosophila melanogaster; Pletcher et al. 2007). The age-related upregulation of inflammation has crucial implications in terms of survival prospects since pro-inflammatory cytokines are reliable predictors of mortality risk in the elderly (van den Biggelaar et al. 2004).
The crucial questions arising from these results are: ‘Why are the elderly more prone to exhibit a pro-inflammatory status?’ and, ‘Is this the product of selective forces acting on the inflammatory response across an individual lifespan?’ The recent increase in longevity seen in some human societies can help provide an answer. Humans have probably experienced relatively constant longevities (approx. 40–50 years) for thousands of years. Selection is, therefore, thought to have optimized immune function with respect to expected longevity. The spectacular increase of longevity in a few generations has considerably extended the length of action of the immune system. This leads to the possibility of accrued oxidative damage that accumulates over an individual lifespan (Licastro et al. 2005). This idea is in line with the disposable soma theory (Kirkwood 1977), which predicts that it does not pay to invest in repair mechanisms that would function well beyond the expected longevity, under the prevailing extrinsic mortality regime.
An alternative explanation for age-related dysfunction of the inflammatory response may reside in the antagonistic pleiotropic effect of inflammation (Williams 1957). Age-related inflammatory disease, with its shift towards chronic inflammation, might be the price paid for an immune system that provides effective protection in early life. This also suggests that the trade-off shaping the optimal inflammatory response is not (at least not only) based on resource allocation, but might involve early good/bad protection versus late mild/severe dysfunction. Here again, time is a crucial component of the trade-off, since the fitness relevance of inflammation-induced damage depends not only on its magnitude but also on the timing of the onset of the damage. Natural selection is probably almost blind to inflammatory diseases that arise during post-reproductive life.
Parasites, of course, play a crucial role in the evolution of optimal defences across age. For instance, the long-lived Caenorhabditis elegans mutant daf-2 with upregulated immune defence, benefits from an increased longevity when kept with bacterial pathogens: the higher the pathogenicity the higher the lifespan increase relative to wild-type worms (Garsin et al. 2003). Interestingly, an interaction between infection risk and the costs/benefits of inflammation has been suggested also for humans. Certain alleles of genes coding for anti-inflammatory cytokines (IL-10) are over-represented in Italian centenarians, suggesting that downregulated inflammation has a positive effect on lifespan (Lio et al. 2002). However, this is likely to be the case only for people who have escaped early exposure to infectious diseases (Caruso et al. 2005). In the current context of reduced antigenic exposure witnessed in western society, it is tempting to predict that genotypes associated with a weak inflammatory response will express a phenotype of extended longevity.
Overall, the available evidence emphasizes the importance of inflammation as a first line of defence, not only to preserve individual integrity in a pathogenic environment, but also its price through oxidative stress and its subsequent disabling effects. A fine tuning of inflammatory response to limit its cost is necessary over two time scales: in the short term, to quickly downregulate the effectors of the acute phase and induce the resolution of the inflammatory response; in the long term, during an individual's lifespan, to avoid delayed costs of an essential component of early protection.
(d) Immune regulation
Regulation of the inflammatory process relies on both cellular and molecular effectors. Anti-inflammatory cytokines are secreted by a number of immune cells, the most important being macrophages and a population of CD4+ lymphocytes called regulatory T-cells (Treg; Belkaid 2007). The vital function of immune regulation is clearly illustrated by the major fitness costs, in terms of immunopathology, paid by individuals who lack regulation (Kim et al. 2007).
To reduce oxidative damage without compromising protection, resolution of inflammation has to be fine graded and properly timed (Raes et al. 2007). This means that resolution must occur promptly after the acute phase of inflammation to limit damage, but not so early as to prevent parasite clearance. This idea is nicely illustrated by the following example. Mice infected with a ‘non-lethal’ strain of Plasmodium yoelii exhibit a peak in TGF-β production at 10–15 days post-infection, once the parasite has been cleared; conversely, mice infected with a ‘lethal’ strain exhibit TGF-β peak at 2–3 days post-infection. This early regulation of the inflammatory response induces the failure of parasite clearance and finally results in 100 per cent host mortality. Experimental neutralization of early production of anti-inflammatory cytokines after infection with the lethal strain largely restores clearance rate and mouse survival (Omer et al. 2003). Therefore, at its own level, immune regulation may be seen as a double-edged sword: a timely and graded regulation is beneficial by reducing the cost of inflammation, while a too early or a too late resolution leads either to failure in parasite clearance or to oxidative damage.
On top of this, to fully capture the selective forces shaping the evolution of inflammation, it is crucial to consider the strategies that parasites adopt to evade this first physiological barrier. It is clear that although regulation has a vital importance for the host, it may also offer a window for parasites to control hosts' defences (Bergstrom & Antia 2006).
