Abstract
Plant biologists have long recognized that host defence against parasites and pathogens can be divided into two conceptually different components: the ability to limit parasite burden (resistance) and the ability to limit the harm caused by a given burden (tolerance). Together these two components determine how well a host is protected against the effects of parasitism. This distinction is useful because it recognizes that hosts that are best at controlling parasite burdens are not necessarily the healthiest. Moreover, resistance and tolerance can be expected to have different effects on the epidemiology of infectious diseases and host–parasite coevolution. However, studies of defence in animals have to date focused on resistance, whereas the possibility of tolerance and its implications have been largely overlooked. The aim of our review is to (i) describe the statistical framework for analysis of tolerance developed in plant science and how this can be applied to animals, (ii) review evidence of genetic and environmental variation for tolerance in animals, and studies indicating which mechanisms could contribute to this variation, and (iii) outline avenues for future research on this topic.
Keywords: immunopathology, infectious disease, resistance, tolerance, virulence
1. Introduction
Once infected, hosts can in principle protect themselves from subsequent harm in two ways: they can directly attack parasites and thereby reduce parasite loads, or they can limit the harm caused by a given parasite burden. Plant biologists have long recognized that defence can be decomposed into these two components (Cobb 1894 cited in Schafer 1971; Caldwell et al. 1958; Clarke 1986), and have called them resistance and tolerance, respectively. Under this view, host health and ultimately fitness thus depend not only on the ability of a host to limit parasite burdens (resistance) but also to limit the damage caused by a given parasite burden (tolerance). This distinction recognizes the important fact, which we discuss below, that hosts that are good at reducing parasite burdens are not necessarily the healthiest: hosts can sometimes be quite healthy despite high parasite burdens, or conversely die with parasite loads which others survive.1 We believe that it is widely accepted in the health sciences that pathogen burden and health are not always well correlated, but that the rather profound logical consequences of this decoupling for biomedicine and animal evolutionary ecology have been largely overlooked. We contend that the concept of tolerance as developed by plant biologists, and most importantly the statistical approach they developed to quantitatively distinguish tolerance and resistance, offers much for understanding animal defences against infectious disease.
Why then is it important to distinguish between resistance and tolerance? From a biomedical perspective, the ability to statistically decompose host defences into those directed at pathogen limitation and those directed at damage limitation should lead to improved understanding of the actual causes of pathology. Furthermore, understanding the relationship between resistance and tolerance may be essential where manipulations of host defences by immune interventions or genetic manipulations are being contemplated; for example, if resistance and tolerance are negatively correlated, enhancing resistance may make host health worse. From an ecological and evolutionary perspective, the critical difference between resistance and tolerance is that resistance protects the host at the expense of the parasite, while tolerance saves the host from harm without having any direct negative effects on the parasite. Owing to this fundamental difference, it has been argued that the ecological and evolutionary consequences of resistance and tolerance should differ. First, evolution of resistance should reduce the prevalence of the parasite in the host population, while tolerance should have a neutral or positive effect on parasite prevalence (Roy & Kirchner 2000; Miller et al. 2006; Boots 2008). Second, because resistance has a negative effect on parasite fitness, it may impose selection on the parasite to overcome this type of host defence, which in turn may impose selection for improved resistance in the host, leading to antagonistic coevolution between host and parasite (Woolhouse et al. 2002). Tolerance, on the other hand, by definition does not have any negative effect on the performance of the parasite, and so there should not be any selection on the parasite to overcome this type of defence. Several authors have therefore argued that evolution of tolerance should not be expected to result in open-ended antagonistic coevolution (Caldwell et al. 1958; Schafer 1971; Clarke 1986; Rausher 2001; Boots 2008; but see §7). Because resistance and tolerance can be expected to have such radically different effects on the epidemiology of infectious diseases and the coevolution of hosts and parasites, elucidating the relative importance of these two types of defence is key to understanding the ecology and evolution of host–parasite interactions.
During the 1990s, plant biologists developed a rigorous statistical framework to measure the relative importance of resistance and tolerance, and have since found genetic (heritable) and environmentally induced variation in both traits (Simms & Triplett 1994; Fineblum & Rausher 1995; Tiffin & Rausher 1999; Stowe et al. 2000; Koskela et al. 2002; Kover & Schaal 2002; Strauss et al. 2002; Kniskern & Rausher 2006; Du et al. 2008). Plant scientists continue to pursue studies of tolerance, both owing to the fundamental scientific interest, and owing to the important implications for plant breeding (Rausher 2001). However, the advances by plant scientists have had almost no impact on those studying animal diseases. Immunologists, microbiologists, parasitologists and animal evolutionary ecologists have typically measured either hosts' ability to limit parasite burdens (i.e. resistance; e.g. Kloosterman et al. 1992; Smith et al. 1999), or the overall ability to maintain health or fitness in the face of infection, irrespective of parasite burden (i.e. the combined effect of resistance and tolerance; e.g. Hill et al. 1991; McGuire et al. 1994; Bisset & Morris 1996; Medina & North 1998). Indeed, these two types of measures are sometimes even used synonymously (Malo & Skamene 1994; Fortin et al. 2002). Only very recently has the possibility of variation in tolerance in the strict sense used in the plant literature received any empirical consideration in studies of animal diseases (Råberg et al. 2007), even though the epidemiological and evolutionary implications of distinguishing between resistance and tolerance have been pointed out by several theoreticians interested in animal diseases (Roy & Kirchner 2000; Fornoni et al. 2004; Restif & Koella 2004).
With the hope of stimulating research on tolerance in animals, including use of its conceptually clear statistical definition, we here (i) describe the statistical framework for the analysis of tolerance developed in the plant literature and how this can be applied to animals, (ii) discuss the semantic problems involved with the term ‘tolerance’, some of which are conceptual, and some of which are an inevitable consequence of attempting to cross disciplines with largely isolated histories, (iii) review the existing evidence for tolerance in animals, and (iv) discuss open questions, particularly the possible costs of tolerance. We conclude with a further discussion of the implications of tolerance for biomedicine and for evolutionary ecology.
2. How to measure resistance and tolerance: a statistical definition of tolerance
Here we outline the statistical framework for analysis of resistance and tolerance developed in plant science. Resistance is typically measured as the inverse of infection intensity (number of parasites per host or per unit host tissue); all else being equal, a lower intensity means an animal is more resistant. Tolerance, on the other hand, is usually operationally defined as the slope of a regression of host fitness against infection intensity; the steeper the slope, the lower the tolerance (Simms & Triplett 1994; Koskela et al. 2002). Thus, a formally more correct verbal definition of tolerance than the one we gave above (‘the ability to limit the damage of a given parasite burden’) is the rate of change in fitness as parasite burden increases. This kind of trait, which describes how individuals of a specified type respond to different environmental conditions (in this case how fitness of a specified host type changes with increasing parasite burden), is commonly known as a ‘reaction norm’ in ecology and evolutionary biology (Schlichting & Pigliucci 1998). Below we give a more explicit statistical description of how the determinants of host fitness in the presence of parasites can be decomposed into resistance and tolerance, and explain why tolerance is best defined in terms of a reaction norm. For the moment we continue to follow the plant literature in discussing tolerance in terms of host fitness. However, the best choice of response variable is context dependent. In many biomedical contexts, health may be of more direct interest than measures of Darwinian fitness (e.g. lifetime reproductive success). Similarly, in veterinary medicine, productivity or yield may be the most relevant choice, while in an epidemiological context, host survival rather than total fitness is most relevant (unless a parasite is vertically transmitted). We discuss the semantics of tolerance below, but note here that because tolerance can be applied to these different response variables, explicit definitions are key.
In its simplest form, the fitness of host type i in the presence of parasites can be described by the following equation:
| (2.1) |
where Wi is fitness of hosts of type i, ai is the intercept (i.e. the fitness when uninfected), I is infection intensity, and bi is the slope of the relationship between W and I, that is, tolerance (cf. Stowe et al. 2000). Thus, the effect of infection on fitness of hosts of type i is determined by the term biI. Variation in I can be determined by both host and parasite factors, as well as external factors such as inoculation dose. If I differs among host types (all else equal), this means there is variation in resistance. This source of variation in I can be estimated from an ANOVA of I against host type. If the slope (b) varies among host types, such that the fitness of some types declines faster with increasing burden than that of others, this means there is variation in tolerance among host types (figure 1a). Formally, this can be detected as a statistical interaction between host type and infection intensity in an ANCOVA (Simms & Triplett 1994; Tiffin & Rausher 1999; Stowe et al. 2000).
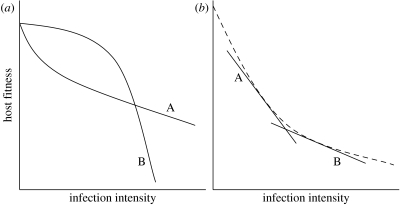
Figure 1.
