Abstract
Predator-mediated coexistence of competitors occurs when a species that is superior in competition is also more vulnerable to a shared predator compared to a poorer competitor. The invasive mosquito Aedes albopictus is usually competitively superior to Ochlerotatus triseriatus. Among second instar larvae, A. albopictus show a lesser degree of behavioral modiWcation in response to waterborne cues from predation by the larval midge Corethrella appendiculata than do O. triseriatus, rendering A. albopictus more vulnerable to predation by C. appendiculata than O. triseriatus. The hypothesis that C. appendiculata predation favors coexistence of these competitors predicts that C. appendiculata abundances will be negatively and positively correlated with A. albopictus and O. triseriatus abundances, respectively, and that coexistence will occur where C. appendiculata are common. Actual abundances of O. triseriatus, A. albopictus, and C. appendiculata in three habitats Wt this prediction. In natural container habitats like tree holes, C. appendiculata were abundant and competitors co-existed at similar densities. In cemeteries and tires, which occur primarily in non-forested, human-dominated habitats, A. albopictus dominated, with abundances twice those found in tree holes, but C. appendiculata and O. triseriatus were rare or absent. We also tested for whether antipredatory behavioral responses of A. albopictus differed among habitats or populations, or were correlated with local C. appendiculata abundances. We could detect no differences in A. albopictus antipredatory behavioral responses to water-borne cues from predation. Tree hole habitats appear to promote co-existence of O. triseriatus and A. albopictus through interactions with predatory C. appendiculata, and this predator effect appears to limit invasion success of A. albopictus in tree holes. There are many studies on predator-mediated coexistence in natural habitats but to our knowledge this is the first study to suggest differential predator-mediated coexistence between natural and man-made habitats.
Keywords: Aedes albopictus, Ochlerotatus triseriatus, Predator-mediated coexistence, Corethrella appendiculata, Deforestation
Introduction
Invasion biology is increasingly perceived as vital for conservation because invasion by exotic species is increasing at an unprecedented rate, and some of these species have enormous economic and ecological impacts (Kolar and Lodge 2001). Native predators could have an effect on the density and population growth of invasive animals, and these effects could impose barriers to successful invasion (Lodge 1993). Predators may preferentially feed on invaders, particularly if invasive species lack appropriate adaptations to escape or to avoid novel predators, thereby impeding invasion and potentially facilitating survival of native species even when the invader is superior to the native species in resource competition (Garvey et al. 2003). Smith (2006) showed that the native predators, eastern newts (Notophthalmus viridescens), promoted co-existence between native toad tadpoles (Bufo terrestris) and invasive Cuban tree frog tadpoles (Osteopilus septentrionalis), whereas in the absence of the predator the invasive Cuban tree frog tadpoles dominated. DeRivera et al. (2005) showed that the native predator, the blue crab (Callinectes sapidus), limits the geographical range of the invasive, European green crab (Carcinus maenas).
Behavioral responses to predators appear to be a major mechanism of prey adaptation to predation in aquatic systems (Sih 1984; Lima and Dill 1990). Prey that alter their behavior in response to predation risk, including reducing movement and foraging, refuge use, and startle displays, can be less vulnerable to predation (Kats et al. 1988; Buskirk et al. 1997; Relyea 2002). Information on the role of prey behavior in invasion systems is lacking but it could be important in understanding the process of invasion and barriers to invasion (Holway and Suarez 1999). Consider a system in which a native prey shows adaptive changes in behavior that reduce risk of predation from the native predator. The success of an invading prey that is also a competitor of the native prey will depend upon whether the invader is a superior competitor to the native prey and also whether the invader shows appropriate behavioral or other facultative responses to the novel predators it encounters as it invades a new area. Without such appropriate responses, the presence of the predator, and its maintenance by populations of native prey, may act as a barrier to invasion, or may facilitate coexistence of invader and native (i.e., keystone predation; Leibold 1996). If however, the invader is both a superior competitor and has more effective behavioral avoidance of predation, the invader may eliminate and replace the native prey. In such an invasion system, it is also possible that selection imposed by the predator on the invading population may cause evolution of predator deterrence or avoidance in the invading species (e.g., Phillips and Shine 2005). If abundance and impact of predators varies among invaded locations, such selection could result in differentiation of invader behavioral responses and local variation in the outcome of the invasion. Most of the studies on predator-mediated biotic resistance have investigated the role of predators in natural habitats (e.g., DeRivera et al. 2005) and there have been only a few studies on how different habitats, especially natural and man-made habitats, influence predator-mediated biotic resistance to invasive species.
The habitats that are compared in the study described here are small container habitats. Rainwater that collects in these small containers supports a diverse, specialized invertebrate community (Kitching 2000). These container systems can be natural (e.g., tree holes) and man made (e.g., cemetery vases and discarded tires). Aedes albopictus (order, Diptera; family, Culicidae) is a container-dwelling invasive mosquito that invaded the USA in the 1980s from Asia (Hawley et al. 1987) and has established itself in the southeastern United States (O’Meara et al. 1995). A. albopictus is a vector of human diseases including West Nile and dengue (Ibanez-Bernal et al. 1997; Turell et al. 2005). Larval A. albopictus are superior competitors to native mosquitoes (e.g., Livdahl and Willey 1991; Novak et al. 1993; Teng and Apperson 2000; Aliabadi and Juliano 2002), and have managed to displace them in some invaded areas (Juliano and Lounibos 2005). Ochlerotatus triseriatus (order, Diptera; family, Culicidae) is a container-dwelling mosquito that is native to North America. A. albopictus is a superior competitor to O. triseriatus under laboratory conditions (Livdahl and Willey 1991; Novak et al. 1993; Teng and Apperson 2000; Aliabadi and Juliano 2002) but populations of O. triseriatus in Florida tree holes have not declined since the invasion of A. albopictus (Lounibos et al. 2001).