4. Inflammation imposes strong selection pressures on pathogens as to escape it
Living organisms respond to the environment they experience by evolving traits that improve their fitness in that specific environment. As prey is selected to run faster to escape a predator, parasites are selected to escape the immune response. This goal is achieved by different strategies that depend on the nature of the threat. When the immune response is based on the recognition of a specific peptidic signature, parasites can evade the immune response by changing the shape of the signature. This is a relatively widespread strategy used by a number of micro-organisms (e.g. Finlay & McFadden 2006). One option that the immune system has to counteract this parasitic strategy is to rely on highly conserved structures that cannot be easily modified by the pathogen without paying a major cost. This is what happens with the so-called PAMPs, such as bacterial LPS and peptidoglycan that trigger the inflammatory response. The non-specific nature of the effectors of inflammation poses an additional problem for the pathogen in finding a way to escape the host's immune response. As already mentioned, ROS and RNS do not differentiate between host and pathogen structures. Antigenic shift, molecular mimicry or any other mechanism that aims at escaping the adaptive immune response of the host will be of little help when facing an inflammatory process. This might confer a selective benefit to the host in the arms race with the parasite, but does this mean that the pathogens are defenceless against inflammation?
(a) Evading inflammation
In spite of the generic nature of the threat imposed by the inflammatory response, pathogens have evolved a number of evasion strategies (Hornef et al. 2002; Sacks & Sher 2002) that can be chronologically ordered as follows (table 1): (i) avoid recognition; (ii) interfere with cellular communication; (iii) avoid phagocytosis (iv) interfere with normal vacuolar activity; (v) disrupt ROS and RNS production, (vi) resist phagocytic digestion; and (vii) repair oxidative damage.
Table 1.
Diversity of strategies adopted by pathogens to escape the innate inflammatory response. (The last column reports a non-exhaustive list of species known to adopt each of the strategies.)
| aim | mechanism to achieve the aim | selected examples |
|---|---|---|
| avoid recognition | structural change in PAMPs (phase variation) | Helicobacter pylori Salmonella |
| inhibit cellular communication and recruitment of inflammatory cells | interfere with pro-inflammatory cytokine production | Cytomegalovirus Mycobacteria Epstein-Barr virus |
| stimulate anti-inflammatory cytokine production | respiratory syncytial virus | |
| express homologue of anti-inflammatory cytokines | Salmonella Schistosoma Brugia | |
| avoid phagocytosis | transfer pathogen material (proteins) that disrupt cytoskeleton | Yersinia |
| escape phagosome | transfer pathogen material (proteins) that disrupt normal vacuolar activity | Listeria Mycobacteria Shigella Salmonella Trypanosoma Leishmania |
| disrupt ROS and RNS production | interfere or inhibit enzymatic activity governing ROS and RNS production | Helicobacter Plasmodium Leishmania |
| resist digestion | express enzymatic and non-enzymatic defences | Salmonella Staphylococcus Bacillus anthracis |
| repair oxidative damage | express proteins that repair oxidative damage of DNA | Salmonella |
(i) Avoid recognition
PAMPs are conserved micro-organism structures used by the host as stimuli to initiate innate immune responses. Bacteria express several PAMPs, including cell wall components such as LPS. Although LPS has a conserved structure across gram-negative bacteria, changes in the acylation and phosphorylation pattern result in variable levels of immune activation. For instance, Helicobacter pylori expresses a LPS with a weaker inflammatory potential (approx. 100-fold weaker) than Salmonella LPS (Rhen et al. 2000). Similarly, mutant Salmonella with lipid A molecule lacking a single fatty acyl chain induce a weaker response both in terms of pro-inflammatory cytokines and iNOS expression (Khan et al. 1998). Antigenic variation is a common strategy used by many pathogens to escape antigen-specific recognition by the adaptive immune response. The functional importance of PAMPs for pathogens certainly constrains the possibility of evasion from the innate immune response by mutating these molecular patterns.
(ii) Interfere with cellular communication
Upon infection, activated immune cells produce and release a number of proteins whose function is to ‘transfer information’ between cells. These proteins, called cytokines, can instruct other immune cells about the current ‘danger’ faced by the organism. Cytokines are essential molecules for the recruitment, proliferation, activation and downregulation of inflammatory cells. As such, parasites can effectively manipulate the strength of the immune response by interfering with cytokine secretion. In particular, intracellular as well as extracellular metazoan parasites can hijack anti-inflammatory cytokines produced by the host, such as IL-10 or TGF-β, to dampen the host immune response (Redpath et al. 2001; Maizels et al. 2004). In addition, some parasites express an IL-10 homologue that interferes with the normal functioning of the immune system (Redpath et al. 2001).
(iii) Avoid phagocytosis
Bacteria use secretion systems to transfer bacterial protein either in the extracellular environment or directly within target cells. The translocation of bacterial mediators can disrupt the cell cytoskeleton and therefore prevent engulfment. Yersinia parasites, responsible for various infectious diseases, such as plague and enterocolitis, encode and deliver three different effector proteins (YopO, YopT and YopE) that interfere with macrophage uptake (Navarro et al. 2005).
(iv) Interfere with normal vacuolar activity
Some intracellular bacteria such as Listeria, Mycobacterium or Shigella, once phagocytized, can disrupt the phagosome membrane and escape into the cytoplasm where they can multiply (Hornef et al. 2002).
Salmonella rely on a type III protein secretion system, called Salmonella pathogenicity island 2 (SPI2), to survive within infected macrophages. Products of the SPI2 gene have been shown to interfere with the trafficking of oxidase-containing vesicles towards the phagosome (Vazquez-Torres et al. 2000). Pathogenic Salmonella can, therefore, achieve survival within macrophages by excluding NADPH oxidase from the phagosome.
