Abstract
We compare anti-parasite defences at the level of multicellular organisms and insect societies, and find that selection by parasites at these two organisational levels is often very similar and has created a number of parallel evolutionary solutions in the host's immune response. The defence mechanisms of both individuals and insect colonies start with border defences to prevent parasite intake and are followed by soma defences that prevent the establishment and spread of the parasite between the body's cells or the social insect workers. Lastly, germ line defences are employed to inhibit infection of the reproductive tissue of organisms or the reproductive individuals in colonies. We further find sophisticated self/non-self-recognition systems operating at both levels, which appear to be vital in maintaining the integrity of the body or colony as a reproductive entity. We then expand on the regulation of immune responses and end with a contemplation of how evolution may shape the different immune components, both within and between levels. The aim of this review is to highlight common evolutionary principles acting in disease defence at the level of both individual organisms and societies, thereby linking the fields of physiological and ecological immunology.
Keywords: physiological immune systems, evolutionary immunology, hygienic behaviour, self/non-self-recognition, levels of selection, immune regulation
1. Introduction
A comparison of immune defence at the level of the individual or society may help to uncover common evolutionary principles affecting the selection pressures imposed by parasites and the solutions by hosts to counteract infection. Both individual and society need to quickly detect and appropriately react to an invading parasite, to prevent the spread of the invader between host cells or group members, and to protect the most valuable cells or individuals, the germ line or the reproductive individuals, from infection. When performing such a multi-level analysis, it is inevitable that generalizations are made, but we feel that avoiding mechanistic detail is necessary to highlight the underlying principles. We stress that we only report phenomenological analogies in defence strategies, and do not make any claims that the underlying mechanisms might even be related—in most cases they will be strikingly different. Despite these simplifications, we hope that our comparative approach will help both immunologists and evolutionary biologists to extract common principles of immune defence at different organisational levels (individual or society), which share the fact that they are ‘reproductive entities’. It may further be used as a guideline to explore either organisational level for the expression of specific defence modes that hitherto are only described for the other.
2. Terminology
Owing to the interdisciplinary character of this review, the same terms may not have the same a priori meaning for all readers. Typically, evolutionary biologists tend to describe observed phenomena, while immunologists may use the same term to refer to a physiological mechanism leading to this phenomenon. These different traditions and different levels of current knowledge in the respective fields have caused frequent misunderstandings and debates, not only between immunologists and evolutionary biologists, but even between vertebrate and invertebrate immunologists. We would therefore like to stress that we use the respective terms only to describe immunological outcomes and do not make any implications on specific underlying mechanisms.
Moreover, we use most terms in a broad sense; for example, when referring to parasites this comprises any type of organism entering the host and causing harm, typically bacteria, fungi, viruses, but also protozoa and multicellular parasites such as worms (helminths). We even include examples from parasitoids, i.e. species that enter the host and ultimately kill (and often consume) it. In addition to the parasites that harm single individuals, there are also specialized parasites of social insect colonies, which force their way into the nest and may then either feed on the brood, such as butterfly larvae, or kill the queen and start producing offspring instead of her, the so called ‘social parasites’ (Schmid-Hempel 1998).
When employing the term individual immunity we include all anti-parasite protection at the level of a (multicellular) organism, achieved by the combination of its hygienic behaviours (e.g. parasite avoidance strategies) and its physiological immune system. When describing physiological immunity, which comprises most of our analogies, we focus on the innate immune systems known from invertebrates such as insects and crustaceans on one hand, and on the innate and acquired systems of vertebrates. We refer mostly to the well-studied jawed vertebrates including mammals such as mice and humans, but also to the immune systems of the jawless lampreys or hagfish that have evolved a parallel system to the jawed vertebrates (Pancer & Cooper 2006; Amemiya et al. 2007). We use the term acquired immunity for the acquired immune functions in vertebrates, achieved by B and T lymphocytes, which in physiological immunology are typically referred to as the adaptive immune component.
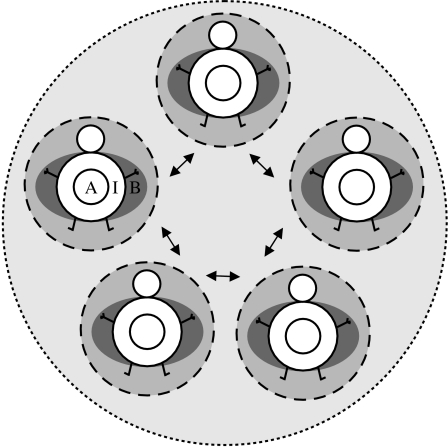
In contrast to individual immunity, social immunity describes colony-level anti-parasite protection, achieved by the cooperation of all group members, collectively avoiding, controlling or eliminating parasitic infections. It lies in the nature of these defences that they cannot be performed efficiently by single individuals, but depend strictly on the cooperation of at least two individuals. Similar to individual defences, social immunity is characterized by both hygienic behaviour and physiological defences, but also has a third major component: spatial organization and contact frequency regulation (Schmid-Hempel 1998; Cremer et al. 2007). As examples of social immunity, we focus on current data for the colonies of social insects—the social bees and wasps, and especially the ants and termites that have evolved large and complex societies. It is important to note that an individual member of any society can perform both individual defences (when alone) and collective defences (when interacting with its group members), and at both levels, the mechanisms of defence can be either based on behaviour or physiology (figure 1). Thus, in social organisms, selection for immunity acts simultaneously on both levels (individual and society), potentially encompassing complex interactions and different selective constraints.
Figure 1.
Immune modules. The collective defence (pale grey, dotted line) of a group comprises all individual defences (medium grey, dashed line) of the group members and their interaction (arrows). Individual defences are composed of anti-parasite behaviours (B, dark grey ellipse) and physiological immune systems that can contain either only the innate (I) immune component (e.g. invertebrates) or also the acquired (A) immune component (e.g. vertebrates). The single immune modules interact with each other during anti-parasite defence and thus shape each other's evolution.
3. The insect society as a ‘superorganism’
While human and primate societies also have elaborate collective disease defence strategies (Nunn & Altizer 2006), we want to focus on the societies of eusocial insects, as they are most similar to a multicellular organism in that they represent a single reproductive unit. They are characterized by an obligate reproductive division of labour with one or several individuals (the queens and males) capitalizing reproduction, while all other colony members are sterile workers (Hölldobler & Wilson 1990; Bourke & Franks 1995). Both castes are highly interdependent, as workers cannot reproduce themselves but rear their sisters and brothers to adulthood, while the queens are highly specialized, often nearly immobile, ‘egg-laying machines’ that need the workers to provide food and defend the colony. In this aspect, the reproductive individuals of a social insect colony resemble the germ line of a multicellular organism, while workers are similar to cells of the somatic tissue. In addition to the reproductive division of labour between sexual individuals and workers, there is even a division of labour within the worker caste, with groups of individuals specialising on different tasks, thus forming organ-like entities. This analogy between social insect colonies and multicellular organisms had already been drawn in the early twentieth century, when insect colonies were referred to as ‘superorganisms’ (Wheeler 1911).
As with many analogies, this comparison might be more useful in some contexts than others, and especially the general validity of the superorganism idea has been vigorously disputed in the past (for a discussion of the pros and cons of the use of this term, see Bourke & Franks (1995) and references therein). While the cells of a body are all descendants of the inseminated egg cell through cell division, the workers of an insect colony are the offspring of one or several mother queens, each having mated with one or several males. With some rare exceptions (e.g. Heinze & Hölldobler 1995), queens produce their offspring via sexual reproduction, leading to an inherently larger difference between the workers of a social insect colony than between the cells in a body (even if non-heritable somatic diversification can increase the diversity of the soma in both organisms and superorganisms). This higher heritable diversity in the colonies' soma leads to a higher conflict potential between individuals of a superorganism than between the cells of an organism (Keller & Reeve 1999). Despite these differences, we consider the superorganism concept a helpful construct to emphasize that—similar to a body—only the colony as a whole, but not any single individual, can produce offspring, which is the fitness-relevant currency in evolution.
4. Life histories of multicellular organisms and superorganisms
Insect societies have a life history remarkably similar to that of individual organisms, especially that of long-lived vertebrates. Other than may be expected from the relatively short lifespan of individual workers, ranging from several weeks to 3 to 4 years, insect colonies as a whole can reach enormous ages of up to several decades, limited only by the lifespan of the queens—individual ant queens were found to live for up to 30 years (Keller & Genoud 1997; L. Sundström 2008, personal communication). The age of the queens' mates is only of importance in termites, as in bees, wasps and ants the males die during or immediately after insemination, and survive only as sperm stored and preserved in the queen's body. In addition, especially the large insect societies of ants and termites are characterized by a growth phase of several months to years, during which exclusively sterile workers are produced. Only after a species-specific worker force has been built up does the colony reach ‘sexual maturity’ and start producing daughter colonies via second generation queens and males (Hölldobler & Wilson 1990).
This particular life history of social insect colonies promotes strong cooperation between workers and sexuals on one hand and between queens and their mates on the other (Schrempf et al. 2005). It also suggests that not only long-lived organisms with a reproductive phase late in life, but also long-lived ant and termite colonies, should invest greatly in anti-parasite defence to survive the growth phase and enter the reproductive phase. Short-lived species with an early-in-life reproduction, on the other hand, may invest less in immunity and react to infection by shifting the onset of reproduction to an even earlier point in time (Hochberg et al. 1992; Forbes 1993). This life-history strategy was also described for short-lived (single season) societies of bumblebees (Moret & Schmid-Hempel 2004).
In addition to these diverse strategies of immune investment between groups of social insects depending on their life history (Boomsma et al. 2005), one also finds differences within species and even between castes within a colony. Short-lived ant males, for example, show lower immune responses than the longer-lived workers and queens (Vainio et al. 2004; Baer et al. 2005). Similarly, within a vertebrate body, not all organs have the same level of immune protection. Critical compartments such as the germ line and the post-mitotic central nervous system are shielded from the circulation by specialized barriers in order to protect them from systemic threats (Janeway et al. 2001).
