Abstract
Although the faculty of memory holds information about the past, it is mostly about the present and the future, because it permits adaptive responses to ongoing events as well as to events yet to come. Since many elements in the future are uncertain, the plasticity machinery that encodes memories in the brain has to operate under the assumption that stored information is likely to require fast and recurrent updating. This assumption is reflected at multiple levels of the brain, including the synaptic and the cellular level. Recent findings cast new light on how combinations of plasticity and metaplasticity mechanisms could permit the brain to balance over time between stability and plasticity of the information stored.
Keywords: long-term memory, anticipation, storage, consolidations, plasticity, metaplasticity
1. Introduction
Human language commonly associates memory with the past. But memories are made mostly for the sake of present and future. Experience-dependent modifications in the individual's behaviour draw on the past to permit better adapted responses to ongoing reality as well as to the reality to come. Being able to anticipate even limited types or aspects of events is expected to endow the species with significant advantages. Under certain contextual and temporal conditions, our brain seems to be able to do just that. This requires that the plasticity machinery in the brain operate under the assumption that the future is uncertain and that information stored is likely to require quick and recurrent updating. This assumption should be reflected at multiple levels of organization of the brain, including the synaptic and cellular level.
2. Defining ‘future’
For the brain, future is anytime between a fraction of a second and a lifetime ahead. Except ageing and ultimately end of life, the further away the future, the less certain it is. The immediate cognitive future is practically inseparable from the present. If 20 ms or so is taken to be an estimate of the duration of cognitive present or the hypothetical ‘cognitive beat’ (Dudai 2002), but even simple actions take longer to complete (Thorpe et al. 1996; Van Tuernnout et al. 1998; Baddeley 2007), then every ongoing behaviour incorporates future tense. Ample evidence indeed indicates that the brain anticipates the world on a momentary basis (Anokhin 1974; e.g. Shima & Tanji 1998; Naya et al. 2001). Furthermore, selectionist (‘Darwinian’) theories of brain function consider stimulus-driven selection of endogenously generated internal pre-representations, some of which fit better than others to respond to the future event, as instrumental in our adaptive interaction with the world (Young 1979; Heidmann et al. 1984). Again, by definition, pre-representations are expected to precede the relevant ‘teaching’ stimulus by a fraction of a second. (See also Bar (2007) on proposed fast priming of memory representations in facilitating perception and cognition, and Fox et al. (2007) for an example of brain imaging data that might be construed to reflect pre-representations.)
In the present discussion, ‘future’ means further away from the present than fractions of a second only. It makes sense to also exclude from the discussion longer time windows that are still short enough to allow continual attentional control. This is because, for all practical purpose in everyday life, the brain may still consider as present or at most ‘present progressive’, a brief ongoing potential narrative that unfolds under attentional control. Hence, the time of operation of working memory, i.e. seconds to tens of seconds, is left out. Furthermore, there is evidence that neurons can still reach decisions on the state of their recently activated synapses even hours after the offset of a stimulus (Frey & Morris 1997). Since these cellular processes could be considered as time-locked to the direct consequence of the stimulus, none is considered here as a manifestation of ‘future’ with regard to that stimulus. All together, although the brain clearly differentiates distinctive post-stimulus time slices at the second, minute and hour range (Frey & Morris 1997; Toib et al. 1998; Coltheart 1999; Fusi et al. 2005; Gilboa et al. 2005; Buzsaki 2006; Smith et al. 2006; Hare et al. 2008; Hasson et al. 2008), this post-stimulus time window is neglected here.
The future in the context of this discussion is considered to start when memory becomes long-term. The assumption made here is that short-term memory (STM) could still readily adapt to ongoing events, but once the brain decides that the information is important enough to be stored in the long-term, the updating of that information becomes a particularly interesting challenge that involves balancing stability with plasticity. But when does long-term memory (LTM) start? The answer to this question depends on the discipline. For neurologists, for example, LTM is that memory that lasts for more than a few minutes, whereas for cellular and molecular neurobiologists who investigate behaving animals, it is memory that lasts more than a few hours, and by convention, over 24 hours (Dudai 2002). Since the discussion below draws heavily on cellular and molecular data, I have selected the molecular neurobiology view on when memory could be considered as long-term. The question posed in the title of this discussion could hence be rephrased as How the cellular machinery of long-term memory anticipates the uncertain future, where the ‘future’ lies at least a day head.
It should also be noted that the present discussion addresses ‘primitives’ of memory, i.e. basic mechanisms shared by different memory systems. It is likely that in addition to the current rich repertoire of taxonomies of memory systems (Tulving & Fergus 2000; Roediger et al. 2007; Tulving 2007), one could easily conceive a taxonomy based on the relative role of ‘present’ or ‘future’ in the alleged goal of the system. In such taxonomy, for example, skill and habit will be more present-oriented than episodic memory, whose function is assumed to involve reconstruction of future scenarios and imagination (Tulving 1983; Suddendorf & Corballis 1997; Atance & O'Neill 2005; Dudai & Carruthers 2005; Addis et al. 2007; Hassabis et al. 2007). However, the claim made here is that regardless of the weight that evolution had assigned to anticipation of change in the function of the specific memory system, in most memory systems, if not in all of them, there is a built-in capacity to anticipate change.