Mycobacteria adopt a strategy of phagosomal arrest. Instead of maturing and fusing with vacuoles containing microbicidal compounds, phagosomes with living Mycobacteria maintain a non-toxic environment that favours the survival and the persistence of the pathogen (Young et al. 2002).
(v) Disrupt ROS and RNS production
Infection by H. pylori results in strong gastric inflammation. This response is triggered and exacerbated by some bacterial proteins that enhance recruitment of inflammatory cells in the gastric mucosa. Whereas on the one hand, this might be beneficial to the parasite (see below for an explanation of this paradox), the pathogen also needs to defend itself from the inflammatory attack. Among other strategies, Helicobacter produces an arginase that, by converting l-arginine to urea and l-ornithine, reduces the availability of the substrate required for NO production (Gobert et al. 2001). This strategy is effective in resisting killing since arginase-defective bacteria are more susceptible to NO-induced mortality, whereas exposure to macrophages of iNOS−/− mice does not affect the survival of arginase-defective Helicobacter (Gobert et al. 2001).
(vi) Resist phagocytic digestion
Highly ROS and RNS can be detoxified and scavenged by a number of enzymatic as well as non-enzymatic mechanisms. The importance of antioxidants has recently been emphasized in the context of improvement of age-associated degenerative diseases in humans. However, it is interesting to note that, although not as diversified as in vertebrates, micro-organisms also rely on a battery of enzymes and other compounds to resist phagocytic ROS and RNS.
Several studies have emphasized the importance of antioxidant enzymes, such as superoxide dismutase (SOD), catalase and alkyl hydroperoxide reductase, for pathogen resistance to phagocytic-based oxidation. Bacterial Cu–Zn-SOD is found at the periplasmic level, which indirectly suggests a role for the scavenging of extracellular oxidants. Manipulation of the pathogen genome allows the establishment of the functional role of the genes of interest. Using a mutated S. typhimurium strain (sodC) deficient in Cu–Zn-SOD, De Groote et al. (1997) showed that the strain is very susceptible to superoxide and nitric oxide, and killing by macrophages.
Staphylococcus aureus is a major opportunistic pathogen responsible for many nosocomial infections. The bacterial genome encodes two proteins with antioxidant properties. Catalase (KatA) has a very specific scavenging effect on hydroperoxide, whereas alkyl hydroperoxide reductase (AhpC) has a less specific scavenging property, including the highly toxic peroxynitrite. Interestingly, the synthesis of the two antioxidants shows a compensatory behaviour, where the inhibition of AhpC leads to an overexpression of KatA and vice versa (Cosgrove et al. 2007).
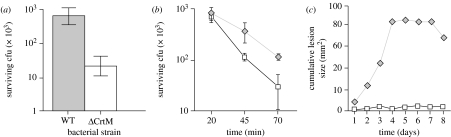
Carotenoids have become a very popular subject of research among evolutionary ecologists (Lozano 1994; von Schantz et al. 1999; Faivre et al. 2003). Staphylococcus aureus takes its name from its golden pigmentation. The golden colour is due to the bacterial synthesis of carotenoids, called staphyxanthin. Recently, it has been suggested that this biosynthetic pathway might confer a selective advantage to the pathogen in the face of phagocytic attack. Using a mutagenesis approach, Liu et al. (2005) produced S. aureus with disrupted carotenoid synthesis. In agreement with the predictions, this mutated strain was less able to resist neutrophil oxidative bursts (figure 5), and was less pathogenic compared with a wild-type strain. Interestingly, the competitive advantage of the wild-type strain disappeared when the bacteria faced neutrophils lacking oxidative burst activity. These results, therefore, show that carotenoids confer a selective benefit during the process of ROS scavenging. Why do not all bacteria exhibit a golden coloration? This is intriguing, since expression of carotenoids in a non-pigmented species (Streptococcus pyogenes) produced the same results: better resistance to neutrophil oxidative burst and increased virulence. Therefore, it seems that as in higher vertebrates, S. aureus has evolved a diversified array of antioxidant tools, both enzymatic and non-enzymatic, to resist immune-mediated oxidative attack.
Figure 5.
Survival of wild-type (WT) and mutant S. aureus (ΔCrtM) lacking carotenogenesis. (a) The mutant (white bar) has a much poorer survival compared with the wild-type (grey bar), when exposed to hydrogen peroxide. (b) Wild-type bacteria (diamonds) also better resist killing by neutrophils and (c) are more virulent (induce more skin lesion) than mutants lacking carotenogenesis (squares) (adapted from Liu et al. 2005).
(vii) Repair oxidative damage
Finally, pathogens can avoid inflammation-induced cost by adopting/performing repair strategies. Bacteria have DNA repair proteins that limit the mutagenic impact of peroxinitrite and other RNS. Salmonella typhimurium expresses two such proteins RecB and RecC (Nathan & Shiloh 2000). RecBC-deficient Salmonella show poor survival in mice expressing normal respiratory burst activity. However, the survival of the RecBC-deficient strain improves in phox−/− hosts and is identical to the wild-type mice lacking both phox and iNOS (Shiloh et al. 1999).
5. Triggering inflammation: when the host response favours the parasite
As already emphasized above, the innate immune response is of vital importance for the vertebrate hosts. Paradoxically, parasites might take advantage of inflammation for their own survival. This is likely to happen through different mechanisms.
