Abstract
The transcription factor ThPOK has been shown to be required and sufficient for CD4+CD8− thymocyte generation, yet the mechanism through which ThPOK orchestrates CD4 helper T cell lineage differentiation remains unclear. Here we utilized reporter mice to track expression of transcription factors in developing thymocytes. Distal promoter-driven Runx3 (Runx3d) expression was restricted to MHC class I-selected thymocytes. In ThPOK-deficient mice, Runx3d expression was de-repressed in MHCII-selected thymocytes, contributing to their redirection to the CD8 T cell lineage. In the absence of both ThPOK and Runx, redirection was prevented and cells potentially belonging to the CD4 lineage, presumably specified independently of ThPOK, were generated. Our results suggest that MHCII-selected thymocytes are directed towards the CD4 lineage independently of ThPOK, but require ThPOK to prevent Runx-dependent differentiation towards the CD8 lineage.
INTRODUCTION
Development of αβ T cells requires rearrangement of the Tcra and Tcrb loci and appropriate signals transduced via interactions between T cell receptors (TCR) on CD4+CD8+ double positive (DP) thymocytes and peptide in the context of major histocompatibility complex (MHC) molecules on thymic stromal cells. Only a small proportion of DP thymocytes is selected for differentiation into mature cells that home to peripheral secondary lymphoid organs. This positive selection process is accompanied or followed by coordinated activation and/or repression of genes that program differentiation of the selected thymocytes to specific lineages of mature T cells. CD4+ helper T cells and CD8+ cytotoxic T cells comprise a majority of mature αβ T lymphocytes in mice and humans. After positive selection, DP cells expressing MHC class I- or MHC class II-restricted TCRs undergo differentiation into CD8+ and CD4+ lineage T cells, respectively; this differentiation involves transcriptional down-regulation of one or the other co-receptor1. CD4 expression is terminated in CD4−CD8+ single positive (CD8SP) thymocytes through activation of the Cd4 silencer, a negative cis-regulatory element in the Cd4 locus; Cd4 silencing is subsequently maintained by epigenetic mechanisms2,3. CD8 expression is transiently down-regulated in both MHCI- and MHCII-restricted thymocytes and is selectively reactivated in MHCI-restricted CD8SP cells through stage-specific enhancers4. In addition to Cd4, Cd8a and Cd8b, other genes are selectively activated or repressed in each mature T cell lineage, and a major goal has been to elucidate how lineage-specific gene regulation is coordinated.
During early hematopoiesis, a balance between distinct lineage-specific transcription factors determines fates of mature cells derived from common multipotent progenitors5. Similar gene regulatory networks may regulate the CD4 versus CD8 lineage decision. The C2H2 type zinc finger transcription factor ThPOK (http://www.informatics.jax.org/javawi2/servlet/WIFetch?page=markerDetail&key=18709), encoded by the Zbtb7b gene, is required and sufficient for generation of CD4SP thymocytes following positive selection6,7. hd/hd (helper deficient) mice, which harbor a mutation in ThPOK, lack CD4+ helper T cells, and thymocytes selected by MHCII are redirected to the CD8+ T cell lineage. Forced expression of ThPOK results in redirection of MHCI restricted thymocytes to the CD4+ T cell lineage6,7.
Whereas ThPOK exerts a dominant influence over the differentiation of CD4SP thymocytes, it is not clear if differentiation of CD8SP thymocytes requires such a pivotal factor or occurs by default if ThPOK is not up-regulated. We previously identified the Runx family of transcriptional regulators as crucial for Cd4 gene silencing associated with differentiation of the CD8+ cytotoxic T cell lineage8. Among the three Runx proteins that form heterodimers with the common subunit CBFβ (http://www.informatics.jax.org/javawi2/servlet/WIFetch?page=markerDetail&key=15686), Runx3 (http://www.informatics.jax.org/javawi2/servlet/WIFetch?page=markerDetail&key=45178) is the primary mediator of heritable Cd4 silencing during development of the CD8SP lineage, and Runx1 (http://www.informatics.jax.org/javawi2/servlet/WIFetch?page=markerDetail&key=45176) also contributes to partial Cd4 silencing in the absence of Runx3 (ref. 8,9). Conditional inactivation of both Runx1 and Runx3 at the DP stage of thymocyte differentiation resulted in a complete loss of CD8SP thymocytes, although generation of CD4SP thymocytes was still observed10. Runx3 protein is detected in CD8SP but not CD4SP thymocytes, even though Runx3 mRNA was up-regulated in both lineages following positive selection10,11. These findings suggest that Runx3 expression is regulated at least in part via post-transcriptional mechanisms. Runx transcripts are initiated at two distinct sites; Runx3 expression from the distal promoter is detected exclusively in CD8SP thymocytes, whereas CD4SP cells express only the proximal promoter-derived transcript that is not sufficient for protein synthesis10. These results, together with our previous finding that Runx1 inactivation alone exerts little influence on development of the CD8+ lineage10, suggest that the CD8+ lineage-specific requirement for Runx function is satisfied primarily by Runx310, and that activation of the Runx3 distal promoter (designated here as Runx3d) is a key event required for CD8 lineage differentiation of MHCI-restricted thymocytes. Even though it is not expressed in naïve peripheral CD4+ T cells, Runx3 protein is up-regulated in interferon (IFN)-γ–producing T helper type 1 (TH1) cells, where it contributes to repression of the interleukin (IL)-4 (Il4) gene, but the mechanism for its regulation in these cells is not known12,13.
ThPOK expression in developing thymocytes is regulated by a cis-acting silencer element required to restrict ThPOK expression to the CD4+ lineage14,15. In the absence of this silencer, ThPOK is prematurely expressed in DP thymocytes, and all positively selected thymocytes differentiate into CD4+ lineage cells14. The ThPOK silencer is bound by Runx complexes, and ThPOK is prematurely expressed in pre-selection DP thymocytes in the absence of both Runx1 and Runx3 (ref. 14). This result implies that ThPOK activity is required for diversion of MHCI-selected Runx-deficient thymocytes to the helper T cell lineage, and suggests that Runx proteins continue to suppress ThPOK expression following positive selection of MHCI-restricted thymocytes. However, a requirement for Runx complexes in lineage-specific ThPOK silencing has been disputed14,15.
To clarify the gene regulatory network governing lineage determination of αβ T cells, we generated knock-in reporter strains that facilitate single cell analysis of ThPOK and Runx3d expression in MHCI- and MHCII-selected thymocytes. By analyzing these reporter mice, we show here that ThPOK prevents up-regulation of Runx3 and other CD8 lineage-specific genes. We provide genetic evidence suggesting that ThPOK stabilizes the fate of MHCII-selected thymocytes that would otherwise be diverted to the CD8SP lineage due to constitutive Runx function. We also show that ThPOK is not required for the appearance of CD4SP cells in an environment in which MHCII-specific thymocytes are positively selected; this latter finding suggests that other factors act upstream of ThPOK to direct CD4 expression following positive selection.
