Abstract
Attention exerts a strong influence over neuronal processing in cortical areas1,2. It selectively increases firing rates2-4 and affects tuning properties1,5, including changing receptive field locations and sizes3,6. Although these effects are well studied, their cellular mechanisms are poorly understood. To study the cellular mechanisms, we combined iontophoretic pharmacological analysis of cholinergic receptors with single cell recordings in V1 while rhesus macaque monkeys (Macaca mulatta) performed a task that demanded top-down spatial attention. Attending to the receptive field of the V1 neuron under study caused an increase in firing rates. Here we show that this attentional modulation was enhanced by low doses of acetylcholine. Furthermore, applying the muscarinic antagonist scopolamine reduced attentional modulation, whereas the nicotinic antagonist mecamylamine had no systematic effect. These results demonstrate that muscarinic cholinergic mechanisms play a central part in mediating the effects of attention in V1.
Attention is a rich and complex psychological and neurobiological construct. In both non-human1-4 and human primates7, researchers have investigated a wealth of paradigms tapping visual attention, showing the selection of behaviourally relevant over behaviourally irrelevant stimuli, and the enhancement of the former’s processing. Further modulation of sensory areas is assumed to be driven by the frontal and parietal cortex7,8 through direct cortico–cortical feedback connections. However, frontal regions also influence sensory areas indirectly, through connections to cholinergic neurons in the basal forebrain that have ascending projections to sensory areas9,10, and there is ample evidence for the involvement of acetylcholine (ACh) in attentional modulation10-14. For instance, depletion of ACh in cortical areas by disrupting cholinergic fibres originating in the basal forebrain results in persistent attentional impairments10,15, and attentional deficits seen in Alzheimer’s disease partly arise from cholinergic dysfunction16. The precise nature of the contribution of ACh to attentional modulation in the cortex is at present unclear. Thus, we recorded the strength of attentional modulation in neurons from V1 of three macaque monkeys, while simultaneously performing pharmacological analysis of cholinergic receptor contributions. Subjects performed a task demanding voluntary allocation of attention5,17 under control conditions and when ACh, or muscarinic or nicotinic receptor antagonists, were iontophoretically applied in the vicinity of the neurons under study5,17.
We used identical task, training, and surgical and neurophysiological procedures as previously described5,17 (see Methods Summary and Supplementary Methods). Neurons were activated by bar stimuli of optimal orientations centred on their receptive fields. Attention was manipulated by a visual cue, which guided attention to be in or away from the neurons’ receptive fields (the attend-receptive-field and attend-away conditions, respectively). The effect of attention on the firing rates of neurons was tested in 15–25 trials in each condition: control, drug-applied and recovery (see Supplementary Methods).
We recorded 156 neurons from 3 monkeys (26 neurons in monkey B, 87 in monkey HU and 43 in monkey HO) in the absence and presence of ACh application (Supplementary Methods provides more details). We tested whether attention, bar length or drug application significantly affected neuronal activity, and whether there was a significant interaction between these factors (three-factor analysis of variance (ANOVA), P < 0.05). We used the response period from 200 ms to 500 ms after stimulus onset for our analysis, as attentional modulation is most prominent during the sustained response in the striate4,5 and extrastriate cortex18.
Neurons were analysed further if attention and drug application significantly affected firing rates, or if a significant interaction between attention and drug application occurred. Strengths of attentional modulation were quantified by calculating the area under the receiver operating characteristic (ROC5) curve on the basis of single-trial responses (given knowledge of the bar length). ROC values of 0.5 indicate that an ideal observer can only perform at chance level in deciding the locus of attention. Higher ROC values indicate greater attentional response enhancement, thus if ACh increased attentional modulation, ROC values should be increased in ACh-applied conditions relative to control conditions.