In Florida, A. albopictus and O. triseriatus co-occur with the larval predator Corethrella appendiculata (order, Diptera; family, Corethrellidae). C. appendiculata are midges and when they are in the larval form they prey upon early instars of A. albopictus and O. triseriatus (Kesavaraju et al. 2007a). C. appendiculata appear to use mechanoreceptors to detect their prey and predominantly hunt at the bottom of the containers (Kesavaraju et al. 2007a). Moving prey at the bottom of containers are at a greater risk of being captured by C. appendiculata compared to motionless prey at the surface of the water (Kesavaraju et al. 2007a). Studies on the antipredatory behavior of O. triseriatus in response to other predators, which is similar to their response to C. appendiculata predation, have shown that this reduced movement also affects the foraging behavior and results in reduced foraging opportunities (Kesavaraju et al. 2007b). Second instar O. triseriatus reduce movement at the bottom of containers in the presence of water-borne cues from C. appendiculata predation, and although second instar A. albopictus show a qualitatively similar response to such cues, the degree of behavioral change is significantly less than that for O. triseriatus (Kesavaraju et al. 2007a). Larvae of A. albopictus are more vulnerable to predation by C. appendiculata than are O. triseriatus (Kesavaraju et al. 2007a; Griswold and Lounibos 2005b). Fourth instar O. triseriatus are relatively invulnerable to predation by C. appendiculata, but despite this, they also reduce movement at the bottom of containers in the presence of waterborne cues from C. appendiculata predation (Kesavaraju et al. 2007a). Behavioral studies comparing antipredator behavior of A. albopictus and O. triseriatus in response to C. appendiculata water-borne cues show that A. albopictus is more vulnerable to predation by C. appendiculata than is O. triseriatus (Kesavaraju et al. 2007a). These data suggest that in habitats where C. appendiculata are abundant they may function as keystone predators (Leibold 1996), facilitating co-existence between competitively dominant, but predator-vulnerable A. albopictus and competitively inferior, but predator-resistant O. triseriatus, and in particular limiting declines of the competitively inferior native species (Griswold and Lounibos 2006).
Selection caused by novel conditions, and resulting evolutionary changes, can occur in response to species invasions (Lee 2002). For example, several native snake species in Australia have shown a reduction in gape size and increase in body size since the arrival of invasive cane toads. These changes prevent ingestion of toads that are large enough to be toxic (Phillips and Shine 2004). In that same system, there is evidence for evolution of invasive cane toad defenses in response to local variation in predation regimes (Phillips and Shine 2005). Invasive A. albopictus have been in both North and South America long enough to evolve modiWed diapause responses (Lounibos et al. 2003), suggesting that local adaptation to some environmental variables is possible for these introduced populations. Controlled laboratory investigations of selection by predation on O. triseriatus have shown that rapid evolution of larval behavior is possible (Juliano and Gravel 2002). Local populations of O. triseriatus differ in behavior patterns (Juliano and Reminger 1992; Juliano et al. 1993; Juliano and Gravel 2002), but that variation is not significantly associated with the large-scale geographic range of predators (Juliano et al. 1993), and quantitative associations of behavior of local populations with measured predator abundances have not been tested. Because A. albopictus has occupied parts of south Florida for >15 years, if there is local variation in predation, then local populations of A. albopictus from habitats where C. appendiculata are abundant may have been selected for greater behavioral response to water-borne predation cues than those from habitats of low abundance of C. appendiculata. If selection for greater antipredator responses also has the correlated cost of reduced competitive ability, this kind of evolutionary response by A. albopictus could contribute to the apparently limited impact of A. albopictus on O. triseriatus in areas where they co-occur with C. appendiculata.
In this research we tested whether: (1) there are any correlations of abundances of the predator C. appendiculata with abundances of the prey A. albopictus and O. triseriatus, and particularly whether coexistence of these mosquitoes is associated with presence of this predator; (2) populations of A. albopictus from habitats or sites with different abundances of C. appendiculata differ in behavioral responses to C. appendiculata water-borne cues from predation.
Materials and methods
Correlation of abundances
Abundances of A. albopictus, O. triseriatus, and C. appendiculata were determined from Weld samples taken between May and October in both 2004 and 2005 in Florida, USA, from cemeteries (Oak Hill, Bartow; Rose Hill, Tampa; Joshua Creek, Arcadia; White City, Fort Pierce), tire sites (M&K Used Auto Parts, Vero Beach; A & A Auto Salvage, Fort Pierce; Snake Road Auto Salvage, Stuart; Action Auto Salvage, Okeechobee) and tree hole sites (Indrio Road, Fort Pierce; Sherwood Hammock, Fort Pierce; Myakka River State Park, near Sarasota; Highlands Hammock State Park, near Sebring) (see Appendix for map of the locations). The months between May and October are the wet season in Florida and the abundances of mosquitoes are higher at these times (M. H. Reiskind, personal communication). All the contents of containers (e.g., water, detritus etc.) and tree holes from these sites were collected and the number of individuals of the three species in each of the containers were identified and counted. Tree hole sites were located in forested areas, with tree holes exclusively in live oak (Quercus virginiana), whereas tire and cemetery vase sites were located in non-forested, human-developed areas ranging from rural to urban. Field collections were conducted during the summers of 2004–2005 and all the sites were sampled at least twice with the exception of two tire sites (A & A Auto Salvage and Action Auto Salvage). From eight to 30 containers were sampled from each site and because the containers from all sites were sampled destructively, we allowed a minimum of 2 months between samples at a given site. A nested multivariate ANOVA (MANOVA) (Scheiner 2001) was used for the analysis with the numbers of A. albopictus, O. triseriatus, and C. appendiculata from a container as dependent variables and habitat types (cemeteries, tires, and tree holes) and sites nested within the habitat types as independent variables. Abundances were log10(y+1) transformed to meet assumptions of normality and homogeneous variances. Standardized canonical coefficients (SCCs; Scheiner 2001) were used to identify the relative contribution of the dependent variables to significant differences among sites and habitat types, and to interpret correlations of mean abundances among habitat types and among sites. Correlations of abundances of A. albopictus, O. triseriatus, and C. appendiculata were further analyzed by testing Pearson correlations among the log10 values of numbers of each species across containers, across habitat types (pooling all sites within a type).