RESULTS
Runx3d is required for Runx3 protein expression in CD8+ T cells
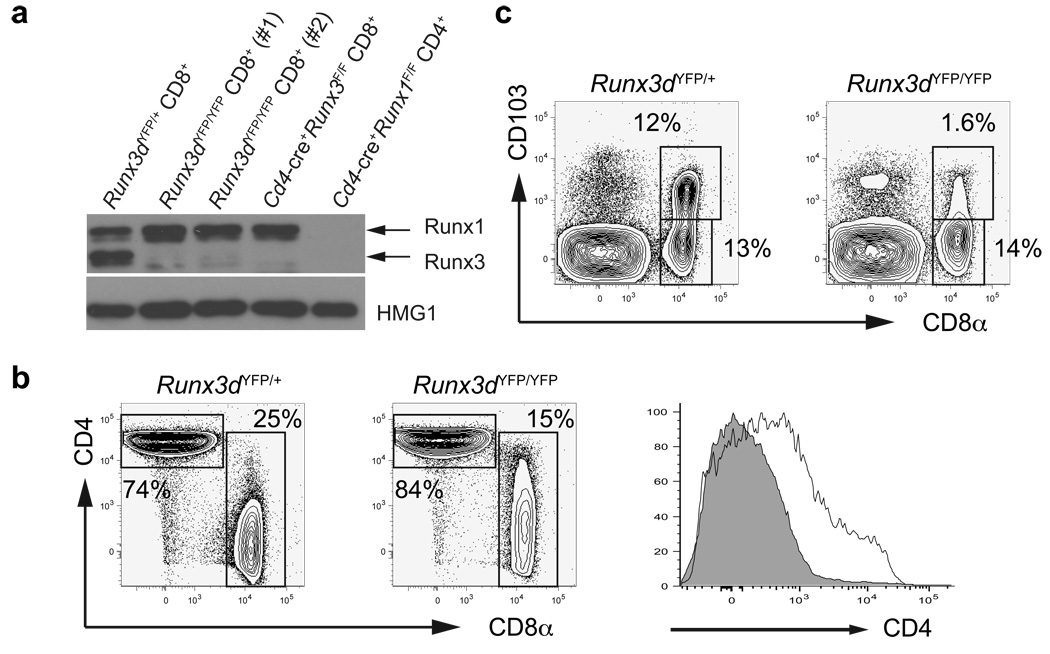
To visualize thymocytes expressing Runx3d mRNA, we generated a reporter allele (Runx3dYFP) by replacing the first coding exon utilized by the Runx3 distal promoter with the yellow fluorescent protein (YFP) coding sequence (Supplementary Fig. 1a, online). In accordance with previous analyses of Runx protein and mRNA expression10, YFP expression was detected in CD8+ T cells, but not in CD4+ T cells or B220+ cells in peripheral blood lymphocytes from Runx3dYFP/+ mice (Supplementary Fig. 1b, online). To determine whether Runx3 distal promoter activity is required for Runx3 protein expression in CD8+ T cells, we generated homozygous Runx3dYFP/YFP mice, in which distal promoter-derived Runx3 expression was eliminated. Whereas germline deletion of both proximal promoter- and distal promoter-derived Runx3 results in neonatal lethality in the 129 or C57BL6 genetic background16,17, Runx3dYFP/YFP mice were viable and fertile, and showed no gross abnormality (data not shown). Runx3 protein was almost undetectable in CD8+ T cells, and was markedly reduced in T helper type (TH1)-polarized activated CD4+ T cells from Runx3dYFP/YFP compared to Runx3dYFP/+ mice (Fig. 1a and Supplementary Fig. 1c,d, online). Runx1 was up-regulated in Runx3-deficient CD8+ T cells from both Runx3F/FCd4-cre+ and Runx3dYFP/YFP mice (Fig. 1a and Supplementary Fig. 1d, online). This result indicates that most of Runx3 protein is derived from the distal promoter-driven transcript both in CD8+ T cells and in TH1-polarized CD4+ T cells, and that only a small amount of Runx3 protein is expressed from the proximal promoter in T cells. In CD8+ T cells from Runx3dYFP/YFP mice, Cd4 silencing was incomplete and there was no up-regulation of CD103 (integrin αE) expression, which was previously shown to be dependent on Runx3 (ref. 18) (Fig. 1b,c). Thus, Runx3 expression from the distal promoter is required for Cd4 silencing and CD103 expression in CD8SP thymocytes.
Figure 1. CD8SP lineage-specific Runx3 expression from its distal promoter is required for Cd4 silencing and CD103 expression in CD8+ T cells.
(a) Runx3 and Runx1 protein expression in purified CD8+ cells from Runx3dYFP/YFP and Runx3dYFP/+ mice is shown with anti-HMG1 blot as loading control. Lysates from Cd4-cre+Runx1F/F CD4+ T cells and Cd4-cre+Runx3F/F CD8+ T cells were used as negative controls for Runx1 and Runx3 expression, respectively. (b,c) CD4 and CD8 (b) or CD8 and CD103 (c) expression in TCRβ+ lymph node (LN) cells from Runx3dYFP/YFP and Runx3dYFP/+ mice. In the right panel in (b), CD4 expression in CD8+ T cells from Runx3dYFP/YFP (open histogram) and Runx3dYFP/+ (shaded histogram) mice is shown. Mean fluorescent intensity: Runx3dYFP/+ 308, Runx3dYFP/YFP 1767. Data shown are representative of more than 3 independent experiments.
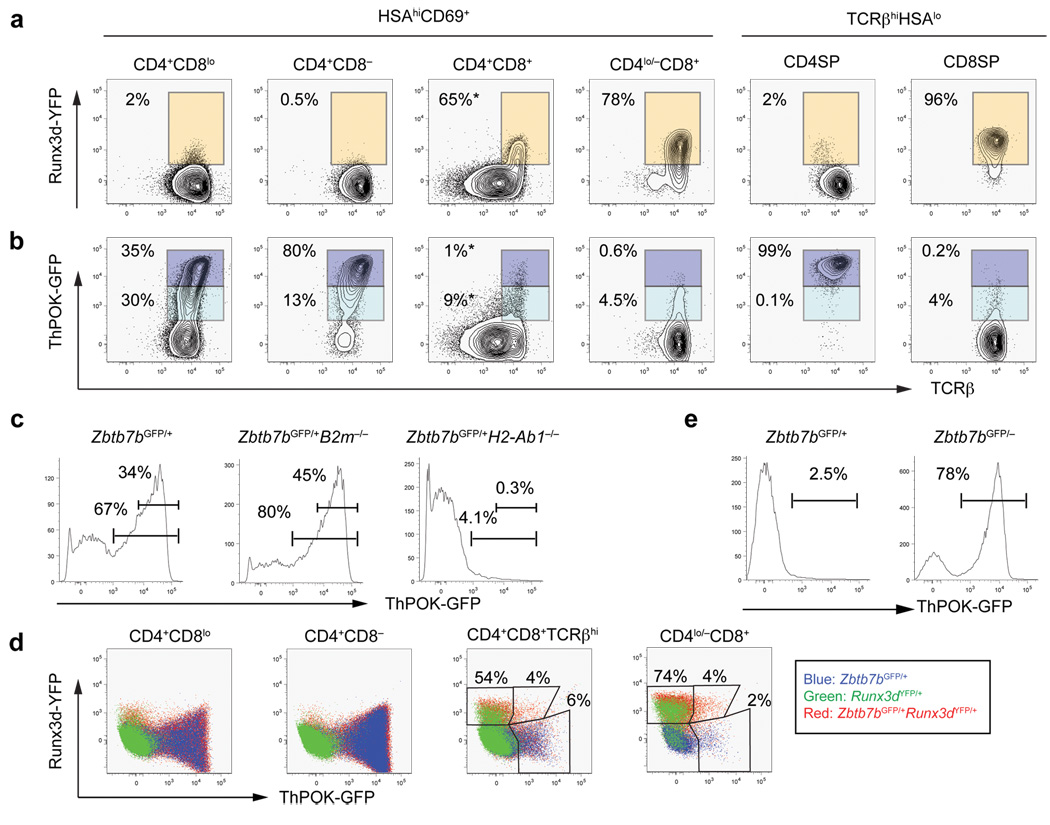
To investigate regulation of Runx3d expression in developing thymocytes, we next examined YFP expression in a series of intermediate thymocyte subsets defined by CD4, CD8, TCRβ, CD69 and CD24 (HSA) expression (Fig. 2a and Supplementary Fig. 2a,b, online). YFP+ cells were not detected in pre-selected CD69− DP thymocytes (data not shown). Following positive selection, MHCI- and MHCII-selected thymocytes both transit through an intermediate CD69+HSAhiTCRβintCD4+CD8lo (referred to hereafter as CD4+CD8lo) stage (Supplementary Fig. 2c, online) 19–25. At this stage, we observed 2–5 % of cells expressing YFP (Fig. 2a and Supplementary Fig. 2d, online). These YFP+ cells were absent in Runx3dYFP/+ mice on a B2m−/− background, indicating that Runx3d transcription is activated only in positively selected MHCI-restricted thymocytes (Supplementary Fig. 2d, online). Following the CD4+CD8lo stage, MHCI-selected thymocytes become CD4+CD8+CD69+TCRβhi, and such cells are absent in mice lacking β2-microglobulin, an essential component of MHCI molecules (B2m−/−) (Supplementary Fig. 2e, online). In contrast, MHCII-restricted thymocytes continue to down-regulate surface CD8 expression to become CD4+CD8− thymocytes before down-regulation of HSA. A majority of CD4+CD8+CD69+TCRβhi thymocytes expressed YFP, and cells with the highest surface TCRβ expression were brightest for YFP (Fig. 2a). Mature HSAlo/− CD8SP thymocytes expressed a uniformly high amount of YFP (Fig. 2a). YFP expression was not detected in the MHCII-restricted CD4+CD8−HSAhi population. These results indicate that Runx3d-YFP expression is highly restricted to MHCI-selected thymocytes, that it occurs largely following transit from the CD4+CD8lo stage, and that it specifically marks developing CD8SP thymocytes.