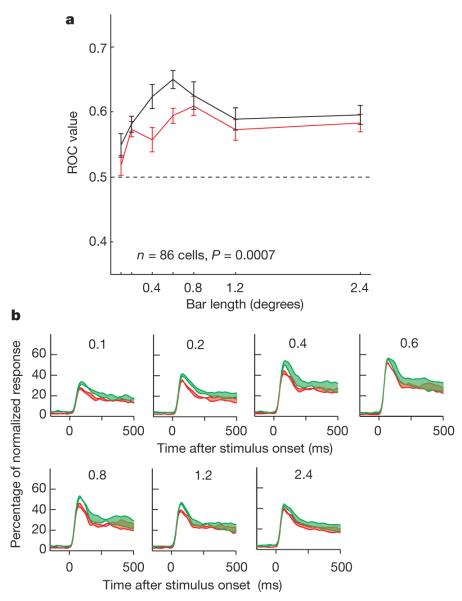
A total of 86 out of 156 neurons (16 in monkey B, 38 from monkey HU and 32 from monkey HO) passed the basic statistical test (three-factor ANOVA) on application of ACh. As seen in Fig. 1a, ACh generally increased attentional modulation. The neuron showed a significant main effect of attention (F1,284 = 24.92, P < 0.001) and a significant effect of drug (F1,284 = 6.7, P = 0.01). ROC values showed that attentional modulation was increased on ACh application (ROC (-ACh): 0.6774 (0.8° bar length); ROC (+ACh): 0.8262; for further example cells, see Supplementary Methods). The population ROC values for the drug and control condition are shown in Fig. 2a. In the presence of ACh, ROC values were increased. A two-factor ANOVA showed that the effect was significant for the population of cells and did not depend on bar length (data were converted to z-scores before testing; samples passed normality and equal variance tests (P > 0.05); factor 1, drug application: F1,874 = 16.58, P < 0.001 (two-factor ANOVA); factor 2, bar length: F6,874 = 7.61, P < 0.001, drug × bar lengths interaction: F6,874 = 1.058, P = 0.386). The effect of ACh application on attentional modulation was significant in monkey HO and monkey HU individually (P < 0.05), but not in monkey B. However, the trend in monkey B was the same as that in the other two monkeys, and the lack of significance was possibly due to the smaller neuronal sample. Figure 2b shows the time course of the effect of ACh on attentional modulation. The upper and lower green curves show normalized population activities when ACh was applied for attention into and away from the receptive fields, respectively. The upper and lower red curves show the same, but without ACh. Thus, the widths of the colour-shaded regions show the evolution of attentional modulation. The fact that the green areas are generally wider than the red ones is an indication of the boosting effect of ACh on attentional modulation.
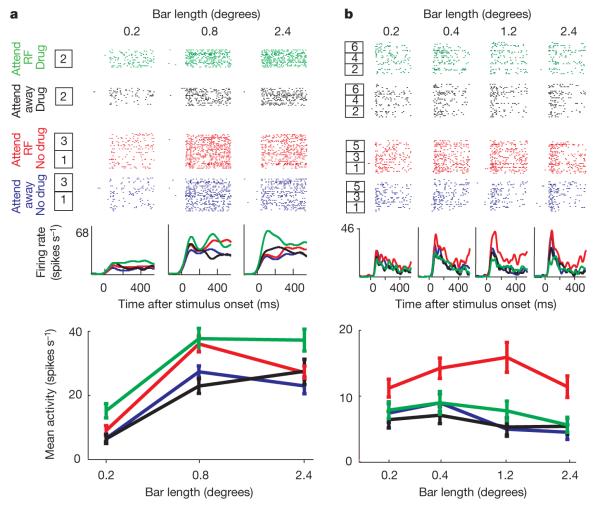
Figure 1. Drug effects on attentional modulation.
a, Attentional enhancement by application of ACh. We recorded the effect of attention on firing rates when no ACh was applied (16 trials) for three bar lengths in the attend-inside receptive field (RF) versus attend-outside (away) condition (box 1). Thereafter we recorded the effect of attention when ACh was applied (16 trials; box 2) followed by recovery (16 trials; box 3). The bottom plot shows the average activity (from 200 ms to 500 ms after stimulus onset) for the different stimulus, attention and drug conditions. ACh increased attentional modulation. b, Effect of scopolamine application on neuronal attentional modulation. All conventions are as in a. Drug application and recovery were repeated three times (boxes 1–6). Scopolamine reduced attentional modulation. Error bars represent s.e.m.