3. The LTM∼f(Growth) paradigm
A conceptual paradigm that has dominated biological models of learning and memory for over a century now, considers learning as a stimulus that triggers a local developmental shift that involves local growth processes in the brain. Memory is the outcome of these local growth processes. Holt (1931) epitomizes this view: ‘Growth and learning are one continuous process, to the earlier phases of which we give the one name, and to the later… phases we give the other’. He was not, of course, the first to suggest the memory–growth analogy. Attempts to translate this idea into specific biological algorithms and mechanisms preceded even the introduction of concepts as ‘neuron’ and ‘synapse’ into the jargon of the brain sciences: ‘For every act of memory’, says Bain (1872), ‘… there is a specific grouping or coordination of sensations and movements, by virtue of specific growth in the cell junctions’. Elaborate experience-dependent growth theories followed (e.g. Kappers 1917; Hebb 1949), paving the way to the proposal (Monne 1949), and then to the discovery (Flexner et al. 1963), that de novo macromolecular synthesis, so characteristic of developmental shifts and growth, is required for LTM. The introduction of the concept of memory consolidation (Muller & Pilzecker 1900) seemed also to fit the idea that memories mature over time, similarly to organs and organisms.
Consolidation refers to the progressive post-acquisition stabilization of the memory trace (McGaugh 2000; Dudai 2004). The term is used to denote hypothetical memory stabilization processes at different levels of brain organization. Molecular neurobiologists refer to post-encoding stabilization of synaptic or cell-wide information storage that occurs over hours or days after encoding (cellular consolidation, Dudai & Morris 2000; Dudai 2004). Systems and cognitive neuroscientists refer to post-encoding reorganization of information in distributed cortico-hippocampal circuits, which requires weeks, months, possibly even years to complete (systems consolidation, Dudai & Morris 2000; Dudai 2004).
The classic consolidation hypothesis connotes two interrelated attributes of LTM. One is irreversibility, the other stability (forgetting and lesions notwithstanding). The textbook account of both cellular and systems consolidation was until recently that consolidation occurs just once per item. Furthermore, it was assumed that once consolidation is over, the memory item becomes resistant to a variety of amnesic agents, such as inhibitors of protein synthesis.
4. Use reinstates fresh plasticity in old memories
The textbook account of memory consolidation has been, however, undergoing significant revisions in recent years. This might not come as a surprise to those who follow the cognitive literature, since the notion that memory items gain stability once consolidated does not sit well with ample evidence from human cognitive psychology that again and again portrayed recollection as constructive, casting doubts on the stability and veracity of retrieved facts and episodes (Bartlett 1932; Loftus & Loftus 1980; Schacter 1995). Unfortunately, the historical dissociation between the practioners of cognitive psychology and brain researchers tends to hinder proper cross-fertilization and cross-migration of concepts (Wixted 2004; Roediger et al. 2007). Over the years, animal studies did contribute evidence that items in LTM are less stable then previously assumed and that they can regain their sensitivity to amnesic agents upon reactivation in retrieval (Misanin et al. 1968; Nader et al. 2000; Sara 2000). But this phenomenon, dubbed ‘reconsolidation’, was somewhat pushed under the rug in view of the dominance of the consolidation dogma. Ultimately, the data made their impact, and in recent years, the study of reconsolidation has become a major focus of interest in both human and animal research (Nader 2003; Dudai 2004; Alberini 2005).
The current majority view in the field of memory research—as judged by bibliometry and definitely not without opposition—is that items in memory become transiently sensitive to a variety of amnesic agents immediately after encoding and then again immediately after retrieval. This transient susceptibility to amnesia is taken to imply that encoding and retrieval trigger in the neuronal substrate of the memory a special process and physical state. This post-activation state (Dudai 2007) is called ‘consolidation’ when it occurs after encoding and ‘reconsolidation’ when it occurs after retrieval. In terms of the cellular and circuit mechanisms involved, reconsolidation is not a faithful replay of consolidation (Bahar et al. 2004; Debiec & LeDoux 2004; Dudai 2004; Lee et al. 2004; Alberini 2005; von Hertzen & Giese 2005). Both processes do, however, share dependence on de novo macromolecular synthesis. It was reported (Parsons et al. 2006) that consolidation requires both protein and mRNA synthesis, whereas reconsolidation requires protein but not mRNA synthesis, but others reported that reconsolidation also depends on mRNA synthesis (Duvarci et al. 2008). The extent to which macromolecular reorganization, whether cell-wide or synapse-specific, takes place in reconsolidation vis-à-vis consolidation is hence yet to be determined. The crucial point, however, is not the identity of the detailed cellular and circuit mechanisms, but rather the finding that upon its reactivation, the long-term trace re-enters an unstable state that shares characteristics with post-encoding consolidation.
An additional difference between consolidation and reconsolidation is that, whereas cellular consolidation is a universal process, i.e. detected in every form of learning and every species tested so far, reconsolidation seems to occur only under certain conditions (Dudai 2004, 2006). To date, several boundary conditions have been identified that constrain reconsolidation, including the dominance of the trace (i.e. its ability to control behaviour after retrieval, Eisenberg et al. 2003), competition with concomitant memory extinction, and, most pertinent to the context of this discussion, conditions that promote new encoding in or immediately after retrieval (Pedreira et al. 2004; Morris et al. 2006).