Figure 2. Runx3d and ThPOK reporter expression in developing thymocytes.
(a) Distal promoter-derived Runx3 expression determined by the Runx3d-YFP reporter in thymocyte subpopulations as defined in Supplementary Fig. 2, online. YFP+ cells are gated with orange rectangles. (b) ThPOK-GFP reporter expression in developing thymocytes. GFPhi and GFPlo populations are gated with dark and light blue rectangles, respectively. Percentages indicate frequencies of YFP+, GFPhi, and GFPlo cells among thymocyte subsets marked at top, except for those in the CD4+CD8+ subpopulation, for which percentages correspond to frequencies of gated cells among CD4+CD8+TCRβhi thymocytes (marked with asterisks). (c) ThPOK-GFP reporter expression in MHCI- and MHCII-restricted CD4+CD8lo thymocyte subpopulations. The percentages of total GFP+ cells (bottom gate) and GFPhi cells (top gate) are shown. (d) Mutually exclusive high ThPOK-GFP and Runx3d-YFP expression during thymocyte differentiation. ThPOK-GFP and Runx3d-YFP expression in developing thymocytes from indicated mice. Numbers indicate percentages of cells within gates. (e) Continued ThPOK-GFP expression in redirected MHCII-restricted ThPOK-deficient CD8+ T cells. ThPOK-GFP expression in CD8+ T cells from Zbtb7bGFP/+ or Zbtb7bGFP/− mice. Numbers indicate percentages of GFP+ cells. Data shown are representative of more than 3 independent experiments.
ThPOK expression specific to MHCII-restricted thymocytes
To visualize ThPOK expression in individual differentiating thymocytes, we generated a green fluorescent protein (GFP) knock-in allele of ThPOK (Zbtb7bGFP) by replacing the entire exon 2 and exon 3 with GFP cDNA (Supplementary Fig. 3a, online). In the periphery, all CD4+ T cells expressed high amounts of GFP, and a small proportion of CD8+ T cells expressed GFP, although in approximately 10-fold lower quantities (Supplementary Fig. 4a, online)6,7. A large proportion of B220+ cells also expressed low amounts of GFP (Supplementary Fig. 4a, online). In the thymus, GFP+ cells were not detected in CD69− pre-selected DP thymocytes (data not shown). Following positive selection, approximately half of the CD4+CD8lo cells and all mature CD4SP thymocytes expressed high quantities of GFP (Fig. 2b). In the B2m−/− background, a larger proportion of the CD4+CD8lo thymocytes expressed GFP due to enrichment of MHCII-restricted cells (Fig. 2c). At the CD4+CD8lo stage, distinct GFPhi and GFPlo populations were observed (Fig. 2b). We noted slightly higher TCRβ expression in GFPhi cells, suggesting that MHCII-selected thymocytes undergo maturation while up-regulating ThPOK during the CD4+CD8lo stage (Fig. 2b). Thus, expression of ThPOK occurs earlier in differentiation than that of Runx3d. Consistent with little GFP expression in peripheral CD8+ T cells, only a small subset of MHCI-selected thymocytes expressed low amounts of GFP. In Zbtb7bGFP/+H2-Ab1−/− mice, which lack MHCII molecules, approximately 5% of CD4+CD8lo, CD4+CD8+CD69+TCRβhi and CD4lo/−CD8+ populations expressed low quantities of GFP (Fig. 2c and data not shown). Approximately 4% of CD4+CD8+CD69+TCRβhi and CD4lo/−CD8+ thymocytes co-expressed Runx3d-YFP and low amounts of ThPOK-GFP (Fig. 2d), but we could not detect cells expressing high amounts of both reporters. Thus, strong expression of ThPOK and Runx3d in positively selected thymocytes may be mutually exclusive.
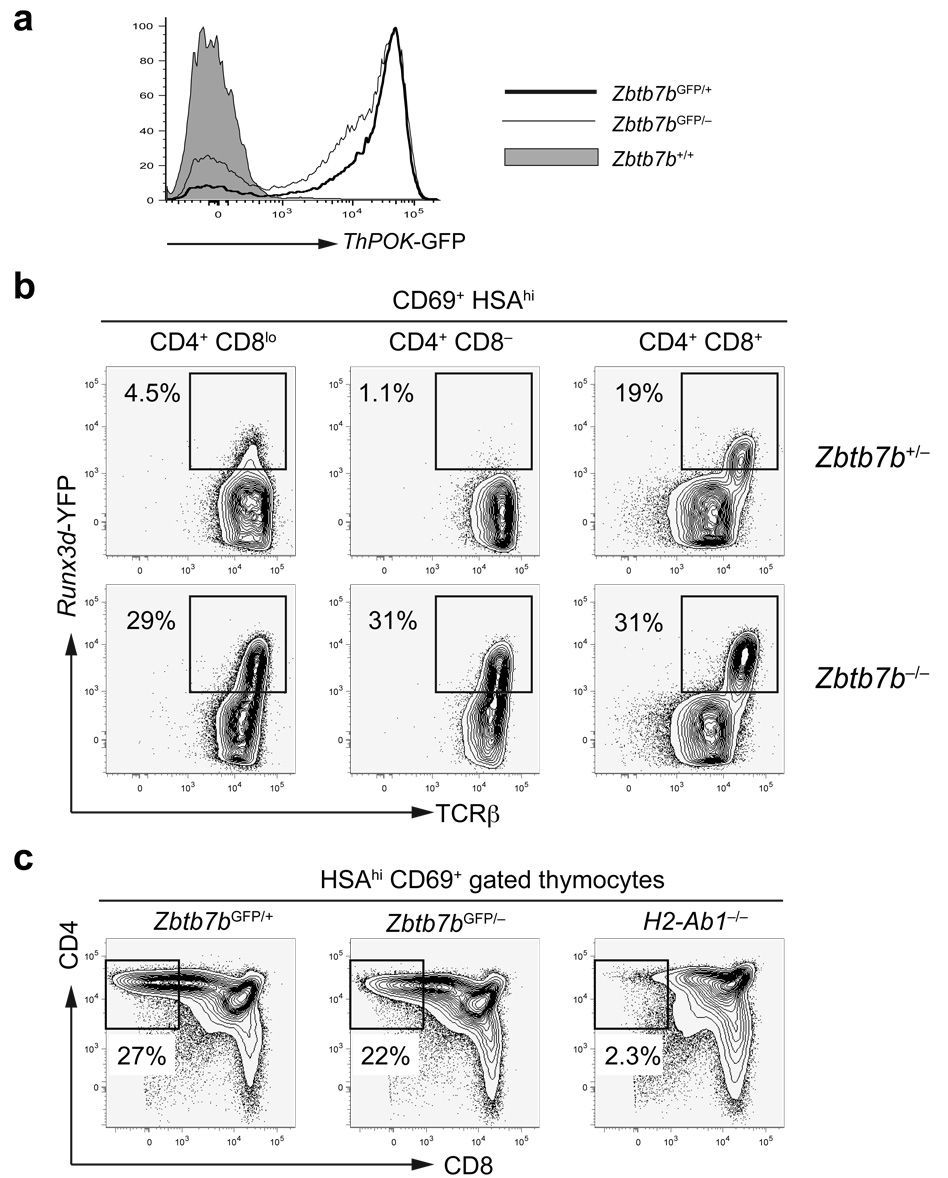
We also generated mice with a null allele of ThPOK (Supplementary Fig. 3b, online) and examined Zbtb7bGFP/− mice that express no ThPOK protein. Zbtb7bGFP/− mice contained very few mature HSAlo/− CD4SP cells, but the total number of TCRhiHSAlo/− thymocytes remained normal (Supplementary Fig. 4b, online). This phenotype is similar to that found in hd/hd mice26. As GFP expression in the CD69+HSAhiCD4+CD8lo/− subpopulations was comparable between Zbtb7bGFP/+ mice and Zbtb7bGFP/− mice, ThPOK up-regulation during positive selection appeared unlikely to be dependent on ThPOK expression itself (Fig. 3a). We observed a substantial amount of GFP expression in approximately two-thirds of CD8SP thymocytes and peripheral CD8+ T cells in Zbtb7bGFP/− mice (Fig. 2e). GFP expression in CD8+GFP+ T cells in the periphery was approximately 3-fold lower compared to that in CD4+ T cells (data not shown). All CD8SP thymocytes in Zbtb7bGFP/−B2m−/− mice expressed GFP, indicating that they were MHCII-restricted cells redirected from the CD4SP lineage (Supplementary Fig. 4c). These findings suggest that MHCII-restricted positive selection is required and sufficient to turn on high ThPOK expression.
