Abstract
New calix[4]pyrroles bearing dipyrrolylquinoxaline as strapping elements have been synthesized and characterized by spectroscopic means. The binding behavior of these receptors at 25 °C was investigated first by proton NMR spectroscopy in CD3CN/DMSO-d6 (9:1 v./v.), as well as by UV-vis spectroscopy and isothermal titration calorimetry (ITC) in CH3CN/DMSO (97:3 v./v.). The receptors displayed a selective colorimetric response when exposed to the fluoride, dihydrogen phosphate, and acetate anions (studied in the form of the corresponding tetrabutylammonium salts) and an enhanced affinity as compared to a comparable calix[4]pyrrole system lacking the dipyrrolylquinoxaline-containing strap.
Keywords: Strapped-calix[4]pyrrole, anion sensing, dipyrrolylquinoxaline, colorimetric sensor
Motivated in part by an increasing appreciation of the importance of anions in the environment and biology, considerable continues to be devoted to the design and synthesis of anion receptors possessing high affinity and selectivity.1,2 A variety of systems that are capable of recognizing anions are now known, and most general classes have been discussed at some length in a variety of recent reviews.3,4 Much of our own work of late has focused on a set of modified calix[4]pyrrole-based receptors known as strapped- or capped-calix[4]pyrroles. These modified calix[4]pyrrole derivatives generally display affinities and selectivities towards anions that are significantly enhanced relative to simple calix[4]pyrroles. They thus offer the attractive possibility of tuning the anion recognition properties of this well-studied class of receptors.5–9 However, to be useful in certain applications, the augmented affinity of the strapped calix[4]pyrroles would need to be coupled with a “read out” element that would allow the anion binding event to be followed easily. Within the context of this general goal, we are particularly interested in developing strapped calix[4]pyrrole-based receptors that are capable of producing a colorimetric response upon anion recognition. In this paper, we wish to report the design, synthesis, and anion binding properties of calix[4]pyrroles that contain dipyrrolylquinoxalines as a part of the strap as shown in Scheme 1. These systems, which produce an easy-to-see visual response in the presence of certain anions, offer a further potential advantage in that they possess additional pyrrole subunits on the strap that are expected to interact with the quinoxaline chromophore via a variety of second order interactions, including through conjugation and anion-pi effects.
Scheme 1.
Synthesis of target compounds
The synthesis of receptor 4 starts with ketone 1, a species that was prepared by the reaction of oxalyl chloride with two equivalents of 2-(3-oxobutyl)pyrrole.11 Once in hand, 1 was reacted with 1,2-diamino-4-nitro-benzene in the presence of acid to afford bisketone 2 in 24% yield.10 Treatment of this latter intermediate with neat pyrrole in the presence of trifluoroacetic acid afforded the bis-dipyrromethane 3 in 74% yield. While this procedure proved effective, attemopts to effect the direct alkylation of 6-nitro-2,3-di(2’-pyrrolyl)quinoxaline with methyl vinyl ketone produced only trace quantities of the desired product 3. Acid-catalyzed condensation of 3 with acetone then gave the desired strapped calix[4]pyrroles 4 in 7% yield. Support for the proposed structures was obtained from NMR spectroscopic and high resolution mass spectrometric (HRMS) analyses.
Initial studies of the anion binding properties of receptor 4 were carried out in CD3CN/DMSO-d6 (9:1 v./v.) using proton NMR spectroscopy (Figure 1). As would be expected for a 1:1 binding process, a complete disappearance of the signals corresponding to the free host was observed after the addition of only one equivalent of fluoride anion (added in the form of the corresponding tetrabutylammonium (TBA) salt) to receptor 4. These signals were replaced by other peaks, with characteristic shifts being observed. For instance, the signals corresponding to the pyrrole NH protons on the calix[4]pyrrole moiety, originally appearing at 7.47 ppm, were found to shift to 12.32 ppm and to undergo a splitting; such behavior is completely consistent with the presence of a centrally bound 19F-containing fluoride anion. Likewise, the pyrrole NH signals on the strap, which were found to resonate originally at 9.92 ppm, were seen to shift to 10.34 ppm but to undergo relatively little, if any, splitting in the process. In addition, the β-pyrrole CH signals on the calix[4]pyrrole moiety were seen to shift from 5.99-5.96 to 5.57-5.54 ppm. Finally, the β-pyrrole CH signals of the strap underwent a shift from 6.54-6.49 and 5.80-5.74 to 6.85-6.80 and 5.92-5.90 ppm, respectively.