If the reactivated long-term trace regains augmented plasticity, does it mean that the original trace can be completely erased? A close look at the data and discussions in the field distinguishes three versions of the reconsolidation hypothesis (Dudai 2004). The ‘strong version’ of the reconsolidation hypothesis posits that the regained plasticity applies to all the elements of the original memory and may indeed end-up in the erasure of that memory. The ‘intermediate version’ posits that there is a core memory that is stable and unaffected by the reconsolidation, but some stored elements of the original trace can still be modified and even erased. The weak version proposes that the original trace is actually unaffected in the process and that the plasticity refers only to new information that is added to the older memory in the context of retrieval. The latter version does not deviate from the classical consolidation hypothesis, as it simply says that new information consolidates; it is not really reconsolidation. It is yet unclear which of the other versions fits reality better, the strong or the intermediate. Anyway, even if upon memory reactivation the core representation becomes sensitive to amnesic agents, related memory associations seem to be spared (Debiec et al. 2006).
It is important to appreciate that the fact that reconsolidation is usually unveiled by the use of amnesic agents, does not imply that in real life, reconsolidation results in the weakening of the trace. Amnesic agents are only a tool to infer function from dysfunction (Dudai 2002). Reconsolidation might also provide an opportunity for the strengthening of the trace (Sara 2000; Frenkel et al. 2005; Tronson et al. 2006). This, together with the finding that reconsolidation is promoted by the induction of an encoding state in the retrieval situation (Morris et al. 2006), raises the possibility that the role of reconsolidation is to update memory, i.e. to adapt the reactivated memory to the new circumstances of the retrieval context (Sara 2000; Dudai 2004). However, whereas the consolidation hypothesis postulates that the original memory is securely consolidated, updating notwithstanding, the reconsolidation hypothesis, even in its intermediate version (see above), assumes that at least part of the original trace regains susceptibility to change. Some data (Rodriguez-Ortiz et al. 2008) but not others (Tronel et al. 2005) support a role for reconsolidation in updating of long-term memories. The current discrepancy on the role of reconsolidation in updating in different systems and paradigms might be related to boundary conditions on reconsolidation, which are not yet completely understood (Dudai 2006).
5. Plastic opportunities in the absence of explicit retrieval
Recent evidence indicates that long-term and remote memory is susceptible to certain amnesic agents even in the absence of explicit memory reactivation. These agents are inhibitors of an atypical isozyme of protein kinase C (PKC), called PKMζ. PKCs are composed of a catalytic subunit and a regulatory subunit, which are attached via a proteineous hinge. The regulatory subunit inhibits the catalytic subunit by a psuedosubstrate domain. In the absence of the regulatory subunit, the enzyme becomes constitutively active or autonomous. PKMζ is an autonomous form of PKCζ, which is formed in the brain by alternative splicing of PKCζ pre-mRNA. PKMζ can be inhibited by a number of selective inhibitors, particularly the cell permeable pseudosubstrate inhibitory peptide, ZIP. PKMζ has been reported to be critical in the maintenance of long-term potentiation (LTP) in the hippocampus (Ling et al. 2002). Two sequential steps are required for the persistent increase in PKMζ activity that maintains LTP (Kelly et al. 2007). One is de novo synthesis of PKMζ from PKMζ mRNA in the dendrite. This is regulated by several enzymes, including pre-existing PKMζ. The other is formation of a complex with the enzyme phosphoinositide-dependent kinase-1 (PDK1): though PKMζ is autonomous in the sense that second messengers required to activate PKC are not required, it must be still phosphorylated by PDK1 for optimal catalytic activity. The persistently active PKMζ phosphorylates synaptic substrates, which modify the microstructure of the synapse. This ultimately leads to a substantial increase in the number of functional post-synaptic AMPA-type glutamate receptors that persistently enhances synaptic transmission (Ling et al. 2006).
Long-term spatial information in the hippocampus, which is subserved by LTP, was shown to critically depend on persistent activity of PKMζ (Pastlakova et al. 2006). This was demonstrated by the micorinfusion of ZIP into the hippocampus of the behaving rat; scrambled ZIP had no effect. Although the hippocampus is well known to play a critical role in some types of memory, it is the neocortex that is considered to serve as the ultimate repository of multiple types of LTM in the mammalian brain (Dudai 2002; Squire & Bayley 2007). Using similar methods, it has indeed been found that microinfusion of ZIP into the neocortex rapidly erases remote memory associations (more than three months old), but not familiarity, in the behaving rat (Shema et al. 2007, 2009). The affected brain area can, however, reacquire readily a new memory association. The above data suggest that PKMζ permanently maintains LTM, and is thus a target for amnesic agents as long as the memory persists. When the enzymatic activity is blocked for a short while (less than 2 hours, and probably minutes only), the experience-dependent synaptic modifications seem to collapse and the memory disappears with them. One possibility is that the target of PKMζ is a ‘tag’ that dephosphorylates rapidly, and in its absence, though the enzymatic activity recovers from the inhibition, the enzyme cannot locate the proper phosphorylation site any more.
Two major conclusions emerge from the recent findings concerning the role of PKMζ in maintaining LTM. First that inhibitors can cause rapid, irreversible amnesia even in the absence of explicit memory reactivation. Thus post-retrieval reconsolidation is not the only window of opportunity in which an item in LTM can be modified, at least in a laboratory setting, once post-encoding consolidation had been completed. And second that neuronal changes which subserve LTM are not an indelible modification of synaptic structure, but remain dependent on ongoing enzymatic activity and, thus, are capable of rapid and dynamic alterations by experimental manipulations.