Figure 3. CD8+ lineage-specific Runx3d-YFP expression is de-repressed in ThPOK-deficient MHCII-restricted thymocytes.
(a) ThPOK up-regulation independently of ThPOK following positive selection. ThPOK-GFP reporter expression in HSAhiCD69+CD4+CD8lo/− thymocytes from Zbtb7b+/+, Zbtb7bGFP/+ and Zbtb7bGFP/− mice. (b) Runx3d-YFP expression in thymocyte subpopulations in the presence or absence of ThPOK. YFP+ cells are gated as indicated by rectangles. (c) CD8 down-regulation following thymocyte positive selection in the absence of ThPOK. CD4 and CD8 expression in HSAhiCD69+ thymocytes from Zbtb7bGFP/+, Zbtb7bGFP/− and H2-Ab1−/− mice is shown. CD4+CD8− cells are gated as shown within rectangles and their frequencies are indicated. Data shown are representative of more than 3 independent experiments.
ThPOK blocks Runx3 expression
To determine whether there is cross regulation of ThPOK and Runx3 during thymocyte lineage specification, we examined expression of the ThPOK and Runx3d reporters in the absence of one or the other factor. ThPOK-GFP expression was unaffected by disruption of the distal promoter-derived Runx3 transcript or by transgenic overexpression of Runx3 (data not shown). In contrast, Runx3d-YFP was ectopically expressed in MHCII-restricted thymocytes in ThPOK-deficient mice, suggesting that ThPOK acts as an upstream negative regulator of Runx3 expression (Fig. 3b). Approximately 30–40% of CD4+CD8− HSAhi thymocytes expressed Runx3d-YFP in the absence of ThPOK; these YFP+ cells expressed the highest amount of TCRβ (Fig. 3b). ThPOK-GFP was up-regulated despite the absence of ThPOK protein in MHCII-restricted CD4+CD8−HSAhi thymocytes, which were present only in MHCII–sufficient mice and were eventually redirected to the CD8SP lineage (Fig. 3a, c). These findings suggest that Runx3d-YFP de-repression is an early indication of lineage redirection of ThPOK-deficient CD4SP “wannabe” cells to the CD8+ T cell lineage.
Lineage redirection due to a hypomorphic ThPOK allele
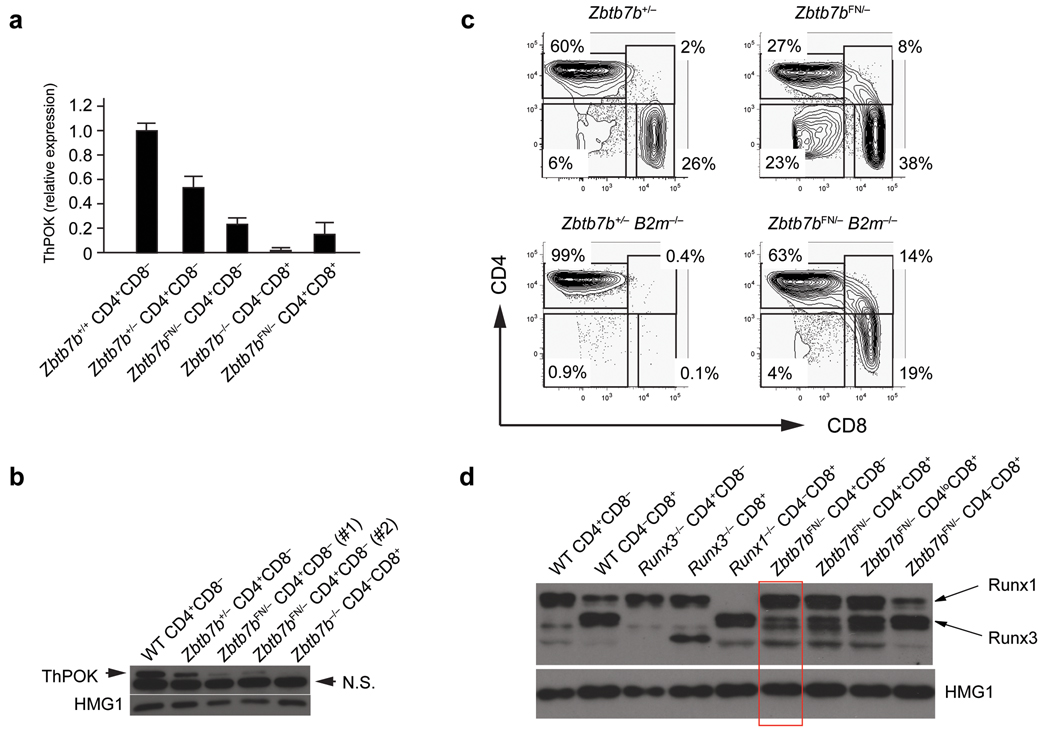
Our data imply that ThPOK expression following MHCII-restricted positive selection prevents expression of Runx3d. However, our analysis of ThPOK-GFP expression showed that a small proportion of CD8+ T cells also expressed ThPOK but differentiated into the cytotoxic lineage with normal Runx3d-YFP expression. This observation suggests that there is a threshold level of ThPOK expression that is required for helper T cell lineage commitment. To study the effect of reduced ThPOK quantities, we employed a hypomorphic Zbtb7b allele, in which the Pgk-neor selection cassette was left in intron 1 of the Zbtb7b locus (Zbtb7bFN allele) (Supplementary Fig. 3, online). CD4+CD8− mature thymocytes and peripheral T cells were still present in Zbtb7bFN/− mice even though ThPOK expression was reduced by 80% compared to control CD4+ T cells as determined by q-RT-PCR, by ThPOK protein expression, and by GFP expression from a Zbtb7bGFP-neo allele (the Zbtb7bGFP allele with the Pgk-neor cassette in the same context as in the Zbtb7bFN allele) (Fig. 4a,b and Supplementary Fig. 3, online). As compared to Zbtb7b+/+, Zbtb7bFN/+, Zbtb7b+/−, or Zbtb7bFN/FN mice, Zbtb7bFN/− mice had fewer CD4+CD8− cells and more CD4−CD8+ cells in HSAlo/− mature thymocyte and peripheral T cell populations, which resulted in an inverted CD4/CD8 ratio (Fig. 4c). CD4−CD8+ mature thymocytes and peripheral T cells, as well as CD4SP cells, were present in Zbtb7bFN/−B2m−/− mice, indicating that some MHCII-selected cells were redirected to the CD8SP lineage in the presence of an attenuated amount of ThPOK (Fig. 4c). In addition to cells expressing CD4 or CD8, we observed atypical populations of mature thymocytes and T cells with a CD4+CD8+ phenotype in Zbtb7bFN/− and Zbtb7bFN/−B2m−/− mice, suggesting that a reduced amount of ThPOK during thymocyte development affected the CD4 versus CD8 lineage decision (Fig. 4c). These DP cells and a fraction of CD4−CD8+ T cells expressed GFP in Zbtb7bFN/GFP mice, consistent with their being MHCII-restricted (Supplementary Fig. 5a, online). These results suggest that during CD4SP thymocyte development a threshold amount of ThPOK expression is needed to mediate CD4SP thymocyte differentiation, and that when ThPOK amounts fall below this threshold, MHCII-restricted cells are diverted to a CD8 lineage fate.