Figure 1.
1H NMR spectral changes of receptor 4 (2.64 mM) seen upon titration with F− (as its tetrabutylammonium salt) in CD3CN/DMSO-d6 (9:1 v./v.) at 25 °C.
In addition to providing support for the expectation that the bound anion is complexed by the calix[4]pyrrole core, the above observations lead us to suggest that the NH protons of the pyrroles on the strap do not interact with the added anions via simple NH-anion hydrogen bonds. In particular, the fact that the pyrrole NH protons on the strap do not undergo an appreciable downfield shift upon the addition of up to ~1 equivalent of F− is consistent with these protons not participating directly in the binding process. The lack of apparent 1H-19F splitting for these signals provides further support for this conclusion. Further, an inspection of molecular models leads to an appreciation that the two pyrrole ring on the strap must be almost perpendicular to the quinoxaline ring in order to accommodate the bound fluoride anion within the cavity.
The rather unusual down-field shift seen for the β-pyrrolic protons of the dipyrrolylquinoxaline strap subunits is also noteworthy; it could reflect an anion-pi interaction between these pyrrole rings and the bound fluoride anion.12 While further study will be required to confirm or refute the validity of this supposition, it is important to note that such anion-pi interaction have recently been observed in functionalized calix[4]pyrrole systems containing aryl groups in “walls”, rather than “straps”.13 In any case, the fact that the NH signals shift, but do not disappear, serves to rule out a significant degree of NH deprotonation, at least under the conditions of fluoride anion binding in this solvent system.
The observation of peaks corresponding to both the bound and unbound forms during the titrations with TBAF leads us to infer that the binding of fluoride anion to receptor 4 is subject to slow complexation/decomplexation kinetics. This made it difficult to quantify the binding interactions using 1H NMR spectroscopy. Accordingly, the fluoride anion binding process was studied using absorption spectroscopy. As shown in Figure 2, addition of tetrabutylammonium fluoride, acetate, or dihydrogen phosphate to solutions of receptor 4 in CH3CN/DMSO (97:3 v./v.) resulted in monotonic changes in the absorption maximum. In fact, naked eye-detectable differences in the color of receptor 4 (1.12 mM in CH3CN/DMSO; 97:3 v./v.) could be seen before and after the addition of several anions (as their respective tetrabutylammonium salts), with the effect being especially noticeable in the case of the fluoride and dihydrogen phosphate anions. Detectable changes could also be seen in the case of acetate anion. On the other hand, the addition of the corresponding chloride, bromide, iodide, hydrogen sulfate, nitrate, or thiocyanate salts did not result in any appreciable color changes.
Figure 2.
(a) Changes in the color of 1.12 mM solutions of receptor 4 in CH3CN/DMSO (97:3 v./v.) seen upon the addition of various anions (100 equiv. each). (b) The spectral changes seen upon the addition of acetate anion (added as TBA-H2PO4) to a 50.1 µM solution of receptor 4.
By following the UV-vis absorption spectra seen upon titration with anions (in CH3CN/DMSO; 97:3 v./v.) and fitting the associated changes to a 1:1 binding profile according to standard methods, it proved possible to calculate the corresponding binding constants (Ka). The values obtained in this way for receptor 4 are collected in Table 1. As can be seen from an inspection of this table, and as would be inferred from Figure 2, receptor 4 binds the fluoride anion quite strongly in this polar solvent system. On the other hand, the affinities for chloride and bromide anion are smaller than those of the octamethyl calix[4]pyrrole. A ~10 fold increase is observed for acetate anion while the affinity for dihydrogen phosphate anion remains the same. A strong selectivity for fluoride over other halogen anions was apparent compared with octamethyl calix[4]pyrrole, as deduced from the observed difference in these affinities.
Table 1.
Association constants for receptor 4 with various anions as calculated from titrations carried out in CH3CN/DMSO (97:3 v./v.) at 25 °C; [4] = 50.1 µM.
| Ka(M−1)a | |||
|---|---|---|---|
| Anion | UV-vis | ITC | Octamethyl calix[4]pyrrole |
| F− | 8.97 × 106 | K1 = 3.72 × 108, K2 = 5.0 × 105 | N. A. |
| Cl− | 1.09 × 104 | 1.94 × 104 | 1.02 × 105 |
| Br− | 3.65 × 102 | N. D. | 1.05 × 103 |
| AcO− | 8.12 × 103 | 1.89 × 104 | 2.17 × 105 |
| H2PO4− | 1.13 × 103 | 1.33 × 103 | 2.65 × 103 |
errors are <10%.