What might the physiological role be of such potential to rapidly erase LTM? Three main possibilities come to mind. First, that in situ, the cellular mechanism that requires persistent phosphorylation by PKMζ is regulated in a more graded and discriminative manner than by ZIP inhibition in the artificial laboratory setting, resulting in real life in restricted fast modulation of local synaptic properties and memory rather than in complete memory erasure. Such rapid, local modulation of long-term synaptic plasticity might, for example, be useful in the course of fast incorporation of new experience into existing associative knowledge schemas in the neocortex (Tse et al. 2007), without necessarily activating all the affected associations at the time of change. Second, rapid inhibition of PKMζ in specific synapses may indeed lead to rapid step-wise shift of synapses to a basal level of efficacy or even to a silent state. This might be useful in conditions in which the previous accumulating modifications culminate in catastrophic ‘freezing’ (e.g. a stable local minimum trap) of the computational abilities of the circuit, a situation that might be remedied by ‘rebooting’. And third, as computational models suggest (Hopfield 1982; Amit 1989), circuits may saturate, potentially requiring erasure to create space for storing new information.
Selective inhibitors of PKMζ are so far the only agents found to be capable of rapidly erasing long-term and remote memory in the mammalian brain in the absence of explicit memory reactivation. Since the phosphorylation of a target protein can be reversed by protein phosphatases, further research on protein phosphatase inhibitors may identify additional types of memory erasers, including, possibly, LTM erasers (Mansuy & Shenolikar 2006). These agents could cast further light on key elements the molecular machinery that keeps LTM going, and on the potential role of erasure in modifying LTM in the behaving brain.
6. On memory metaphors
Freezing and rebooting, as used above, are computer-age metaphors. More accurately, they are palimpsest-type metaphors anchored in technological and cultural contexts much older than the computer age. Metaphors are abundant in the science of memory (Roediger 1980; Dudai 2002). The problem with them is that although they help us in organizing our thoughts, they are also potentially misleading and promote fixation of conceptual paradigms (Dudai 2002). Storehouse, a dominant metaphor in memory research, epitomizes the problem. The storehouse metaphor connotes stability, whereas memory is dynamic. The data on reconsolidation as well as on the ability to rapidly erase LTM associations without damaging new learning, only augment the dynamic nature of memory.
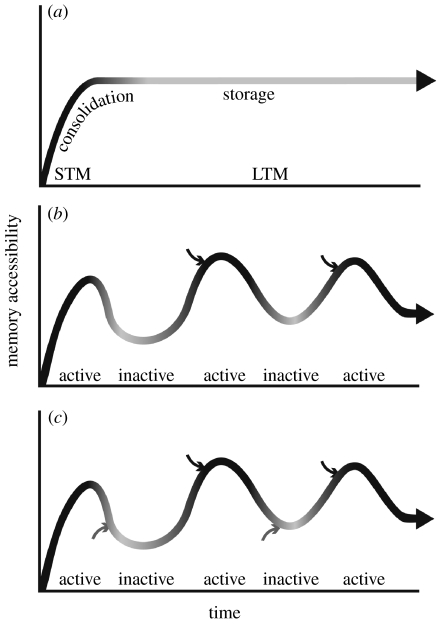
The classic time-based classification of memory distinguishes STM from LTM. This classification is known as the ‘dual trace’ hypothesis. It posits a transient, reverberating short-term trace, ‘that carries the information until the growth change is made’ (Hebb 1949), and a long-term, stabilized trace, which is the outcome of the postulated growth change (for other terms used to describe the same or similar ideas, such as primary versus secondary memory, see Dudai 2002). Combined with the consolidation hypothesis (see above), the orthodox version of the dual trace hypothesis could be construed to depict the memory trace, once consolidated, as being stored as is over time, used, then re-deposited until next use (figure 1a; for an example of a more relaxed version of the dual-trace hypothesis in the context of systems consolidation, see Nadel & Moscovitch 1997). An alternative conceptual framework was proposed that portrays memory items in two alternating states: active, and inactive (figure 1b; Lewis 1979). Active is the state of the trace on and immediately after encoding and retrieval. Occasionally, the trace might also become activated independent of encoding and retrieval. Otherwise, the trace is inactive. The trace fluctuates between the active–inactive states, or cycles. This hypothesis could be dubbed as ‘the cyclic model’. The data on consolidation and reconsolidation combined indicate that whenever active, the trace enters a special state (post-activation state, Dudai 2007), in which it is highly plastic and susceptible to interference by amnesic agents. This is different from the classic dual trace hypothesis that does not predict augmented plasticity after retrieval, i.e. reconsolidation. Whereas the dual trace model depicts the ontogeny of a memory item as a step function in which consolidation is the transition from one step to the other, the reconsolidation hypothesis is in line with the cyclic model. A modified version of the cyclic model takes into account the reports that LTM is susceptible to certain amnesic agents even in the absence of explicit reactivation of the trace. This modified cyclic model raises the possibility that the long-term trace can be updated whether active or inactive (figure 1c).
Figure 1.