Figure 4. CD4+ T cell differentiation in the presence of reduced amounts of ThPOK.
(a,b) ThPOK expression in CD4+CD8− T cells from Zbtb7bFN/− mice. ThPOK expression was examined by qRT-PCR (a) and immunoblotting (b). qRT-PCR data are shown as averages and standard deviations from three independent samples. (c) Lineage redirection of MHCII-restricted T cells to the CD8 lineage in the presence of reduced amounts of ThPOK. CD4 and CD8 expression in gated HSAlo/−TCRβhi mature thymocytes from Zbtb7b+/−, Zbtb7bFN/−, Zbtb7b+/−B2m−/− and Zbtb7bFN/−B2m−/− mice is shown. Percentages of CD4SP, CD8SP, CD4+CD8+ and CD4−CD8− cells are indicated. (d) De-repressed Runx3 protein expression in CD4+CD8− T cells from Zbtb7bFN/− mice. Runx1 and Runx3 expression (indicated by arrows) was examined by immunoblotting with anti-pan-Runx. Anti-HMG1 was used as loading control. Data shown are representative of more than 3 independent experiments.
Unstimulated peripheral CD4+ T cells do not normally express Runx3 protein. However, peripheral CD4+CD8− T cells from Zbtb7bFN/− mice constitutively expressed Runx3, and CD4+CD8− T cells from Zbtb7bFN/−Runx3dYFP/+ mice expressed YFP (Fig. 4d and Supplementary Fig. 5b, online). Runx3 protein expression was higher in CD4+CD8+ cells, and GFP expression from the Zbtb7bGFP-neo allele, which reflects ThPOK mRNA expression from the Zbtb7bFN allele, was inversely correlated with Runx3 protein expression (Fig. 4d and Supplementary Fig. 5, online). These results indicate that a high quantity of ThPOK is required to block Runx3 up-regulation and diversion of MHCII-restricted thymocytes to the CD8SP lineage.
CD4SP cells can be generated in the absence of ThPOK
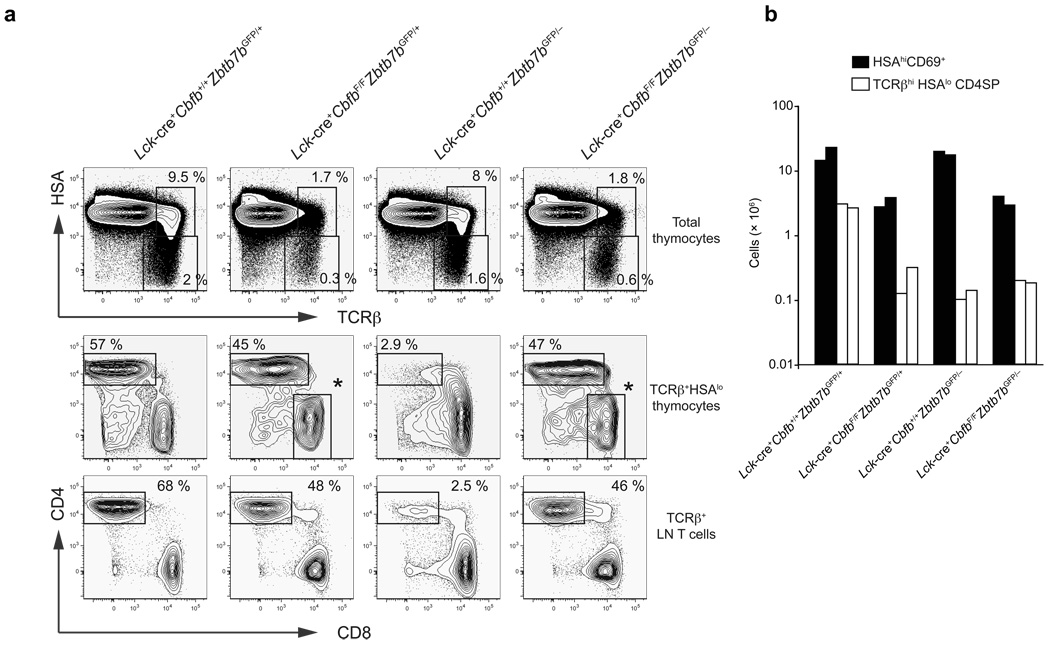
Our data indicated a correlation between Runx3 de-repression and redirection of MHCII-restricted thymocytes to the cytotoxic lineage. To determine whether Runx complexes are required for lineage redirection occurring in the absence of ThPOK, we generated mice doubly deficient for ThPOK and CBFβ. We used the Lck-cre transgene to conditionally inactivate Cbfb because its later inactivation in DP thymocytes with Cd4-cre allows generation of CD8+ mature thymocytes (which de-repress CD4)12, most likely due to long half-life of the CBFβ protein. Conditional inactivation of Cbfb with Lck-cre allows thymocytes to proceed through beta selection and phenocopies loss of both Runx1 and Runx3 at the DP stage. HSAhiCD69+ and HSAhiTCRβhi positively selected thymocytes were reduced by approximately 5-fold in Lck-cre+CbfbF/F mice and Lck-cre+CbfbF/FZbtb7bGFP/− mice as compared to littermate control or ThPOK-deficient mice (Fig. 5a,b). Similar to our previous observation in Runx1F/FRunx3F/FCd4-cre+ mice10, CD4−CD8+ mature thymocytes with intact CD4 silencing, that had escaped Cre-mediated inactivation of Cbfb, were present in Lck-cre+CbfbF/F mice. Although almost all of the mature thymocytes were redirected to the CD8SP subset in Lck-cre+ Cbfb+/+Zbtb7bGFP/− mice, a majority of the mature HSAlo thymocytes in Lck-cre+ CbfbF/FZbtb7bGFP/− mice were CD4+CD8− and were not redirected to the CD8 lineage (Fig. 5a). We also observed a CD4+CD8− T cell population not redirected to the CD8 lineage in lymph nodes of Lck-cre+CbfbF/FZbtb7bGFP/− mice (Fig. 5a). The number of CD4+CD8− thymocytes and peripheral T cells were reduced compared to wild-type mice but were similar in Lck-cre+CbfbF/FZbtb7bGFP/+ and Lck-cre+CbfbF/FZbtb7bGFP/− mice (Fig. 5b). We do not believe that these cells represent a rare population of unconventional CD4SP thymocytes, as iNKT cells do not develop in the absence of Runx complexes27 and Foxp3+ regulatory T cells are severely reduced in both CBFβ-deficient mice and ThPOK-deficient mice (unpublished results). In addition, CD40L (CD154) was up-regulated upon stimulation of CD4+ T cells lacking both transcription factors, consistent with their being helper lineage cells (Supplementary Fig. 6, online). Thus these findings indicate that Runx complexes are required for redirection of MHCII-restricted thymocytes towards the CD8SP lineage in the absence of ThPOK, and ThPOK is not required for CD4 expression in mature MHCII-restricted thymocytes and CD4+ peripheral T cells.
Figure 5. ThPOK is dispensable for differentiation of CD4SP thymocytes.
(a) HSA and TCRβ expression in total thymocytes (top), and CD4 and CD8 expression in HSAlo/− TCRβhi mature thymocytes (middle) and TCRβ+B220− peripheral T cells (bottom) in the absence of ThPOK, CBFβ, or both. The CD4−CD8+ cells shown with asterisks in Lck-cre+CbfbF/F and Lck-cre+ CbfbF/FZbtb7bGFP/− panels are those that escaped Cre-mediated inactivation of Cbfb and hence have normal Cd4 silencing. Data shown here are representative from two independent experiments with similar results. (b) Absolute numbers of HSA+CD69+ positively selected thymocytes and HSAlo/− mature CD4SP thymocytes in the absence of ThPOK, CBFβ, or both. Each column shows cell numbers in a mouse with each of the genotypes.