Reaction norms for fitness of two host genotypes (A and B) across a parasite burden range. The dots represent individual hosts of genotype A (unfilled) or B (filled). (a) Host genotypes differ in tolerance; A is more tolerant. (b) Host genotypes differ in ‘general vigour’, but not in tolerance.
Equation (2.1) and the division of defence into resistance and tolerance take a host-centred view on infection. The term ‘virulence’ is often used to describe the reduction in host fitness caused by infection, and in parasite-centric views is seen as a parasite trait, and in more holistic accounts, as an outcome of the host–parasite interaction (Read et al. 1999). We want to emphasize that tolerance is not just the inverse of virulence (or 1−virulence). Rather, host resistance and tolerance jointly affect the virulence of infection. In terms of equation (2.1), the virulence of a particular parasite genotype j in a particular host type i is biIij, where Iij is the infection intensity of parasite genotype j in host type i.
With tolerance defined as a slope, it follows that this trait (by contrast with resistance) cannot be measured on a single animal; instead it must be measured across individuals of a given host type. Thus if one is interested in genetic variation in tolerance, it is necessary to measure fitness of genetically distinct groups of hosts that harbour different numbers of parasites and compare the slopes among the genetic units (e.g. strains or breeds of laboratory or domestic animals, sib groups of outbred wild animals, or individuals carrying a particular allele at a specific locus).
Why then is it important to define tolerance as a reaction norm? The primary reason is that it is only by defining tolerance in this way that one can conclude that fitness differences between host types actually arise, because hosts differ in their ability to limit the damage per parasite. If instead tolerance is measured for a single host as, for example, the residual deviation from a regression of host fitness across a range of infection intensities, one cannot rule out that variation among hosts is caused by factors other than tolerance. To see this, consider the case shown in figure 1b. Here, host types vary in the residual deviation from a common regression line, but the individual regression lines are parallel so there is no interaction between host type and parasite burden. In other words, host types differ in intercept rather than slope. Since the difference in fitness is constant across infection intensities (even when the parasite burden is zero), it can have nothing to do with defence against the parasite in question. Instead the fitness difference must be caused by variation in host traits other than those involved in defence against this particular parasite (Stowe et al. 2000). These other traits can be summarized under the somewhat metaphysical label of ‘general vigour’ (Fry 1993; Stowe et al. 2000), closely related to the concepts ‘resource acquisition’ (van Noordwijk & de Jong 1986; Houle 1991) and ‘condition’ (Rowe & Houle 1996), which are more commonly used in the animal literature. Only by testing for a statistical interaction between host genotype and parasite burden can this important and well-known source of variation be ruled out. As a concrete example, suppose one aims to investigate the tolerance of different cattle breeds to a particular parasite, where the parameter of interest is a production trait such as weight gain. It seems likely that breeds may vary in weight gain even in the complete absence of infection. Thus, variation in weight gain among breeds at a particular parasite load could arise simply owing to this background difference. Comparing the weight gain at a particular parasite load is therefore not informative about the relative ability of breeds to tolerate the parasite. The crucial question is instead, how does the difference in weight gain between breeds change with parasite burden? A significant statistical interaction between load and breed would mean that this difference changes with increasing burden, that is, some breeds are better able to tolerate the parasite.
The reaction norm approach also has another important advantage: it offers the possibility to investigate the shape of the relationship between fitness and infection intensity. Indeed, there is no a priori reason to assume that this relationship should be linear. Importantly, if the relationship between fitness and infection intensity is nonlinear, there may be large differences in fitness between host types even if there is little difference in parasite burden, or vice versa. An analysis that compares the mean fitnesses and infection intensities of different host types can then give the false impression that host types vary in tolerance, when they are in fact just at different positions along a common reaction norm. By using the reaction norm approach, it is possible to control statistically for this potentially confounding factor (this is most readily done by including a quadratic infection intensity term in the regression; see further §5).
3. Concepts and terminology
How does this statistical decomposition of the host determinants of health into resistance and tolerance accord with what is known about the mechanistic basis of animal defences? Mechanisms of host defence responsible for reducing parasite burdens (resistance) are well known, and include innate and adaptive immunity. Mechanisms of tolerance are likely to include tissue repair as well as immunological mechanisms. The latter mechanisms can be of several different types. First, tolerance may involve immune responses that are not directed at the parasite itself, but rather at toxins and other harmful substances produced by the parasite (often referred to as ‘anti-disease immunity’ or ‘anti-toxin immunity’; Playfair et al. 1990). Second, it may involve mechanisms that damp down inappropriate host responses and/or limit collateral damage (‘immunopathology’) from otherwise well-directed immune responses. Thus tolerance, as well as resistance, may involve a great variety of mechanisms. Moreover, a particular mechanism may affect both resistance and tolerance. For example, some antibody responses in an individual host's repertoire may target the parasite itself, while others target toxins. In fact, a particular mechanism may affect both resistance and tolerance simultaneously. For instance, pro-inflammatory cytokines may stimulate immune responses that attack the parasite (i.e. enhance resistance) but at the same time lead to increased collateral damage (thus reducing tolerance), as discussed in more detail below (see §4c).
The division of host defence into resistance and tolerance should be seen as complementary, rather than alternative, to a division based on mechanistic details. While a division of host defence strategies based on mechanisms (e.g. innate versus adaptive immunity; B versus T cell-mediated immunity) is clearly useful for many purposes, the more conceptual division into resistance and tolerance is arguably more helpful for understanding the health consequences of infection, and the ecological and evolutionary interactions between hosts and parasites.
The term tolerance is used by many in the biomedical community to mean things other than what we and the plant science community mean. For example, in studies of trypanosomes in cattle, different breeds are often classified as tolerant or non-tolerant, but here tolerance describes the overall effect of infection on disease severity and host fitness, irrespective of parasite burden (Naessens 2006). In immunology, tolerance is often taken to mean immunological non-responsiveness: an individual is tolerant to a particular antigen if s/he does not mount an immune response to it. By this definition, most hosts are tolerant of self-antigens (Roitt et al. 1993), as well as parasite antigens when non-responsiveness is mediated by, for example, T-regulatory cells (Schwartz 2005). Yet a different definition is the ability to avoid immunopathology (Boutlis et al. 2006). Although the current usage of tolerance and other related terms in biomedicine sometimes approaches the meaning we propose, there is a crucial difference: in none of these cases has tolerance been explicitly defined as the ability to limit the health or fitness consequences of a given parasite burden. This latter definition is useful because it makes tolerance and resistance two different but complementary traits that together determine how well a host is protected against parasites.
Given that tolerance already has divergent definitions in biomedicine, none of which align with our definition, it is reasonable to ask why we do not use another word. We do not for the following reasons. First, the plant science definition of tolerance has a long pedigree, and it is now very precisely and quantitatively defined. Second, tolerance already means a variety of things to different animal disease biologists, so we are adding negligible additional confusion (indeed, we think the statistical definition is adding novel precision). Third, obvious alternatives (e.g. resilience, endurance) already have other meanings.
Actually, this semantic problem is a particular instance of a general problem in host–parasite science: critical words are often used by different scientific communities in contrasting ways. For instance, virulence to animal evolutionary biologists is reduction in host fitness following infection, while to plant biologists, it is the ability to replicate in host tissue (for other uses of virulence see Read et al. (1999)). Similar problems apply to ‘susceptibility’, ‘immunopathology’, ‘resistance’, ‘sensitivity’, ‘pathogenicity’ and even ‘disease’. This terminological chaos arises in large part owing to the quite separate traditions, educational cultures and historical development of medicine, immunology, microbiology, plant science, parasitology, ecology and evolution. None of this makes interdisciplinary communication easy, but we do not think it otherwise ultimately matters much, so long as each contentious term is defined rigorously in any given context. Above we have given such a definition of our usage of tolerance (it is the b in equation (2.1)). We note that precise, mathematical definitions are rare in biomedicine, and so in a sense the label one attaches to that term is not terribly important. The label we choose to attach is the one used by plant biologists, who have priority.
4. Empirical evidence
Here we review studies showing evidence for variation in tolerance in animals. We focus on genetic variation, but also provide an example of an environmental factor that can affect tolerance. In §4c we review gene knockout studies, which not only demonstrate tolerance but also give some hints about possible mechanisms.
(a) Genetic variation
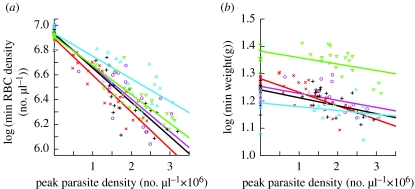
Among the most well-studied parasites are protozoans of the genus Plasmodium, which cause malaria in a wide range of animals, including mammals, birds and reptiles. A recent study by Råberg et al. (2007) applied the statistical framework for analysis of tolerance developed by plant biologists to a rodent malaria model, Plasmodium chabaudi in laboratory mice. The study used five different strains of mice that had previously been shown to differ in resistance. To generate variation in infection intensity, and thereby increase the statistical power to detect a host genotype-by-parasite burden interaction, mice were infected with one of three different parasite clones that vary in the infection intensity they induce, or left uninfected. Resistance was measured as the inverse of peak parasite density. The most often measured effects of infection on host health in this system are anaemia and weight loss, and both are good predictors of mortality (Mackinnon & Read 2004). Hence, tolerance was measured as the slope of a regression of anaemia or weight loss against peak parasite density. In the case of both anaemia and weight loss, the slopes of these regressions differed between mouse strains, revealing variation in tolerance among mouse strains (figure 2).