Schematic models of memory states and stability over time. (a). The dual trace model classifies memory into a transient, short-term phase (STM) and a stable, long-term trace (LTM). The latter is generated by synaptic and cell-wide growth-like processes in the course of post-encoding consolidation. Amnesic agents can disrupt the memory trace during consolidation, but lose their effectiveness once consolidation is over. Consolidation according to this model occurs just once per item. This type of model does not refer specifically to the fate of the trace after retrieval, and assumes, usually implicitly, that when new information is interwoven into old knowledge, the new information undergoes consolidation without altering plasticity in the older, consolidated memory. (b). The cyclic model depicts two states of memory, active (black) and inactive (grey), which alternate over time. Activity is time-locked to encoding or retrieval. This type of model still predicts an initial consolidation period (on or immediately after encoding of the new memory item) but then allows for consolidation-like processes to occur more than once per item, i.e. allows reconsolidation. Recent data suggest that reconsolidation is not a faithful recapitulation of consolidation. One possibility is that reinstated plasticity in reactivated memory allows some sort of memory reorganization or updating (in the scheme, arrows representing new information merge with the old information only when the memory is in an active state). (c). A modified version of the cyclic model that takes into account recent reports that LTM is susceptible to certain amnesic agents even in the absence of explicit reactivation of the trace. This model raises the possibility that the long-term trace can be updated, whether active or inactive (again, as in (b), arrows represent new information merging with existing to generate an updated representation).
It is noteworthy that the active–inactive type of models does not nullify the existence of some type of consolidation, i.e. does not preclude an initial maturation phase for each item in memory. As noted above, studies that compare consolidation to reconsolidation show that reconsolidation is not a faithful recapitulation of consolidation, and studies on the role of PKMζ in neural plasticity and memory show that memories are not sensitive to PKMζ inhibitors in the first hours after training. All this implies that the properties of a fresh memory are different from those of an old memory (see also Berman & Dudai 2001). But once LTM is established, active–inactive models assume that the memory is still malleable and not stored as an indelible consolidated item. Coming back to metaphors, whereas the combination of the dual trace model with the consolidation hypothesis connotes the storehouse metaphor, the more recent data on the high plasticity of the long-term trace and the cyclic models that stem from these data favour a Phoenix metaphor: occasionally, items in memory may get the opportunity to be born anew.
7. On being just stable enough
In an influential account, Marr (1982) distinguished three levels of description or analysis in information processing machines: (a) the level of the computational theory, i.e. what are the goals of the computations and the logic of the strategy to carry them out, (b) the levels of representations and algorithms, i.e. how can the computations be implemented in terms of ‘input’ and ‘output’ representations and of the algorithms for the transformation of input into output, and (c) the level of hardware implementation, i.e. the way the representations and algorithms are implemented in the hardware of the machine, which, in the case of the brain, is the biological material, spanning from molecules to cells, circuits and brain organs.
Taking the anthropocentric adaptationist approach, which posits that biological systems have evolved to achieve a goal and, furthermore, that we can identify that goal (and see Gould & Lewontin 1979 for a critique), one could start the analysis of memory systems by defining their goal. Such an approach is likely to culminate in assignment of different goals to different memory systems, since, as noted above, systems as different as emotional or motor conditioning, skill, priming, semantic or episodic memory, had probably evolved under different selection pressures for different purposes. Yet one could still simplify and generalize, by proposing a common goal for all memory systems. It is tempting to propose that this universal goal is to optimize adaptive responses to stimuli in the changing milieu of the individual.
The algorithmic and hardware routes taken to the aforementioned goal seem to navigate among conflicting pressures. On the one hand, once a proper response is installed, either by the species' experience (i.e. innate response programs) or by individual experience or, usually, by their combination, stability is advantageous since it ensures fast response and saves on the energy needed to learn anew. On the other hand, since the milieu changes, plasticity should permit fast changes in the existing response, should the conditions require such changes. On top of it, anticipating future events and trends permits preparative steps and fast-adaptive response. Summation of these requirements probably underlies basic plastic properties of the memory trace of the type described above. Hence, following encoding, initial consolidation converts the trace into a state that is just stable enough, so that it will last till the next encounter with the proper stimulus, but at same time is amenable to change once the proper stimuli or contexts change significantly. Under these constraints, a system that opens windows of augmented plasticity only when effective cues concerning the relevant specific situation become available could be beneficial, since it could reduce the risk of unwarranted change, restricting change to when it is needed only. Reconsolidation provides such a cue-locked restricted window of opportunity. The existence of privileged plasticity windows is a type of metaplasticity, i.e. the plasticity of neural plasticity (Abraham & Tate 1997). As other variants of metaplasticity, it reflects a dynamic balance between the need to change, to resist excessive change, and the metabolic price of both (Dudai 2002).
The question could then be raised why is it that the trace can be rapidly changed or even erased with a PKMζ inhibitor outside the consolidation or reconsolidation windows. The possibility should not be excluded that this reflects inherent mechanistic shortcoming of the system and not adaptivity (e.g. Gould & Lewontin 1979). In other words, that the susceptible part of the cellular long-term plasticity machinery that collapses as a consequence of transient interruption of the persistent kinase activity is not a target for cellular regulation in vivo. However, several hypothetical possibilities that assume physiological regulation of this site were raised above. Of these, the one most appealing in my view is the possibility of facilitating fast incorporation of new experience into existing associative knowledge schemas in the neocortex (Tse et al. 2007) in the absence of superfluous activation of indirect associations (Debiec et al. 2006).
In conclusion, biological memory systems have evolved the basic capacity to anticipate an uncertain future by combining neuronal plasticity and metaplasticity mechanisms so that they can encode experience in a reasonably robust way on the one hand, but update it quickly on the other. It would be of interest to determine whether this capacity is exploited differentially in different memory systems, according to the relative weight of the requirement for stability versus anticipation of change in each system. Such differential reliance on plasticity that results in experience-dependent stability and on metaplasticity that permits future destabilization may account also for differences in the veracity of long-term items in different memory systems.