5. Individual and social anti-parasite defence mechanisms
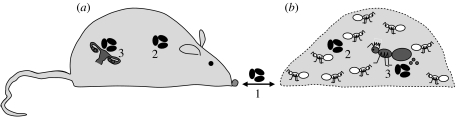
In the following, we will parallel anti-parasite defence mechanisms of multicellular organisms and insect societies, and identify similar evolutionary solutions against parasite threats at the individual and colony level. Following the scheme in figure 2, we will differentiate between three steps of parasite invasion and host defence: (i) border defence to avoid parasite intake, (ii) soma defence to oppose establishment and multiplication of the parasite in the host somatic tissue or worker force and (iii) germ line defence to prevent an infection of reproductive tissues or individuals, thereby protecting the daughter generation. For all three lines of defence, we will review first individual and then social defences, and will then extract the common evolutionary principles.
Figure 2.
(a) Steps of infection and anti-parasite defence of individual organisms and (b) insect societies. (1) Border defence: parasites (black ovals) are prevented from entering the body or colony by avoidance behaviour and consolidated physiological defences in the epithelia or at nest entrances. (2) Soma defence: immune defence acts to prevent infection of somatic tissue (cells in the body and sterile workers in insect colonies). (3) Germ line and offspring defence: the reproductive tissue (ovaries) of organisms and the reproductive individuals (queens) of insect colonies are subject to specific immune privileges to prevent offspring infection.
(a) Border defence
Hosts can reduce the probability of contracting an infection by avoiding parasite-rich areas and by preventing parasite intake by either ingestion or other entrance routes of the parasite into the body or colony. This border defence is characterized by an interplay of behavioural and physiological defence mechanisms that differ in their specificity (Schmid-Hempel & Ebert 2003). One mostly finds prophylactic hygiene measures, but also danger-dependent upregulation.
(i) Individual disease avoidance and hygienic behaviour
As we know from our own ‘intuitive microbiology’ and feelings of disgust against, mouldy food or excrements (Curtis 2007), for example, humans and animals often preventively avoid close contact to potential sources of infection such as self-produced litter or infected conspecifics (Moore 2002). The importance of such avoidance behaviour becomes obvious in humans with anosmia, the inability to smell, who suffer overproportionally from food poisoning (Schiffman 1997). Individuals typically show specific parasite-avoidance strategies. For example, when individual foraging ants detect phorid flies hovering in the air—parasitoids that lay their eggs into their hosts that are later decapitated by the emerging offspring—they immediately hide under stones, only continuing to forage when the fly is gone and the danger of being parasitized is over (Orr et al. 1995). Also their choice of food items is regulated by parasite presence: ants typically feed on carcasses, but despise parasite-infected corpses (Marikovsky 1962; Zhou et al. 2002).
Vomiting is a typical reaction by mammals to ingestion of contaminated material (Nesse & Williams 1994). Despite its potential clinical relevance, it is not well investigated if such a response is a useful self-decontamination act of the host, manipulation by the parasite to promote its spreading or just a meaningless by-product (Ewald 1994). As with most adaptive behaviour, avoidance reactions are subject to learning and conditioning: a specific meal or drink that caused disease due to contamination often results in life-long disgust by association (Garb & Stunkard 1974; Harris & Ross 1987). Such reactions and the degrees to which parasites are avoided are very flexible and do not depend only on the parasite pressure, but also on the status of the individuals, e.g. its infection status, as shown for sheep (see Hughes & Cremer (2007) and references therein), or reproductive status. During early pregnancy, when foetal development is especially sensitive to environmental toxins, hypersensitivity and enhanced disgust are common (Profet 1988).
Another defence against parasite infections is ‘self-medication’, i.e. the collection and ingestion or application of antimicrobial substances, such as plant secondary compounds (Lozano 1998; Moore 2002, and references therein). For example, monkeys rub toxic centipedes onto their skin and some birds pick up ants, squeeze them and apply the formic acid to their plumage or even take regular baths in ant hives. Some animals such as elephants take regular baths, while other species, such as dogs and cats and also ants, constantly inspect and clean their body surface by licking or grooming.
(ii) Individual physiological border defence
Not only do animals collect and apply substances with antimicrobial properties, they also may produce them themselves in glands, as is found in ants that have evolved a specific gland with a large opening to the body surface (Brown 1968). Physiological and behavioural defences are often tightly linked, e.g. the antimicrobial gland secretions are spread over the body by leg movements. The intensity of this spreading behaviour is flexible and is upregulated upon parasite challenge (Fernández-Marín et al. 2006). Moreover, infectious particles that are groomed off by the ants from their body surface are filtered out in the mouth cavity not to enter the gut, and killed off by labial gland substances (Kermarrec et al. 1986; Jaccoud et al. 1999).
The skin itself forms a physical barrier against parasite entry, and the thickness and degree of sclerotisation of the cuticle have been shown to correlate with resistance against parasites in insects (Armitage & Siva-Jothy 2005). In vertebrates, epithelial surfaces are not only tightly sealed by junctional complexes but often use cilia as transport systems that drain internal cavities. Epithelia are rich in antimicrobial peptides and mucosal immunoglobulins that specifically tag, immobilize and eliminate pathogens. Exposed and sensitive areas such as mucosal surfaces often create hostile environments for parasites by low pH and secretion of antimicrobial milieus as found in saliva and mucus. Most epithelia are densely seeded with resident immune cells (mast cells, specialized lymphocytes, macrophages and dendritic cells) that are in place to phagocytose parasites or kill them indirectly via the secretion of soluble factors (Medzhitov & Janeway 1998, 2000). Every serious insult of the integument further triggers leakage of antimicrobial plasma proteins from the blood vessels into the tissue, subsequent infiltration of additional immune cells and finally a wound healing response that reseals the surface (von Andrian & Mackay 2000). Also in invertebrates, haemolymph flushes out of the body after wounding, likely sweeping out most of the penetrated parasites. This is followed by immediate wound clotting, during which remaining parasites are trapped in the local area, preventing their further distribution through the body (Bohn 1986; Gupta 1991; Theopold et al. 2002).
(iii) Collective disease avoidance and hygiene
Similar to the specialization of different maintenance tasks of a body by different organs, insect colonies are characterized by a strong division of labour, with most tasks (e.g. brood care and nest maintenance) being performed inside the nest. Only a few individuals—the foragers—leave the protected nest and later distribute the food to the intra-nest workers and the queen after having stored the food first in their ‘social stomach’, the crop (Hölldobler & Wilson 1990). This nutrient distribution throughout the colony resembles the transport of nutrients and oxygen within a body via the blood flow. As not every individual has to leave the clean and protected nest and may encounter parasites outside, the chance of colony infection is lowered by a ‘selfish herd effect’ (Hamilton 1987). The social organization of insect colonies in itself thus limits the risk of contracting an infection (Schmid-Hempel & Schmid-Hempel 1993; Cremer et al. 2007). It also seems adaptive that it is the old individuals leaving the nest to forage, while young workers stay in the centre of the colony tending the queen and rearing the brood.
How can individuals that have detected a parasite warn their group members so that they do not contract the disease? Upon exposure, termites perform a ‘pathogen alarm’, i.e. they produce vibrations that cause colony members to stay away (Rosengaus et al. 1999; Myles 2002). After ants encounter a passive parasite such as fungal spores in the vicinity of a nest entrance, they close this particular nest entrance so that their nestmates do not come into contact with the parasite (Diehl-Fleig & Lucchese 1991). When parasites enter the nest actively, as do the slave-making ants that break into the colony of closely related ant species, rob their brood and enslave their workers, the host colony tries to block the nest entrance. In some species, workers with specifically evolved square heads jointly and very efficiently plug the nest entrance (Hölldobler & Wilson 1990), similar to a wound clot.
Special care is also taken to prevent contaminated material from being brought into the nest. Leaf-cutting fungus-growing ants harvest many leaves per day and carry them into the colony. They have evolved a specific caste of tiny workers that hitchhike on the leaves that are carried back to the nest by their larger sisters. A recent study demonstrated that these small workers not only prevent flies laying their eggs into the large workers when busy during leaf transport, but also clean the leaves of fungal spores (Vieira-Neto et al. 2006). This selectivity of what or who can enter the nest may not always directly benefit the infected individuals. Honeybees have special bouncer individuals guarding the nest entrance, sometimes barring entrance to incoming infected individuals (Waddington & Rothenbuhler 1976; Drum & Rothenbuhler 1985). Being cast out from the society means the death of the infected individual, but may be a successful strategy at the colony level by preventing a disease outbreak with many more casualties (Cremer et al. 2007).
Not only do social insects carefully control individuals and material entering the colony, they moreover perform a similar ‘self-medication’ described for individual organisms. Ants and bees collect and enrich their nest with antimicrobial substances such as tree resin, which reduces bacterial and fungal growth and reduces infection of colony members (Gilliam et al. 1988; Christe et al. 2003; Chapuisat et al. 2007). They also impregnate their nests with self-produced antimicrobial substances from glands (Brown 1968; Turillazzi et al. 2006), and even rear bacteria on their body that help them produce antibiotics against parasites in the colonies (Currie et al. 1999).
(iv) Common principles in individual and social defence
Preventive defence measures against picking up a disease from the environment have evolved consistently at both the individual and the colony level, certainly because the cheapest defence against a parasite is not to contract it in the first place. Cooperation between individuals and warning systems may over-proportionally reduce infection risks due to the higher levels of vigilance achieved by several cooperating versus single individuals. Most hygienic measures involve behaviours, either acting alone or fine-tuning physiological responses. Behaviours are more flexible and can be quickly adjusted to both changes in the parasite pressure and the status of the individual or colony (e.g. life phases, reproductive status, etc). Physiological defences appear to be more important for soma and germ line defence than border defence.