Intracellular pathogens infecting macrophages might benefit from the ability of these cells to extravasate and spread from the site of the infection into the systemic circulation. In other words, these pathogens use macrophages as vehicles to circulate and spread within the organism. Salmonella have been shown to exploit macrophages in this way (Vazquez-Torres et al. 1999). Ebola virus infects monocytes and macrophages, and stimulates the production of pro-inflammatory cytokines, such as TNF-α and interleukin-1β, as well as ROS and RNS. The activation of the pro-inflammatory pathway is thought to be of prime importance for the spread of the virus, since infected phagocytes move from the site of infection to lymph nodes (Zampieri et al. 2007). Pro-inflammatory cytokines attract and recruit even more macrophages which provide further susceptible hosts for viral replication and dissemination through the organism.
Shigella-infected macrophages do not live for long (1–2 hours). Following the death of the macrophage, released Shigella can expand the infection by entering other macrophages or surrounding enterocytes. Shigella infection of macrophages and epithelial cells induces a strong host-mediated inflammation characterized by the production of pro-inflammatory cytokines. This leads to further recruitment of neutrophils to the inflamed site. Neutrophil migration is favourable to the bacterium since neutrophils disrupt the junctions between epithelial cells, making access to these cells easier for the pathogen (Ogawa & Sasakawa 2006). However, neutrophil migration and local recruitment can also limit the spread of the infection by exerting substantial mortality on the bacteria. There exists, therefore, a subtle equilibrium between inflammation-derived benefits for the parasites in terms of facilitated access to host cells, and costs in terms of mortality.
Triggering the inflammatory response can also be beneficial to the parasites in terms of nutrients released by the host. Helicobacter pylori lives in a particularly nutrient-poor environment (between the gastric epithelium and the mucous layer). Infection by Helicobacter is followed by the recruitment of inflammatory cells in the lamina propria of the gastric mucosa. This recruitment and activation is in part driven by two bacterial proteins (cag and neutrophil-activating protein). The recruitment and activation of inflammatory cells might make nutrients available for bacterial growth (Baldari et al. 2005).
(a) Using inflammation as a weapon against competitors
Establishing an infection often results in competition with other micro-organisms that live within the host. This is particularly true for intestinal bacteria, acquired through the oral-faecal route, that need to bypass the pre-existing biofilm of commensal bacteria covering the intestinal wall. A dense biofilm can effectively protect the host from colonization by pathogenic organisms and this form of resistance has been termed ‘colonization resistance’. In some cases, perturbation of the commensal bacterial community can lead to enhanced risk of infection by pathogens. Recently, it has been suggested that parasites can achieve this goal by using the host inflammatory response as a weapon to overcome colonization resistance (Stecher et al. 2007; Brown et al. 2008). In a series of elegant experiments, Stecher et al. (2007) compared the colonization potential of virulent (wild type) and avirulent (avir) Salmonella enterica Typhimurium. The capacity of the wild type to trigger inflammation conferred a selective advantage during competition with commensal bacteria that was lacking to the avir strain. To confirm this, infection with the avir strain proved successful if inflammation was experimentally triggered. These results provide firm evidence for the concept that host inflammation can be diverted by parasites and used as a weapon against pre-established competitors.
6. Conclusion
When we think of immune defences we usually have in mind the sophisticated and subtle capacity of T and B lymphocytes to recognize specific pathogen-derived peptides and build on a response with memory. Vertebrate ecological immunology has tackled the problem of the costs and benefits of immunity mostly from this perspective, leaving little room for the innate response (but see Råberg et al. 2002). This has been a mistake for several important reasons. First, the activation of innate immune effectors is essential for deployment of adaptive immunity. Most importantly, all else being equal, early defences always confer a better selective advantage compared to late defences (but see Hamilton et al. 2008). Innate inflammatory responses are usually expressed within minutes of the detection of the intruder. Selection pressures are, therefore, expected to act strongly on inflammation.
The two main features of the innate inflammatory response (fast and non-specific) open the way to potential devastating costs if inflammation gets out of control. Natural selection seems to have shaped an effective regulatory network that operates to resume inflammation once the danger signal has disappeared. In spite of this regulation, chronic inflammatory diseases are among the most widespread pathologies affecting human societies, in particular among elderly people. Is chronic inflammation the unavoidable price of effective innate protection? This is perhaps the case, especially for age-related inflammatory diseases. Benefits of protection in early life largely outweigh the cost paid during post-reproductive life. Genes that regulate the inflammatory response with antagonistic pleiotropic effects should therefore be selected for.
As for adaptive immunity, pathogens have evolved an amazing diversity of strategies to evade the inflammatory response. Similar to host resistance mechanisms, we should expect that parasite strategies that escape the immune response at earlier stages of the interaction should be favoured by selection. The first step during a host–parasite interaction is obviously recognition. Thus, strategies that avoid pathogen recognition by the host immune response should enjoy a selective advantage. In sharp contrast with this prediction, PAMPs are universally recognized by cell surface receptors. Although structural alteration of LPS is observed in some bacteria, this is a matter of amount of immune activation (some LPS are more immunogenic than others) but not of complete camouflage. Why is this so? This example illustrates how constraints limit the shape of possible adaptive landscapes. LPS and other PAMPs are molecules with vital function for bacteria. They can minimally change their structure but cannot live without them.