Figure 2. Acetylcholine effects on attentional modulation.
a, Quantification of attentional modulation by mean population ROC for different bar length with (black line) or without (red line) ACh application (86 cells; error bars denote s.e.m.). b, Normalized population response depending on ACh application, stimulus (bar length; indicated at the top of each subplot) and attention condition. Activity levels were normalized relative to the peak activity of each neuron and averaged across the population. The red lines indicate activity without ACh application; green lines represent activity with ACh application. The upper line of each colour plot shows the normalized activity when attention was directed to the neuron’s receptive field, the lower line when it was directed away from the receptive field. Widths of the coloured areas show strength of attentional modulation. ACh increased attentional modulation.
ACh slightly increased the overall firing rates (Fig. 2b), making it possible that increased ROCs were simply caused by multiplicative scaling of firing rates. However, it can be shown that ROC values are invariant to proportional rescaling of distributions (see Supplementary Methods). Furthermore, we calculated a modulation index for each cell, drug condition and bar length: modulation index = (activityattend receptive field – activityattend away)/(activityattend receptive field + activityattend away) which normalizes explicitly for firing rate. In line with the ROC analysis, we found that ACh significantly increased the population modulation index (F1,874 = 9.49, P = 0.002, two-factor ANOVA), and that this was independent of bar length (F6,874 = 0.42, P = 0.869).
Having established that ACh application increases attentional modulation, we were interested in the receptors that mediate this effect. Thus we recorded 118 cells (46 from monkey B and 72 from monkey HU) while monkeys performed the same task, but we applied the muscarinic antagonist scopolamine iontophoretically (see Supplementary Methods). Out of the 118 cells, 41 showed a significant attention and a significant drug effect, or a significant interaction between these two (20 cells from monkey B and 21 cells from monkey HU).
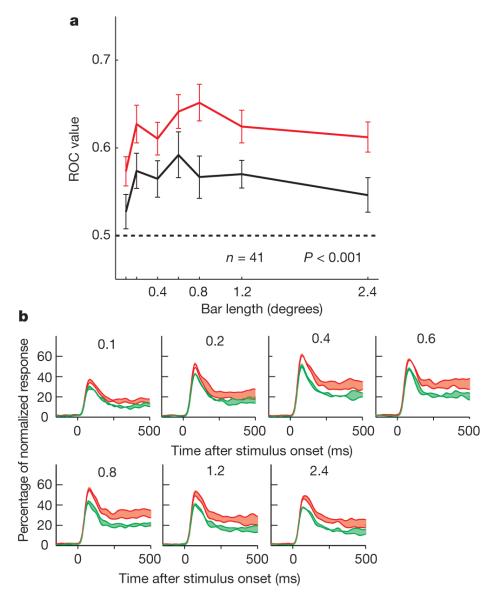
Scopolamine generally reduced attentional modulation. An example cell is shown in Fig. 1b. Attention (F1,464 = 33.34, P < 0.001), drug application (F1,464 = 18.13, P < 0.001) and bar length (F3,464 = 3.13, P = 0.025) all had a significant effect on firing rates (three-factor ANOVA). Attentional modulation was significantly reduced in the presence of scopolamine (drug × attention interaction: F3,464 = 13.5, P < 0.001). We quantified the effect of scopolamine application on attentional modulation by calculating the ROC for each cell and bar length. Population ROCs are shown in Fig. 3a. Scopolamine significantly reduced attentional modulation (F1,466 = 27.744, P < 0.001, two-factor ANOVA). The effect of scopolamine on ROCs was independent of bar length (drug × bar length interaction: F6,466 = 0.217, P = 0.971). The reduction in attentional modulation with scopolamine application was significant for each monkey individually (P < 0.001).