Behavioral difference among sites
Behavior of second instar A. albopictus larvae from different sites was recorded in the absence and presence of cues from predation. A. albopictus used in the experiment originated as Weld-collected larvae from the four cemetery and the four tire sites, and three of the tree hole sites (Indrio Road, Sherwood Hammock, and Highlands Hammock State Park). Field-collected larvae were reared to adulthood and blood fed using chickens (University of Florida Institutional Animal Care and Use Committee protocol no. VB-17) to obtain eggs. Resulting progeny, one generation removed from the Weld, were used in behavioral assays.
Following Kesavaraju et al. (2007a), the predation treatment was prepared by holding ten second instar A. albopictus with three fourth instar C. appendiculata larvae for 5 days in 10 ml deionized water in polystyrene disposable cups, and the control treatment was prepared by holding ten second instar A. albopictus alone in similar containers. Dead, eaten, and pupated individuals were replaced daily. The test subjects, F1 generation A. albopictus from the different sites, were hatched and held in 5 ml of water in 4 dram vials and fed with 1 ml of liver powder suspension prepared by stirring 0.3 g of liver powder in 1,000-ml beaker with 1,000 ml water on a stir plate and transferred using an Eppendorf pipette (Juliano and Gravel 2002; Kesavaraju and Juliano 2004). A single feeding was sufficient for A. albopictus to develop to second instar.
Test larvae were starved for 24 h in 10-ml cups with 10 ml of water before being transferred to prepared water for behavior recording. All larvae of both predator and prey were removed from the prepared water, leaving behind only cues (e.g., dissolved substances, uneaten body parts, feces, etc.), before adding the test subjects for video recording. One second instar A. albopictus larva was placed in each cup of prepared water and its behavior was recorded on a computer using Winfast XP 2000 PCI card for 15 min. Each video clip included four cups (two control and two predation).
Behavior analysis
Behaviors were classified into activities and positions (Juliano and Reminger 1992). Activities were: (1) browsing—mouthparts in contact with the container surfaces, (2) filtering—moving through the water propelled by feeding movements of the mouthparts, (3) thrashing—moving with vigorous lateral flexion of body, (4) resting—not exhibiting any of the above activities. Positions were: (1) surface—siphon in contact with water surface; (2) wall—within 1 mm of the sides; (3) bottom—within 1 mm of the bottom; and (4) middle—more than 1 mm from the sides, bottom, and surface.
Activity and position of the test larvae were recorded every 30 s for 15 min upon playback of the video clips. Frequencies of behaviors were then converted to proportions (total number of observations per replicate = 30) for each replicate larva. The number of variables per replicate was reduced with principal component analysis (PCA). Principal components (PCs) with eigen values >1 were retained and analyzed by MANOVA, with the sites nested within habitat type (tree hole, tire, and cemetery). SCCs were used to evaluate the relative contribution of the PCs to significant effects (Scheiner 2001). A second analysis tested for any differences among sites, ignoring type.
Because previous studies have shown that resting and surface are the least risky behaviors of mosquito prey compared to other behaviors (Juliano and Reminger 1992), we estimated the difference between the means of PC1 (which primarily quantified proportion of time spent resting at the surface) for control and predation treatments for each site as a means of quantifying the degree of change in behavior in response to predator cues. We tested the correlation of this difference with the mean abundance of C. appendiculata for the site. We also tested the correlation between means PC1 for control and predation treatments (i.e, quantification of the frequency of resting at the surface) for each site with C. appendiculata abundance.
Results
Correlation of abundances
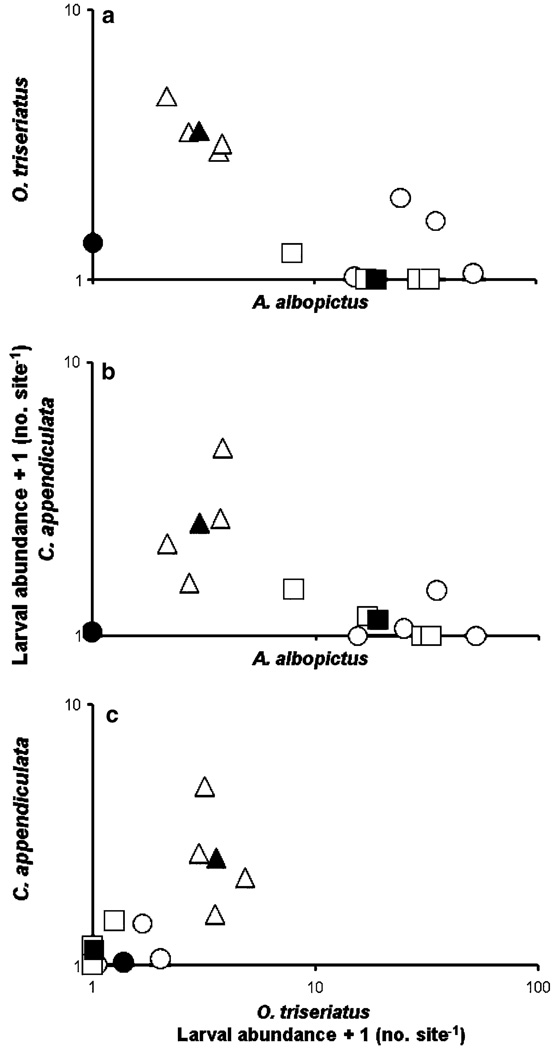
There was a significant habitat type effect (Pillai’s trace = 1.198, df = 6, 16, P = 0.0125) indicating that the abundances of the prey and predators differed among tree hole, tire, and cemetery sites. Abundance of A. albopictus was lowest in tree hole sites and greater in cemetery and tire sites (Fig. 1). In contrast, abundances of C. appendiculata and O. triseriatus were greatest in tree hole sites, and lower in tire and cemetery sites (Fig. 1). SCCs indicated that the site type mean abundances of O. triseriatus (SCC = −0.376) and C. appendiculata (SCC = −0.298) were negatively correlated with the site type mean abundances of A. albopictus (SCC = 0.293) (Fig. 1a, b), and were positively correlated with each other (Fig. 1c). There was also a significant site nested within type [site(type)] effect (Pillai’s trace = 0.347, df = 27, 1,173, P = < 0.0001) indicating that sites within the types also differed in their abundances prey and predators. Correlations among the site mean abundances of the three species were generally similar to the correlations observed across site types, with C. appendiculata and O. triseriatus positively related to each other and both negatively related to A. albopictus (Fig. 1a–c).