(b) Soma defence
Once parasites enter the body or are able to ‘break the fortress’ of the social insect colony (Schmid-Hempel 1998), anti-parasite defence is restricted to reducing the damage caused by the parasite and to ensuring host survival by protecting the somatic tissue of the body, or the worker force of an insect colony, against infection. This is achieved by preventing the establishment of the parasite and its spread between the cells of the body or between the individuals of the colony by immediate local actions at the site of entry, and—if insufficient—followed by a systemic response involving the whole organism or colony.
(i) Individual soma defence
Individual immunity mounts responses at multiple levels to prevent systemic spreading of a parasite. This begins with a gradual extension of the local border defence strategies that have been described above and leads to systemic reactions that simultaneously affect most tissues. Every surface insult, no matter whether it is infectious or sterile, triggers the quick recruitment and local accumulation of innate immune cells (von Andrian & Mackay 2000). These cells can directly phagocytose the parasite or kill it by the local secretion of toxic substances (Stuart & Ezekowitz 2008). The basis of such responses is the reliable discrimination between invader and own tissue, which is described in §6.
If invaders cannot be directly removed but manage to persist locally, a common reaction of the local tissue is the formation of a granuloma that quarantines the parasite from the tissue. Here phagocytes often acquire epithelial characteristics and extracellular matrix deposition seals off the local infection. In some cases this can lead to local calcification, the most robust form of encapsulation in vertebrates, whereas invertebrates melanize the granuloma (Janeway et al. 2001; Kumar et al. 2003). Metazoans also have cell autonomous methods of preventing the spread of infection. Especially, intracellular pathogens are fought by creating an intracellular environment that is hostile for the parasite or prevents its replication. In the ultimate case, the cell sacrifices itself by undergoing programmed cell death. This can occur after the cell itself has sensed the pathogen but also after instruction by the acquired immune system. In any case, the remnants of self-destruction are again removed by phagocytes (Janeway et al. 2001).
Both vertebrates and invertebrates have protective systems that act at the systemic level and are mostly mediated by soluble factors. Invertebrates release cytotoxic substances and antimicrobial peptides into their haemolymph (Gillespie et al. 1997). The complement system of vertebrates consists of ubiquitously distributed factors that detect and attack the absence of self-structures while immunoglobulins selectively tag non-self-structures. Another example is the diverse family of acute phase proteins that are released into the circulation upon infection. They act at multiple levels and mostly create hostile environments for the parasite as exemplified by the depletion of available cofactors, such as iron, required for the parasite (Janeway et al. 2001). A similar effect is achieved by raising the body temperature to presumably non-parasite-friendly temperatures. While warm-blooded vertebrates can directly increase their body temperature, cold-blooded vertebrates such as reptiles and invertebrates show ‘behavioural fever’, in which they seek areas of higher temperature (Kluger et al. 1975).
(ii) Social soma defence
At the level of the colony, soma defence mechanisms are also employed both locally and by a systemic response mode. Similar to granuloma formation in individual organisms, insect colonies build small prisons around incoming parasites. These can consist either of workers in analogy to immune cells or of stiff material in parallel to the connective tissue encapsulation material. So far, these behaviours have only been described for the honeybee that quarantines the parasite from the colony by a layer of propolis (‘social encapsulation’; Neumann et al. 2001), or by surrounding them in a ‘ball’ of worker bees. Killing occurs through sweltering and suffocation, as the ‘ball bees’ produce heat and constrict the enclosed individual tightly inside the ball, preventing it from moving and effective breathing (Ono et al. 1995; Papachristoforou et al. 2007; Stabentheiner et al. 2007).
The reaction towards infected group members is best described as a ‘care-kill dichotomy’ (P. Schmid-Hempel 2007, personal communication). Infected individuals are often allogroomed intensively by their group members, which drastically increases their survival after parasite exposure (e.g. Hughes et al. 2002). On the other hand, infected workers may be sacrificed similarly to infected cells within an organism. To date, no clear case of ‘social apoptosis’ has been described, yet ‘natural killer insect workers’ that may kill infected group members do exist, as is described for termites that imprison infected individuals behind walls of faeces, which moreover have antimicrobial properties (Klein 1990; Rosengaus et al. 1998). Also the brood is regularly checked for infection. Specialized honeybee workers patrol the brood combs, uncap brood cells in which a larvae or pupae is detected to be parasitized, and exclude them from the colony (Spivak & Gilliam 1998a,b).
Nest hygiene of insect societies further involves disposal of infectious material such as garbage and dead nestmates that are brought to specific garbage dumps or funerals outside the nest. They are typically located at a considerable distance from, as well as downhill of the nest, to prevent flushing of the material back into the colony during rain (Howard & Tschinkel 1976; Hart & Ratnieks 2001). Honeybees also produce a systemic ‘social fever’. Simultaneous wing-fanning by many worker bees in the colony increases the hive temperature by several degrees, a defence that is employed against infections of the colony with fungal spores (Starks et al. 2000).
(iii) Common principles in individual and social defence
The first line of anti-parasite defence in the soma of host organisms and societies is to prevent spread of the parasite from the local site of infection to the whole body or colony. Most described soma defences are thus local defences such as granuloma production and social encapsulation. A second general principle seems to be that sacrificing cells or individuals can benefit the whole organism or group. It is possible that the modular organization of insect societies favours the sacrifice of infected individuals, as (i) they are not physically connected and can thus easily be removed, and (ii) a great number of workers are dispensable for the colony (Bourke & Franks 1995). Still, there are only very few reports of infected individuals being socially excluded or killed, while intensive care and allogrooming seem to be the preferred reactions under most parasite attacks (Cremer et al. 2007).
(c) Germ line defence
Border and soma defences per se do not ensure host fitness, as evolutionary success depends on the number of offspring or gene copies carried into the next generation. Individual organisms as well as social insect colonies have therefore evolved mechanisms for special protection of their reproductive tissues and individuals, the germ cells and queens (and kings), to ensure the production of healthy offspring. In both individuals and societies, the germ line is subject to specific immune privilege and the offspring are protected both before and after birth.
(i) Individual germ line defence
In vertebrates, the organs of the germ line are surrounded by a specific blood–ovary/testis barrier, as well as a high number of immune cells. This combination protects the reproductive tissues against infection and particularly against the effects of immunopathology (Fijak & Meinhardt 2006). The blood–ovary/testis barrier is much less intensively studied than the blood–brain barrier, for example, which is why many interesting details of germ line protection still await clarification. In mammals, immunoglobulins are transferred via the placenta to the embryo, the protective effect of which persists for several months after birth. Mothers even transfer mucosal immunoglobulins to their offspring after birth via their milk during the lactation phase (Janeway et al. 2001). A similar transgenerational protection occurs in bird eggs (Grindstaff et al. 2003), as well as in bumblebees, where immune-challenged mothers produce eggs with better protection than that of control mothers (Sadd & Schmid-Hempel 2007).
(ii) Social germ line defence
In social insect colonies, the queens (and kings) are typically found in the centre of the nest, in termites even within a hard-shelled royal chamber. They are exclusively tended by young individuals that have never left the protective nest and are therefore unlikely to be infected by parasites (‘centrifugal polyethism’; Schmid-Hempel & Schmid-Hempel 1993; Bourke & Franks 1995). In addition to this prophylactic defence through colony organization, contact rates are adjusted in the case of an infection. If queen-tending honeybee workers contract an infection, they stop caring for the queen and may change their behaviour to perform a task further away from the queen or even outside the colony (Wang & Moeller 1970; Schmid-Hempel 1998). In addition to the reproductive individuals themselves, the offspring are protected against infection, e.g. by spraying the brood with venom containing antimicrobial properties as is found in fire ants (Siebeneicher et al. 1992). Moreover, immune-challenged workers in bumblebee colonies were shown to raise sexual offspring with a higher constitutive immune response than sexual offspring reared in untreated control colonies (Moret & Schmid-Hempel 2001).
(iii) Common principles in individual and social defence
Germ line and offspring defence provides an effective protection of the next generation, and thus ensures the fitness of the mother generation. Inside organisms and superorganisms, prophylactic and induced measures protect the reproductive tissue or individuals, as well as the embryos. In addition, both also show a long-term protective effect that continues after birth. This transgenerational protection is often specific to parasites encountered by the mother during her lifetime, and which are likely still found in the habitat at the time when the offspring is born or young. Such an ‘inherited’ immune protection against local parasites is highly flexible and adaptive.
6. Parasite detection and self/non-self-discrimination
One important function of the soma is the detection and elimination of dangerous intruders and the reduction of the harm caused by a misguided immune response on the host tissue or individuals. How is efficient detection of intruders and distinction from own tissue or colony members achieved?
(a) Individual discrimination of parasites and self-tissue
Four interdependent recognition principles are used within a vertebrate body to distinguish a potentially dangerous invader from self-tissue: (i) detection of conserved parasite patterns by, e.g. pattern recognition receptors (exogenous alarm signals), (ii) detection of the presence of tissue damage (endogenous alarm signals), (iii) detection of the absence of self-structures (natural killer cells and the complement system), and (iv) detection of the presence of non-self-structures (acquired immune systems).
Recognition receptors of the acquired immune system are the most advanced development for identifying specific pathogens and therefore also serve best to discuss organization principles. The main feature of the cells of the acquired immune system is their somatically rearranged repertoire of T- and B-cell receptors which creates an almost unlimited variety of specificities. Such somatic diversification systems evolved in parallel in the immune systems of Agnatha and Gnathostomata (Pancer & Cooper 2006). As the emerging repertoire is unpredictable, every individual has to learn during ontogenesis how self looks, as in a process of quality control self-reactive cell clones are eliminated (Boehm 2006) and failure to do so results in severe autoimmunity (Mathis & Benoist 2004).