Figure 3. Effect of the muscarinic antagonist scopolamine on attentional modulation.
a, Quantification of attentional modulation by mean population ROC when scopolamine was (black line) and was not (red line) applied (n = 41 cells, error bars represent s.e.m.). ROC values were significantly reduced on scopolamine application, demonstrating that muscarinic receptors are involved in mediating the effects of attention in V1.
b, Normalized population response depending on scopolamine application, bar length (indicated at the top of each subplot) and attention. The widths of the red and green shaded areas show the strengths of attentional modulation as a function of scopolamine application (green lines represent activity with scopolamine application; red lines denote activity without scopolamine application). Attentional modulation was reduced when scopolamine was applied.
Figure 3b shows that the effect of scopolamine on the evolution of the population response in the same form as in Fig. 2b. The green shaded regions (scopolamine application) are now thinner than the red regions during the sustained response, indicating that attentional modulation was stronger in the absence of scopolamine application. Scopolamine also reduced firing rates, so, as for ACh, we also calculated the firing-rate-normalized modulation index. Confirming the results from the ROC analysis, modulation indexes were significantly reduced on scopolamine application (F1,466 = 11.15, P = 0.0009; see Supplementary Methods).
ACh also acts on nicotinic receptors, which might equally contribute to attentional modulation in V1. We therefore tested attentional modulation in the presence and absence of the nicotinic antagonist mecamylamine, whilst recording 151 V1 cells (113 cells from monkey HU and 39 cells from monkey HO). Significant effects of attention and of mecamylamine application, or an interaction, were found in 65 cells (47 cells from monkey HU and 18 cells from monkey HO). Figure 4 shows the mean ROC values for these cells as a function of bar length for the control and the mecamylamine-applied condition. A two-factor ANOVA did not show significant effects of drug application (F1,744 = 0.54, P = 0.465), whereas there was a significant effect of bar length (F6,744 = 2.98, P = 0.0069) on the size of the ROC values. There was no interaction between mecamylamine applied/not-applied and bar length (F6,744 = 0.40, P = 0.881). These findings were consistent for the two animals. The same outcome was true when the modulation index was used to quantify the effects of mecamylamine application on attentional modulation (F1,744 = 1.41, P = 0.236, two-factor ANOVA).
Figure 4. Effect of the nicotinic antagonist mecamylamine on attentional modulation.
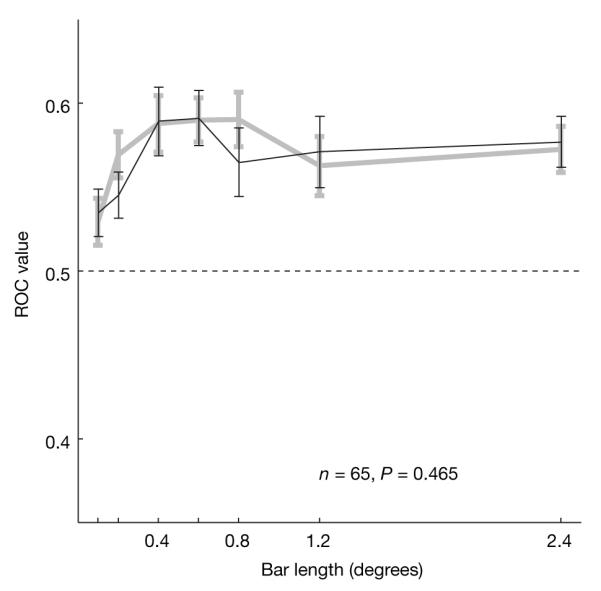
Attentional modulation was quantified by calculating the population ROC for the different bar lengths when mecamylamine was (black line) and was not (grey line) applied (n = 65 cells). ROC values were not significantly affected by mecamylamine application. Error bars show the s.e.m.
None of the effects of ACh, scopolamine or mecamylamine were due to differences in eye position induced by drug application (see Supplementary Methods).