Fig. 1.
Abundances (back-transformed means) of three species of mosquito larvae, Aedes albopictus, Ochlerotatus triseriatus and Corethrella appendiculata, in cemeteries (circles), automobile tires (squares), or tree holes (triangles) at 11 sites in Florida, USA, May–October 2004 and 2005. a O. triseriatus and A. albopictus, b C. appendiculata and A. albopictus, c C. appendiculata and O. triseriatus. Open symbols are least squares means for individual sites within each type of site and closed symbols are least square means for the type
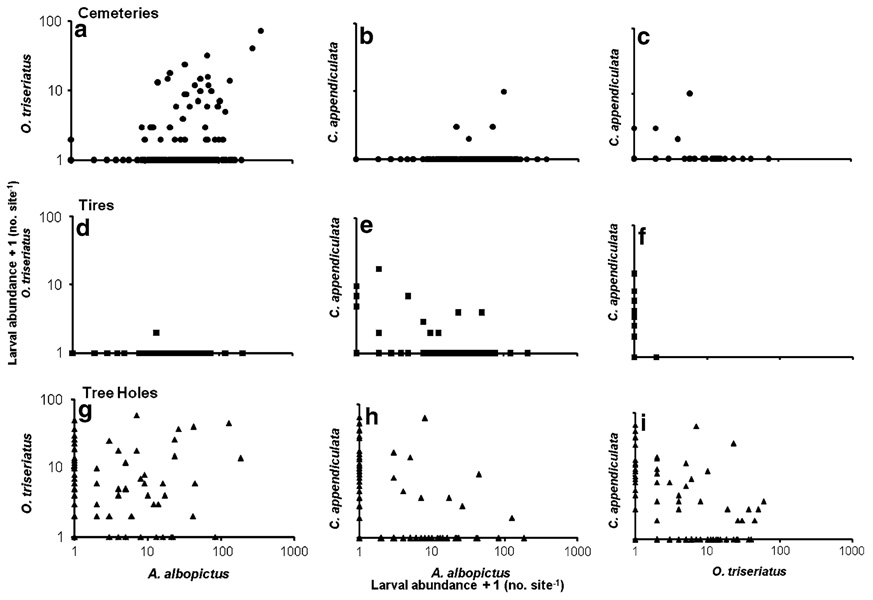
The abundances of C. appendiculata and O. triseriatus were greatest in tree hole sites but lower in tire and cemetery sites. Among containers within habitat types, patterns of correlation among species abundances were not consistent with correlations at the type or site level. In cemeteries, A. albopictus and O. triseriatus abundances were significantly positively correlated (Fig. 2a), and neither prey abundance was significantly related to abundance of C. appendiculata (Fig. 2b, c,). In tires, A. albopictus and C. appendiculata abundances were significantly negatively correlated (Fig. 2e), whereas abundances of A. albopictus and C. appendiculata were not significantly related to abundance of O. triseriatus, which was very rare in tires (Fig. 2d, f). In tree holes, O. triseriatus and A. albopictus abundances were both significantly negatively correlated with C. appendiculata abundances (Fig. 2h, i), and were not significantly correlated with each other (Fig. 2g). Ignoring abundances and considering only presence vs. absence, a contingency table test of association between species in tree holes showed that A. albopictus and C. appendiculata were significantly negatively associated (χ2 = 14.08, P = 0.0001), but O. triseriatus and C. appendiculata were not significantly associated (χ2 = 2.77, P = 0.0960), though the trend was for a negative association.
Fig. 2.
Correlation of log10 (y + 1) values of total number of each species of mosquito per container from a–c cemeteries, d–f tires and g–i tree holes. Cemeteries a O. triseriatus and A. albopictus [correlation coeffcient (CC) = 0.233, P = 0.0004], b C. appendiculata and A. albopictus (CC = 0.083, P = 0.2283), c C. appendiculata and O. triseriatus (CC = 0.121, P = 0.0782); tires d O. triseriatus and A. albopictus (CC = −0.016, P = 0.8760), e C. appendiculata and A. albopictus (CC = −0.433, P = <0.0001), f C. appendiculata and O. triseriatus (CC = −0.033, P = 0.7475); tree holes g O. triseriatus and A. albopictus (CC = 0.190, P = 0.0669), h C. appendiculata and A. albopictus (CC = −0.314, P = 0.0021), i C. appendiculata and O. triseriatus (CC = −0.271, P = 0.0085). Each point in the graph represents the total number present in a container
Behavioral difference among sites
The correlated response variables were reduced to three uncorrelated PCs with eigen values >1, which together summarized 89% of the variation in behavior frequencies. A greater positive score on PC1 indicated that larvae spent more time resting at the surface and a negative score indicated they spent more time browsing at the wall and bottom. A greater score on PC2 indicated that larvae spent more time thrashing in the middle and a negative score indicated they spent more time browsing. A greater score on PC3 indicated that larvae spent more time filtering in the middle and a negative score indicated they spent more time in other behaviors (Table 1).
Table 1.