ThPOK represses genes specific for the CD8SP lineage
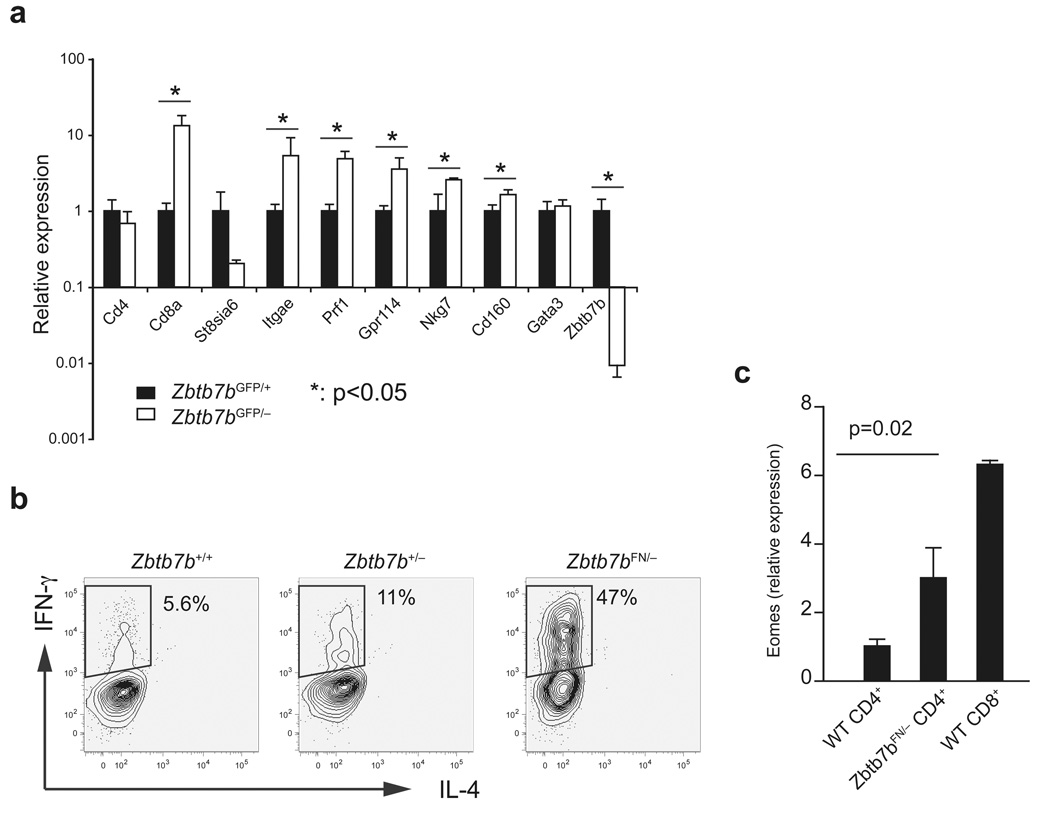
The finding of Runx3d de-repression in ThPOK-deficient MHCII-restricted thymocytes raises the possibility that other CD8+ lineage-specific genes may also be de-repressed in these cells. To assess this possibility, we compared gene expression between CD4+CD8−HSAhiGFP+ thymocytes from Zbtb7bGFP/− and Zbtb7bGFP/+ mice. qRT-PCR analysis showed that Zbtb7bGFP/− cells expressed significantly higher amounts of transcripts that are normally expressed in the CD8SP lineage of wild-type mice, including Cd8a, Itgae, Nkg7, Cd160 and Prf1 (Fig. 6a). In addition, unlike wild-type CD4+ T cells, a majority of Zbtb7bFN/− CD4+ T cells produced IFN-γ when stimulated with anti-CD3 and anti-CD28 in the absence IL-12 (Fig. 6b). Expression of the CD8 lineage-specific IFN-γ–regulating transcription factor Eomes was significantly elevated in Zbtb7bFN/− compared to wild-type CD4+ T cells (Fig. 6c). These findings suggest that a high amount of ThPOK expression is required during development of CD4+ T cells to block the CD8SP lineage-specific gene expression program.
Figure 6. De-repression of CD8+ lineage-specific genes in MHCII-restricted cells in the absence of ThPOK or in the presence of insufficient amount of ThPOK.
(a) MHCII-restricted HSAhiCD69+CD4+CD8lo/− ThPOK-GFPhi thymocytes from Zbtb7bGFP/+ (black columns) or Zbtb7bGFP/− (white columns) were purified using gates as shown in Fig. 3, and expression of CD4+ lineage-specific genes (Cd4, St8si6, Zbtb7b and Gata3) and CD8+ lineage-specific genes (Cd8a, Itgae, Prf1, Gpr114, Nkg7 and Cd160) was examined by qRT-PCR. mRNA expression of individual genes was normalized against Hprt1 expression and average expression in Zbtb7bGFP/+ cells was set as 1. (b) Intracellular staining for IFN-γ and IL-4 in Zbtb7bFN/− CD4+CD8− T cells following 3 days of stimulation with anti-CD3 and anti-CD28 in the absence of IL-12. (c) Expression of the CD8+ lineage-specific IFN-γ regulator Eomes in CD4+ T cells from wild-type and Zbtb7bFN/− mice and in wild-type CD8+ T cells, as quantified by q-RT-PCR. Hprt1-normalized Eomes mRNA expression is shown as average and standard deviations from three independent samples. Statistical difference was tested by two-tailed T test with assumption of unequal variance. Genes that showed a P value smaller than 0.05 are marked with asterisks in (a). Actual P values for individual genes were as follows: Cd4: 0.28, St8sia6: 0.13, Zbtb7b: 0.01, Gata3: 0.5, Cd8a: 0.04, Itgae: 0.04, Prf1: 0.01, Gpr114: 0.09, Nkg7: 0.04, Cd160: 0.02.
Discussion
Despite extensive effort over the past two decades, the mechanism by which DP cells undergoing positive selection commit to either the CD4+ or CD8+ lineage is not yet understood. The discovery of the hd mouse, which harbors a mutation in the Zbtb7b gene that encodes ThPOK, clearly demonstrated that positive selection could occur independently of lineage commitment26,28. Subsequent studies have suggested that ThPOK serves as a “master regulator” that directs the differentiation of helper T cells6,7. This conclusion was based on findings that forced expression of ThPOK redirected MHCI-selected thymocytes to the CD4+ lineage, and that MHCII-selected cells adopted the CD8+ lineage fate in the absence of functional ThPOK. Furthermore, a defect in a Runx-dependent silencer within the Zbtb7b gene resulted in de-repression of ThPOK in DP cells and in redirection of MHCI-selected thymocytes14. Here we showed that ThPOK is up-regulated in positively selected CD4+CD8lo thymocytes before induction of Runx3 in cells destined to become CD8+ T cells, that ThPOK represses expression of Runx3 in MHCII-selected cells transiting from the CD4+CD8lo to the CD4+CD8− stage, and, intriguingly, that MHCII-selected thymocytes can become CD4SP cells even in the absence of ThPOK and, hence, may be specified to the helper lineage independently of ThPOK. These results argue that ThPOK may not be a “master regulator” but rather that it reinforces the CD4 lineage decision, at least in part by repressing Runx3 and thus preventing cells from expressing CD8+ lineage-specific genes.
Among the three Runx proteins, Runx3 is the only one specifically expressed in the CD8 lineage, where it is transcribed from its distal promoter. We previously demonstrated that conditional inactivation of Runx3 resulted in de-repression of the Cd4 gene in CD8 lineage T cells, but not in loss of CD8SP thymocytes or in redirection of MHCI-restricted thymocytes to the CD4SP lineage 10. This is most likely explained by functional redundancy achieved upon compensatory up-regulation of Runx1, and, accordingly inactivation of both Runx1 and Runx3 in DP thymocytes resulted in complete loss of CD8 lineage cells and in lineage redirection10,14. The results thus imply that, in the absence of genetic manipulation, activation of the Runx3 distal promoter is critical for initiation of CD8SP development. In the current study, we showed that ThPOK suppresses Runx3d expression in MHCII-selected cells. Following positive selection, a large proportion of MHCII-restricted thymocytes within the CD4+CD8lo population expressed the ThPOK-GFP reporter, but the Runx3d-YFP reporter was not expressed by MHCI-restricted thymocytes until they differentiated further. ThPOK-deficient MHCII-restricted thymocytes, in contrast, de-repressed Runx3d after they up-regulated ThPOK-GFP reporter expression in CD4+CD8lo/− subsets. This observation suggests that CD4+ T cell differentiation requires high ThPOK expression that is initiated prior to Runx3 up-regulation. In the presence of insufficient amounts of ThPOK, MHCII-restricted thymocytes fail to block Runx3 expression and are redirected to the CD8SP lineage. These findings suggest that Runx3d transcription can be activated regardless of MHC restriction but is normally blocked by ThPOK in a CD4+ lineage-specific manner. Analysis of regulation of the Runx3 distal promoter may clarify the potential cross regulation between these two transcription factors.