In addition to early evasion, selection should favour multiple escape tactics. Multiple strategies obviously limit the risk of failure and minimize the chance that hosts have evolved the appropriate counterattack. A few pathogens have, indeed, evolved a sequential array of defence mechanisms against inflammation and immunological oxidative stress starting from minimal activation to DNA repair. Evolutionary biologists have mastered the concept that hosts and parasites are involved in a never-ending arms race. Whereas constraints can set a limit to the possible outcome of the interactions, it is clear that we are far from a complete picture of the strategies of immune protection and immune escape. For instance, the recent finding that the pathogens can divert host inflammatory response as a weapon against commensal intestinal bacteria opens a new area of investigation on immune-mediated parasite competition.
Acknowledgments
This work has been supported by the ANR, NT05-2-45491.
Footnotes
One contribution of 11 to a Theme Issue ‘Ecological immunology’.
References
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. doi:10.1016/j.cell.2006.02.015 [DOI] [PubMed] [Google Scholar]
- Alonso-Alvarez C., Bertrand S., Devevey G., Prost J., Faivre B., Chastel O., Sorci G. An experimental manipulation of life-history trajectories and resistance to oxidative stress. Evolution. 2006;60:1913–1924. doi:10.1554/05-644.1 [PubMed] [Google Scholar]
- Ames B.N., Swirsky Gold L., Willett W.C. The causes and prevention of cancer. Proc. Natl Acad. Sci. USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. doi:10.1073/pnas.92.12.5258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annane D., Bellissant E., Cavaillon J.-M. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. doi:10.1016/S0140-6736(04)17667-8 [DOI] [PubMed] [Google Scholar]
- Anstey N.M., et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 1996;184:557–567. doi: 10.1084/jem.184.2.557. doi:10.1084/jem.184.2.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong P.B., Quikley J.P. α2-Macroglobulin: an evolutionarily conserved arm of the innate immune system. Dev. Comp. Immunol. 1999;23:375–390. doi: 10.1016/s0145-305x(99)00018-x. doi:10.1016/S0145-305X(99)00018-X [DOI] [PubMed] [Google Scholar]
- Baldari C.T., Lanzavecchia A., Telford J.L. Immune subversion by Helicobater pylori. Trends Immunol. 2005;26:199–207. doi: 10.1016/j.it.2005.01.007. doi:10.1016/j.it.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Bayne C.J. Origins and evolutionary relationships between the innate and adaptive arms of immune systems. Integr. Comp. Biol. 2003;43:293–299. doi: 10.1093/icb/43.2.293. doi:10.1093/icb/43.2.293 [DOI] [PubMed] [Google Scholar]
- Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 2007;7:875–888. doi: 10.1038/nri2189. doi:10.1038/nri2189 [DOI] [PubMed] [Google Scholar]
- Bergstrom C.T., Antia R. How do adaptive immune systems control pathogens while avoiding autoimmunity? Trends Ecol. Evol. 2006;21:22–28. doi: 10.1016/j.tree.2005.11.008. doi:10.1016/j.tree.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Bertrand S., Criscuolo F., Faivre B., Sorci G. Immune activation increases the susceptibility to oxidative tissue damage in zebra finches. Funct. Ecol. 2006;20:1022–1027. doi:10.1111/j.1365-2435.2006.01191.x [Google Scholar]
- Bogdan C., Röllinghoff M., Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. doi:10.1016/S0952-7915(99)00052-7 [DOI] [PubMed] [Google Scholar]
- Brown S.P., LeChat L., Taddei F. Evolution of virulence: triggering host inflammation allows invading pathogens to exclude competitors. Ecol. Lett. 2008;11:44–51. doi: 10.1111/j.1461-0248.2007.01125.x. doi:10.1111/j.1461-0248.2007.01125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulet P., Stöcklin R., Menin L. Anti-microbial peptides: from invertebrates to vertebrates. Immunol. Rev. 2004;198:169–184. doi: 10.1111/j.0105-2896.2004.0124.x. doi:10.1111/j.0105-2896.2004.0124.x [DOI] [PubMed] [Google Scholar]
- Caruso C., Candore G., Colonna-Romano G., Lio D., Franceschi C. Inflammation and life-span. Science. 2005;307:208. doi: 10.1126/science.307.5707.208. doi:10.1126/science.307.5707.208 [DOI] [PubMed] [Google Scholar]