Figure 2.
Inbred strains of laboratory mice differ in tolerance to the rodent malaria parasite P. chabaudi. (a) Tolerance measured as the slope of minimum red blood cell (RBC) density (log-transformed) against peak parasite density. (b) Tolerance measured as the slope of minimum weight (log-transformed) against peak parasite density. In the case of both minimum RBC density and weight, ANCOVAs revealed highly significant interactions between mouse strain and parasite density. The ranking of the slopes for minimum RBC density and weight is the same, indicating that the two forms of tolerance are positively correlated (rs=1.0, p<0.05). From Råberg et al. (2007). Up triangles, DBA/1 mice; down triangles, NIH; circles, A/J; pluses, CBA; crosses, C57.
We are unaware of any other studies that have used the reaction norm approach to test for tolerance in animals. Indeed, there are to the best of our knowledge no other studies that have explicitly looked for tolerance in the sense of the ability of different host genotypes to limit the effect of a given burden. Still, a number of studies of other host–parasite systems show results that are consistent with genetic variation for tolerance. Perhaps the most convincing evidence for genetic variation for tolerance in a natural host–parasite system comes from studies of human malaria. Several different monogenic disorders in humans have been shown to protect against malaria. These include, among other mechanisms, various types of haemoglobinopathies, such as HbS (which causes sickle cell disease in its homozygous state) and α+-thalassaemia (Williams 2006). As for HbS, there is good evidence that the protective effect is due to resistance: individuals carrying the mutation have lower parasite densities (Aidoo et al. 2002; Williams et al. 2005a). In contrast, α+-thalassaemia does not affect parasite densities, but nevertheless reduces the incidence of severe disease (including, e.g. cerebral malaria; Allen et al. 1997; Mockenhaupt et al. 2004; Williams et al. 2005b), probably at least partly because anaemia at high parasite loads is alleviated (Wambua et al. 2006). In other words, it seems HbS increases resistance, while α+-thalassaemia enhances tolerance. Even though these studies strongly suggest that α+-thalassaemia causes variation for tolerance, one cannot yet definitely rule out alternative explanations, mainly because the classification of ‘severe malaria’ is based on both measurement of infection intensity and clinical manifestation of disease symptoms; thus resistance and tolerance are partly confounded in the currently published analyses.
Another important protozoan parasite is Trypanosoma, which causes Chagas' disease and sleeping sickness in humans (Trypanosoma cruzi and Trypanosoma brucei, respectively), and nagana in cattle (T. brucei and Trypanosoma congolense). Studies of both T. cruzi and T. congolense have found infection intensity, disease severity (measured as anaemia or weight loss) and mortality to be correlated across mouse strains (Graefe et al. 2003) and cattle breeds (Paling et al. 1991), indicating that resistance is an important determinant of host health and fitness. However, crossing and gene mapping studies in mice and cattle have shown that parasitaemia and mortality are at least partly controlled by different loci (Degee et al. 1988; Hanotte et al. 2003). Hence, it seems likely there is genetic variation not only for resistance but also for tolerance. Similarly, a gene mapping study of susceptibility to Toxoplasma gondii (a protozoan zoonotic disease) in laboratory mice showed that completely different loci were associated with resistance (measured as number of cysts in the brain) and survival (Johnson et al. 2002).
Beyond the protozoa, there are other apparent examples of genetic variation for tolerance. For instance, several different species of the bacteria genus Borrelia cause Lyme disease, one of the most important vector borne diseases of humans in temperate regions. Studies of mouse models of this disease have found that mouse strains differ in resistance. However, there is also evidence of variation in tolerance. For example, C57BL/6N and C3H/He mice have similar levels of spirochetes in tissues, but C3H/He develop much more severe pathology (arthritis; Ma et al. 1998).
A recent paper on fruit flies provides strong evidence that invertebrates may also show variation for tolerance. Corby-Harris et al. (2007) infected eleven different lines of Drosophila melanogaster with the bacteria Pseudomonas aeruginosa, a common insect pathogen. There were highly significant differences among lines for both bacterial load and survival time. However, these two traits were completely uncorrelated across lines, suggesting that tolerance rather than resistance determines survival.
(b) Environmental variation
A potentially important environmental determinant of tolerance to a given infection is the simultaneous presence of other infections. Coinfecting parasites impact upon each other in many ways. Immune-mediated interactions are common and can affect parasite densities and host health independently (Page et al. 2006). Helminths, for example, often induce suppressive types of immune responses that benefit their own survival (Maizels et al. 2004) but have so-called ‘bystander effects’ on responses to other infections (Hartgers & Yazdanbakhsh 2006; Kamal & Khalifa 2006). Helminths can thus reduce the resistance of mice to microparasites (Helmby et al. 1998; Su et al. 2005). Crucially, immune responses to helminth co-infection can also decrease (Buendia et al. 2002; Furze et al. 2006) or increase (Marshall et al. 1999; Graham et al. 2005b) the severity of microparasite-induced disease without altering microparasite density. Helminth co-infection therefore has the potential to cause variation in tolerance. In fact, it seems that in some cases, helminth co-infected hosts may be at once less resistant to and more tolerant of microparasitic infection. For example, helminth co-infected hosts may be more prone to high malaria densities (i.e. less resistant), but less prone to the immunopathological symptoms of cerebral malaria (i.e. more tolerant; Specht & Hoerauf 2007). Indeed, these opposing effects of co-infection on the determinants of host health may be the crux of the recent debate about how concurrent helminth infection affects the course of human malaria (Specht & Hoerauf 2007). It may therefore be important to decide whether medical interventions would do better to promote resistance or tolerance in co-infected populations.
(c) Genetic knockouts
Some of the best evidence that animals have defences against infections which involve not only resistance but also tolerance come from infection experiments with genetically engineered animals, for example ‘knockout’ mice, where deletion of a particular gene leads to altered disease severity without concurrent changes in parasite intensities (table 1). Such data do not of course reveal natural genetic variation, but they do demonstrate that animals possess mechanisms of disease control which do not simply involve reductions in parasite burden. These data also provide some strong hints of the sort of biological mechanisms that can underpin tolerance.
Table 1.
Infection experiments with knockout mice where gene deletion apparently affects tolerance. (We included studies where gene deletion has either (i) an effect on host health/fitness but no effect on resistance (measured as the inverse of infection intensity), or (ii) opposing effects on health/fitness and resistance.)
| knocked out gene | function | parasite | effect of knockout on resistance | effect of knockout on host health/fitness | reference |
|---|---|---|---|---|---|
| IL-10 | reduces inflammation | Plasmodium chabaudi | 0 | − (increased mortality) | Li et al. (1999) |
| IL-10 | Trypanosoma cruzi | + | − (increased mortality) | Hölscher et al. (2000) | |
| IL-10 | Borrelia burgdorferi | + | − (increased arthritis) | Brown et al. (1999) | |
| MyD88 | propagates intracellular signals when PAMPs bind TLRs | Plasmodium chabaudi | 0 | + (reduced weight loss) | Franklin et al. (2007) |
| 5-lipoxygenase | regulates pro-inflammatory cytokines (IL-12 & IFN-γ) | Toxoplasma gondii | + | − (increased mortality) | Aliberti et al. (2002) |
| TNF receptor p55 | promotes formation of granulomas around parasites in tissue | Mycobacterium avium | 0 | − (increased mortality) | Ehlers et al. (1999) |
| inducible nitric oxide synthase (iNOS) | produces NO, a potent effector molecule | Mycobacterium avium | + | − (increased mortality) | Ehlers et al. (2001) |
| IFN-γ | promotes inflammation | respiratory syncytical virus | 0 or − | + (reduced weight loss) | Ostler et al. (2002) |
| haem oxygenase-1 | detoxifies haem, a by-product of the oxidation of haemoglobin | Plasmodium berghei | 0 | − (increased mortality from cerebral malaria) | Pamplona et al. (2007) |
| urokinase plasminogen activator (uPA) and its receptor (uPAR) | enhances adhesion of platelets | Plasmodium berghei | 0 | + (reduced mortality from cerebral malaria) | Piguet et al. (2000) |
In many human diseases, a considerable proportion of the harm is due to the host's immune response rather than a direct effect of the parasite replicating in host tissue (Graham et al. 2005a). If the extent of such immunopathology for a given parasite burden varies among host genotypes, this could result in variation in tolerance. This scenario is well supported by infection experiments with knockout mice. A number of such studies have shown that deletion of particular immune defence genes may have an effect on host health or fitness, even though there is no measurable effect on parasite burden. For example, Li et al. (1999) found that mice deficient in IL-10 (interleukin-10; an anti-inflammatory cytokine) and wild-type mice had similar burdens of P. chabaudi, but knockouts had much higher mortality rates. Thus, there was no difference in resistance, but IL-10 knockouts nonetheless suffered more from the infection. In some cases, gene deletion has even been found to have opposing effects on resistance and health/fitness (table 1). For instance, in an infection experiment with T. cruzi, Hölscher et al. (2000) found that mice deficient in IL-10 had lower infection intensity but higher mortality than normal mice. These results strongly indicate that presence/absence of IL-10 causes variation for tolerance. Similar results have been obtained in knockout studies of other genes that, like IL-10, are directly involved in regulating inflammation: IFN-γ (Ostler et al. 2002), 5-lipoxygenase (Aliberti et al. 2002) and iNOS (Ehlers et al. 2001).