For the neuromodulator dopamine it has been reported that only an optimal dose benefits neuronal and behavioural performance19, whereby too much and too little dopamine is detrimental for cognitive performance. We found evidence for a similar pattern of results for ACh in V1: that is, only small doses of extra ACh increased attentional modulation (for details, see Supplementary Methods).
Systemic applications of cholinergic drugs or large injections into higher cortical areas have been shown to affect performance in humans20 and macaque monkeys21. The highly local character of iontophoretic drug application used in our study made it unlikely that performance would be affected, as even systemic infusions result in relatively small changes to reaction times20. In line with this expectation, neither scopolamine nor mecamylamine application affected reaction times significantly. However, when ACh was applied we found a significant interaction between drug application and the locus of attention on reaction times (F1,31827 = 4.56, P = 0.038, three-factor ANOVA). ACh increased reaction times in the attend-away condition and decreased reaction times in the attend-receptive-field condition. This effect was significant across the three monkeys, but only individually in monkey HO (F1,5588 = 4.77, P = 0.029, three-factor ANOVA). Similar trends were found in monkey HU and monkey B, but they did not reach significance (interaction between ACh application and locus of attention on reaction times: monkey HU, F1,20334 = 3.03, P = 0.081; monkey B, F1,5905 = 3.06, P = 0.080). These results indicate that ACh boosts aspects of attention when the animals attend to the stimulus in the receptive field, thus decreasing reaction times. The increase in reaction times in the attend-away condition could be due to improved neuronal processing at the application site making it more difficult to disengage attention from the irrelevant location, thus slightly increasing reaction times (for more information, see Supplementary Methods).
We found that ACh contributes to attentional modulation in V1, of the macaque monkey through muscarinic, but not nicotinic, receptor mechanisms. Fewer excitatory cells compared to inhibitory cells are subject to muscarinic modulation in V1 (ref. 22), raising the possibility that our recordings were mostly from inhibitory neurons. Analysis of the ‘spike’ waveform, however, suggests that recordings were mostly from excitatory neurons. Thus, the effects may have been mediated through local cortical circuitry, perhaps through an effect on the sort of oscillatory activity that has been linked to attentional effects23. Alternatively, the fact that attentional effects are stronger in V2 and V4 (ref. 24), where there are also more muscarinic receptors22 on excitatory cells, suggests that the effects may have been more direct and not necessarily mediated by changes in the overall network state.
Nicotinic receptors in macaque V1 are mostly located presynaptically on thalamocortical terminals in layer 4C, where they affect the neuronal gain25. Although attention affects firing rates in simple cells of V1 (ref. 26), where nicotinic receptors would be expected to have the most direct effect25, our result indicates that this is not mediated at the thalamocortical synaptic input stage. Although we found no evidence of nicotinic receptor contribution to attentional modulation in V1 it is probable that they contribute to attentional modulation in higher areas27, perhaps through the feed-forward cortico–cortical pathway.
An important question for neuromodulatory accounts of attention28 is reconciling the highly localized and fast effects of spatial attention with the apparent (though perhaps arguable29) coarseness of the ACh innervation. One possibility is that an interaction between ACh and glutamatergic feedback mediates attentional processing in sensory areas. Increased amounts of ACh alter the strengths of the connections in V1 and the biophysical state of sensory neurons which may then allow spatially specific glutamatergic feedback to enhance specific incoming information. Other possible sources of highly localized cholinergic contribution to attentional modulation are the recently described cholinergic cortical interneurons30.
Future studies will be necessary to clarify these issues. Nevertheless, the first step in understanding attentional modulation is to unpick its cortical determinants; our findings directly address this.
METHODS SUMMARY
We recorded extracellular neuronal activity from three hemispheres in three male macaque monkeys (Macaca mulatta), whilst applying ACh, scopolamine or mecamylamine iontophoretically on selected trials. Animals were implanted with a custom made head-holding device and recording chambers made of Tecapeek GF for compatibility in functional magnetic resonance imaging settings. Surgical procedures were performed under aseptic conditions and general anaesthesia. Experiments and surgeries were performed in accordance with the European Communities Council Directive 1986 (86/609/EEC), the National Institutes of Health (Guidelines for Care and Use of Animals for Experimental Procedures), the Society for Neurosciences Policies on the Use of Animals and Humans in Neuroscience Research, and the UK Animals Scientific Procedures Act.