Rotated factor patterns to test the differences in behavioral responses of Aedes albopictus across different sites. The three principal components (PCs) explained 89% of the variation. Values >40 are in bold
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| Resting | 98 | −20 | −8 |
| Browsing | −88 | −45 | −12 |
| Thrashing | 24 | 99 | −11 |
| Filtering | 2 | 8 | 99 |
| Surface | 98 | 19 | −1 |
| Wall | −79 | −39 | −7 |
| Middle | 26 | 93 | 42 |
| Bottom | −56 | −23 | −17 |
| Interpretation | Resting, surface vs. browsing, wall, bottom | Thrashing, middle vs.browsing | Filtering, middlevs. other |
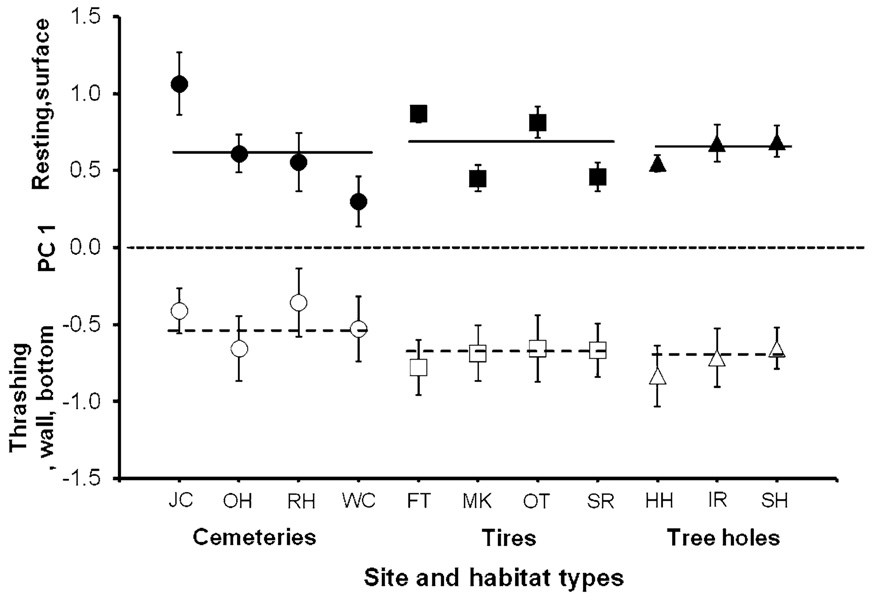
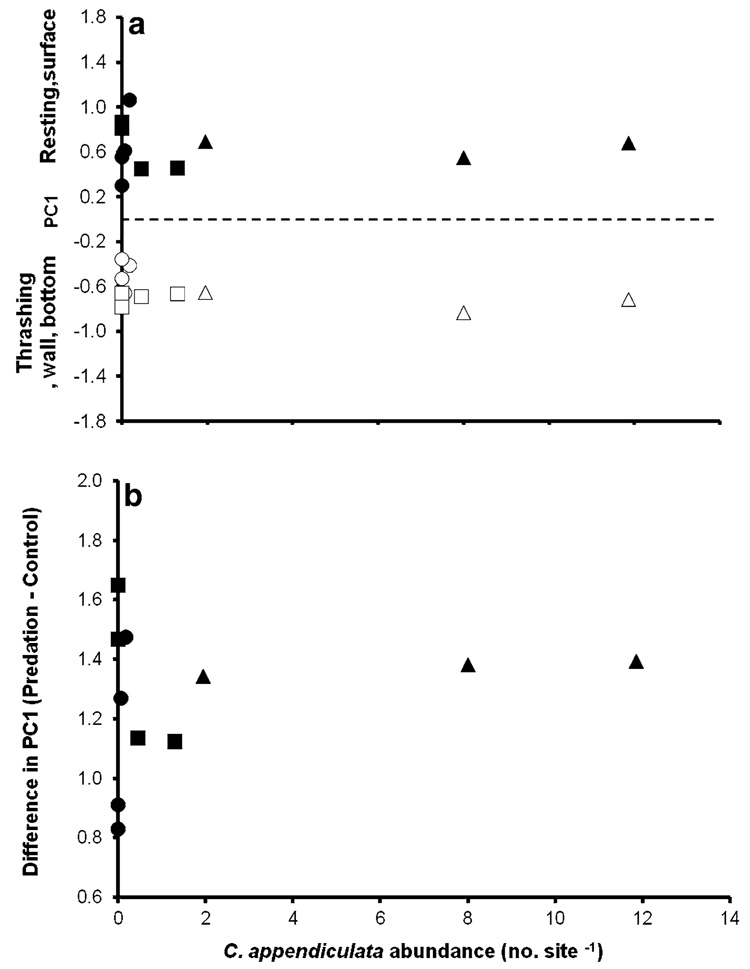
Treatment (control, predation) was significant but habitat type (tree hole, tire, and cemetery), and the treatment by habitat type interaction were not significant (Table 2; Fig. 3). Because habitat type was not significant, we dropped it from the analysis and tested for site effects among all 11 tested sites, in order to detect any differentiation in behavior among sites. The site and the interaction of site and treatment were also not significant (Table 2; Fig. 3). Thus, there were no differences in A. albopictus’ response to control and predation treatments among the habitat types or sites. Correlation analysis indicated that there was no significant correlation between the magnitude of A. albopictus’ behavioral response (quantified by divergence in PC1 values between control and predation treatments) and C. appendiculata mean abundance (Fig. 4b). There were also no significant correlations between means of PC1 for control and predation water treatments for each site and C. appendiculata abundance at that site (Fig. 4a).
Table 2.
Multivariate ANOVA table to test the differences in behavioral responses of A. albopictus across different sites. Significant effects are in bold. Num Numerator, Den denominator
| Variables | Num df | Den df | Pillai’s trace | P | Standardized canonical coefficients |
||
|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | |||||
| Part 1a | |||||||
| Type | 6 | 14 | 0.269 | 0.8905 | 0.526 | −0.726 | −0.112 |
| Treatment | 3 | 6 | 0.977 | <0.0001 | 1.036 | 0.491 | 0.064 |
| Type × Treatment | 6 | 14 | 0.738 | 0.2941 | 0.396 | 0.848 | 0.818 |
| Part 2b | |||||||
| Site | 30 | 1209 | 0.071 | 0.5042 | −0.023 | −0.474 | 0.941 |
| Treatment | 3 | 401 | 0.614 | <0.0001 | 1.351 | 0.749 | −0.139 |
| Treatment × Site | 30 | 1209 | 0.091 | 0.1641 | 0.723 | 0.671 | 0.715 |
With type effect
Without type effect
Fig. 3.