Immune genes encoding receptors such as major histocompatibility complex (MHC), whose role is to recognize parasite-derived molecules, could also affect tolerance. A host with the right receptor (allele) for a particular parasite could probably mount a more specific response, which should reduce immunopathology caused by collateral damage. Evidence for this scenario comes from a study of Theiler's murine encephalomyelitis virus. This virus infects the central nervous system and is used as an animal model of multiple sclerosis. Drescher et al. (1998) found that transgenic mice possessing a particular human MHC gene showed much reduced pathology (breakdown of nervous tissue) compared with controls, even though transgenes and controls had similar virus titres.
Other factors that may affect tolerance include mechanisms involved in the scavenging of damaging molecules and the repair of tissue destroyed by parasites (or host immune responses). In the case of malaria, a possible example of such a mechanism is haem oxygenase-1 (HO-1) (table 1): the destruction of red blood cells during malaria infection results in release of free haem, which is cytotoxic. HO-1 transforms haem into non-toxic compounds. A study with knockout mice lacking the gene encoding HO-1 showed that this enzyme protects the host against cerebral malaria without affecting parasite densities (Pamplona et al. 2007). However, the immune system is also indirectly involved in this case: haem causes disruption of the blood–brain barrier, which activates leucocytes (specifically CD8+ T cells), which in turn may lead to cerebral malaria (Pamplona et al. 2007). Thus, genes that are not primarily immune defence genes may affect tolerance via inflammatory processes.
Taken together these infection experiments with knockout mice indicate that genes that are directly or indirectly involved in immune responses can affect tolerance. Many of the genes in these pathways are known to be polymorphic in humans (Hill 2001), so it is clearly possible that they may also contribute to tolerance variation in natural populations. Either way, their very existence, revealed by these sorts of experiments, demonstrates that a substantial component of host defence may be involved in tolerance rather than resistance.
Knockout studies have also revealed the existence of tolerance mechanisms in invertebrates. Ayres et al. (2008) infected over 1000 mutant lines of D. melanogaster with the intracellular bacterial pathogen Listeria monocytogenes. Eighteen mutants were more likely to die from infection than the wild-type. Infection intensities in 12 of these were elevated, suggesting these mutants had defective resistance pathways. The remaining six were more likely to die from infection in the absence of elevated pathogen titres. These mutants therefore seem likely to have defects in tolerance pathways. The powerful tools available to fly geneticists should soon reveal what these pathways are.
5. Practical issues in the measurement of tolerance
We argued in §2 that the reaction norm approach is the best way to demonstrate variation in tolerance. But even with that approach, there are some factors that can lead to spurious variation for tolerance; we here discuss how these potential problems can be addressed. To simplify the discussion, we assume that the experimental aim is the elucidation of genetic variation in tolerance, and hence that comparisons of tolerance are being made across host genotypes. The issues are the same when comparison of tolerance is being made across any other host types (e.g. hosts raised in different conditions, or different ages or gender).
First, it is important to measure parasite burden in such a way that it accurately reflects host resistance; otherwise it may not be possible to unambiguously distinguish between resistance and tolerance. In experimental studies, the most commonly used measure of parasite burden is the density at the peak of infection. However, measuring the ability to control peak parasite density does not necessarily take all aspects of resistance into account. For example, there may also be variation in clearance rate, which is clearly a component of resistance. Importantly, if host genotypes vary in clearance rate, but only peak density is measured, variation in resistance may be interpreted as tolerance. This problem can be overcome if an appropriate summary measure of parasite burden during the course of the infection can be found. This might for example be the average parasite density or the total number of parasites accumulated up to the time point when disease severity or fitness is measured.
Second, it is crucial to test whether reaction norms are linear or nonlinear. If reaction norms are nonlinear, as revealed, for example, by a statistically significant quadratic infection intensity term, a couple of interesting complexities arise:
In the case of nonlinear reaction norms, a statistical interaction between host genotype and either the linear or quadratic term in the regression would indicate genetic variation for tolerance. If the interaction only involves the linear infection intensity term, tolerance can still be quantified as the linear coefficient. However, if the interaction involves the quadratic term, it is less straightforward to quantify a host genotype's tolerance (figure 3a). One solution is to scale the reaction norms for different host genotypes so that they have the same intercept (to avoid the general vigour problem), and then use the area under the curve as a measure of tolerance (where a larger area means higher tolerance; Pilson 2000).
If the reaction norm is nonlinear and the average infection intensity differs between host genotypes (which could occur if genotypes differ in resistance), fitting only a linear term in the regression can produce both a falsely significant interaction and a spurious correlation between tolerance and resistance (Tiffin & Inouye 2000; figure 3b). To be able to rule out this potential problem, it is crucial to have a large overlap in parasite burden between host genotypes. In studies of plant tolerance to herbivory, this can be achieved by experimentally imposing fixed levels of damage (Tiffin & Inouye 2000). However, when working with parasites, it is rarely possible to experimentally control infection intensities at fixed levels (because intensities are determined by dynamic interactions between the host and parasite). Still, it may often be possible to experimentally enhance the variation in infection intensity by using, for instance, several different parasite clones that vary in the infection intensity they induce (Råberg et al. 2007), chemotherapy to artificially reduce burdens in some animals, or to infect animals with different doses of the same parasite strain (Timms et al. 2001).
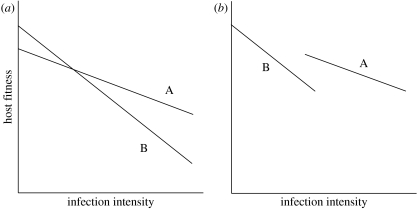
Figure 3.
(a) Reaction norms of different host genotypes (A and B) differ in shape, that is, there is a statistical interaction between host genotype and the quadratic infection intensity term. In such cases, it may be difficult to rank the tolerance of different host types. For illustration purposes, we show an extreme example where one host genotype has a convex reaction norm while the other has a concave norm; more subtle differences are perhaps more likely in reality. (b) If the range of parasite burdens differs between host genotypes (A and B), and the overall reaction norm (dashed curve) is nonlinear, an analysis that only considers linear relationships may yield the incorrect conclusion that host genotypes vary in tolerance (in this case that B is more tolerant than A) (Tiffin & Inouye 2000).
Third, when infection intensities cannot be experimentally controlled at fixed levels (which they usually cannot; see above), there is a risk that estimates of tolerance are biased by an unmeasured factor that affects both infection intensities and fitness independently of each other (Tiffin & Inouye 2000). As long as any such factor affects all host genotypes in the same way, this will not influence estimates of genetic variation for tolerance. However, it may inflate estimates of genetic variation for tolerance if there is a genotype-by-environment (G×E) interaction for the host's response to the unmeasured factor (Tiffin & Inouye 2000). This could be a problem when the magnitude of the genotype-by-environment interaction for this unmeasured variable is large relative to the effect of infection intensity on fitness; otherwise the effect on estimates of tolerance should be small (Tiffin & Inouye 2000). This potential problem has not received much attention in the plant literature, so further work is clearly required before it is possible to judge how important it is. For the moment we suggest that the best one can do to avoid or at least minimize this potential problem is to (i) conduct studies in homogenous environments, ideally in the laboratory (to limit the magnitude of any interactions between host genotype and unknown environmental variables) and (ii) enhance the variation in infection intensities experimentally, for example by using a range of different parasite genotypes or inoculation doses (because the more of the variation that is due to factors controlled by the experimenter, the less the scope for confounding effects of unmeasured environmental variables).
6. Open questions
In this section, we provide a list of issues that seem to us to be the most important for further empirical work.
(a) Do animals generally show variation in tolerance?