A perfect distinction between self and non-self appears useful at first sight. Self is an approximation to non-dangerous because self-antigens are usually not a threat (tumour-associated neoantigens might be a rare exception). Tolerating self and fighting everything that is different would be one way to defend the body against all possible invaders. However, such distinction is not practicable as non-self only rarely means dangerous. Examples of non-dangerous non-self-structures are commensals of the epithelial flora and food antigens that have to be tolerated and if mistakenly attacked lead to serious self-damage such as allergic reactions and other immunopathologies (Pamer 2007). To avoid such over-reactions, T cells never attack their antigen directly upon first recognition but are under the strict control of dendritic cells that present antigens on major histocompatibility complex (MHC) molecules. Dendritic cells interpret the context in which they took up the antigen and instruct the antigen-specific T cell to become either unresponsive, one of the various types of effector cells, or active silencers of other effector cells (Reis e Sousa 2006). Hence, dendritic cells are the decision makers that shape the immune response according to the presence or absence of endogenous or exogenous danger signals (Matzinger 2007). Only upon secondary (spatial and temporal) encounter with the same antigen can the T cell act autonomously and activate a quick systemic recall response (Janeway et al. 2001).
This ability to learn from previous experience was long considered a hallmark of acquired immune systems. However, recently, invertebrates have also been shown to exert specificity and memory upon secondary exposure to the same parasite, despite the absence of the cells of the acquired immune system (Kurtz & Franz 2003; Little et al. 2003; Sadd et al. 2005; Sadd & Schmid-Hempel 2006). Even T- and B-cell-depleted vertebrates were still able to show specific memory (O'Leary et al. 2006). To date, the degree of specificity in the invertebrate immune system is largely unexplored and the underlying mechanisms are not yet understood. It may be that alternative splicing of an immune molecule (Dscam) might mediate specificity in phagocytosis in invertebrates (Watson et al. 2005; Dong et al. 2006). Therefore, it is as yet unclear whether the enhanced immune response of invertebrates to the second exposure of the same parasite is due to enhanced sensitivity to damage caused by the parasite or specific antigen recognition as in the case of T cell-mediated immune memory in vertebrates.
(b) Social discrimination of parasites and colony members
Individual immune systems are thus characterized by high plasticity and a continuous and lifelong learning process of what the self and the non-dangerous template look like and how dangerous invaders can be identified. In a similar process, members of insect societies learn and constantly update their reference of self—the colony odour.
Whilst cells carry a repertoire of molecules on their outer membrane, the body surface of insects carries cuticular waxes consisting of hydrocarbons, which are non-volatile chemical ‘odour’ compounds. Cuticular hydrocarbons are the major cues for nest-mate recognition in social insects (Lahav et al. 1999), and show colony-specific patterns (Vander Meer & Morel 1998; Howard & Blomquist 2005). The colony odour is composed of the genetically encoded hydrocarbon patterns of all colony members, but also changes over time in response to environmental fluctuations in, food intake and nest site materials, for example (see, e.g. Liang & Silverman 2000). Individuals that are separated from their group and are therefore excluded from the continuous updating process deviate more and more from the overall colony odour and will no longer be accepted by the other group members after a long isolation period (Boulay et al. 2000). Not only is the chemical profile itself subject to continuous change, also the template that the individuals are learning and which they use as a self-reference is flexible and varies depending on the colony composition (Tsutsui et al. 2003). This means that, similarly to immune cells in a vertebrate body, individual social insects learn tolerance during their lifetime.
Despite this detailed knowledge on the self/non-self-recognition system of social insects, not much is known about how parasite infestations are detected. Parasite infection can alter the chemical profile of ants (Trabalon et al. 2000), but how self/non-self-recognition and parasite detection interact in social insects is far from clear. Still, it is likely that odour plays an important role as, for example, honeybees that are effective in removing infected brood from the combs (‘hygienic bees’) possess better olfactory sensitivity than other individuals not performing such tasks (Gramacho & Spivak 2003). Moreover, ‘danger signals’ are likely to be common after infection of colonies, and include sick or dead individuals as well as the parasites themselves. In fact, the presence of parasites has been shown to be detected immediately and to lead to prompt behavioural changes in both exposed individuals and non-infected group members (Ugelvig & Cremer 2007).
Insect societies also acquire protection against parasites and show a ‘collective immune memory’ during secondary exposure of the colony. In both termites and ants, social contact with infected workers provides resistance to nest-mate workers upon later infection with the same parasite (Traniello et al. 2002; Ugelvig & Cremer 2007). The mechanisms of this ‘collective memory’ are still to be explored.
(c) Maintaining individual integrity
One prominent example of effective non-self-recognition is the immunological rejection of non-MHC matched transplants of allogeneic donors. From a teleological point of view, the enormous efficiency of transplant rejections is somewhat astonishing as it has no obvious biological sense. It is widely assumed that the reactivity of approximately 1–10 per cent of all T-cell receptors to foreign MHC molecules is a by-product of the mechanisms of how T-cell clones are selected during ontogeny: in the thymus new T cells first undergo positive selection, meaning that all T-cell receptors that do not recognize own MHC molecules are deleted. In a subsequent round of negative selection, all T cells that respond to complexes of MHC and self-peptides are deleted. A by-product of such selection is that foreign MHC molecules, no matter whether they are presenting self or non-self-peptides, can activate many T-cell clones that have not been deleted by negative selection (Janeway et al. 2001).
If one assumes that allo-reactivity is a by-product, it is puzzling that organisms without an acquired immune component, such as invertebrates, also effectively reject allo-transplants (De Tomaso 2006), though there may exist some variation in the extent of rejection across taxa. Is it thus possible that allo-reactivity serves a biological function? We consider this likely as acquired immunity without strong allo-reactivity would be imaginable in principle: if the variable region of the T-cell receptor were to bind, not to the combined MHC–peptide structure, but exclusively to the presented peptide antigen, whereas MHC and T-cell receptor interacted via invariable regions, parasite detection would be functional, while allo-reactivity and the need for positive selection would be eliminated. Indeed, it seems likely that recognition of allo-MHC molecules is not a necessary molecular design feature, as sole detection of antigens is realized in B-cell receptors and the T-cell receptors of the gamma/delta T-cell lineage (Boehm 2006).
The question arises about whether recognizing cells of other individuals has adaptive value. In tunicates, allogeneic tissue was indeed shown to be a threat. The primitive colonial chordate Botryllus schlosseri lives in constant danger that its soma will be infiltrated by conspecifics that try to replace its germ line with their germ cells (De Tomaso 2006). Currently no such examples of ‘germ line slavery’ are known in higher vertebrates and it remains to be investigated whether such a fundamental threat of individuality may have influenced the evolution of immune systems. A less dramatic form of inter-individual cell transfer exists: ‘transmittable transplants’ are infectious tumours that can be transmitted by licking or biting. Examples have been reported in dogs, and one such tumour, which is obviously escaping allo-recognition, is currently threatening the population of the Tasmanian devil (Dingli & Nowak 2006; Murgia et al. 2006). It is unknown whether transmittable tumours are just a rare exception or a potential driver of immune evolution.
(d) Maintaining colony integrity
Germ line slavery and ‘social tumours’ also threaten the integrity of social insect colonies. Faultless self/non-self-recognition would be an essential and sufficient protection of an insect society against exploitation by so called ‘social parasites’ (Bourke & Franks 1995). These are other social insects, typically sister species, that gain access to the colony and take over reproduction. It is often only a single parasite queen that enters the colony, kills the queen by, for example, strangling her to death and starts laying eggs in her place. Parasite queens evade detection of the host colony by the initial absence of self-odour and take over the host colony's odour over time (D'Ettorre et al. 2002).
In Cape honeybees, even workers of the same species have evolved the capacity to enter foreign colonies and produce royal offspring (Jordan et al. 2008). This single clonal lineage was shown to infect and harm many bee colonies (Neumann & Hepburn 2002). In addition to this ‘infectious social tumour’, tumour formation can also occur within a single colony. Even if typically functionally sterile, the workers in some species of social insects, with the honeybee as the best known example, sometimes start laying eggs, thereby trying to increase their own direct fitness. As such behaviour is ‘antisocial’ and reduces colony efficiency and the fitness of the other colony members, those cheating individuals are punished in a process called ‘worker policing’ (Ratnieks & Visscher 1989). Likewise, emerging tumours within vertebrate bodies are suppressed by immune cells (Nunney 1999).
7. Regulation of immune responses
Local detection of the parasites at the site of entrance into a body or colony results in a decentralized process of local immune defence that allows quick reaction at the precise area of infection. Like specific cell populations of the innate immune system which continuously patrol the tissues of the body searching for signs of infection, some workers of insect societies always patrol the nest. Upon detection of infected brood, corpses or the parasites themselves, they start performing hygienic behaviour. Individuals differ greatly in their threshold value to execute this behavioural switch, i.e. they require more or less stimuli to start the task of, for example, uncapping brood cells or removing corpses. Their sensitivity to the stimuli varies depending on their genotype and/or developmental stage (Hughes et al. 2003).
There is little knowledge on how social immunity is regulated, and whether, which and how information is transferred between individuals. A vibration alarm was found to occur in termites, which apparently prevents nestmates contracting the contagion (Rosengaus et al. 1999; Myles 2002), but whether also helpers may be attracted to clean up the site of infection, and by which means this communication may occur, are still unexplored. Within vertebrate organisms, information transfer typically occurs via soluble signalling molecules, the cytokines, which guide immune cells to the site of infection and trigger them to produce cytokines themselves, leading to a positive feedback loop. A similar process is known from the chemical communication in social insects, where trails of volatile chemical compounds are laid to food sources. Whenever an individual follows the route, it applies additional chemical cues, increasing the odour intensity and thus its attractiveness for others (Hölldobler & Wilson 1990). These characteristics of social insect communication have guided optimal capacity utilization algorithms for network use by British Telecom, for example. It may well be that chemical cues also effect communication regarding parasite infections inside the nest.
Vertebrates have spatial centres for information exchange, the lymph nodes, each of which is drained by innate immune cells and soluble antigens from a different area of the body surface. Here, the antigen presenting cells of the innate immune component are visited by a high number of T cells in a short time. When a match between the highly specific T-cell receptor and the presented antigen is found, the acquired immune response gets kick-started (Janeway et al. 2001). The microanatomy of the lymph node is shaped to maximize contact rates between the cells of the innate and the acquired immune systems, thus creating an efficient environment for information transfer, which is indispensable for a well-organized immune response (Lämmermann & Sixt 2008). Some parasites have managed to specifically target these host information hubs and abuse them for their own transmission (Piguet & Steinman 2007).