Interestingly, exposure of 4 to the dihydrogen phosphate anion gives rise to a significant color change than fluoride anion, even though this latter anion is characterized by a higher binding affinity. This leads us to suggest that the binding mode of dihydrogen phosphate anion is different from that for spherical anions (see supporting information). In the event, it is clear that the anion affinity of receptor 4 decreases as the size of the anion increases.
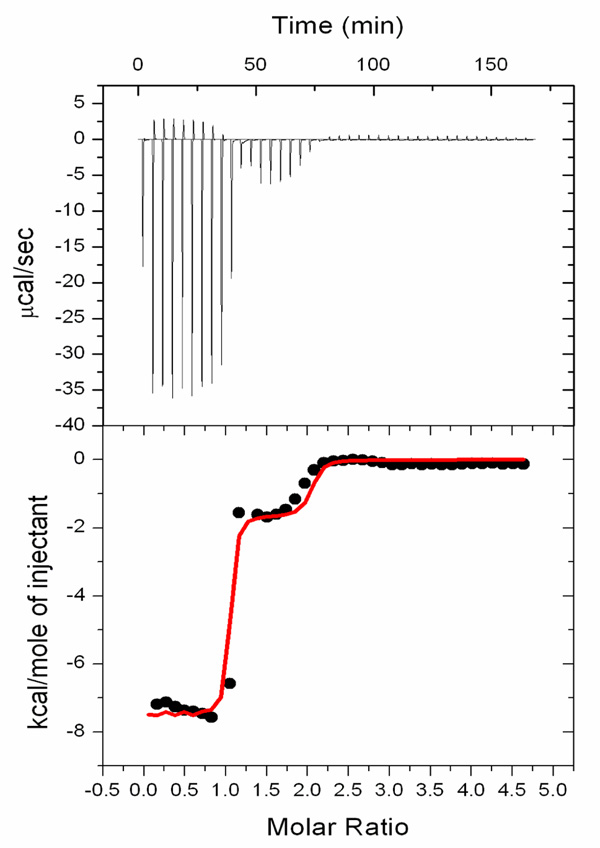
In order to verify the binding constants obtained from absorption spectroscopy, additional quantitative studies of the anion binding properties of receptor 4 were made using isothermal titration calorimetry (ITC). This is a method that has been applied extensively of late to the study of calix[4]pyrrole-based anion binding.14 Table 1 summarizes the equilibrium association constants Ka, measured by ITC for the binding of various anions to receptor 4 as determined in CH3CN/DMSO (97:3 v./v.). Gratifyingly, the binding constants obtained by these two different methods were for the most parts in good agreement with one another. The one exception was fluoride anion. In this case, the addition of excess anion produced changes in the ITC curves that are best interpreted in terms of an additional binding isotherm (Figure 3). Such findings are certainly not a proof of the binding mode suggested in Scheme 2, but are fully consistent with it in that at high fluoride concentrations additional interactions involving the pyrrole NH protons of the strap would be possible. The calculated second binding constant is K2 = 5.0 ×105 M−1.
Figure 3.
Binding isotherm corresponding to the ITC titration between TBAF and receptor 4 ([4] = 0.41 mM in CD3CN/DMSO; 97:3 v./v. at 30 °C).
Scheme 2.
Proposed binding mode for the interaction of fluoride anion with receptor 4 as inferred from 1H NMR spectroscopic analyses. Additional interactions, involving the binding of a second equivalent of fluoride anion to the dipyrrolylquinoxaline pyrrolic NH protons, are considered likely at high fluoride-to-receptor ratios.
In summary, we have shown that strapping a calix[4]pyrrole core with a dipyrrolylquinoxaline moiety can be used to produce a chromogenic sensor (4) for certain anionic species, notably for fluoride and dihydrogen phosphate anion, that functions well in organic media. The anion-induced changes are particularly dramatic in the case of the fluoride and dihydrogen phosphate anions, albeit a detectable signal can be observed in the case of acetate anion. Based on 1H NMR spectroscopic analyses, it is considered likely that the dipyrrolylquinoxaline-containing strap part is tilted such that the bound anions can interact with the dipyrrolylquinoxaline NH protons, perhaps through anion-pi interactions. To the extent these proposed ancillary effects can be generalized, it is considered likely that the specific choice of strapping element could be used as a means for modulating the intrinsic anion affinities of calix[4]pyrroles as we have recently shown in the case of CH- vs. NH-anion hydrogen bonding interactions.15 Current work is focused on exploring various putative second order binding effects, as well as on the design of other strapped systems bearing built-in chromophores, including ones that might display analyte selectivity very different from those displayed by receptor 4.