Acknowledgments
I am grateful to Omri Barak and Joseph E. LeDoux for discussion of memory models. My research is supported by grants from the Israeli Science Foundation (ISF), the US-Israel Binational Science Foundation (BSF), the Nella and Leon Benoziyo Center for Neurological Diseases and the Minerva Foundation.
Footnotes
One contribution of 18 to a Theme Issue ‘Predictions in the brain: using our past to prepare for the future’.
References
- Abraham W.C., Tate W.P. Metaplasticity: a new vista across the field of synaptic plasticity. Prog. Neurobiol. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. doi:10.1016/S0301-0082(97)00018-X [DOI] [PubMed] [Google Scholar]
- Addis D.R., Wong A.T., Schacter D.L. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. doi:10.1016/j.neuropsychologia.2006.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini C.M. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. doi:10.1016/j.tins.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Amit D.J. Cambridge University Press; Cambridge, UK: 1989. Modeling brain function. The world of attractor model networks. [Google Scholar]
- Anokhin P.K. Pergamon Press; Oxford, UK: 1974. Biology and neurophysiology of the conditioned reflex and its role in adaptive behavior. [Google Scholar]
- Atance C.M., O'Neill D.K. The emergence of episodic future thinking in humans. Learn. Motiv. 2005;36:126–144. doi:10.1016/j.lmot.2005.02.003 [Google Scholar]
- Baddeley A. Oxford University Press; New York, NY: 2007. Working memory, thought, and action. [Google Scholar]
- Bahar A., Dorfman N., Dudai Y. Amygdalar circuits required for either consolidation or extinction of taste aversion memory are not required for reconsolidation. Eur. J. Neurosci. 2004;19:1115–1118. doi: 10.1111/j.0953-816x.2004.03215.x. doi:10.1111/j.0953-816X.2004.03215.x [DOI] [PubMed] [Google Scholar]
- Bain A. Henry King; London, UK: 1872. Mind and body: the theories of their relation. [Google Scholar]
- Bar M. The predictive brain: using analogies and associations to generate predictions. Trends Cogn. Sci. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. doi:10.1016/j.tics.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Bartlett E.C. Cambridge University Press; London, UK: 1932. Remembering. A study in experimental and social psychology. [Google Scholar]
- Berman D.E., Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. doi:10.1126/science.1058165 [DOI] [PubMed] [Google Scholar]
- Buzsaki G.Rhythms of the brain2006Oxford University Press; New York, NY [Google Scholar]
- Coltheart, V. (ed.) 1999 Fleeting memories. Cognition of brief visual stimuli, Cambridge, MA: MIT Press.
- Debiec J., LeDoux J.E. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdale. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. doi:10.1016/j.neuroscience.2004.08.018 [DOI] [PubMed] [Google Scholar]
- Debiec J., Doyere V., Nader K., LeDoux J.E. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc. Natl Acad. Sci. USA. 2006;103:3428–3433. doi: 10.1073/pnas.0507168103. doi:10.1073/pnas.0507168103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. Oxford University Press; Oxford, UK: 2002. Memory from A to Z. Keywords, concepts, and beyond. [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram. Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. doi:10.1146/annurev.psych.55.090902.142050 [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Curr. Opin. Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. doi:10.1016/j.conb.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Dudai Y. Post-activation state: a critical rite of passage of memories. In: Bontempi B., Silva A.J., Christen Y., editors. Memories: molecules and circuits, research and perspectives in neurosciences. Springer; Heidelberg, Germany: 2007. pp. 69–82. [Google Scholar]
- Dudai Y., Carruthers M. The Janus face of Mnemosyne. Nature. 2005;434:567. doi: 10.1038/434567a. doi:10.1038/434567a [DOI] [PubMed] [Google Scholar]
- Dudai Y., Morris R.G.M. To consolidate or not to consolidate: what are the questions? In: Bolhuis J.J., editor. Brain, perception, memory. Advances in cognitive sciences. Oxford University Press; Oxford, UK: 2000. pp. 149–162. [Google Scholar]
- Duvarci, S. Nader, & Ledoux, J. E. 2008 De novo mRNA synthesis is required for both consolidation and reconsolidation of fear memories in the amygdala 15, 747–755. (doi:10.1101/lm.1027208) [DOI] [PMC free article] [PubMed]
- Eisenberg M., Kobilo T., Berman D.E., Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. doi:10.1126/science.1086881 [DOI] [PubMed] [Google Scholar]
- Flexner J.B., Flexner L.B., Stellar E. Memory in mice as affected by intracerebral puromycin. Science. 1963;141:57–59. doi: 10.1126/science.141.3575.57. doi:10.1126/science.141.3575.57 [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. doi:10.1016/j.neuron.2007.08.023 [DOI] [PubMed] [Google Scholar]
- Frenkel L., Maldonado H.H., Delorenzi A. Memory strengthening by a real-life episode during reconsolidation: an outcome of water deprivation via brain angiotensin II. Eur. J. Neurosci. 2005;22:1757–1766. doi: 10.1111/j.1460-9568.2005.04373.x. doi:10.1111/j.1460-9568.2005.04373.x [DOI] [PubMed] [Google Scholar]
- Frey U., Morris R.G.M. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. doi:10.1038/385533a0 [DOI] [PubMed] [Google Scholar]