Whether or not such immune information routes and central hubs also exist in social insects is unknown. Recent network studies indicate that disease transmission within social insect colonies is highly dependent on network density and individual contact rates (Otterstatter & Thomson 2007). Contact is a double-edged sword, with the potential to both enhance or decrease transmission, and optimal contact frequencies may be asymmetric, depending on whether one considers the infected individual or an uninfected group member. While infected individuals may greatly benefit from being taken care of by nestmates, the helpers run the risk of infection and the performance of hygienic behaviours may thus increase disease transmission through the colony (Kramm et al. 1982; Feffermann et al. 2007). Some parasites of social insects have even adapted to specifically exploit the hygienic behaviours of their social insect host and misuse helpers as their disease transmission vectors (Schmid-Hempel 1998). At the same time, social contact with infected individuals can also protect the helper individual by conferring a ‘social vaccination’ (Traniello et al. 2002; Ugelvig & Cremer 2007). The evolution of contact rates and their fine-tuning probably depends on many variables determined by both parasites and host societies, the general patterns of which are not yet well understood.
An optimal immune response is not only characterized by its quick and efficient activation; an adequate downregulation is just as important as it limits the collateral damage on the host. Immune responses should therefore be restricted temporally and spatially to affect as little uninfected tissue or neighbouring individuals as possible. Within multicellular organisms, many mechanisms exist to reduce immunopathology and autoimmunity. In vertebrates, some inflammatory cytokines, such as tumour necrosis factor, can have both activating and dampening effects depending on whether they act locally and acutely or systemically and chronically, respectively (Janeway et al. 2001). Also invertebrates use specific molecules to keep their cytotoxic immune effectors locally confined in the open body cavity (Siva-Jothy et al. 2006). Still, collateral damage cannot always be completely prevented, as exemplified by the occurrence of sepsis and autoimmune diseases, allergic reactions and paraneoplastic syndromes, or tissue damage around infections (Janeway et al. 2001; Brandt et al. 2004; Sadd & Siva-Jothy 2006). Overshooting immune reactions can have very high costs, sometimes creating more harm than the parasite itself, as observed for sepsis that often leads to death.
It is not yet clear how precisely insect societies can trigger their social defences, and whether they sometimes may reject or co-immure individuals that are not infected. The decision of whether or not to care for or kill a specific individual may depend not only on its infection load, but also on its future value for the colony, which differs greatly between individuals, e.g. the queen or a worker (Cremer et al. 2007). Counter-parasite measures can be very costly for the colony, for example when colonies abscond their nest completely leaving behind the infected individuals but probably also many more individuals in the move (Oi & Pereira 1993). Those drastic measures may only be an option for large societies that can still be efficient after the loss of many individuals, while smaller societies may try harder to save all their individuals. While immunopathology may play a role in insect societies, there is so far no evidence for social autoimmunity.
8. Interaction and evolution of immune modules
We have so far described behavioural, physiological and organisational defence mechanisms, mostly as different, rather independent, components of the immune defence. We would like to emphasize that the different defence modules are tightly interwoven (figure 1). First of all, at the interface between the different components of individual immunity, potential trade-offs may have shaped the fine-tuned interaction between innate and acquired immunity: depending on the efficiency of innate immunity in removing incoming parasites, the robustness of T-cell priming varies because increased persistence and spread of the pathogen boosts priming (Rotta et al. 2008). Second, interdependency also occurs between behavioural and physiological immune components, as effective behavioural disease avoidance may render the physiological response unnecessary. Lastly, at the interface between social and individual immunity, several findings indicate that a strong social defence may replace to a certain extent the need for a sophisticated individual immune system. It has recently been shown that in ants, in which foragers enrich the nest with antimicrobial tree resin (Christe et al. 2003), this social defence reduces the investment of individual group members in their own physiological defences (Castella et al. 2008). The finding that the social honeybee possesses a strikingly lower number of immune genes than several solitary insects (Evans et al. 2006) may be consistent with this idea.
When selection acts on an organism or superorganism, it simultaneously shapes all the interacting components at all levels of selection. This also means that in insect colonies, selection not only acts at the level of the colony, but concurrently at the level of the individuals that comprise this entity (Keller 1999). Social immunity can thus only evolve if overall it enhances colony reproductive output, even if some individual interests may be violated. Conflicts of interest are more common within societies with greater genetic diversity than in clonal societies or in individuals that consist of clones of cells. In this work, we have only considered societies of insects, and have used humans only as examples for individual defences. Still, human societies also perform a great variety of social anti-parasite defences, have evolved hospitals and health care systems as their extended phenotype (Dawkins 1999) and create their own social environment. The increase of hygiene and frequent drug use may also shape selection pressures and lead to changes in our individual immune systems, as exemplified by the increasing frequency of allergies that were set in context with our loss of worm parasites (Maizels 2005).
9. Conclusions and outlook
We suggest that similar parasite-linked selection pressures have shaped many parallel evolutionary solutions in the immune response at different organisational levels, i.e. at the individual (multicellular organism) and the society level (social insect colony). Analogies are sometimes eye-catching in specific mechanisms, such as for example granuloma formation and social encapsulation, or fever in both organisms and superorganisms. However, we would like to draw the attention of the reader towards the common underlying principles of anti-parasite defence: in the above cases the joint strategy of local disarming of the parasite and systemic host responses. We also found many similarities in self/non-self-recognition and the regulation and evolution of different immune modules at the two organisational levels, even if many processes are not yet fully understood, especially in the much less well-studied social immunity.
We hope that our review can point to interesting questions in the organization of immunity and in the evolution of defences at different levels which may be worth addressing in the future. For example, even if the general existence of an immune privilege of the germ line is established in individual immunity, our understanding is far from complete and it may be highly valuable to search for comparable mechanisms as described in social immunity. On the other hand, while activation and regulation of immune effector cells such as T and B cells by the antigen presenting cells is well studied in individual immunity, it is completely unclear whether social insect workers actively recruit and guide others to the site of infection, or whether each individual performing hygienic behaviours acts autonomously. Also, whether hygienic behaviour in social insects can be elicited only by the single signal of an incoming parasite or whether it may require multiple signals such as additional danger signals is far from understood.
Moreover, we think that the two fields cannot benefit from each other only by extracting specific open questions in either field, but also by adopting general approaches to solve these questions. The strength of evolutionary biology lies in the development and experimental testing of theoretical models, in integrating several interacting levels of selection or functional models and in incorporating potential trade-offs between them. Immunology, on the other hand, typically uses more sophisticated technical approaches with which specific mechanisms can be exactly defined. We think that the field of EvoDevo constitutes a promising alliance, answering ultimate questions by proximate mechanisms, as only the knowledge of the underlying mechanisms and constraints can functionally link genetics and physiology and higher levels of selection (Carroll et al. 2001; West-Eberhard 2003).
We have compared individual and social immunity in the light of their analogies, but the opposite approach—pinpointing the differences between the two—might also be very valuable, as it may highlight evolutionary or organisational constraints, differences in selection pressures or simply alternative routes to the same problems, similar to the two systems of somatic diversification in the immune systems of jawed and jawless vertebrates (Pancer & Cooper 2006). For example, we are not aware of any ‘vomiting response’ in social insects, i.e. the flushing out of infectious particles after intake. Instead, it seems that social insects may be more efficient in avoiding intake in the first place, which is achieved by sentinel individuals guarding the intake into the colony (Drum & Rothenbuhler 1985). In case of a heavy infection of the nest, the colony has an alternative strategy to separate sick nestmates and infectious material from healthy individuals, which is structurally impossible for a multicellular organism: the healthy individuals abandon the infected nest and move to a new, clean area (Royce et al. 1991; Drees et al. 1992).
Lastly, we would like to emphasize that insect societies should not only be a useful analogy to multicellular organisms, but are probably also a valuable model for epidemiological questions in human societies. The comparison of how insect versus human societies respond to disease can therefore be of interest to the field of evolutionary medicine (Ewald 1994; Nesse & Williams 1994; Stearns & Koella 2008). Societies of social insects have existed for more than 35 Myr (Hölldobler & Wilson 1990), and presumably in all this time have coevolved with their parasites. Studying these old societies in more detail may uncover even more sophisticated collective behavioural, physiological and organisational anti-parasite defences, which have not (yet?) evolved in humans but which may also be good strategies in human societies.
Despite their obvious differences in organisational level, their age and evolutionary history, human and insect societies have in common that both recently became subject to the same changes induced by globalization. These are, for example, the introduction of exotic species, landscape fragmentation, urbanization and climate change. Importantly, the recent drastic increase of global travel activities has most probably compromised host–parasite local adaptations, which also shaped earlier human history (Diamond 2005). These disturbances of our ecosystems through man-made actions set new and often unpredicted dynamics in motion, such as the emergence of new diseases or epidemics. In order to improve predictions as to the effects of environmental changes on disease dynamics in societies, insect colonies may prove highly useful, because they can be easily monitored in nature and approached experimentally. Testing predictions from epidemiological models on insect societies can be performed, for example, by experimental evolution studies that, due to the much shorter developmental time of insects, can produce quantitative data in short periods of time. Lastly, another similarity between insect and human societies may be of particular interest: they both create their own social environment that can buffer effects from the biotic and abiotic environment.
Acknowledgments
We thank the editors of this special issue, especially Hinrich Schulenburg, and two anonymous referees as well as Simon Tragust, for comments on the manuscript. Funding for this work was obtained from the Peter Hans Hofschneider Foundation for Experimental Biomedicine (to M.S.) and from the German Science Foundation (to S.C. and M.S.).
Footnotes
One contribution of 11 to a Theme Issue ‘Ecological immunology’.