As reviewed above (§4), a number of studies indicate that animals may show genetic as well as environmental variation in tolerance to a wide range of parasites. However, with the exception of the study on rodent malaria by Råberg et al. (2007), these studies have not aimed to study tolerance, and have therefore usually not addressed potentially confounding factors. In many of the cases, it is therefore difficult to completely rule out alternative explanations for the observed patterns. Moreover, most of the studies above concern laboratory or domestic animals and are therefore not necessarily informative about the amount of tolerance variation in nature. More studies explicitly testing for tolerance by employing the statistical framework developed in plant biology are clearly needed before it is possible to draw any firm conclusions regarding the contribution of genes and various environmental factors to variation in tolerance in animals. In particular, we eagerly await studies of tolerance in natural host–parasite systems. Beyond merely demonstrating statistically significant variation for tolerance among host genotypes or phenotypes, it would also be interesting to investigate the relative importance of resistance and tolerance, that is, how much of the variation in health or fitness during infection can be attributed to each of these components of defence?
(b) Does tolerance have costs?
If tolerance is heritable, what prevents hosts from evolving perfect tolerance (so that b=0 in equation (2.1))? One factor that could constrain the evolution of tolerance is if it has not only fitness benefits, but also costs to the host (Strauss & Agrawal 1999; Stowe et al. 2000; Fornoni et al. 2004; Miller et al. 2006). In principle, such costs could come about in several different ways.
First, higher tolerance may lead to reduced fitness in the absence of parasites. In the plant literature, this is often referred to as ‘allocation costs’ (Stowe et al. 2000). Allocation costs are expected to arise simply because maintenance of the physiological mechanisms that enhance tolerance requires resources, which means resources have to be allocated from other fitness enhancing traits. This type of cost can be detected as a negative genetic correlation between tolerance and fitness in the absence of parasites, that is, a negative correlation between the intercept and slope in equation (2.1) (see figure 4a). Several plant studies have found evidence for this type of cost, in the context of tolerance to parasites and pathogens (Simms & Triplett 1994; Koskela et al. 2002), as well as herbivores (Tiffin & Rausher 1999). It is important to note that it is not straightforward to obtain a correct estimate of the correlation between the intercept and slope, because these two parameters are not independent (Tiffin & Rausher 1999).
Figure 4.
Schematic of different types of costs of tolerance. (a) Tolerance is costly in the sense that a more tolerant genotype (A) has lower fitness at low or zero parasite burdens. (b) Tolerance is costly in the sense that a more tolerant genotype (A) has lower resistance.
Second, costs of tolerance may be expressed in the presence of parasites in the form of a negative genetic correlation between tolerance and resistance, that is, more resistant genotypes are less tolerant and vice versa (figure 4b). A negative genetic correlation may be a result of either antagonistic pleiotropy or linkage disequilibrium between different loci (Stowe et al. 2000). Antagonistic pleiotropy means that a single gene has opposing effects on different traits. Linkage disequilibrium may arise when correlational selection favours particular combinations of two traits. This form of selection can build up and maintain linkage disequilibria resulting in genetic correlations between traits (although recombination will make these correlations decay rapidly if selection is relaxed; Sinervo & Svensson 2002). Correlational selection may operate in the present context because resistance and tolerance are mutually redundant traits; a completely resistant host cannot improve its defence by enhancing its tolerance, and vice versa. Thus, natural selection should be expected to favour either high resistance and low tolerance, or high tolerance and low resistance, or some combination of intermediate values of both traits, but not maximal (or minimal) values of both resistance and tolerance (Fornoni et al. 2004; Restif & Koella 2004). It is important to elucidate the underlying cause of a negative genetic correlation, because a correlation caused by antagonistic pleiotropy represents a more severe constraint on adaptive evolution than one caused by linkage disequilibrium (Rausher 1996). In plants, a negative genetic correlation between resistance and tolerance has so far only been demonstrated in the context of defence against herbivory, and only in two studies (Fineblum & Rausher 1995; Stowe 1998). The relative importance of antagonistic pleiotropy and correlational selection for generating these correlations is as yet unknown.
Both allocation costs and negative genetic correlations between tolerance and resistance may be important in animal tolerance. The study of human malaria in Kenya provides some indication that higher tolerance may carry a cost in the absence of parasites (i.e. an allocation cost): Wambua et al. (2006) found that individuals hetero- or homozygous for α+-thalassaemia had lower haemoglobin concentration when uninfected compared with individuals homozygous for the wild-type (see fig. 2 in Wambua et al. 2006). Evidence that tolerance may be costly in the currency of resistance comes from the study of rodent malaria in laboratory mice by Råberg et al. (2007). Here, there was a negative correlation between tolerance and resistance across mouse strains (i.e. a genetic correlation). The fact that several infection experiments with knockout mice have found that deletion of particular immune defence genes may have opposing effects on resistance and health/fitness (table 1) suggest that this correlation may be a result of antagonistic pleiotropy.
(c) What is the genetic basis for tolerance in animals?
In plants, where tolerance has been studied for a long time, genes conferring tolerance have not yet been identified at a molecular level, despite their commercial importance (Rausher 2001). The availability of a wide range of knockout and transgenic mice offers a unique possibility to elucidate the genetic basis of tolerance variation in animals. The knockout studies reviewed above suggest that genes that have the potential to affect tolerance are usually at least indirectly involved in immune function. This pattern may be real, or may be a result of the fact that infection experiments with knockout mice have, for natural reasons, largely focused on these types of genes. We think it is likely that a range of other mechanisms will also be involved, particularly those affecting the speed of tissue repair. For instance, genetic differences in the rate of erythropoeisis will surely underpin at least some of the variations in anaemia caused by malaria.
(d) Can patterns of resistance and tolerance be interpreted in an adaptive framework?
A natural hypothesis is that the fitness benefits and costs of resistance and tolerance vary across environmental conditions, favouring different combinations of these two components of defence under different circumstances. For example, it has been argued that a high rate of infection but low virulence should select for host tolerance, whereas the opposite should favour resistance (Restif & Koella 2004). Similar adaptive scenarios can be envisaged for variation in host reproductive status, body condition, etc., which could lead to phenotypic plasticity in tolerance. How much of the variation in tolerance within and across populations can be interpreted in terms of such adaptive variation? This question could be addressed with comparative analyses as well as experimental manipulations of host phenotypes.
(e) How should parasite factors be incorporated?
Throughout, we have taken the host-centric viewpoint that tolerance is determined primarily by host factors, so that it makes sense to speak of more or less tolerant hosts. Yet it is quite possible that there might be some kind of interaction between host genotype and parasite genotype, so that a particular host genotype that is tolerant to one parasite strain is less tolerant of another and vice versa. Decomposing the causal elements of host health into host resistance, host tolerance, various parasite factors, and the interactions between all these elements is a major challenge.
7. CODA
Experiments analogous to those of Råberg et al. (2007) could relatively easily address many of the questions we have raised above. If tolerance turns out to be an important feature of animal defence, as we suspect, it will become important to investigate the ecological and evolutionary consequences of tolerance versus resistance in more detail. In particular, our understanding of how tolerance affects the coevolution between host and parasites is still rudimentary. As briefly mentioned in §1, a number of authors have argued that since tolerance does not have any direct negative effects on the performance of the parasite, it should not select for countermeasures in the parasite (Caldwell et al. 1958; Schafer 1971; Clarke 1986; Rausher 2001). Consequently, tolerance, unlike resistance, should not fuel antagonistic coevolution. This verbal argument has been around for half a century, yet this question has to our knowledge not been thoroughly analysed even in theory. In fact, there are reasons to suspect that this view is not entirely correct. One could imagine that evolution of tolerance may impose selection on the parasite after all, because tolerance reduces the chances a virulent strain will truncate its infectious period by killing its host. Assuming virulence is correlated with transmission rate, which it often is (Mackinnon et al. 2008), tolerance should therefore favour more virulent parasites (Miller et al. 2006). More virulent parasites could, in turn, select for further increases in tolerance. Thus, it is after all not inconceivable that evolution of tolerance may result in host–parasite coevolution. Nonetheless, we note that, by contrast with coevolution involving resistance, this tolerance ‘coevolution’ is not antagonistic in the sense of countermeasure matching countermeasure. We suspect that, if tolerance-virulence coevolution occurs, it will therefore result in a new stable equilibrium rather than the open-ended non-equilibrium dynamics typical of antagonistic coevolution. However, this issue requires further theoretical work: intuition is often an alarmingly poor guide in host–parasite dynamics (Read et al. 1999).