Principal component 1 (PC1) (means ± SE) for control (open symbols) and predation (closed symbols) treatments at each site with mean PC1 for cemeteries (circles), tires (squares) and tree holes (triangles) indicated by the horizontal line (dotted lines control, solid lines predation)
Fig. 4.
Correlation between C. appendiculata abundance (no. site−1) and a mean PC1 of control and predation treatments (control, CC = −0.446, P = 0.1483; predation, CC = −0.0487, P = 0.8870) and b difference in PC1 (i.e., change in behavior) for each site (CC = −0.229, P = 0.4960) with habitat types identified as cemeteries (circles), tires (squares) or tree holes (triangles). For abbreviations, see Fig. 2 and Fig. 3
Discussion
Competitive interactions between prey species can be altered by differential prey responses to predators (Werner and Anholt 1996; Relyea 2000). Classic studies (Morin 1981) and more recent work (Ciros-Perez et al. 2004) show that coexistence of a competitively inferior species with a superior species is aided by selective predation on the competitively superior species. Field studies have shown that A. albopictus has displaced the competitively inferior species, A. aegypti, in many areas of northern and central Florida, particularly in tire and cemetery vase sites (O’Meara et al.1995; Juliano and Lounibos 2005). We see in our present data that these classes of sites are largely predator free, and that displacement is indicative of the simple effect of interspecific competition in largely predator-free habitats. Both laboratory and Weld studies have shown that A. albopictus is the superior resource competitor to O. triseriatus, particularly when food availability is very low (Novak et al. 1993; Lounibos et al. 2001; Aliabadi and Juliano 2002). Despite this, Lounibos et al. (2001) reported that O. triseriatus have not been replaced by A. albopictus in the Weld, especially in tree holes in wooded habitat. Behavioral responses of A. albopictus to water-borne cues to risk of predation are either absent (Kesavaraju and Juliano 2004) or of much smaller magnitude than those of native O. triseriatus (Kesavaraju et al. 2007a). This likely contributes to greater vulnerability of A. albopictus to predation by C. appendiculata (and to other predators like Toxorhynchites rutilus (order, Diptera; family, Culicidae) compared to O. triseriatus (Lounibos et al. 2001; Griswold and Lounibos 2005a; Griswold and Lounibos 2005b).
Among habitat types, C. appendiculata and O. triseriatus abundances were negatively correlated with abundances of A. albopictus, with both native species abundant in tree holes compared to cemetery and tire sites (Fig. 1). In cemeteryand tire sites, where C. appendiculata and O. triseriatus abundances were low, A. albopictus dominated and their abundances were twice those found in tree hole habitats (Fig. 1). The combination of our distribution data for these species and previous data on competition (Teng and Apperson 2000; Lounibos et al. 2001; Aliabadi and Juliano 2002), and vulnerability to predation (Lounibos et al. 2001;Griswold and Lounibos 2005a, b; Kesavaraju et al. 2007a) strongly suggest predator-mediated coexistence via predation by C. appendiculata (i.e., C. appendiculata are keystone predators; Leibold 1996) in the tree hole systems. By limiting the abundances of competitively superior, but predation-vulnerable A. albopictus within sites, C. appendiculata prevent O. triseriatus from being competitively excluded by invading A. albopictus. The data on individual containers clearly show that C. appendiculata abundance is negatively related to abundances of both prey species, particularly in tree holes (Fig. 2), as would be expected for the local relationship of predator abundance to prey abundance. It is also clear that A. albopictus is rarely present in tree holes with C. appendiculata (ten of 40 tree holes with C. appendiculata had A. albopictus; Fig. 3h), whereas O. triseriatus is more frequently observed in tree holes with C. appendiculata (25 of 40 tree holes with C. appendiculata had O. triseriatus; Fig. 2i). The contingency table analysis shows that in tree holes, where C. appendiculata are most abundant, A. albopictus is significantly and strongly negatively associated with C. appendiculata whereas O. triseriatus showed no significant association with C. appendiculata. These patterns also suggest that O. triseriatus Wnds a refuge from competition with A. albopictus in sites and containers where C. appendiculata is present.
Adults of species in the genus Corethrella take blood meals from frogs, using protein to produce eggs (McKeever 1977; McKeever and Hartberg 1981; Bernal et al. 2006). These flies show species-specific attraction to mating calls of frogs (Bernal et al. 2006). C. appendiculata collected during the Weld survey in Florida, have taken blood meals from Hyla cinerea (green tree frog) in the laboratory (B. Kesavaraju, unpublished data). Amphibians are very sensitive to disturbances and their abundances are often lower in urban areas (e.g., cemetery and tire sites) when compared to forested areas (e.g., tree hole sites) (Knutson et al.1999). Low abundances of C. appendiculata in the cemetery/tire sites could be related, in part, to low abundances of frogs upon which C. appendiculata depend for blood meals.
It is tempting to infer from the limited behavioral responses of A. albopictus to North American predators like T. rutilus and C. appendiculata that this invader must have had limited evolutionary history with such predators in its native range. Such an inference is likely untrue although quantitative data are lacking. Toxorhynchites spp. are distributed worldwide in the tropics and subtropics, and are found in temperate Asia, where North American A. albopictus originated (Hawley 1988). Less is known about distribution of the genus Corethrella, although members of this genus are found in Japan (Miyagi 1974). Thus it seems probable that A. albopictus has an evolutionary history with congenerics of the North American predators it encounters most, though specifics of microhabitat overlap remain undocumented. The limited behavioral response to these predators by A. albopictus may thus reflect an alternative suite of antipredator adaptations in this mosquito (e.g., life history adaptations, habitat choice).