References
- Amemiya C.T., Saha N.R., Zapata A. Evolution and development of immunologial structures in the lamprey. Curr. Opin. Immunol. 2007;19:535–541. doi: 10.1016/j.coi.2007.08.003. doi:10.1016/j.coi.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage S.A.O., Siva-Jothy M.T. Immune function responds to selection for cuticular colour in Tenebrio molitor. Heredity. 2005;94:650–656. doi: 10.1038/sj.hdy.6800675. doi:10.1038/sj.hdy.6800675 [DOI] [PubMed] [Google Scholar]
- Baer B., Krug A., Boomsma J.J., Hughes W.O.H. Examination of the immune responses of males and workers of the leaf-cutting ant Acromyrmex echinatior and the effect of infection. Insect. Soc. 2005;52:298–303. doi:10.1007/s00040-005-0809-x [Google Scholar]
- Boehm T. Quality control in self/nonself discrimination. Cell. 2006;125:845–858. doi: 10.1016/j.cell.2006.05.017. doi:10.1016/j.cell.2006.05.017 [DOI] [PubMed] [Google Scholar]
- Bohn H. Hemolymph clotting in insects. In: Brehélin M., editor. Immunity in invertebrates. Springer; Heidelberg, Germany: 1986. pp. 189–207. [Google Scholar]
- Boomsma J.J., Schmid-Hempel P., Hughes W.O.H. Life histories and parasite pressure across the major groups of social insects. In: Fellowes M., Holloway G., Rolff J., editors. Insect evolutionary ecology. CABI; Wallingford, UK: 2005. pp. 139–175. [Google Scholar]
- Boulay R., Hefetz A., Soroker V., Lenoir A. Camponotus fellah colony integration: worker individuality necessitates frequent hydrocarbon exchanges. Anim. Behav. 2000;59:1127–1133. doi: 10.1006/anbe.2000.1408. doi:10.1006/anbe.2000.1408 [DOI] [PubMed] [Google Scholar]
- Bourke A.F.G., Franks N.R. Princeton University Press; Princeton, NJ: 1995. Social evolution in ants. [Google Scholar]
- Brandt S.M., Dionne M.S., Khush R.S., Pham L.N., Vidgal T.J., Schneider D.S. Secreted bacterial effectors and host-produced Eiger/TNF drive death in a Salmonella-infected fruit fly. PLoS Biol. 2004;2:2067–2075. doi: 10.1371/journal.pbio.0020418. doi:10.1371/journal.pbio.0020418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W.L. An hypothesis concerning the function of the metapleural glands in ants. Am. Nat. 1968;102:188–191. doi:10.1086/282536 [Google Scholar]
- Carroll S., Grenier J., Wetherbee S. Blackwell Science; Malden, MA: 2001. From DNA to diversity: molecular genetics and the evolution of animal design. [Google Scholar]
- Castella G., Chapuisat M., Moret Y., Christe P. The presence of conifer resin decreases the use of the immune system in wood ants. Ecol. Entomol. 2008;33:408–412. doi:10.1111/j.1365-2311.2007.00983.x [Google Scholar]
- Chapuisat M., Oppliger A., Magliano P., Christe P. Wood ants use resin to protect themselves against pathogens. Proc. R. Soc. B. 2007;274:2013–2017. doi: 10.1098/rspb.2007.0531. doi:10.1098/rspb.2007.0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christe P., Oppliger A., Bancala F., Castella G., Chapuisat M. Evidence for collective medication in ants. Ecol. Lett. 2003;6:19–22. doi:10.1046/j.1461-0248.2003.00395.x [Google Scholar]
- Cremer S., Armitage S.A.O., Schmid-Hempel P. Social immunity. Curr. Biol. 2007;17:R693–R702. doi: 10.1016/j.cub.2007.06.008. doi:10.1016/j.cub.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Currie C.R., Scott J.A., Summerbell R.C., Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 1999;398:701–704. doi:10.1038/19519 [Google Scholar]
- Curtis V.A. Dirt, disgust and disease: a natural history of hygiene. J. Epidemiol. Community Health. 2007;61:660–664. doi: 10.1136/jech.2007.062380. doi:10.1136/jech.2007.062380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ettorre P., Mondy N., Lenoir A., Errard C. Blending in with the crowd: social parasites integrate into their host colonies using a flexible chemical signature. Proc. R. Soc. B. 2002;269:1911–1918. doi: 10.1098/rspb.2002.2110. doi:10.1098/rspb.2002.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins R. Oxford University Press; Oxford, UK: 1999. The extended phenotype. [Google Scholar]
- De Tomaso A.W. Allorecognition polymorphism versus parasitic stem cells. Trends Genet. 2006;22:485–490. doi: 10.1016/j.tig.2006.07.001. doi:10.1016/j.tig.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. W. W. Norton & Company; New York, NY: 2005. Guns, germs, and steel. The fates of human societies. [Google Scholar]
- Diehl-Fleig E., Lucchese M.E. Reacoes comportamentais de operarias de Acromyrmex striatus (Hymenoptera, Formicidae) na presenca de fungos entomopatogenicos. Rev. Bras. Entomol. 1991;35:101–107. [Google Scholar]
- Dingli D., Nowak M.A. Cancer biology: infectious tumour cells. Nature. 2006;443:35–36. doi: 10.1038/443035a. doi:10.1038/443035a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Taylor H.E., Dimopoulos G. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol. 2006;4:1137–1146. doi: 10.1371/journal.pbio.0040229. doi:10.1371/journal.pbio.0040229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees B.M., Miller R.W., Vinson S.B., Georgis R. Susceptibility and behavioral response of red imported fire ant (Hymenoptera: Formicidae) to selected entomogenous nematodes (Rhabditida: Steinernematidae & Heterorhabditidae) J. Econ. Entomol. 1992;85:365–370. doi: 10.1093/jee/85.2.365. [DOI] [PubMed] [Google Scholar]
- Drum N.H., Rothenbuhler W.C. Differences in non-stinging aggressive responses of worker honeybees to diseased and healthy bees in May and July. J. Apic. Res. 1985;24:184–187. [Google Scholar]
- Evans J.D., et al. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006;15:645–656. doi: 10.1111/j.1365-2583.2006.00682.x. doi:10.1111/j.1365-2583.2006.00682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald P.W. Oxford University Press; Oxford, UK; New York, NY: 1994. Evolution of infectious disease. [Google Scholar]
- Feffermann N.H., Traniello J.F.A., Rosengaus R.B., Calleri D.V. Disease prevention and resistance in social insects: modeling the survival consequences of immunity, hygienic behavior, and colony organization. Behav. Ecol. Sociobiol. 2007;61:565–577. doi:10.1007/s00265-006-0285-y [Google Scholar]
- Fernández-Marín H., Zimmermann J.K., Rehner S.A., Wcislo W.T. Active use of the metapleural glands by ants in controlling fungal infections. Proc. R. Soc. B. 2006;273:1689–1695. doi: 10.1098/rspb.2006.3492. doi:10.1098/rspb.2006.3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijak M., Meinhardt A. The testis in immune privilege. Immunol. Rev. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. doi:10.1111/j.1600-065X.2006.00438.x [DOI] [PubMed] [Google Scholar]
- Forbes M.R.L. Parasitism and host reproductive effort. Oikos. 1993;67:444–450. doi:10.2307/3545356 [Google Scholar]
- Garb J.L., Stunkard A.J. Taste aversions in man. Am. J. Psychiatry. 1974;131:1204–1207. doi: 10.1176/ajp.131.11.1204. [DOI] [PubMed] [Google Scholar]
- Gillespie J.P., Kanost M.R., Trenczek T. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. doi:10.1146/annurev.ento.42.1.611 [DOI] [PubMed] [Google Scholar]
- Gilliam M., Taber S., III, Lorenz B.J., Prest D.B. Factors affecting development of chalkbrood disease in colonies of honey bees, Apis mellifera, fed pollen contaminated with Ascosphaera apis. J. Invertbr. Pathol. 1988;52:314–325. doi:10.1016/0022-2011(88)90141-3 [Google Scholar]
- Gramacho K.P., Spivak M. Differences in olfactory sensitivity and behavioural responses among honey bees bred for hygienic behavior. Behav. Ecol. Sociobiol. 2003;54:472–479. doi:10.1007/s00265-003-0643-y [Google Scholar]
- Grindstaff J.L., Brodie E.D., Ketterson E.D. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. B. 2003;270:2309–2319. doi: 10.1098/rspb.2003.2485. doi:10.1098/rspb.2003.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.P. Insect immunocytes and other hemocytes: roles in cellular and humoral immunity. In: Gupta A.P., editor. Immunology of insects and other arthropods. CRC Press; Boca Raton, FL: 1991. pp. 19–118. [Google Scholar]
- Hamilton W.D. Kinship, recognition, disease and intelligence. In: Ito Y., Brown J.L., Kikkawa J., editors. Animal societies: theories and facts. Japan Scientific Societies Press; Tokyo, Japan: 1987. pp. 81–102. [Google Scholar]
- Harris M., Ross E.B., editors. Food and evolution: toward a theory of human food habits. Temple University Press; Philadelphia, PA: 1987. [Google Scholar]
- Hart A.G., Ratnieks F.L.W. Task partitioning, division of labour and nest compartimentalisation collectively isolate hazardous waste in the leafcutting ant Atta cephalotes. Behav. Ecol. Sociobiol. 2001;49:387–392. doi:10.1007/s002650000312 [Google Scholar]
- Heinze J., Hölldobler B. Thelytokous parthenogenesis and dominance hierarchies in the ponerine ant, Platythyrea punctata. Naturwissenschaften. 1995;82:40–41. [Google Scholar]
- Hochberg M.E., Michalakis Y., Meeus T. Parasitism at a constraint on the rate of life-history evolution. J. Evol. Biol. 1992;5:491–504. doi:10.1046/j.1420-9101.1992.5030491.x [Google Scholar]