Experimental
Proton NMR spectra were recorded using TMS as the internal standard. High and Low resolution FAB mass spectra were obtained by high-resolution mass spectrometer. Column chromatography was performed over silica gel (Merck, 230–400 mesh). Pyrrole was distilled at atmospheric pressure from CaH2. Both CH2Cl2 and CHCl3 (reagent grade) were distilled from K2CO3 to eliminate traces of acid. Compound 1 was synthesized according to a literature procedure.10 All other reagents were obtained from Aldrich and used as received unless noted otherwise. Isothermal titration calorimetery (ITC) measurements were performed as follows: Solutions of the chosen receptor in acetonitrile/DMSO_(97:3 v./v.) were made up so as to provide a receptor concentration range of 0.1~1.0 mM. These solutions were then individually titrated with the appropriate alkylammonium salts at 30 ± 0.01 °C. The original heat pulses were normalized using reference titrations carried out using the same salt solution but pure solvent, as opposed to a solution containing the receptor. The values recorded in Table 1 represent the average of at least three separate titrations carried out at least two at different concentrations.
2,3-Bis[(5-(3-oxobutyl)-1H-pyrrol-2-yl)]ethanedione (1)
Oxalyl chloride (0.14 mL, 1.6 mmol) was dissolved in CH2Cl2 (2 mL) under annitrogen atmosphere. After cooling to −78 °C, dry pyridine (0.34 mL, 4.0 mmol) was added. To this cooled solution was added a solution of 4-(2-pyrrolyl)butan-1-one (0.44 g, 3.2 mmol) in CH2Cl2 (5 mL). The reaction was allowed to stir for 15 min at −60 °C before hydrochloric acid (5 M solution, 5 mL) was slowly added to quench the reaction. The resulting mixture was then extracted with CH2Cl2 (50 mL × 3) and the organic layer was dried over anhydrous sodium sulfate. The organic solutions were evaporated under reduced pressure and the residue was purified by column chromatography over silica gel (eluent: CH2Cl2 / EtOAc, 3:1) to afford 1 (0.31 g, 56%) as a yellowish solid: 1H NMR (400 MHz, CDCl3) δ 11.44 (brs, 2H), 6.94 (brs, 2H), 6.00-5.98 (m, 2H), 2.93-2.90 (m, 4H), 2.86-2.82 (m, 4H), 2.16 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 207.26, 180.44, 142.59, 128.58, 122.158, 109.48, 42.51, 29.84, 21.64; EI MS Calcd. for C18H20N2O4 exact mass 328.1423, Found 328.1423 (M+).
6-Nitro-2,3-bis[(5-(3-oxobutyl)-1H-pyrrol-2-yl)]quinoxaline (2)
Compound 1 (1.85 g, 5.6 mmol) and 4-nitro-1,2-phenylenediamine were dissolved in glacial acetic acid (30 mL). The resulting mixture was heated at reflux for 90 min under a nitrogen atmosphere. Solvent was removed, while hot, under reduced pressure and the remaining solid was dissolved in CH2Cl2 (50 mL) and washed with aqueous NaOH (0.1 N, 40 mL). The organic layer was dried over anhydrous sodium sulfate and the solvent was removed under reduced pressure. The resulting residue was purified by column chromatography over silica gel (eluent: CH2Cl2 / EtOAc, 9:1) to afford 2 (0.6 g, 24%) as a yellowish solid: 1H NMR (400 MHz, CDCl3) δ 9.86 (brs, 1H), 9.79 (brs, 1H), 8.67 (d, J = 2.5 Hz, 1H), 8.25-8.22 (m, 1H), 7.86 (d, J = 9.1 Hz, 1H), 7.07-7.00 (m, 2H), 5.96-5.94 (m, 2H), 2.98-2.95 (m, 4H), 2.89-2.86 (m, 4H), 2.22 (d, J = 1.3 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 208.45, 208.43, 146.41, 145.32, 144.95, 142.57, 138.15, 137.64, 136.88, 128.68, 127.84, 127.61, 124.02, 121.76, 115.72, 114.75, 108.37, 107.96, 43.36, 43.30, 30.08, 21.61, 21.59; MALDI-TOF MS Calcd. for C24H23N5O4 445.1750, Found 446.3195 (M++1).