- Cooper M.D., Alder M.N. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. doi:10.1016/j.cell.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Cosgrove K., Coutts G., Jonsson I.M., Tarkowski A., Kokai-kun J.F., Mond J.J., Foster S.J. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence and nasal colonization in Staphylococcus aureus. J. Bacteriol. 2007;189:1025–1035. doi: 10.1128/JB.01524-06. doi:10.1128/JB.01524-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. doi:10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins C.E., Finch E.M. Infection, inflammation, height, and longevity. Proc. Natl Acad. Sci. USA. 2006;103:498–503. doi: 10.1073/pnas.0501470103. doi:10.1073/pnas.0501470103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis I.C., Zajac A.J., Nolte K.B., Botten J., Hjelle B., Matalon S. Elevated generation of reactive oxygen/nitrogen species in hantavirus cardiopulmonary syndrome. J. Virol. 2002;76:8347–8359. doi: 10.1128/JVI.76.16.8347-8359.2002. doi:10.1128/JVI.76.16.8347-8359.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote M.A., et al. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl Acad. Sci. USA. 1997;94:13 997–14 001. doi: 10.1073/pnas.94.25.13997. doi:10.1073/pnas.94.25.13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzo A.M., Platz E.A., Sutcliffe S., Xu J., Grönberg H., Drake C.G., Nakai Y., Isaacs W.B., Nelson W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer. 2008;7:256–269. doi: 10.1038/nrc2090. doi:10.1038/nrc2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre B., Grégoire A., Préault M., Cézilly F., Sorci G. Immune activation rapidly mirrored in a secondary sexual trait. Science. 2003;300:103. doi: 10.1126/science.1081802. doi:10.1126/science.1081802 [DOI] [PubMed] [Google Scholar]
- Fang F.C. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. doi:10.1038/nrmicro1004 [DOI] [PubMed] [Google Scholar]
- Finch C.E., Crimmins E.M. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. doi:10.1126/science.1092556 [DOI] [PubMed] [Google Scholar]
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. doi:10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Finlay B.B., McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;24:767–782. doi: 10.1016/j.cell.2006.01.034. doi:10.1016/j.cell.2006.01.034 [DOI] [PubMed] [Google Scholar]
- Garsin D.A., Villanueva J.M., Begun J., Kim D.H., Sifri C.D., Calderwood S.B., Ruvkun G., Ausubel F.M. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. doi:10.1126/science.1080147 [DOI] [PubMed] [Google Scholar]
- Giunta S. Is inflammaging an auto[innate]immunity subclinical syndrome? Immun. Ageing. 2006;3:12–13. doi: 10.1186/1742-4933-3-12. doi:10.1186/1742-4933-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert A.P., McGee D.J., Akhtar M., Mendz G.L., Newton J.C., Cheng Y., Mobley H.L.T., Wilson K.T. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc. Natl Acad. Sci. USA. 2001;98:13 844–13 849. doi: 10.1073/pnas.241443798. doi:10.1073/pnas.241443798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.L., Allen J.E., Read A.F. Evolutionary causes and consequences of immunopathology. Annu. Rev. Ecol. Evol. Syst. 2005;36:373–397. doi:10.1146/annurev.ecolsys.36.102003.152622 [Google Scholar]
- Halliwell B. Phagocyte-derived reactive species: salvation or suicide? Trends Biochem. Sci. 2006;31:509–515. doi: 10.1016/j.tibs.2006.07.005. doi:10.1016/j.tibs.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Hamilton R., Siva-Jothy M., Boots M. Two arms are better than one: parasite variation leads to combined inducible and constitutive innate immune responses. Proc. R. Soc. B. 2008;275:937–945. doi: 10.1098/rspb.2007.1574. doi:10.1098/rspb.2007.1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;2:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hobbs M.R., et al. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet. 2002;360:1468–1475. doi: 10.1016/S0140-6736(02)11474-7. doi:10.1016/S0140-6736(02)11474-7 [DOI] [PubMed] [Google Scholar]
- Hollyfield J.G., Bonilha V.L., Rayborn M.E., Yang X., Shadrach K.G., Lu L., Ufret R.L., Salomon R.G., Perez V.L. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008;14:194–198. doi: 10.1038/nm1709. doi:10.1038/nm1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornef M.W., Wick M.J., Rhen M., Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat. Immunol. 2002;3:1033–1040. doi: 10.1038/ni1102-1033. doi:10.1038/ni1102-1033 [DOI] [PubMed] [Google Scholar]
- Imlay J.A., Linn S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J. Bacteriol. 1986;166:519–527. doi: 10.1128/jb.166.2.519-527.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. doi:10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Scofield V.L., Yan M., Qiang W., Liu N., Reid A.J., Lynn W.S., Wong P.K.Y. Retrovirus-induced oxidative stress with neuroimmunodegeneration is suppressed by antioxidant treatment with a refined monosodium α-luminol (Galavit) J. Virol. 2006;80:4557–4569. doi: 10.1128/JVI.80.9.4557-4569.2006. doi:10.1128/JVI.80.9.4557-4569.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.A., et al. A lethal role for lipid a in Salmonella infections. Mol. Microbiol. 1998;29:571–579. doi: 10.1046/j.1365-2958.1998.00952.x. doi:10.1046/j.1365-2958.1998.00952.x [DOI] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. doi:10.1038/ni1428 [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B.L. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. doi:10.1038/270301a0 [DOI] [PubMed] [Google Scholar]