A question that is related to the issue about tolerance and coevolution is what the evolutionary dynamics of tolerance are. One of the most interesting implications of tolerance is that, as a defence mechanism, it is theoretically more likely to go to fixation than a resistance mechanism (Roy & Kirchner 2000). This is because resistance mechanisms work by eliminating parasites, thus reducing the very selection pressures that favoured them in the first place. Consequently, as a particular resistance mechanism nears fixation in a population, parasites should die out or change, rendering the resistance mechanism in question either unnecessary or useless. By contrast, tolerance does not eliminate the selective pressure that favoured it (parasites) and so it can more easily go to fixation (although variation can still be maintained if tolerance is costly enough; see §6b). This insight raises an important possibility: are the majority of host defence mechanisms that have occurred during evolution in fact tolerance mechanisms? Perhaps a very important reason for hosts not getting sicker following infection is because an endless succession of tolerance mechanisms has become fixed in populations. Is the focus on resistance mechanisms not simply because parasite killing is more readily observed but also because also any particular resistance mechanism is less likely to go to fixation and so is more frequently variable and detectable? Tolerance may in fact be more important over evolutionary time.
Work on tolerance should also be relevant beyond academic evolutionary ecology. As Rausher (2001) has pointed out in the context of plant diseases, the possibly different coevolutionary outcomes prompted by tolerance and resistance raise the prospect that different manipulations of host defences may be more or less evolution-proof. By not imposing selection for pathogen countermeasures, public or animal health interventions that increase tolerance may be less likely to be eroded by pathogen evolution than interventions that increase resistance. In the agricultural sector, attempts to select for increased yield in the face of parasite challenge may come to nothing (or even make things worse) if there is a trade-off between resistance and tolerance. And finally, a key issue in disease epidemiology is the existence of ‘superspreaders’, that is, hosts that are responsible for a disproportionately large number of secondary cases (Lloyd-Smith et al. 2005; Matthews et al. 2006). Identifying and removing superspreaders has a massive impact on disease incidence. Are superspreaders tolerant hosts in largely resistant populations?
Acknowledgments
L.R. was supported by a grant from the Swedish Research Council (VR). A.L.G. is a fellow of the UK Biotechnology and Biological Sciences Research Council. We thank Tom Little, Dave Shuker, Martin Stjernman and Andy Stephenson for discussion, and Tom Gosden and Erik Svensson for comments on the manuscript.
Endnote
Throughout, we use the terms pathogen and parasite interchangeably, to make the point that we are inclusively talking about all infectious agents, irrespective of the discipline that studies them. Microbiologists, ecologists and parasitologists also use different terms to mean the number of pathogens/parasites per host or per unit of host tissue, so we interchangeably use titre, load, burden and intensity, again to avoid the implication we are talking about any particular group of infectious agents.
One contribution of 11 to a Theme Issue ‘Ecological immunology’.
References
- Aidoo M., Terlouw D.J., Kolczak M., McElroy P.D., ter Kuile F.O., Kariuki S., Nahlen B.L., Lal A.A., Udhayakumar V. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–1312. doi: 10.1016/S0140-6736(02)08273-9. doi:10.1016/S0140-6736(02)08273-9 [DOI] [PubMed] [Google Scholar]
- Aliberti J., Serhan C., Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J. Exp. Med. 2002;196:1253–1262. doi: 10.1084/jem.20021183. doi:10.1084/jem.20021183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S.J., O'Donnell A., Alexander N.D.E., Alpers M.P., Peto T.E.A., Clegg J.B., Weatherall D.J. α+-Thalassemia protects children against disease caused by other infections as well as malaria. Proc. Natl Acad. Sci. USA. 1997;94:14 736–14 741. doi: 10.1073/pnas.94.26.14736. doi:10.1073/pnas.94.26.14736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres J., Freitag N., Schneider D. Identification of Drosophila mutants altering defense to and endurance of Listeria monocytogenes infection. Genetics. 2008;178:1807–1815. doi: 10.1534/genetics.107.083782. doi:10.1534/genetics.107.083782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset S.A., Morris C.A. Feasibility and implications of breeding sheep for resilience to nematode challenge. Int. J. Parasitol. 1996;26:857–868. doi: 10.1016/s0020-7519(96)80056-7. doi:10.1016/S0020-7519(96)80056-7 [DOI] [PubMed] [Google Scholar]
- Boots M. Fight or learn to live with the consequences? Trends Ecol. Evol. 2008;23:248–250. doi: 10.1016/j.tree.2008.01.006. doi:10.1016/j.tree.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Boutlis C., Yeo T., Anstey N.M. Malaria tolerance—for whom the cell tolls? Trends Parasitol. 2006;22:371–377. doi: 10.1016/j.pt.2006.06.002. doi:10.1016/j.pt.2006.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.P., Zachary J.F., Teuscher C., Weis J.J., Wooten R.M. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buendia A.J., Fallon P.G., Del Rio L., Ortega N., Caro M.R., Gallego M.C., Salinas J. Previous infection with the nematode Nippostrongylus brasiliensis alters the immune specific response against Chlamydophila abortus infection. Microb. Pathog. 2002;33:7–15. doi: 10.1006/mpat.2002.0507. doi:10.1006/mpat.2002.0507 [DOI] [PubMed] [Google Scholar]
- Caldwell R., Compton L., Patterson F. Tolerance to cereal leaf rusts. Science. 1958;128:714–715. doi: 10.1126/science.128.3326.714. doi:10.1126/science.128.3326.714 [DOI] [PubMed] [Google Scholar]
- Clarke D. Tolerance of parasites and disease in plants and its significance in host–parasite interactions. Adv. Plant Pathol. 1986;5:161–197. [Google Scholar]
- Cobb N. Contributions to an economic knowledge of Australian rusts (Uredineae) Agric. Gaz. NSW. 1894;5:239–250. [Google Scholar]
- Corby-Harris V., Habel K., Ali F., Promislow D. Alternative measures of response to Pseudomonas aeruginosa infection in Drosophila melanogaster. J. Evol. Biol. 2007;20:526–533. doi: 10.1111/j.1420-9101.2006.01267.x. doi:10.1111/j.1420-9101.2006.01267.x [DOI] [PubMed] [Google Scholar]
- Degee A.L.W., Levine R.F., Mansfield J.M. Genetics of resistance to the African trypanosomes. 6. Heredity of resistance and variable surface glycoprotein-specific immune responses. J. Immunol. 1988;140:283–288. [PubMed] [Google Scholar]
- Drescher K., Nguyen L., Taneja V., Coenen M., Leibowitz J., Strauss G., Hammerling G., David C., Rodriguez M. Expression of the human histocompatibility leukocyte antigen DR3 transgene reduces the severity of demyelineation in a murine model of multiple sclerosis. J. Clin. Invest. 1998;101:1765–1774. doi: 10.1172/JCI167. doi:10.1172/JCI167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du D., Winsor J., Smith M., DeNicco A., Stephenson A. Resistance and tolerance to herbivory changes with inbreeding and ontogeny in a wild gourd (Cucurbitaceae) Am. J. Bot. 2008;95:84–92. doi: 10.3732/ajb.95.1.84. doi:10.3732/ajb.95.1.84 [DOI] [PubMed] [Google Scholar]
- Ehlers S., Benini J., Kutsch S., Endres R., Rietschel E.T., Pfeffer K. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect. Immun. 1999;67:3571–3579. doi: 10.1128/iai.67.7.3571-3579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S., Benini J., Held H.D., Roeck C., Alber G., Uhlig S. αβ T cell receptor-positive cells and interferon-γ, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology. J. Exp. Med. 2001;194:1847–1859. doi: 10.1084/jem.194.12.1847. doi:10.1084/jem.194.12.1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineblum W., Rausher M. Tradeoff between resistance and tolerance to herbivore damage in a morning glory. Nature. 1995;377:517–520. doi:10.1038/377517a0 [Google Scholar]