6-Nitro-2,3-bis[(5-(3,3-bis(1H-pyrrol-2-yl)butyl)-1H-pyrrol-2-yl)]quinoxaline (3)
To the solution of 2 (0.70 g, 1.57 mmol) and pyrrole (20 mL) was added trifluoroacetic acid (120 µL, 1.57 mmol). The whole mixture was stirred for 4.5 h at 70 °C. Then the mixture was combined with methylene chloride and washed with aqueous NaOH solution (0.1 N, 50 mL), and water (50 mL). The organic layer was evaporated under reduced pressure and the residue was purified by column chromatography on silica (CH2Cl2 / EtOAc = 95 / 5) to afford 3 (0.78 g, 74%) as an orange solid: 1H NMR (300 MHz, CDCl3) δ 9.36 (brs, 1H), 9.29 (brs, 1H), 8.62 (d, J = 2.4 Hz, 1H), 8.27-8.23 (m, 1H), 7.85 (s, 1H), 7.82 (brs, 4H), 7.16-7.14 (m, 2H), 6.69-6.66 (m, 4H), 6.19-6.16 (m, 8H), 5.99-5.96 (m, 2H), 2.64-2.59 (m, 4H), 2.42-2.36 (m, 4H), 1.69 (d, J = 1.1 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 146.16, 145.66, 145.33, 142.66, 139.49, 138.68, 137.63, 137.56, 128.37, 127.62, 123.72, 121.55, 117.08, 116.168, 115.108, 108.04, 107.59, 104.56, 39.05, 26.18, 26.12, 23.35, 23.30; MALDI-TOF MS Calcd. for C40H39N9O2 677.7968, Found 678.4298 (M++1).
6-Nitro-dipyrrolylquinoxaline-strapped calix[4]pyrrole (4)
To a solution of compound 3 (0.51 g, 0.75 mmol) in acetone (300 mL) was added BF3·OEt2 (190 µL, 1.51 mmol). The resulting reaction mixture was stirred for 3.5 h at room temperature, then TEA (6 mL) was added. The solvent was removed under reduced pressure. The residue was purified by column chromatography on silica (CH2Cl2) to afford 4 (39 mg, 7%) as a yellowish solid: 1H NMR (300 MHz, CDCl3) δ 8.83 (d, J = 2.5 Hz, 1H), 8.78 (brs, 1H), 8.72 (brs, 1H), 8‥38-8.34 (m, 1H), 8.01 (d, J = 9.1 Hz, 1H), 7.05 (brs, 4H), 6.60-6.54 (m, 2H), 6.03-5.99 (m, 8H), 5.82-5.79 (m, 2H), 2.53-2.49 (m, 4H), 2.24-2.16 (m, 4H), 1.60 (s, 6H), 1.50 (s, 6H), 1.49 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 146.90, 145.55, 145.06, 143.13, 138.84, 138.07, 137.79, 137.43, 135.69, 129.35, 127.60, 127.36, 124.60, 122.36, 115.27, 114.29, 108.81, 108.402, 105.24, 104.28, 43.02, 42.92, 39.64, 39.57, 35.80, 30.82, 29.04, 28.01, 28.00, 23.56; MALDI-TOF MS Calcd. for C46H48N8 757.9245, Found 757.3912 (M++1).
Supplementary Material
Synthetic details of all the compounds, spectroscopic data are available with free of charge via the internet at http://pubs.acs.org
Acknowledgments
This work was supported by a grant (R01-2006-000-10001-0) from the Basic Research Program of the Korea Science and Engineering Foundation. The Vascular System Research Center (VSRC) is also acknowledged for support. The work in Austin was supported by the National Institutes of Health (grant no. GM 58907).