- Hölldobler B., Wilson E.O. Harvard University Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Howard D.F., Tschinkel W.R. Aspects of necrophoric behavior in the red imported fire ant, Solenopsis invicta. Behaviour. 1976;56:158–180. doi:10.1163/156853976X00334 [Google Scholar]
- Howard R.W., Blomquist G.J. Ecological, behavioural, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005;50:371–393. doi: 10.1146/annurev.ento.50.071803.130359. doi:10.1146/annurev.ento.50.071803.130359 [DOI] [PubMed] [Google Scholar]
- Hughes D.P., Cremer S. Plasticity in anti-parasite behaviours and its suggested role in invasion biology. Anim. Behav. 2007;74:1593–1599. doi:10.1016/j.anbehav.2006.12.025 [Google Scholar]
- Hughes W.O.H., Eilenberg J., Boomsma J.J. Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc. R. Soc. B. 2002;269:1811–1819. doi: 10.1098/rspb.2002.2113. doi:10.1098/rspb.2002.2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W.O.H., Sumner S., Van Borm S., Boomsma J.J. Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proc. Natl Acad. Sci. USA. 2003;100:9394–9397. doi: 10.1073/pnas.1633701100. doi:10.1073/pnas.1633701100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccoud D.B., Hughes W.O.H., Jackson C.W. The epizootiology of a Metarhizium infection in mini-nests of the leaf-cutting ant Atta sexdens rubropilosa. Entomol. Exp. Appl. 1999;93:51–61. doi:10.1023/A:1003830625680 [Google Scholar]
- Janeway C.A., Travers P., Walport M., Shlomchik M. Garland Publishing; New York, NY; London, UK: 2001. Immunobiology. [Google Scholar]
- Jordan L.A., Allsopp M.H., Oldroyd B.P., Wossler T.C., Beekman M. Cheating honeybee workers produce royal offspring. Proc. R. Soc. B. 2008;275:345–351. doi: 10.1098/rspb.2007.1422. doi:10.1098/rspb.2007.1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L., editor. Levels of selection in evolution. Princeton University Press; Princeton, NJ: 1999. [Google Scholar]
- Keller L., Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. doi:10.1038/40130 [Google Scholar]
- Keller L., Reeve H.K. Dynamics of conflicts within insect societies. In: Keller L., editor. Levels of selection in evolution. Princeton University Press; Princeton, NJ: 1999. pp. 153–175. [Google Scholar]
- Kermarrec A., Febvay G., Decharme M. Protection of leaf-cutting ants from biohazards: is there a future for microbiological control? In: Lofgren C.S., Vander Meer R.K., editors. Fire ants and leaf-cutting ants: biology and management. Westview Press; Boulder, CO: 1986. pp. 339–356. [Google Scholar]
- Klein M.G. Efficacy against soil-inhabiting insect pests. In: Gaugler R., Kaya H.K., editors. Enthomopathogenic nematode biological control. CRC Press; Boca Raton, FL: 1990. pp. 195–214. [Google Scholar]
- Kluger M.J., Ringler D.J., Anver M.R. Fever and survival. Science. 1975;188:166–168. doi:10.1126/science.1114347 [PubMed] [Google Scholar]
- Kramm K.R., West D.F., Rockenbach P.G. Termite pathogens: transfer of the entomopathogen Metarhizium anisopliae between Reticulitermes sp. termites. J. Invertbr. Pathol. 1982;40:1–6. doi:10.1016/0022-2011(82)90029-5 [Google Scholar]
- Kumar S., Christophides G.K., Cantera R., Charles B., Han Y.S., Meister S., Dimopoulos G., Kafatos F.C., Barillas-Mury C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc. Natl Acad. Sci. USA. 2003;100:14 139–14 144. doi: 10.1073/pnas.2036262100. doi:10.1073/pnas.2036262100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J., Franz K. Evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. doi:10.1038/425037a [DOI] [PubMed] [Google Scholar]
- Lahav S., Soroker V., Hefetz A., Vander Meer R.K. Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften. 1999;86:246–249. doi:10.1007/s001140050609 [Google Scholar]
- Lämmermann T., Sixt M. The microanatomy of T-cell responses. Immunol. Rev. 2008;221:26–43. doi: 10.1111/j.1600-065X.2008.00592.x. doi:10.1111/j.1600-065X.2008.00592.x [DOI] [PubMed] [Google Scholar]
- Liang D., Silverman J. “You are what you eat” : diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften. 2000;87:412–416. doi: 10.1007/s001140050752. doi:10.1007/s001140050752 [DOI] [PubMed] [Google Scholar]
- Little T.J., O'Connor B., Colegrave N., Watt K., Read A.F. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 2003;13:489–492. doi: 10.1016/s0960-9822(03)00163-5. doi:10.1016/S0960-9822(03)00163-5 [DOI] [PubMed] [Google Scholar]
- Lozano G.A. Parasitic stress and self-medication in wild animals. In: Moller A.P., Milinski M., Slater P.J.B., editors. Stress and behaviour. Advances in the study of behaviour. Academic Press; London, UK: 1998. pp. 291–317. [Google Scholar]
- Maizels R.M. Infections and allergy—helminths, hygiene and host immune regulation. Curr. Opin. Immunol. 2005;17:656–661. doi: 10.1016/j.coi.2005.09.001. doi:10.1016/j.coi.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Marikovsky P.I. On some features of behavior of the ants Formica rufa L. infected with fungous disease. Insect. Soc. 1962;9:173–179. doi:10.1007/BF02224263 [Google Scholar]
- Mathis D., Benoist C. Back to central tolerance. Immunity. 2004;20:509–516. doi: 10.1016/s1074-7613(04)00111-6. doi:10.1016/S1074-7613(04)00111-6 [DOI] [PubMed] [Google Scholar]
- Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat. Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. doi:10.1038/ni0107-11 [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr Innate immune recognition and control of adaptive immune responses. Semin. Immunol. 1998;10:351–353. doi: 10.1006/smim.1998.0136. doi:10.1006/smim.1998.0136 [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr Innate immunity. New Engl. J. Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. doi:10.1056/NEJM200008033430506 [DOI] [PubMed] [Google Scholar]
- Moore J. Oxford Series in Ecology and Evolution. Oxford University Press; Oxford, UK: 2002. Parasites and the behavior of animals. [Google Scholar]
- Moret Y., Schmid-Hempel P. Immune defence in bumble-bee offspring. Nature. 2001;414:506. doi: 10.1038/35107138. doi:10.1038/35107138 [DOI] [PubMed] [Google Scholar]
- Moret Y., Schmid-Hempel P. Social life-history response to individual immune challenge of workers of Bombus terrestris L.: a possible new cooperative phenomenon. Ecol. Lett. 2004;7:146–152. doi:10.1046/j.1461-0248.2003.00561.x [Google Scholar]
- Murgia C., Pritchard J.K., Kim S.Y., Fassati A., Weiss R.A. Clonal origin and evolution of a transmissible cancer. Cell. 2006;126:477–487. doi: 10.1016/j.cell.2006.05.051. doi:10.1016/j.cell.2006.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles T.G. Alarm, aggregation, and defense by Reticulitermes flavipes in response to a naturally occurring isolate of Metarhizium anisopliae. Sociobiology. 2002;40:243–255. [Google Scholar]
- Nesse R.M., Williams G.C. Phoenix; London, UK: 1994. Evolution and healing: new science of Darwinian medicine. [Google Scholar]
- Neumann P., Hepburn R. Behavioural basis for social parasitism of Cape honeybees (Apis mellifera capensis) Apidologie. 2002;33:165–192. doi:10.1051/apido:2002008 [Google Scholar]
- Neumann P., Pirk C.W.W., Hepburn H.R., Solbrig A.J., Ratnieks F.L.W., Elzen P.J., Baxter J.R. Social encapsulation of beetle parasites by Cape honeybee colonies (Apis mellifera capensis Esch.) Naturwissenschaften. 2001;88:214–216. doi: 10.1007/s001140100224. doi:10.1007/s001140100224 [DOI] [PubMed] [Google Scholar]
- Nunn C.L., Altizer S. Oxford Series in Ecology and Evolution. Oxford University Press; Oxford, UK: 2006. Infectious disease in primates. [Google Scholar]
- Nunney L. Lineage selection: natural selection for long-term benefit. In: Keller L., editor. Levels of selection in evolution. Princeton University Press; Princeton, NJ: 1999. pp. 238–252. [Google Scholar]
- O'Leary J.G., Goodarzi M., Drayton D.L., von Andrian U.H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006;7:437–439. doi: 10.1038/ni1332. doi:10.1038/ni1332 [DOI] [PubMed] [Google Scholar]
- Oi D.H., Pereira R.M. Ant behavior and microbial pathogens (Hymenoptera: Formicidae) Florida Entomol. 1993;76:63–74. doi:10.2307/3496014 [Google Scholar]
- Ono M., Igarashi T., Ohno E., Sasaki M. Unusual thermal defence by a honeybee against mass attack by hornets. Nature. 1995;377:334–336. doi:10.1038/377334a0 [Google Scholar]
- Orr M.R., Seike S.H., Benson W.W., Gilbert L.E. Flies suppress fire ants. Nature. 1995;373:292–293. doi:10.1038/373292a0 [Google Scholar]
- Otterstatter M.C., Thomson J.D. Contact networks and transmission of an intestinal pathogen in bumble bee (Bombus impatiens) colonies. Oecologia. 2007;154:411–421. doi: 10.1007/s00442-007-0834-8. doi:10.1007/s00442-007-0834-8 [DOI] [PubMed] [Google Scholar]
- Pamer E.G. Immune responses to commensal and environmental microbes. Nat. Immunol. 2007;8:1173–1178. doi: 10.1038/ni1526. doi:10.1038/ni1526 [DOI] [PubMed] [Google Scholar]
- Pancer Z., Cooper M.D. The evolution of adaptive immunity. Annu. Rev. Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. doi:10.1146/annurev.immunol.24.021605.090542 [DOI] [PubMed] [Google Scholar]