- Kun J.F., Mordmüller B., Perkins D.J., May J., Mercereau-Puijalon O., Alpers M., Weinberg J.B., Kremsnen P.G. Nitric oxide synthase 2Lambaréné (G-954C), increased nitric oxide production, and protection against malaria. J. Infect. Dis. 2001;184:330–336. doi: 10.1086/322037. doi:10.1086/322037 [DOI] [PubMed] [Google Scholar]
- Levesque M.C., et al. Nitric oxide synthase type 2 promoter polymorphisms, nitric oxide production, and disease severity in Tanzanian children with malaria. J. Infect. Dis. 1999;180:1994–2002. doi: 10.1086/315140. doi:10.1086/315140 [DOI] [PubMed] [Google Scholar]
- Libert S., Chao Y., Chu X., Pletcher S.D. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFκB signaling. Aging Cell. 2006;5:533–543. doi: 10.1111/j.1474-9726.2006.00251.x. doi:10.1111/j.1474-9726.2006.00251.x [DOI] [PubMed] [Google Scholar]
- Licastro F., Candore G., Lio D., Porcellini E., Colonna-Romano G., Franceschi C., Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun. Ageing. 2005;2:8–21. doi: 10.1186/1742-4933-2-8. doi:10.1186/1742-4933-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio D., Scola L., Crivello A., Colonna-Romano G., Candore G., Bonafé M., Cavallone L., Franceschi C., Caruso C. Gender-specific association between-1082 IL-10promoter polymorphism and longevity. Genes Immun. 2002;3:30–33. doi: 10.1038/sj.gene.6363827. doi:10.1038/sj.gene.6363827 [DOI] [PubMed] [Google Scholar]
- Liu G.Y., Essex A., Buchanan J.T., Datta V., Hoffman H.M., Bastian J.F., Fierer J., Nizet V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005;202:209–215. doi: 10.1084/jem.20050846. doi:10.1084/jem.20050846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano G.A. Carotenoids, parasites, and sexual selection. Oikos. 1994;70:309–311. doi:10.2307/3545643 [Google Scholar]
- Mackintosh C.L., Beeson J.G., Marsh K. Clinical features and pathogenesis of severe malaria. Trends Parasitol. 2004;20:597–603. doi: 10.1016/j.pt.2004.09.006. doi:10.1016/j.pt.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Maizels R.M., Balic A., Gomez-Escobar N., Nair M., Taylor M.D., Allen J.E. Helminth parasites: masters of regulation. Immunol. Rev. 2004;201:89–115. doi: 10.1111/j.0105-2896.2004.00191.x. doi:10.1111/j.0105-2896.2004.00191.x [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Vazquez-Torres A., Fang F.C., Xu Y., Khan S., Hormaeche C.E., Dougan G. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 2000;192:237–247. doi: 10.1084/jem.192.2.237. doi:10.1084/jem.192.2.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A., et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol. 2005;6:587–592. doi: 10.1038/ni1200. doi:10.1038/ni1200 [DOI] [PubMed] [Google Scholar]
- McGonagle D., McDermott M.F. A proposed classification of the immunological diseases. PLoS Med. 2006;3:1242–1248. doi: 10.1371/journal.pmed.0030297. doi:10.1371/journal.pmed.0030297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. doi:10.1016/S0092-8674(00)80412-2 [DOI] [PubMed] [Google Scholar]
- Nathan C., Shiloh M.U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl Acad. Sci. USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. doi:10.1073/pnas.97.16.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L., Alto N.M., Dixon J.E. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr. Opin. Microbiol. 2005;8:21–27. doi: 10.1016/j.mib.2004.12.014. doi:10.1016/j.mib.2004.12.014 [DOI] [PubMed] [Google Scholar]
- Ogawa M., Sasakawa C. Intracellular survival of Shigella. Cell. Microbiol. 2006;8:177–184. doi: 10.1111/j.1462-5822.2005.00652.x. doi:10.1111/j.1462-5822.2005.00652.x [DOI] [PubMed] [Google Scholar]
- Omer F.M., Brian de Souza J., Riley E.M. Differential induction of TGF-β regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J. Immunol. 2003;171:5430–5436. doi: 10.4049/jimmunol.171.10.5430. [DOI] [PubMed] [Google Scholar]
- Pace G.W., Leaf C.D. The role of oxidative stress in HIV disease. Free Radic. Biol. Med. 1995;19:523–528. doi: 10.1016/0891-5849(95)00047-2. doi:10.1016/0891-5849(95)00047-2 [DOI] [PubMed] [Google Scholar]
- Pletcher S.D., Kabil H., Partridge L. Chemical complexity and the genetics of aging. Annu. Rev. Ecol. Evol. Syst. 2007;38:299–326. doi: 10.1146/annurev.ecolsys.38.091206.095634. doi:10.1146/annurev.ecolsys.38.091206.095634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W., et al. Activation of transcription factor Nrf-2 and its downstream targets in response to Moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J. Virol. 2004;78:11 926–11 938. doi: 10.1128/JVI.78.21.11926-11938.2004. doi:10.1128/JVI.78.21.11926-11938.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W., et al. Astrocytes survive chronic infection and cytopathic effects of retrovirus MoMuLV-ts1 by restoring thiol redox status via upregulation of antioxidant defenses. J. Virol. 2006;80:3273–3284. doi: 10.1128/JVI.80.7.3273-3284.2006. doi:10.1128/JVI.80.7.3273-3284.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L., Grahn M., Hasselquist D., Svensson E. On the adaptive significance of stress induced immunosuppression. Proc. R. Soc. B. 1998;265:1637–1641. doi: 10.1098/rspb.1998.0482. doi:10.1098/rspb.1998.0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L., Vestberg M., Hasselquist D., Holmdahl R., Svensson E., Nilsson J.A. Basal metabolic rate and the evolution of the adaptive immune system. Proc. R. Soc. B. 2002;269:817–821. doi: 10.1098/rspb.2001.1953. doi:10.1098/rspb.2001.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes G., Beschin A., Hassanzadeh Ghassabeh G., De Baetselier P. Alternatively activated macrophages in protozoan infections. Curr. Opin. Immunol. 2007;19:454–459. doi: 10.1016/j.coi.2007.05.007. doi:10.1016/j.coi.2007.05.007 [DOI] [PubMed] [Google Scholar]