- Fusi S., Drew P.J., Abbott L.F. Cascade models of synaptically stored memories. Neuron. 2005;45:599–611. doi: 10.1016/j.neuron.2005.02.001. doi:10.1016/j.neuron.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Gilboa G., Chen R., Brennner N.N. History-dependent multiple-time-scale dynamics in a single-neuron model. J. Neurosci. 2005;25:6479–6489. doi: 10.1523/JNEUROSCI.0763-05.2005. doi:10.1523/JNEUROSCI.0763-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S.J., Lewontin R.C. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. B. 1979;203:581–598. doi: 10.1098/rspb.1979.0086. doi:10.1098/rspb.1979.0086 [DOI] [PubMed] [Google Scholar]
- Hare T.A., O'Doherty J., Camerer C.F., Schultz W., Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and predictions errors. J. Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. doi:10.1523/JNEUROSCI.1309-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D., Kumaran D., Vann S.D., Maguire E.A. Patients with hippocampal amnesia cannot imagine new experiences. Proc. Natl Acad. Sci. USA. 2007;104:1726–1731. doi: 10.1073/pnas.0610561104. doi:10.1073/pnas.0610561104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Yang E., Vallines I., Heeger D.J., Rubin N. A hierarchy of temporal receptive windows in human cortex. J. Neurosci. 2008;28:2539–2550. doi: 10.1523/JNEUROSCI.5487-07.2008. doi:10.1523/JNEUROSCI.5487-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D.O. Wiley; New York, NY: 1949. The organization of behavior: a neuropsychological theory. [Google Scholar]
- Heidmann A., Heidmann T.M., Changeux J.-P. Stabilization selective de representation neuronals per resonance entre ‘prerepresentations’ spontanees du raseau cerebral et ‘percepts’ evoques par interactions avec le monde exterieur. Compt. Rend. Acad. Sci. Paris S. III. 1984;299:839–843. [PubMed] [Google Scholar]
- Holt E.B. Holt; New York, NY: 1931. Animal drive and the learning process. [Google Scholar]
- Hopfield J.J. Neural networks and physical systems with emergent collective computational abilities. Proc. Natl Acad. Sci. USA. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. doi:10.1073/pnas.79.8.2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers C.U.A. Further considerations on neurobiotaxis. IX. An attempt to compare the phenomenon of neurobiotaxis with other phenomena of taxis and tropism. The dynamic polarization of the neurone. J. Comp. Neurol. 1917;27:261–298. doi:10.1002/cne.900270302 [Google Scholar]
- Kelly M.T., Crary J.F., Sacktor T.C. Regulation of protein kinase Mzeta synthesis by multiple kinases in long-term potentiation. J. Neurosci. 2007;27:3439–3444. doi: 10.1523/JNEUROSCI.5612-06.2007. doi:10.1523/JNEUROSCI.5612-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.L.C., Everitt B.J., Thomas K.L. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. doi:10.1126/science.1095760 [DOI] [PubMed] [Google Scholar]
- Lewis D.J. Psychobiology of active and inactive memory. Psychol. Bull. 1979;86:1054–1083. doi:10.1037/0033-2909.86.5.1054 [PubMed] [Google Scholar]
- Ling D.S., Benardo L.S., Serrano P.A., Blace N., Kelly M.T., Crary J.F., Sacktor T.C. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat. Neurosci. 2002;5:295–296. doi: 10.1038/nn829. doi:10.1038/nn829 [DOI] [PubMed] [Google Scholar]
- Ling D.S., Benardo L.S., Sacktor T.C. Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. doi:10.1002/hipo.20171 [DOI] [PubMed] [Google Scholar]
- Loftus E.F., Loftus G.R. On the permanence of stored information in the human brain. Am. Psychol. 1980;35:409–420. doi: 10.1037//0003-066x.35.5.409. doi:10.1037/0003-066X.35.5.409 [DOI] [PubMed] [Google Scholar]
- Mansuy I.M., Shenolikar S. Protein serine/threonine phophatases in neuronal plasticity and disorders of learning and memory. Trends Neuroci. 2006;29:679–686. doi: 10.1016/j.tins.2006.10.004. doi:10.1016/j.tins.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Marr D. Freeman; San Francisco, CA: 1982. Vision. [Google Scholar]
- McGaugh J.L. A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. doi:10.1126/science.287.5451.248 [DOI] [PubMed] [Google Scholar]
- Misanin J.R., Miller R.R., Lewis D.J. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;159:554–555. doi: 10.1126/science.160.3827.554. doi:10.1126/science.160.3827.554 [DOI] [PubMed] [Google Scholar]
- Monne L. Structure and function of neurones in relation to mental activity. Biol. Rev. Camb. Philos. Soc. 1949;24:297–315. doi: 10.1111/j.1469-185x.1949.tb00578.x. doi:10.1111/j.1469-185X.1949.tb00578.x [DOI] [PubMed] [Google Scholar]
- Morris R.G.M., Inglis J., Ainge J.A., Olverman H.J., Tulloch J., Dudai Y., Kelly P.A.T. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. doi:10.1016/j.neuron.2006.04.012 [DOI] [PubMed] [Google Scholar]
- Muller G.E., Pilzecker A. Experimentelle Beitrgae zur Lehre und Gedachtnis. Zeit. Psychol. 1900;1:1–300. [Google Scholar]
- Nadel L., Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. doi:10.1016/S0959-4388(97)80010-4 [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. doi:10.1016/S0166-2236(02)00042-5 [DOI] [PubMed] [Google Scholar]
- Nader K., Schafe G.E., LeDoux J.E. Fear memories require protein synthesis in the amygdala for reocnsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. doi:10.1038/35021052 [DOI] [PubMed] [Google Scholar]