In mice lacking both ThPOK and Runx complexes, lineage redirection of MHCII-restricted CD4SP cells no longer occurred, suggesting that the CD4 lineage can be specified even in the absence of ThPOK. Consistent with this interpretation, in preliminary studies we found that CD40L (CD154), which is expressed on the surface of wild-type activated CD4+ but not CD8+ T cells, was up-regulated in CD4+CD8− T cells lacking both ThPOK and CBFβ. This result appears at first to be inconsistent with observations of previous studies on lineage regulation by ThPOK and Runx. For example, de-repression of ThPOK in the absence of Runx complexes in DP cells resulted in diversion of MHCI-restricted cells to the CD4+ lineage14. However, this effect was not demonstrated to be dependent on ThPOK. Similar lineage diversion observed upon targeted deletion of the ThPOK silencer may have resulted in higher-than-normal amounts of ThPOK in MHCI-selected cells; this elevated ThPOK expression may thus have effectively phenocopied forced ThPOK expression in transgenic mice, and may have bypassed the transcriptional mechanisms that normally precede ThPOK up-regulation during CD4 lineage specification14.
Our results therefore suggest that signals initiated in DP thymocytes upon TCR interaction with peptide-loaded MHCII molecules specify differentiation to the CD4+ lineage prior to the activity of ThPOK, which is expressed in this lineage upon release of its silencing and, possibly, activation of lineage-restricted positive regulatory elements14. The signals and factors involved in specification of the CD4+ lineage and in induction of ThPOK remain to be defined. GATA3 another transcription factor known to be required for the differentiation of CD4+ lineage cells, is up-regulated earlier than ThPOK, at the CD69+TCRlo DP stage29. In the absence of GATA3, MHCII-selected thymocytes are arrested at the CD4+CD8lo stage30. GATA3 or other unknown CD4 specification factors may be required for ThPOK up-regulation, although a GATA3 consensus binding sequence in the ThPOK silencer appears to be dispensable15.
These findings are consistent with the notion that there is reciprocal regulation of ThPOK and Runx complexes. Runx1 and Runx3 are required for ThPOK silencing in DP cells, although their role in ThPOK regulation following positive selection remains unclear. Specific expression of Runx3 in the CD8SP lineage raises the possibility that ThPOK silencing is maintained by Runx3 in MHCI-restricted thymocytes, and that ThPOK silencing is ‘released’ in MHCII-selected thymocytes that do not express Runx3. However, as ThPOK up-regulation in MHCII-selected thymocytes occurs earlier than Runx3 up-regulation in MHCI-selected thymocytes, it is unlikely that Runx3 determines lineage-specific ThPOK expression. Alternatively, it is possible that Runx1 may modulate ThPOK silencer activity following positive selection signals either by acting alone or in cooperation with other CD4SP specifying factors, such as GATA3. Indeed it has been demonstrated that Runx and GATA factors potentially interact with each other and synergistically regulate blood cell lineage decisions in Drosophila31,32.
The roles of ThPOK and Runx factors in the helper versus cytotoxic T cell lineage decision appear to be asymmetric. Whereas ThPOK represses Runx3 expression following positive selection, the Runx proteins do not appear to prevent ThPOK expression except at the DP stage. In contrast to the effect of forced expression of ThPOK, transgenic overexpression of Runx proteins fails to redirect MHCII-specific cells to the CD8 lineage. Therefore, following induction of ThPOK in CD4 lineage thymocytes, its expression appears to become resistant to Runx-mediated repression. This may be due to epigenetic changes or to active inhibition of the repressive effect of Runx complexes, which have been reported to remain associated with the ThPOK silencer even in CD4+ lineage cells14.
ThPOK is likely to function not only to repress Runx3 expression, but also to repress other factors involved in cytotoxic lineage commitment or to insulate genes involved in CD4+ lineage commitment from the effects of Runx expression. Thus, in the absence of ThPOK, not only Runx proteins but also other ThPOK target genes are likely involved in the diversion to the CD8+ lineage. Following intrathymic differentiation, ThPOK and Runx3 protein can be co-expressed in activated peripheral CD4+ T cells under TH1 polarizing conditions12,13 (data not shown). The mechanism which permits Runx3 expression in mature cells in the presence of ThPOK is unknown, but may involve a dominant role for the transcription factor T-bet, which is required for Runx3 expression in TH1 cells13. Effector helper T cell functions may depend on expression of both transcription factors.
The “kinetic signaling” model of CD4 vs. CD8 lineage choice33,34 proposes that, during the post-positive selection CD4+CD8lo stage, extended signaling due to interaction of CD4 with MHCII results in helper lineage specification. Our results are potentially consistent with this model, and are the first to demonstrate that the CD4+CD8lo thymocyte subpopulation is heterogeneous with respect to expression of ThPOK. It is hence possible that only those cells with prolonged signaling up-regulate ThPOK. These may also be cells that preferentially up-regulate GATA3 in response to MHCII29.
In summary, we have provided genetic evidence suggesting that ThPOK may not be essential for specification of the CD4SP lineage, while Runx complexes are required for lineage redirection in the absence of ThPOK. ThPOK plays an essential role to prevent commitment to the CTL lineage following MHCII-restricted selection, at least in part by inhibiting up-regulation of Runx3 from the distal promoter. Future studies are needed to identify factors necessary for CD4+ lineage specification prior to ThPOK induction, to determine if there is a basal differentiation state following positive selection by either MHCI or MHCII, and to elucidate the gene regulatory network governing thymocyte lineage diversification.
METHODS
Mice
Cbfb, Runx3, and Runx1 conditional knockout mice were described previously8,12. B2m−/− mice35, H2-Ab1−/− mice36, Cd4-cre and Lck-cre transgenic mice37 were purchased from Taconic Farms, and EIIa-cre transgenic mice38 were from the Jackson Laboratory. For targeted insertion of YFP into the Runx3 locus, genomic fragments for homologous arms were PCR amplified from a BAC clone (RP24-309N18) encompassing the Runx3 locus. A targeting vector was constructed such that a 46 nt Runx3 coding sequence starting with an initiating codon from the distal promoter transcript was replaced with the YFP coding sequence. The NotI linearized targeting vector was electroporated into embryonic stem cells and G418 and gancyclovir double resistant colonies were screened by PCR for homologous recombination at the 3’ end. Positive clones were validated by Southern blot with BamHI digestion. Genomic fragments encompassing the Zbtb7b locus were excised from a BAC clone (RP23-126P10) and cloned into pBluescript. For targeted deletion or conditional targeting of Zbtb7b, a targeting vector was constructed to flank exon 2 and exon 3, containing the entire coding sequence of Zbtb7b, with loxP sites. The neomycin resistance cassette was inserted approximately 1.1 kb upstream of exon 2 in a forward orientation with an additional loxP site at its 5’ end. SacII-linearized targeting vector was transfected into ES cells by electroporation. G418 and gancyclovir double resistant colonies were screened by Southern blotting. For generation of Zbtb7bF/+ mice, Zbtb7bFN/+ ES cells were transiently transfected by pMC-Cre to remove the neomycin resistance gene. The Zbtb7b− allele was obtained by crossing Zbtb7bFN/+ mice to EIIa-cre transgenic mice. All mice were maintained under specific pathogen free conditions in the Skirball Institute Animal Facility. All experiments were performed in accordance with the protocol approved by the IACUC at the NYU School of Medicine.
Flow cytometry
All monoclonal antibodies were purchased from eBioscience or BD Bioscience. Clone names for individual antibodies used in this study are as follows: anti-CD4 (RM4-5), anti-CD8α (53–6.7), anti-TCRβ (H57–597), anti-HSA (M1/69), anti-CD69 (H1.2F3), anti-CD103 (2E7), anti-CD154 (MR1). anti-IL-4 (11B11), anti-IFN-γ (XMG1.2). Single cell suspensions were stained with the antibodies and DAPI, and analyzed with a LSRII flow cytometer (BD Biosciences) equipped with 355 nm, 405 nm, 488 nm and 633 nm lasers. 510/20, 545/35, 495LP, and 525LP filters were used to separate GFP and YFP signals. Data were analyzed using Flowjo software (Tree Star). Cell sorting was performed with a FACS Aria (BD Biosciences). The purity of sorted samples was higher than 99%.