- Rajan T.V., Porte P., Yates J.A., Keefer L., Shultz L.D. Role of nitric oxide in host defense against an extracellular, metazoanparasite, Brugia malayi. Infect. Immun. 1996;64:3351–3353. doi: 10.1128/iai.64.8.3351-3353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath S., Ghazal P., Gascoigne N.R.J. Hijacking and exploitation of IL-10 by intracellular parasites. Trends Microbiol. 2001;9:86–92. doi: 10.1016/s0966-842x(00)01919-3. doi:10.1016/S0966-842X(00)01919-3 [DOI] [PubMed] [Google Scholar]
- Rhen M., Eriksson S., Pettersson S. Bacterial adaptation to host innate immunity responses. Curr. Opin. Microbiol. 2000;3:60–64. doi: 10.1016/s1369-5274(99)00052-1. doi:10.1016/S1369-5274(99)00052-1 [DOI] [PubMed] [Google Scholar]
- Sacks D., Sher A. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 2002;3:1041–1047. doi: 10.1038/ni1102-1041. doi:10.1038/ni1102-1041 [DOI] [PubMed] [Google Scholar]
- Sarkar D., Fisher P.B. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. doi:10.1016/j.canlet.2005.04.009 [DOI] [PubMed] [Google Scholar]
- Sebbane F., Lemaıˆtre N., Sturdevant D.E., Rebeil R., Virtaneva K., Porcella S.F., Hinnebusch J. Adaptive response of Yersinia pestis to extracellular effectors of innate immunity during bubonic plague. Proc. Natl Acad. Sci. USA. 2006;103:11 766–11 771. doi: 10.1073/pnas.0601182103. doi:10.1073/pnas.0601182103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. ASM Press; Washington, DC: 2001. Immunology, immunopathology, and immunity. [Google Scholar]
- Shiloh M.U., MacMicking J.D., Nicholson S., Brause J.E., Potter S., Marino M., Fang F., Dinauer M., Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. doi:10.1016/S1074-7613(00)80004-7 [DOI] [PubMed] [Google Scholar]
- Stecher B., et al. Salmonella enterica Serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. doi:10.1371/journal.pbio.0050244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle E.J., Metcalfe D.D. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory process. Immunol. Rev. 2007;217:185–205. doi: 10.1111/j.1600-065X.2007.00513.x. doi:10.1111/j.1600-065X.2007.00513.x [DOI] [PubMed] [Google Scholar]
- Titheradge M.A. Nitric oxide in septic shock. Biochim. Biophys. Acta. 1999;1411:437–455. doi: 10.1016/s0005-2728(99)00031-6. doi:10.1016/S0005-2728(99)00031-6 [DOI] [PubMed] [Google Scholar]
- van den Biggelaar A.H.J., Huizinga T.W.J., de Craen A.J.M., Gussekloo J., Heijmans B.T., Frölich M., Westendorp R.G.J. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp. Gerontol. 2004;39:1407–1414. doi: 10.1016/j.exger.2004.06.009. doi:10.1016/j.exger.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A., Fang F.C. Oxygen-dependent anti-Salmonella activity of macrophages. Trends Microbiol. 2001;9:29–33. doi: 10.1016/s0966-842x(00)01897-7. doi:10.1016/S0966-842X(00)01897-7 [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A., et al. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–808. doi: 10.1038/44593. doi:10.1038/44593 [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A., Xu Y., Jones-Carson J., Holden D.W., Lucia S.C., Dinauer M.C., Mastroeni P., Fang F.C. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. doi:10.1126/science.287.5458.1655 [DOI] [PubMed] [Google Scholar]
- von Schantz T., Bensch S., Grahn M., Hasselquist D., Wittzell H. Good genes, oxidative stress and condition-dependent sexual signals. Proc. R. Soc. B. 1999;266:1–12. doi: 10.1098/rspb.1999.0597. doi:10.1098/rspb.1999.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G.C. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. doi:10.2307/2406060 [Google Scholar]
- Young D., Hussel T., Dougan G. Chronic bacterial infections: living with unwanted guests. Nat. Immunol. 2002;3:1026–1032. doi: 10.1038/ni1102-1026. doi:10.1038/ni1102-1026 [DOI] [PubMed] [Google Scholar]
- Zampieri C.A., Sullivan N.J., Nabel G.J. Immunopathology of highly virulent pathogens: insights from Ebola virus. Nat. Immunol. 2007;8:1159–1164. doi: 10.1038/ni1519. doi:10.1038/ni1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J.C., Lin C., Lin D., Wogan G.N. Mutagenesis associated with nitric oxide production in macrophages. Proc. Natl Acad. Sci. USA. 1998;95:8286–8291. doi: 10.1073/pnas.95.14.8286. doi:10.1073/pnas.95.14.8286 [DOI] [PMC free article] [PubMed] [Google Scholar]