- Naya Y., Yoshida M., Miyashita Y. Backward spreading of memory-retrieval signal in the primate temporal cortex. Science. 2001;291:661–664. doi: 10.1126/science.291.5504.661. doi:10.1126/science.291.5504.661 [DOI] [PubMed] [Google Scholar]
- Parsons P.R., Gafford G.M., Baruch D.E., Riedner B.A., Helmestetter F.J. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur. J. Neurosci. 2006;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. doi:10.1111/j.1460-9568.2006.04723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E., Serrano P., Pinkhasova D., Wallace E., Fenton A.A., Sacktor T.C. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. doi:10.1126/science.1128657 [DOI] [PubMed] [Google Scholar]
- Pedreira M.E., Perez-Questa L.M., Maldonado H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn. Mem. 2004;11:579–585. doi: 10.1101/lm.76904. doi:10.1101/lm.76904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ortiz C.J., Garcia_DeLaTorre P., Benavidez E., Ballesteros M.A., Bermudez-Rattoni F. Intrahippocampal anisomycin infusions disrupt previously consolidated spatial memory only when memory is updated. Neurobiol. Learn. Mem. 2008;89:352–359. doi: 10.1016/j.nlm.2007.10.004. doi:10.1016/j.nlm.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Roediger H.L., III Memory metaphors in cognitive psychology. Mem. Cogn. 1980;8:231–246. doi: 10.3758/bf03197611. [DOI] [PubMed] [Google Scholar]
- Roediger H.L., Dudai Y., Fitzpatrick S.M., editors. Science of memory: concepts. Oxford University Press; New York, NY: 2007. [Google Scholar]
- Sara S.J. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn. Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. doi:10.1101/lm.7.2.73 [DOI] [PubMed] [Google Scholar]
- Schacter, D. L. (ed.) Memory distortions Cambridge, MA: Harvard University Press.
- Shema R., Sacktor T.C., Dudai Y. Rapid erasure of long-term memory associations in cortex by an inhibitor of PKMζ. Science. 2007;317:951–953. doi: 10.1126/science.1144334. doi:10.1126/science.1144334 [DOI] [PubMed] [Google Scholar]
- Shema R., Hazvi S., Sacktor T.C., Dudai Y. Boundary conditions for the maintenance of memory by PKMζ in neocortex. Learn. Mem. 2009;16:122–128. doi: 10.1101/lm.1183309. doi:10.1101/lm.1183309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K., Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. doi:10.1126/science.282.5392.1335 [DOI] [PubMed] [Google Scholar]
- Smith M.A., Ghazizadeh A., Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:1035–1043. doi: 10.1371/journal.pbio.0040179. doi:10.1371/journal.pbio.0040179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L.R., Bayley P.J. The neuroscience of remote memory. Curr. Opin. Neurobiol. 2007;17:185–196. doi: 10.1016/j.conb.2007.02.006. doi:10.1016/j.conb.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T., Corballis M.C. Mental time travel and the evolution of the human mind. Genet. Soc. Gen. Psychol. Monogr. 1997;123:133–167. [PubMed] [Google Scholar]
- Thorpe S., Fize D., Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. doi:10.1038/381520a0 [DOI] [PubMed] [Google Scholar]
- Toib A., Lyakhov V., Marom S. Interaction between duration of activity and time course of recovery from slow inactivation in mammalian brain Na+ channels. J. Neurosci. 1998;18:1893–1903. doi: 10.1523/JNEUROSCI.18-05-01893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S., Milekic M.H., Alberini C.M. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 2005;3:1630–1638. doi: 10.1371/journal.pbio.0030293. doi:10.1371/journal.pbio.0030293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson N.C., Wiseman S.L., Olausson P., Taylor J.R. Bidirectional behavioural plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat. Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. doi:10.1038/nn1628 [DOI] [PubMed] [Google Scholar]
- Tse D., Langston R.F., Kakeyama M., Bethus I., Spooner P.A., Wood E.R., Witter M.P., Morris R.G.M. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. doi:10.1126/science.1135935 [DOI] [PubMed] [Google Scholar]
- Tulving E. Oxford University Press; Oxford, UK: 1983. Elements of episodic memory. [Google Scholar]
- Tulving E. Are there 256 kinds of memory? In: Nairne J.S., editor. The foundations of remembering. Essays in honor of Henry L. Roediger, III. Psychology Press; New York, NY: 2007. pp. 39–52. [Google Scholar]
- Tulving E., Fergus F.I.M., editors. The Oxford handbook of memory. Oxford University Press; New York, NY: 2000. [Google Scholar]
- van Turennout M., Hagoort P., Brown C.M. Brain activity during speaking: from syntax to phonology in 40 milliseconds. Science. 1998;280:572–574. doi: 10.1126/science.280.5363.572. doi:10.1126/science.280.5363.572 [DOI] [PubMed] [Google Scholar]
- von Hertzen L.S.J., Giese K.P. Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J. Neurosci. 2005;25:1935–1942. doi: 10.1523/JNEUROSCI.4707-04.2005. doi:10.1523/JNEUROSCI.4707-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted J. The psychology and neuroscience of forgetting. Annu. Rev. Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. doi:10.1146/annurev.psych.55.090902.141555 [DOI] [PubMed] [Google Scholar]
- Young J.Z. Learning as a process of selection and amplification. J. R. Soc. Med. 1979;72:801–814. doi: 10.1177/014107687907201103. [DOI] [PMC free article] [PubMed] [Google Scholar]