Real time PCR for gene expression analysis
Total RNA was prepared from sorted thymocytes or lymphocytes using Trizol (Invitrogen). cDNA was synthesized with Superscript reverse transcriptase (Invitrogen). Real time PCR analysis was performed as described previously10. Primers and Taqman probes for Gata3 and Zbtb7b were described previously6,39. Other primer sequences are listed in Supplementary Table 1, online. Gene expression was examined using 3 independent samples and statistical difference was tested using two-tailed T test with unequal variance assumption. P values smaller than 0.05 were considered significant.
Immunoblotting
FACS purified cells were washed once with PBS and lysed in buffer containing 150 mM NaCl, 2 mM EDTA, 20mM Tris-HCl (pH7.5), 10% glycerol and 1% NP-40. The lysate was cleared by centrifugation, denatured in 1 × Laemmli buffer and separated by SDS-PAGE. Anti-pan-Runx recognizing the C-terminus of Runx1 and Runx3 was described previously10. Anti-ThPOK was generated by immunizing rabbits with a recombinant GST-fusion protein containing amino acids 423–543 of ThPOK. Immunoblot was performed using crude serum. Anti-HMG1 was used to estimate loading.
T cell stimulation and intracellular staining
Purified CD4+CD8−CD25−CD62L+CD44lo naïve T cells were stimulated with plate-bound anti-CD3 (145-2C11, 1 µg/ml) and anti-CD28 (37.5.1, 5 µg/ml) for 3 days in the presence of recombinant mouse IL-2 (20 U/ml) and in the absence of IL-12. The cells were restimulated with PMA and ionomycin for 4 hours in the presence of GolgiPlug (BD Biosciences), fixed with 2% paraformaldehyde, permeabilized with 0.3% saponin, and intracellularly stained for IFN-γ and IL-4 in the presence of 0.03% saponin.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Rockefeller University Gene Targeting Facility for generation of ThPOK mutant mice with B6-C2J ES cells, and J.H. Dong (Memorial Sloan Kettering Cancer Center), A. Auerbach, and W. Ho (NYU Cancer Institute) for microinjection. We thank members of the laboratory for discussion, and J. Huh, M. Chong, A. Collins and S. Schwab for critical reading of the manuscript. This study was supported by a Special Fellowship from the Leukemia and Lymphoma Society (T.E.) and the Howard Hughes Medical Institute (D.R.L.). The authors declare no competing financial interest.
References
- 1.Kappes DJ, He X, He X. CD4-CD8 lineage commitment: an inside view. Nat Immunol. 2005;6:761–766. doi: 10.1038/ni1230. [DOI] [PubMed] [Google Scholar]
- 2.Zou YR, et al. Epigenetic silencing of CD4 in T cells committed to the cytotoxic lineage. Nat Genet. 2001;29:332–336. doi: 10.1038/ng750. [DOI] [PubMed] [Google Scholar]
- 3.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 4.Kioussis D, Ellmeier W. Chromatin and CD4, CD8A and CD8B gene expression during thymic differentiation. Nat Rev Immunol. 2002;2:909–919. doi: 10.1038/nri952. [DOI] [PubMed] [Google Scholar]
- 5.Laslo P, et al. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 6.He X, et al. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 7.Sun G, et al. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol. 2005;6:373–381. doi: 10.1038/ni1183. [DOI] [PubMed] [Google Scholar]
- 8.Taniuchi I, et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- 9.Woolf E, et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato T, et al. Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity. 2005;22:317–328. doi: 10.1016/j.immuni.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Naoe Y, et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djuretic IM, et al. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 14.Setoguchi R, et al. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science. 2008;319:822–825. doi: 10.1126/science.1151844. [DOI] [PubMed] [Google Scholar]
- 15.He X, et al. CD4-CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity. 2008;28:346–358. doi: 10.1016/j.immuni.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Levanon D, et al. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. Embo J. 2002;21:3454–3463. doi: 10.1093/emboj/cdf370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li QL, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–124. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 18.Grueter B, et al. Runx3 regulates integrin alpha E/CD103 and CD4 expression during development of CD4-/CD8+ T cells. J Immunol. 2005;175:1694–1705. doi: 10.4049/jimmunol.175.3.1694. [DOI] [PubMed] [Google Scholar]
- 19.Barthlott T, Kohler H, Pircher H, Eichmann K. Differentiation of CD4(high)CD8(low) coreceptor-skewed thymocytes into mature CD8 single-positive cells independent of MHC class I recognition. Eur J Immunol. 1997;27:2024–2032. doi: 10.1002/eji.1830270829. [DOI] [PubMed] [Google Scholar]
- 20.Chan S, Correia-Neves M, Dierich A, Benoist C, Mathis D. Visualization of CD4/CD8 T cell commitment. J Exp Med. 1998;188:2321–2333. doi: 10.1084/jem.188.12.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidos CJ, Danska JS, Fathman CG, Weissman IL. T cell receptor-mediated negative selection of autoreactive T lymphocyte precursors occurs after commitment to the CD4 or CD8 lineages. J Exp Med. 1990;172:835–845. doi: 10.1084/jem.172.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kydd R, Lundberg K, Vremec D, Harris AW, Shortman K. Intermediate steps in thymic positive selection. Generation of CD4-8+ T cells in culture from CD4+8+, CD4int8+, and CD4+8int thymocytes with up-regulated levels of TCR-CD3. J Immunol. 1995;155:3806–3814. [PubMed] [Google Scholar]
- 23.Lucas B, Germain RN. Unexpectedly complex regulation of CD4/CD8 coreceptor expression supports a revised model for CD4+CD8+ thymocyte differentiation. Immunity. 1996;5:461–477. doi: 10.1016/s1074-7613(00)80502-6. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg K, Heath W, Kontgen F, Carbone FR, Shortman K. Intermediate steps in positive selection: differentiation of CD4+8int TCRint thymocytes into CD4-8+TCRhi thymocytes. J Exp Med. 1995;181:1643–1651. doi: 10.1084/jem.181.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki H, Punt JA, Granger LG, Singer A. Asymmetric signaling requirements for thymocyte commitment to the CD4+ versus CD8+ T cell lineages: a new perspective on thymic commitment and selection. Immunity. 1995;2:413–425. doi: 10.1016/1074-7613(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 26.Keefe R, Dave V, Allman D, Wiest D, Kappes DJ. Regulation of lineage commitment distinct from positive selection. Science. 1999;286:1149–1153. doi: 10.1126/science.286.5442.1149. [DOI] [PubMed] [Google Scholar]
- 27.Egawa T, et al. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Dave VP, Allman D, Keefe R, Hardy RR, Kappes DJ. HD mice: a novel mouse mutant with a specific defect in the generation of CD4(+) T cells. Proc Natl Acad Sci U S A. 1998;95:8187–8192. doi: 10.1073/pnas.95.14.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19:83–94. doi: 10.1016/s1074-7613(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 30.Pai SY, et al. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- 31.Waltzer L, Ferjoux G, Bataille L, Haenlin M. Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis. Embo J. 2003;22:6516–6525. doi: 10.1093/emboj/cdg622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fossett N, Hyman K, Gajewski K, Orkin SH, Schulz RA. Combinatorial interactions of serpent, lozenge, and U-shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis. Proc Natl Acad Sci U S A. 2003;100:11451–11456. doi: 10.1073/pnas.1635050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarafova SD, et al. Modulation of coreceptor transcription during positive selection dictates lineage fate independently of TCR/coreceptor specificity. Immunity. 2005;23:75–87. doi: 10.1016/j.immuni.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Singer A, Bosselut R. CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv Immunol. 2004;83:91–131. doi: 10.1016/S0065-2776(04)83003-7. [DOI] [PubMed] [Google Scholar]
- 35.Zijlstra M, et al. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 36.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 37.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 38.Lakso M, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grogan JL, et al. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.