References
- 1.(a) Sessler JL, Gale PA, Cho WS. Anion Receptor Chemistry. Cambridge: Royal Society of Chemistry; 2006. [Google Scholar]; (b) Gale PA. Coord. Chem. Rev. 2001;213:79–128. [Google Scholar]; (c) Gale PA. Coord. Chem. Rev. 2000;199:181–233. [Google Scholar]
- 2.(a) Beer PD, Gale PA. Angew. Chem, Int. Ed. 2001;40:486–516. [PubMed] [Google Scholar]; (b) Gale PA, García-Garrido SE, Garric J. Chem. Soc. Rev. 2008;37:151–190. doi: 10.1039/b715825d. [DOI] [PubMed] [Google Scholar]
- 3.de Silva AP, Guanarante HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE. Chem. Rev. 1997;97:1515–1566. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]
- 4.Schmidtchen FP, Berger M. Chem. Rev. 1997;97:1609–1646. doi: 10.1021/cr9603845. [DOI] [PubMed] [Google Scholar]
- 5.(a) Lee CH, Miyaji H, Yoon DW, Sessler JL. Chem. Comm. 2008:24–35. doi: 10.1039/b713183f. [DOI] [PubMed] [Google Scholar]; (b) Lee CH, Na HK, Yoon DW, Cho WS, Lynch V, Sessler JL. J. Am. Chem. Soc. 2003;125:7301–7306. doi: 10.1021/ja029175u. [DOI] [PubMed] [Google Scholar]
- 6.Panda PK, Lee CH. Org. Lett. 2004;6:671–674. doi: 10.1021/ol0360750. [DOI] [PubMed] [Google Scholar]
- 7.(a) Lee CH, Lee JS, Na HK, Yoon DW, Miyaji H, Cho WS, Sessler JL. J. Org. Chem. 2005;70:2067–2074. doi: 10.1021/jo0487146. [DOI] [PubMed] [Google Scholar]; (b) Miyaji H, Kim HK, Sim EK, Lee CK, Cho WS, Sessler JL, Lee CH. J. Am. Chem. Soc. 2005;127:12510–12512. doi: 10.1021/ja053612y. [DOI] [PubMed] [Google Scholar]
- 8.Miyaji H, Hong SJ, Jeong SD, Yoon DW, Na HK, Hong J, Ham S, Sessler JL, Lee CH. Angew. Chem. Int. Ed. 2007;46:2508–2511. doi: 10.1002/anie.200604161. [DOI] [PubMed] [Google Scholar]
- 9.(a) Yoon DW, Jeong SD, Song MY, Lee CH. Supramol. Chem. 2007;19:265–270. [Google Scholar]; (b) Jeong SD, Yoo J, Na HK, Chi DY, Lee CH. Supramol. Chem. 2007;19:271–275. [Google Scholar]
- 10.(a) Black CB, Andrioletti B, Try AC, Ruiperez C, Sessler JL. J. Am. Chem. Soc. 1999;121:10438–10439. [Google Scholar]; (b) Anzenbacher P, Jr, Try AC, Miyaji H, Jursikova K, Lynch VM, Marquez M, Sessler JL. J. Am. Chem. Soc. 2000;122:10268–10272. [Google Scholar]
- 11.Yadav JS, Abraham S, Reddy BVS, Sabitha G. Tetrahedron Lett. 2001;42:8063–8065. [Google Scholar]
- 12.Schottel BL, Chifotides HT, Dunbar KR. Chem. Soc. Rev. 2008;37:68–83. doi: 10.1039/b614208g. [DOI] [PubMed] [Google Scholar]
- 13.Gil-Ramirez G, Escudero-Adan EC, Benet-Buchholz J, Ballester P. Angew. Chem. Int. Ed. 2008;47:4114–4118. doi: 10.1002/anie.200800636. [DOI] [PubMed] [Google Scholar]
- 14.(a) Sessler JL, Gross DE, Cho W-S, Lynch VM, Schmidtchen FP, Bates GW, Light ME, Gale PA. J. Am. Chem. Soc. 2006;128:12281–12288. doi: 10.1021/ja064012h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yoon DW, Hwang H, Lee CH. Angew. Chem. Int. Ed. 2002;41:1757–1759. doi: 10.1002/1521-3773(20020517)41:10<1757::aid-anie1757>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]; (c) Schmidtchen FP. Org. Lett. 2002;4:431–434. doi: 10.1021/ol017137u. [DOI] [PubMed] [Google Scholar]; (d) Gross DE, Schmidtchen FP, Antonius W, Gale PA, Lynch VM, Sessler JL. Chem. Eur. J. 2008;26:7822–7827. doi: 10.1002/chem.200800899. [DOI] [PubMed] [Google Scholar]
- 15.Yoon DW, Gross DE, Lynch VM, Sessler JL, Hay BP, Lee CH. Angew. Chem. Int. Ed. 2008;47:5038–5042. doi: 10.1002/anie.200801426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthetic details of all the compounds, spectroscopic data are available with free of charge via the internet at http://pubs.acs.org