- Papachristoforou A., Rortais A., Zafeiridou G., Theophilidis G., Garnery L., Thrasyvoulou A., Arnold G. Smothered to death: hornets asphyxiated by honeybees. Curr. Biol. 2007;17:R795–R796. doi: 10.1016/j.cub.2007.07.033. doi:10.1016/j.cub.2007.07.033 [DOI] [PubMed] [Google Scholar]
- Piguet V., Steinman R.M. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 2007;28:503–510. doi: 10.1016/j.it.2007.07.010. doi:10.1016/j.it.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profet M. The evolution of pregnancy sickness as protection to the embryo against Pleistocene teratogens. Evol. Theory. 1988;8:177–190. [Google Scholar]
- Ratnieks F.L.W., Visscher P.K. Worker policing in the honeybee. Nature. 1989;342:796–797. doi:10.1038/342796a0 [Google Scholar]
- Reis e Sousa C. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006;6:476–483. doi: 10.1038/nri1845. doi:10.1038/nri1845 [DOI] [PubMed] [Google Scholar]
- Rosengaus R.B., Guldin M.R., Traniello J.F.A. Inhibitory effect of termite fecal pellets on fungal spore germination. J. Chem. Ecol. 1998;24:1697–1706. doi:10.1023/A:1020872729671 [Google Scholar]
- Rosengaus R.B., Jordan C., Lefebvre M.L., Traniello J.F.A. Pathogen alarm behaviour in a termite: a new form of communication in social insects. Naturwissenschaften. 1999;86:544–548. doi: 10.1007/s001140050672. doi:10.1007/s001140050672 [DOI] [PubMed] [Google Scholar]
- Rotta G., Matteoli G., Mazzini E., Nuciforo P., Colombo M.P., Rescigno M. Contrasting roles of SPARC-related granuloma in bacterial containment and in the induction of anti-Salmonella typhimurium immunity. J. Exp. Med. 2008;205:657–667. doi: 10.1084/jem.20071734. doi:10.1084/jem.20071734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce L.A., Rossignol P.A., Burgett D.M., Stringer B.A. Reduction of tracheal mite parasitism of honey bees by swarming. Phil. Trans. R. Soc. B. 1991;331:123–129. doi: 10.1098/rstb.1991.0003. doi:10.1098/rstb.1991.0003 [DOI] [PubMed] [Google Scholar]
- Sadd B.M., Schmid-Hempel P. Insect immunity shows specificity upon secondary pathogen exposure. Curr. Biol. 2006;16:1206–1210. doi: 10.1016/j.cub.2006.04.047. doi:10.1016/j.cub.2006.04.047 [DOI] [PubMed] [Google Scholar]
- Sadd B.M., Schmid-Hempel P. Facultative but persistent transgenerational immunity via the mother's eggs in bumblebees. Curr. Biol. 2007;17:R1046–R1047. doi: 10.1016/j.cub.2007.11.007. doi:10.1016/j.cub.2007.11.007 [DOI] [PubMed] [Google Scholar]
- Sadd B.M., Siva-Jothy M.T. Self-harm caused by an instect's innate immunity. Proc. R. Soc. B. 2006;273:2571–2574. doi: 10.1098/rspb.2006.3574. doi:10.1098/rspb.2006.3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadd B.M., Kleinlogel Y., Schmid-Hempel R., Schmid-Hempel P. Trans-generational immune priming in a social insect. Biol. Lett. 2005;1:386–388. doi: 10.1098/rsbl.2005.0369. doi:10.1098/rsbl.2005.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman S.S. Taste and smell losses in normal aging and disease. JAMA. 1997;278:1357–1362. doi:10.1001/jama.278.16.1357 [PubMed] [Google Scholar]
- Schmid-Hempel P. Monographs in Behavior and Ecology. Princeton University Press; Princeton, NJ: 1998. Parasites in social insects. [Google Scholar]
- Schmid-Hempel P., Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 2003;18:27–32. doi:10.1016/S0169-5347(02)00013-7 [Google Scholar]
- Schmid-Hempel P., Schmid-Hempel R. Transmission of a pathogen in Bombus terrestris, with a note on division of labour in social insects. Behav. Ecol. Sociobiol. 1993;33:319–327. doi:10.1007/BF00172930 [Google Scholar]
- Schrempf A., Heinze J., Cremer S. Sexual cooperation: mating increases longevity in ant queens. Curr. Biol. 2005;15:267–270. doi: 10.1016/j.cub.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Siebeneicher S.R., Vinson S.B., Kenerley C.M. Infection of the red imported fire ant by Beauveria bassiana through various routes of exposure. J. Invertebr. Pathol. 1992;59:280–285. doi:10.1016/0022-2011(92)90133-O [Google Scholar]
- Siva-Jothy M.T., Moret Y., Rolff J. Insect immunity: an evolutionary ecology perspective. Adv. Insect Physiol. 2006;32:1201–1248. [Google Scholar]
- Spivak M., Gilliam M. Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa mites. Part I: hygienic behaviour and resistance to American foulbrood. Bee World. 1998a;79:124–134. [Google Scholar]
- Spivak M., Gilliam M. Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa mites. Part II: hygienic behaviour and resistance to American foulbrood. Bee World. 1998b;79:165–182. [Google Scholar]
- Stabentheiner A., Kovac H., Schmaranzer S. Thermal behaviour of honeybees during aggressive interactions. Ethology. 2007;113:995–1006. doi: 10.1111/j.1439-0310.2007.01403.x. doi:10.1111/j.1439-0310.2007.01403.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starks P.T., Blackie C.A., Seeley T.D. Fever in honey bee colonies. Naturwissenschaften. 2000;87:229–231. doi: 10.1007/s001140050709. doi:10.1007/s001140050709 [DOI] [PubMed] [Google Scholar]
- Stearns S.C., Koella J.C., editors. Evolution in health and disease. Oxford University Press; Oxford, UK: 2008. [Google Scholar]
- Stuart L.M., Ezekowitz R.A. Phagocytosis and comparative innate immunity: learning on the fly. Nat. Rev. Immunol. 2008;8:131–141. doi: 10.1038/nri2240. doi:10.1038/nri2240 [DOI] [PubMed] [Google Scholar]
- Theopold U., Li D., Fabbri M., Scherfer C., Schmidt O. The coagulation of insect hemolymph. Cell Mol. Life Sci. 2002;59:363–372. doi: 10.1007/s00018-002-8428-4. doi:10.1007/s00018-002-8428-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabalon M., Plateaux L., Peru L., Bagneres A.G., Hartmann N. Modification of morphological characters and cuticular compounds in worker ants Leptothorax nylanderi induced by endoparasites Anomotaenia brevis. J. Insect Physiol. 2000;46:169–178. doi: 10.1016/s0022-1910(99)00113-4. doi:10.1016/S0022-1910(99)00113-4 [DOI] [PubMed] [Google Scholar]
- Traniello J.F.A., Rosengaus R.B., Savoie K. The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc. Natl Acad. Sci. USA. 2002;99:6838–6842. doi: 10.1073/pnas.102176599. doi:10.1073/pnas.102176599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui N.D., Suarez A.V., Grosberg R.K. Genetic diversity, asymmetrical aggression, and recognition in a widespread invasive species. Proc. Natl Acad. Sci. USA. 2003;100:1078–1083. doi: 10.1073/pnas.0234412100. doi:10.1073/pnas.0234412100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turillazzi S., et al. Dominulin A and B: two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/TOF, and ESI-ion trap. Am. Soc. Mass Spectr. 2006;17:376–383. doi: 10.1016/j.jasms.2005.11.017. doi:10.1016/j.jasms.2005.11.017 [DOI] [PubMed] [Google Scholar]
- Ugelvig L.V., Cremer S. Social prophylaxis: group interaction promotes collective immunity in ant colonies. Curr. Biol. 2007;17:1967–1971. doi: 10.1016/j.cub.2007.10.029. doi:10.1016/j.cub.2007.10.029 [DOI] [PubMed] [Google Scholar]
- Vainio L., Hakkarainen H., Rantala M.J., Sorvari J. Individual variation in immune function in the ant Formica exsecta; effects of the nest, body size and sex. Evol. Ecol. 2004;18:75–84. doi:10.1023/B:EVEC.0000017726.73906.b2 [Google Scholar]
- Vander Meer R.K., Morel L. Nestmate recognition in ants. In: Winston M.L., editor. Pheromone communication in social insects. Westview Press; Boulder, CO: 1998. pp. 79–103. [Google Scholar]
- Vieira-Neto E.H.M., Mundim F.M., Vasconcelos H.L. Hitchhiking behaviour in leaf-cutting ants: an experimental evaluation of three hypotheses. Insect. Soc. 2006;53:326–332. doi:10.1007/s00040-006-0876-7 [Google Scholar]
- von Andrian U.H., Mackay C.R. T-cell function and migration. Two sides of the same coin. New Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. doi:10.1056/NEJM200010053431407 [DOI] [PubMed] [Google Scholar]
- Waddington K.D., Rothenbuhler W.C. Behaviour associated with hairless-black syndrome of adult honeybees. J. Apicult. Res. 1976;15:35–41. [Google Scholar]
- Wang D.I., Moeller F.E. The division of labor and queen attendance behavior of Nosema-infected worker honey bees. J. Econ. Entomol. 1970;63:1539–1541. [Google Scholar]
- Watson F., Püttmann-Holgado R., Thomas F., Lamar D.L., Hughes M., Kondo M., Rebel V.I., Schmucker D. Extensive diversity of Ig-superfamily in the immune system of insects. Science. 2005;309:1874–1878. doi: 10.1126/science.1116887. doi:10.1126/science.1116887 [DOI] [PubMed] [Google Scholar]
- West-Eberhard M.J. Oxford University Press; New York, NY: 2003. Developmental plasticity and evolution. [Google Scholar]
- Wheeler W.M. The ant-colony as an organism. J. Morphol. 1911;22:307–325. doi:10.1002/jmor.1050220206 [Google Scholar]
- Zhou X., Kaya H.K., Heungens K., Goodrich-Blair H. Response of ants to deterrent factor(s) produced by the symbiotic bacteria of entomopathogenic nematodes. Appl. Environ. Microbiol. 2002;68:6202–6209. doi: 10.1128/AEM.68.12.6202-6209.2002. doi:10.1128/AEM.68.12.6202-6209.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]