- Fornoni J., Nuñez-Farfan J., Valverde P.L., Rausher M. Evolution of mixed strategies of plant defense allocation against natural enemies. Evolution. 2004;58:1685–1695. doi: 10.1111/j.0014-3820.2004.tb00454.x. doi:10.1554/03-510 [DOI] [PubMed] [Google Scholar]
- Fortin A., Stevenson M.M., Gros P. Complex genetic control of susceptibility to malaria in mice. Genes Immun. 2002;3:177–186. doi: 10.1038/sj.gene.6363841. doi:10.1038/sj.gene.6363841 [DOI] [PubMed] [Google Scholar]
- Franklin B.S., et al. MyD88-dependent activation of dendritic cells and CD4+ T lymphocytes mediates symptoms, but is not required for the immunological control of parasites during rodent malaria. Microbes Infect. 2007;9:881–890. doi: 10.1016/j.micinf.2007.03.007. doi:10.1016/j.micinf.2007.03.007 [DOI] [PubMed] [Google Scholar]
- Fry J.D. The general vigor problem—can antagonistic pleiotropy be detected when genetic covariances are positive? Evolution. 1993;47:327–333. doi: 10.1111/j.1558-5646.1993.tb01224.x. doi:10.2307/2410143 [DOI] [PubMed] [Google Scholar]
- Furze R.C., Hussell T., Selkirk M.E. Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infect. Immun. 2006;74:1924–1932. doi: 10.1128/IAI.74.3.1924-1932.2006. doi:10.1128/IAI.74.3.1924-1932.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graefe S.E.B., Meyer B.S., Muller-Myhsok B., Ruschendorf F., Drosten C., Laue T., Steeg C., Nurnberg P., Fleischer B. Murine susceptibility to Chagas' disease maps to chromosomes 5 and 17. Genes Immun. 2003;4:321–325. doi: 10.1038/sj.gene.6363972. doi:10.1038/sj.gene.6363972 [DOI] [PubMed] [Google Scholar]
- Graham A.L., Allen J.E., Read A.F. Evolutionary causes and consequences of immunopathology. Annu. Rev. Ecol. Syst. 2005a;36:373–397. doi:10.1146/annurev.ecolsys.36.102003.152622 [Google Scholar]
- Graham A.L., Lamb T., Read A.F., Allen J.E. Malaria–filaria coinfection in mice makes malarial disease more severe unless filarial infection achieves patency. J. Infect. Dis. 2005b;191:410–421. doi: 10.1086/426871. doi:10.1086/426871 [DOI] [PubMed] [Google Scholar]
- Hanotte O., et al. Mapping of quantitative trait loci controlling trypanotolerance in a cross of tolerant west African N'Dama and susceptible east African Boran cattle. Proc. Natl Acad. Sci. USA. 2003;100:7443–7448. doi: 10.1073/pnas.1232392100. doi:10.1073/pnas.1232392100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartgers F.C., Yazdanbakhsh M. Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunol. 2006;28:497–506. doi: 10.1111/j.1365-3024.2006.00901.x. doi:10.1111/j.1365-3024.2006.00901.x [DOI] [PubMed] [Google Scholar]
- Helmby H., Kullberg M., Troye-Blomberg M. Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infect. Immun. 1998;66:5167–5174. doi: 10.1128/iai.66.11.5167-5174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.V.S. The genomics and genetics of human infectious disease susceptibility. Annu. Rev. Genom. Hum. Genet. 2001;2:373–400. doi: 10.1146/annurev.genom.2.1.373. doi:10.1146/annurev.genom.2.1.373 [DOI] [PubMed] [Google Scholar]
- Hill A.V.S., et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. doi:10.1038/352595a0 [DOI] [PubMed] [Google Scholar]
- Hölscher C., Mohrs M., Dai W.J., Köhler G., Ryffel B., Schaub G.A., Mossmann H., Brombacher F. Tumour necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect. Immun. 2000;68:4075–4083. doi: 10.1128/iai.68.7.4075-4083.2000. doi:10.1128/IAI.68.7.4075-4083.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle D. Genetic covariance of fitness correlates: what genetic correlations are made of and why it matters. Evolution. 1991;45:630–648. doi: 10.1111/j.1558-5646.1991.tb04334.x. doi:10.2307/2409916 [DOI] [PubMed] [Google Scholar]
- Johnson J., Suzuki Y., Mack D., Mui E., Estes R., David C., Skamene E., Forman J., McLeod R. Genetic analysis of influences on survival following Toxoplasma gondii infection. Int. J. Parasitol. 2002;32:179–185. doi: 10.1016/s0020-7519(01)00321-6. doi:10.1016/S0020-7519(01)00321-6 [DOI] [PubMed] [Google Scholar]
- Kamal S.M., Khalifa K.E. Immune modulation by helminthic infections: worms and viral infections. Parasite Immunol. 2006;28:483–496. doi: 10.1111/j.1365-3024.2006.00909.x. doi:10.1111/j.1365-3024.2006.00909.x [DOI] [PubMed] [Google Scholar]
- Kloosterman A., Parmentier H.K., Ploeger H. Breeding cattle and sheep for resistance to gastrointestinal nematodes. Parasitol. Today. 1992;8:330–335. doi: 10.1016/0169-4758(92)90066-b. doi:10.1016/0169-4758(92)90066-B [DOI] [PubMed] [Google Scholar]
- Kniskern J., Rausher M.D. Environmental variation mediates the deleterious effects of Coleosporium ipomoeae on Ipomoea purpurea. Ecology. 2006;87:675–685. doi: 10.1890/05-1327. doi:10.1890/05-1327 [DOI] [PubMed] [Google Scholar]
- Koskela T., Puustinen S., Salonen V., Mutikainen P. Resistance and tolerance in a host plant–holoparasitic plant interaction: genetic variation and costs. Evolution. 2002;56:899–908. doi: 10.1111/j.0014-3820.2002.tb01403.x. doi:10.1111/j.0014-3820.2002.tb01403.x [DOI] [PubMed] [Google Scholar]
- Kover P.X., Schaal B.A. Genetic variation for disease resistance and tolerance among Arabidopsis thaliana accessions. Proc. Natl Acad. Sci. USA. 2002;99:11 270–11 274. doi: 10.1073/pnas.102288999. doi:10.1073/pnas.102288999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Corraliza I., Langhorne J. A defect in interleukin-10 leads to enhanced malaria disease in Plasmodium chabaudi chabaudi infection in mice. Infect. Immun. 1999;67:4435–4442. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Smith J.O., Schreiber S.J., Kopp P.E., Getz W.M. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. doi:10.1038/nature04153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Seiler K.P., Eichwald E.J., Weis J.H., Teuscher C., Weis J.J. Distinct characteristics of resistance to Borrelia burgdorferi induced arthritis in C57BL/6N mice. Infect. Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon M.J., Read A.F. Virulence in malaria: an evolutionary viewpoint. Phil. Trans. R. Soc. B. 2004;359:965–986. doi: 10.1098/rstb.2003.1414. doi:10.1098/rstb.2003.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon M.J., Gandon S., Read A.F. Virulence evolution in response to vaccination: the case of malaria. Vaccine. 2008;26(Suppl.):C42–C52. doi: 10.1016/j.vaccine.2008.04.012. doi:10.1016/j.vaccine.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels R.M., Balic A., Gomez-Escobar N., Nair M., Taylor M.D., Allen J.E. Helminth parasites—masters of regulation. Immunol. Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. doi:10.1111/j.0105-2896.2004.00191.x [DOI] [PubMed] [Google Scholar]
- Malo D., Skamene E. Genetic control of host resistance to infection. Trends Genet. 1994;10:365–371. doi: 10.1016/0168-9525(94)90133-3. doi:10.1016/0168-9525(94)90133-3 [DOI] [PubMed] [Google Scholar]
- Marshall A.J., Brunet L.R., van Gessel Y., Alcaraz A., Bliss S.K., Pearce E.J., Denkers E.Y. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-α and early death in C57BL/6 mice. J. Immunol. 1999;163:2089–2097. [PubMed] [Google Scholar]
- Matthews L., et al. Heterogeneous shedding of Escherichia coli O157 in cattle and its implications for control. Proc. Natl Acad. Sci. USA. 2006;103:547–552. doi: 10.1073/pnas.0503776103. doi:10.1073/pnas.0503776103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire W., Hill A.V.S., Allsopp C.E.M., Greenwood B.M., Kwiatkowski D. Variation in the TNF-α promoter region associated susceptibility to cerebral malaria. Nature. 1994;371:508–511. doi: 10.1038/371508a0. doi:10.1038/371508a0 [DOI] [PubMed] [Google Scholar]
- Medina E., North R.J. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology. 1998;93:270–274. doi: 10.1046/j.1365-2567.1998.00419.x. doi:10.1046/j.1365-2567.1998.00419.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.R., White A., Boots M. The evolution of parasites in response to tolerance in their hosts: the good, the bad, and apparent commensalism. Evolution. 2006;60:945–956. doi:10.1554/05-654.1 [PubMed] [Google Scholar]
- Mockenhaupt F.P., Ehrhardt S., Gellert S., Otchwemah R.N., Dietz E., Anemana S.D., Bienzle U. α+-Thalassemia protects African children from severe malaria. Blood. 2004;104:2003–2006. doi: 10.1182/blood-2003-11-4090. doi:10.1182/blood-2003-11-4090 [DOI] [PubMed] [Google Scholar]