Populations that regularly co-occur with predators may evolve adaptations that help alleviate risk of predation, and those adaptations may be limited or absent in populations in predator-free habitats (Downes and Adams 2001). A. albopictus populations from tree hole habitats with high C. appendiculata abundances would thus be expected to show a greater degree of reduced movement in response to water-borne predator cues compared to populations collected from cemetery and tire habitats that are largely predator free. All the populations of A. albopictus in this study showed similar reduced movement and increased resting at the surface when they encountered water-borne cues from predation. Further, there was no difference in baseline patterns of behavior (i.e., no population effect) (Fig. 3). There was also no cross-site correlation in C. appendiculata abundance and the behavioral responses of A. albopictus, suggesting no local adaptation of A. albopictus behavior to predator density (Fig. 4). It is possible that the absence of differentiation indicates lack of sufficient time for evolution of population differentiation, or lack of suficient isolation between populations for local differentiation in antipredator behavior. However, populations of A. albopictus have been in Florida sufficiently long, with sufficient isolation, for evolution of large-scale geographic differences (e.g., in diapause; Lounibos et al. 2003). In other predator–prey systems, the intensity of antipredator behaviors was dependent on proximity and gene flow between populations sympatric and allopatric with predators (Storfer and Sih 1998). The lack of divergence in A. albopictus antipredator behavior between populations of high and low predator abundance could arise because these populations are not sufficiently isolated and are experiencing considerable gene flow between them.
Previous studies have shown that A. albopictus is more vulnerable to C. appendiculata predation than is O. triseriatus (Griswold and Lounibos 2005a, Griswold and Lounibos 2005b; Kesavaraju et al. 2007a), but follow up studies on how vulnerability affects distribution in the field were lacking. Our study shows that in natural container habitats like tree holes, C. appendiculata is more abundant than in artiWcial container habitats, and may be impeding dominance by A. albopictus. In the artificial container habitats like tires and cemeteries, C. appendiculata abundance is lower and A. albopictus dominates. Recent experiments conducted in tires have indicated that addition of C. appendiculata reduces colonization by A. albopictus, but that the presence of O. triseriatus alone does not (S. A. Juliano and L. P. Lounibos, personal communication). These results point out the importance of multiple species interactions for determining success and impact of invasive species. This conclusion suggests that maintaining a diverse array of relatively undisturbed natural ecosystems (e.g., forests) with their associated fauna at multiple trophic levels may provide valuable protection against invasion by undesirable non-native species.
Supplementary Material
Electronic supplementary material The online version of this article (doi:10.1007/s00442-007-0935-4) contains supplementary material, which is available to authorized users.
Acknowledgements
We thank R. Escher for providing C. appendiculata., the Department of Environmental Protection, Florida for permission to collect mosquitoes, L. P. Lounibos, Florida Medical Entomology Laboratory, Vero Beach, Florida for providing lab space, and L. P. Lounibos, C. F. Thompson, W. L. Perry, D. L. Byers, and two anonymous referees for useful comments on the manuscript. This research was supported by grants from the National Science Foundation (DEB no. #0507015), National Institute of Allergy and Infectious Disease (R01-AI44793, Illinois State University subcontract), and the Illinois State University Beta Lambda chapter of Phi Sigma.
Contributor Information
Banugopan Kesavaraju, Email: banu@rci.rutgers.edu, Department of Biology, Illinois State University, Normal, IL 61790, USA; Center for Vector Biology, Rutgers University, 180 Jones Avenue, New Brunswick, NJ 08901, USA.
Kavitha Damal, Department of Biology, Illinois State University, Normal, IL 61790, USA.
Steven A. Juliano, Department of Biology, Illinois State University, Normal, IL 61790, USA
References
- Aliabadi BW, Juliano SA. Escape from gregarine parasites affects the competitive interactions of an invasive mosquito. Biol Invasions. 2002;4:283–297. doi: 10.1023/A:1020933705556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal XE, Rand AS, Ryan MJ. Acoustic preferences and localization performance of blood-sucking flies (Corethrella Coquillett) to tungara frog calls. Behav Ecol. 2006;17:709–715. [Google Scholar]
- Buskirk JV, McCollum SA, Werner EE. Natural selection for environmentally induced phenotypes in tadpoles. Evolution. 1997;51:1983–1992. doi: 10.1111/j.1558-5646.1997.tb05119.x. [DOI] [PubMed] [Google Scholar]
- Ciros-Perez J, Carmona MJ, Lapesa S, Serra M. Predation as a factor mediating resource competition among rotifer sibling species. Limnol Oceanogr. 2004;49:40–50. [Google Scholar]
- DeRivera CE, Ruiz GM, Hines AH, Jivoff P. Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology. 2005;86:3364–3376. [Google Scholar]
- Downes SJ, Adams M. Geographic variation in antisnake tactics: the evolution of scent-mediated behavior in a lizard. Evolution. 2001;55:605–615. doi: 10.1554/0014-3820(2001)055[0605:gviatt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Garvey JE, Rettig JE, Stein RA, Lodge DM, Klosiewski SP. Scale-dependent associations among fish predation, littoral habitat, and distributions of crayfish species. Ecology. 2003;84:3339–3348. [Google Scholar]
- Griswold M, Lounibos LP. Competitive outcomes of aquatic container Diptera depend on predation and resource levels. Ann Entomol Soc Am. 2005a;98:673–681. doi: 10.1603/0013-8746(2005)098[0673:COOACD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol Entomol. 2005b;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold M, Lounibos LP. Predator identity and additive effects in a treehole community. Ecology. 2006;87:987–995. doi: 10.1890/0012-9658(2006)87[987:piaaei]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1988;4:1–40. [PubMed] [Google Scholar]
- Holway DA, Suarez AV. Animal behavior: an essential component of invasion biology. Trends Ecol Evol. 1999;14:328–330. doi: 10.1016/s0169-5347(99)01636-5. [DOI] [PubMed] [Google Scholar]
- Ibanez-Bernal BBS, Mutebi JP, Argot E, Rodriguez G. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med Vet Entomol. 1997;11:305–309. doi: 10.1111/j.1365-2915.1997.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Juliano SA, Gravel ME. Predation and the evolution of prey behavior: an experiment with tree hole mosquitoes. Behav Ecol. 2002;13:301–311. [Google Scholar]
- Juliano SA, Hechtel LJ, Waters J. Behavior and risk of predation in larval tree hole mosquitoes: effects of hunger and population history of predation. Oikos. 1993;68:229–241. [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Reminger L. The relationship between vulnerability to predation and behavior of larval tree-hole mosquitoes: geographic and ontogenetic differences. Oikos. 1992;63:465–467. [Google Scholar]
- Kats LB, Petranka JW, Sih A. Antipredator defenses and the persistence of amphibian larvae with fishes. Ecology. 1988;69:1865–1870. [Google Scholar]
- Kesavaraju B, Alto BW, Lounibos LP, Juliano SA. Behavioural responses of larval container mosquitoes to a size-selective predator. Ecol Entomol. 2007a;32:262–272. doi: 10.1111/j.1365-2311.2006.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Damal K, Juliano SA. Threat-sensitive behavioral responses to concentrations of water-borne cues from predation. Ethology. 2007b;113:199–206. doi: 10.1111/j.1439-0310.2006.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitching RL. The natural history and ecology of phytotelmata. Cambridge: Cambridge University Press; 2000. Food webs and container habitats. [Google Scholar]
- Knutson MG, Sauer JR, Olsen DA, Mossman MJ, Hemesath LM, Lannoo MJ. Effects of landscape composition and wetland fragmentation on frog and toad abundance and species richness in Iowa and Wisconsin, USA. Conserv Biol. 1999;13:1437–1446. [Google Scholar]
- Kolar CS, Lodge DM. Progress in invasion biology: predicting invaders. Trends Ecol Evol. 2001;16:199–204. doi: 10.1016/s0169-5347(01)02101-2. [DOI] [PubMed] [Google Scholar]
- Lee CE. Evolutionary genetics of invasive species. Trends Ecol Evol. 2002;17:386–391. [Google Scholar]
- Leibold MA. A graphical model of keystone predators in food webs: trophic regulation of abundance, incidence, and diversity patterns in communities. Am Nat. 1996;147:784–812. [Google Scholar]
- Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 1990;68:619–640. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lodge DM. Biological invasions: lessons for ecology. Trends Ecol Evol. 1993;8:133–136. doi: 10.1016/0169-5347(93)90025-K. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos RE, Juliano SA. Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biol Invasions. 2001;3:151–166. [Google Scholar]
- Lounibos LP, Escher RL, Lourenco-de-Oliveria R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Ann Entomol Soc Am. 2003;96:512–518. [Google Scholar]
- McKeever S. Observations of Corethrella feeding on tee frogs (Hyla) Mosquito News. 1977;37:522–523. [Google Scholar]
- McKeever S, Hartberg WK. An effective method for trapping adult female Corethrella (Diptera, Chaoboridae) Mosquito News. 1981;40:111–112. [Google Scholar]
- Miyagi I. On a blood-sucking Corethrella sp. collected in Nagasaki, Japan (Diptera: Chaoboridae) Trop Med. 1974;16:89–93. [Google Scholar]
- Morin PJ. Predatory salamanders reverse the outcome of competition among 3 species of anuran tadpoles. Science. 1981;212:1284–1286. doi: 10.1126/science.212.4500.1284. [DOI] [PubMed] [Google Scholar]
- Novak MG, Higley LG, Christianssen CA, Rowley WA. Evaluating larval competition between Aedes albopictus and A. triseriatus (Diptera, Culicidae) through replacement series experiments. Environ Entomol. 1993;22:311–318. [Google Scholar]
- O’Meara GF, Evans LF, Getman AD, Cuda JP. Spread of Aedes albopictus and decline of Aedes aegypti (Diptera, Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Phillips BL, Shine R. Adapting to an invasive species: toxic cane toads induce morphological change in Australian snakes. Proc Natl Acad Sci USA. 2004;101:17150–17155. doi: 10.1073/pnas.0406440101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BL, Shine R. The morphology, and hence impact, of an invasive species (the cane toad, Bufo marinus): changes with time since colonisation. Anim Conserv. 2005;8:407–413. [Google Scholar]
- Relyea RA. Trait-mediated indirect effects in larval anurans: reversing competition with the threat of predation. Ecology. 2000;81:2278–2289. [Google Scholar]
- Relyea RA. Local population differences in phenotypic plasticity: predator-induced changes in wood frog tadpoles. Ecol Monogr. 2002;72:77–93. [Google Scholar]
- Scheiner SM. MANOVA: multiple response variables and multispecies interaction. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. 2nd edn. New York: Oxford University Press; 2001. pp. 99–115. [Google Scholar]
- Sih A. The behavioral response race between predator and prey. Am Nat. 1984;123:143–150. [Google Scholar]
- Smith KG. Keystone predators (eastern newts, Notophthalmus viridescens) reduce the impacts of an aquatic invasive species. Oecologia. 2006;148:342–349. doi: 10.1007/s00442-006-0370-y. [DOI] [PubMed] [Google Scholar]
- Storfer A, Sih A. Gene flow and ineffective antipredator behavior in a stream-breeding salamander. Evolution. 1998;52:558–565. doi: 10.1111/j.1558-5646.1998.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperature. J Med Entomol. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera : Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Werner EE, Anholt BR. Predator-induced behavioral indirect effects: consequences to competitive interactions in anuran larvae. Ecology. 1996;77:157–169. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material The online version of this article (doi:10.1007/s00442-007-0935-4) contains supplementary material, which is available to authorized users.