- Naessens J. Bovine trypanotolerance: a natural ability to prevent severe anaemia and haemophagocytic syndrome? Int. J. Parasitol. 2006;36:521–528. doi: 10.1016/j.ijpara.2006.02.012. doi:10.1016/j.ijpara.2006.02.012 [DOI] [PubMed] [Google Scholar]
- Ostler T., Davidson W., Ehl S. Virus clearance and immunopathology by CD8+ T cells during infection with respiratory syncytial virus are mediated by IFN-gamma. Eur. J. Immunol. 2002;32:2117–2123. doi: 10.1002/1521-4141(200208)32:8<2117::AID-IMMU2117>3.0.CO;2-C. doi:10.1002/1521-4141(200208)32:8<2117::AID-IMMU2117>3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- Page K.R., Scott A.L., Manabe Y.C. The expanding realm of heterologous immunity: friend or foe? Cell. Microbiol. 2006;8:185–196. doi: 10.1111/j.1462-5822.2005.00653.x. doi:10.1111/j.1462-5822.2005.00653.x [DOI] [PubMed] [Google Scholar]
- Paling R., Moloo S., Scott J., Gettinby G., Mcodimba F., Murray M. Susceptibility of N'Dama and Boran cattle to sequential challenges with tsetse-transmitted clones of Trypanosoma congolese. Parasite Immunol. 1991;13:427–445. doi: 10.1111/j.1365-3024.1991.tb00295.x. doi:10.1111/j.1365-3024.1991.tb00295.x [DOI] [PubMed] [Google Scholar]
- Pamplona A., et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 2007;13:703–710. doi: 10.1038/nm1586. doi:10.1038/nm1586 [DOI] [PubMed] [Google Scholar]
- Piguet P.F., Da Laperrousaz C., Vesin C., Tacchini-Cottier F., Senaldi G., Grau G.E. Delayed mortality and attenuated thrombocytopenia associated with severe malaria in urokinase- and urokinase receptor-deficient mice. Infect. Immun. 2000;68:3822–3829. doi: 10.1128/iai.68.7.3822-3829.2000. doi:10.1128/IAI.68.7.3822-3829.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilson D. The evolution of plant response to herbivory: simultaneously considering resistance and tolerance in Brassica rapa. Evol. Ecol. 2000;14:457–489. doi:10.1023/A:1010953714344 [Google Scholar]
- Playfair J., Taverne J., Bate C., de Souza J. The malaria vaccine: anti-parasite or anti-disease? Immunol. Today. 1990;11:25–27. doi: 10.1016/0167-5699(90)90007-v. doi:10.1016/0167-5699(90)90007-V [DOI] [PubMed] [Google Scholar]
- Råberg L., Sim D., Read A.F. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. doi:10.1126/science.1148526 [DOI] [PubMed] [Google Scholar]
- Rausher M.D. Genetic analysis of coevolution between plants and their natural enemies. Trends Genet. 1996;12:212–217. doi: 10.1016/0168-9525(96)10020-2. doi:10.1016/0168-9525(96)10020-2 [DOI] [PubMed] [Google Scholar]
- Rausher M.D. Co-evolution and plant resistance to natural enemies. Nature. 2001;411:857–864. doi: 10.1038/35081193. doi:10.1038/35081193 [DOI] [PubMed] [Google Scholar]
- Read A.F., et al. What can evolutionary biology contribute to understanding virulence? In: Stearns S., editor. Evolution in health and disease. Oxford University Press; Oxford, UK: 1999. pp. 205–215. [Google Scholar]
- Restif O., Koella J.C. Concurrent evolution of resistance and tolerance to pathogens. Am. Nat. 2004;164:E90–E102. doi: 10.1086/423713. doi:10.1086/423713 [DOI] [PubMed] [Google Scholar]
- Roitt I., Brostoff J., Male D. Mosby; London, UK: 1993. Immunology. [Google Scholar]
- Rowe L., Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. B. 1996;263:1415–1421. doi:10.1098/rspb.1996.0207 [Google Scholar]
- Roy B.A., Kirchner J.W. Evolutionary dynamics of pathogen resistance and tolerance. Evolution. 2000;54:51–63. doi: 10.1111/j.0014-3820.2000.tb00007.x. doi:10.1111/j.0014-3820.2000.tb00007.x [DOI] [PubMed] [Google Scholar]
- Schafer J. Tolerance to plant disease. Annu. Rev. Phytopathol. 1971;9:235–252. doi:10.1146/annurev.py.09.090171.001315 [Google Scholar]
- Schlichting C., Pigliucci M. Sinauer Associates; Sunderland, MA: 1998. Phenotypic plasticity: a reaction norm perspective. [Google Scholar]
- Schwartz R. Natural history of regulatory T cells and self-tolerance. Nat. Immunol. 2005;6:327–330. doi: 10.1038/ni1184. doi:10.1038/ni1184 [DOI] [PubMed] [Google Scholar]
- Simms E.L., Triplett J. Costs and benefits of plant responses to disease: resistance and tolerance. Evolution. 1994;48:1973–1985. doi: 10.1111/j.1558-5646.1994.tb02227.x. doi:10.2307/2410521 [DOI] [PubMed] [Google Scholar]
- Sinervo B., Svensson E. Correlational selection and the evolution of genomic architecture. Heredity. 2002;89:329–338. doi: 10.1038/sj.hdy.6800148. doi:10.1038/sj.hdy.6800148 [DOI] [PubMed] [Google Scholar]
- Smith J.A., Wilson K., Pilkington J.G., Pemberton J.M. Heritable variation in resistance to gastro-intestinal nematodes in an unmanaged mammal population. Proc. R. Soc. B. 1999;266:1283–1290. doi: 10.1098/rspb.1999.0776. doi:10.1098/rspb.1999.0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht S., Hoerauf A. Does helminth elimination promote or prevent malaria? Lancet. 2007;369:446–447. doi: 10.1016/S0140-6736(07)60210-4. doi:10.1016/S0140-6736(07)60210-4 [DOI] [PubMed] [Google Scholar]
- Stowe K. Experimental evolution of resistance in Brassica rapa: correlated response of tolerance in lines selected for glucosinolate content. Evolution. 1998;52:703–712. doi: 10.1111/j.1558-5646.1998.tb03695.x. doi:10.2307/2411265 [DOI] [PubMed] [Google Scholar]
- Stowe K., Marquis R., Hochwender C., Simms E.L. The evolutionary ecology of tolerance to consumer damage. Annu. Rev. Ecol. Syst. 2000;31:565–595. doi:10.1146/annurev.ecolsys.31.1.565 [Google Scholar]
- Strauss S.Y., Agrawal A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999;14:179–185. doi: 10.1016/s0169-5347(98)01576-6. doi:10.1016/S0169-5347(98)01576-6 [DOI] [PubMed] [Google Scholar]
- Strauss S.Y., Rudgers J.A., Lau J.A., Irwin R.E. Direct and ecological costs of resistance to herbivory. Trends Ecol. Evol. 2002;17:278–285. doi:10.1016/S0169-5347(02)02483-7 [Google Scholar]
- Su Z., Segura M., Morgan K., Loredo-Osti J.C., Stevenson M.M. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infect. Immun. 2005;73:3531–3539. doi: 10.1128/IAI.73.6.3531-3539.2005. doi:10.1128/IAI.73.6.3531-3539.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin P., Inouye B.D. Measuring tolerance to herbivory: accuracy and precision of estimates made using natural versus imposed damage. Evolution. 2000;54:1024–1029. doi: 10.1111/j.0014-3820.2000.tb00101.x. doi:10.1554/0014-3820(2000)054[1024:MTTHAA]2.3.CO;2 [DOI] [PubMed] [Google Scholar]
- Tiffin P., Rausher M.D. Genetic constraints and selection acting on tolerance to herbivory in the common morning glory Ipomoea purpurea. Am. Nat. 1999;154:700–716. doi: 10.1086/303271. doi:10.1086/303271 [DOI] [PubMed] [Google Scholar]
- Timms R., Colegrave N., Chan B.H.K., Read A.F. The effect of parasite dose on disease severity in the rodent malaria Plasmodium chabaudi. Parasitology. 2001;123:1–11. doi: 10.1017/s0031182001008083. doi:10.1017/S0031182001008083 [DOI] [PubMed] [Google Scholar]
- van Noordwijk A., de Jong G. Acquisition and allocation of resources: their influence on variation in life-history tactics. Am. Nat. 1986;128:137–142. doi:10.1086/284547 [Google Scholar]
- Wambua S., et al. The effect of α+-thalassemia on the incidence of malaria and other diseases in children living on the coast of Kenya. PLoS Med. 2006;3:0643–0651. doi: 10.1371/journal.pmed.0030158. doi:10.1371/journal.pmed.0030158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.N. Human red blood cell polymorphisms and malaria. Curr. Opin. Microbiol. 2006;9:388–394. doi: 10.1016/j.mib.2006.06.009. doi:10.1016/j.mib.2006.06.009 [DOI] [PubMed] [Google Scholar]
- Williams T.N., Mwangi T.W., Wambua S., Alexander N.D., Kortok M., Snow R.W., Marsh K. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J. Infect. Dis. 2005a;192:178–186. doi: 10.1086/430744. doi:10.1086/430744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.N., Wambua S., Uyoga S., Macharia A., Mwacharo J.K., Newton C.R.J.C., Maitland K. Both heterozygous and homozygous α+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005b;106:368–371. doi: 10.1182/blood-2005-01-0313. doi:10.1182/blood-2005-01-0313 [DOI] [PubMed] [Google Scholar]
- Woolhouse M.E.J., Webster J.P., Domingo E., Charlesworth B., Levin B. Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat. Genet. 2002;32:569–577. doi: 10.1038/ng1202-569. doi:10.1038/ng1202-569 [DOI] [PubMed] [Google Scholar]