The monkey’s task was to detect a small change in luminance at a cued (attended) location, while ignoring a change that occurred at a non-cued location and fixating a central fixation spot throughout the trial. After a fixation-only period, two identical stimuli were presented (test stimuli): one centred on the receptive field and the other at the same eccentricity in the opposite hemi-field. After 500–800 ms (randomized in 1 ms steps) a patch appeared at the centre of one of the bars. If presented in the cued location the monkey had to release the touch bar within 500 ms to receive a juice reward. If presented in the un-cued location the monkey had to continue to hold the touch bar and maintain fixation until target appearance. This occurred 1,000–1,300 ms (randomized in 1 ms steps) after the distracter appeared. Thus, we recorded activity when animals attended to the receptive field of the neuron under study and when they attended away from it. We then compared activity levels for these attentional conditions with and without drug application.
For all further information about the paradigm, the neuronal recordings, iontophoresis and data analysis, see Supplementary Methods.
Supplementary Material
Acknowledgements
The work was supported by the BBSRC (BBS/B/09325), the Wellcome Trust (070380/Z/03/Z) and the Gatsby Charitable Foundation.
References
- 1.Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- 2.Treue S, Maunsell JHR. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J. Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roelfsema PR, Lamme VA, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–381. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- 5.Roberts M, Delicato LS, Herrero J, Gieselmann MA, Thiele A. Attention alters spatial integration in macaque V1 in an eccentricity-dependent manner. Nature Neurosci. 2007;10:1483–1491. doi: 10.1038/nn1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Womelsdorf T, Anton-Erxleben K, Pieper F, Treue S. Dynamic shifts of visual receptive fields in cortical area MT by spatial attention. Nature Neurosci. 2006;9:1156–1160. doi: 10.1038/nn1748. [DOI] [PubMed] [Google Scholar]
- 7.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 8.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 9.Russchen FT, Amaral DG, Price JL. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. J. Comp. Neurol. 1985;242:1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- 10.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J. Comp. Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- 13.Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson CL, Sarter M, Bruno JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 15.McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J. Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nobili L, Sannita WG. Cholinergic modulation, visual function and Alzheimer’s dementia. Vision Res. 1997;37:3559–3571. doi: 10.1016/S0042-6989(97)00076-X. [DOI] [PubMed] [Google Scholar]
- 17.Thiele A, Delicato LS, Roberts MJ, Gieselmann MA. A novel electrode-pipette design for simultaneous recording of extracellular spikes and iontophoretic drug application in awake behaving monkeys. J. Neurosci. Methods. 2006;158:207–211. doi: 10.1016/j.jneumeth.2006.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- 19.Goldman-Rakic PS, Muly EC, III, Williams GV. D1 receptors in prefrontal cells and circuits. Brain Res. Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 20.Witte EA, Davidson MC, Marrocco RT. Effects of altering brain cholinergic activity on covert orienting of attention: comparison of monkey and human performance. Psychopharmacology (Berl.) 1997;132:324–334. doi: 10.1007/s002130050352. [DOI] [PubMed] [Google Scholar]
- 21.Davidson MC, Marrocco RT. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. J. Neurophysiol. 2000;83:1536–1549. doi: 10.1152/jn.2000.83.3.1536. [DOI] [PubMed] [Google Scholar]
- 22.Disney AA, Domakonda KV, Aoki C. Differential expression of muscarinic acetylcholine receptors across excitatory and inhibitory cells in visual cortical areas V1 and V2 of the macaque monkey. J. Comp. Neurol. 2006;499:49–63. doi: 10.1002/cne.21096. [DOI] [PubMed] [Google Scholar]
- 23.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 24.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J. Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 25.Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque V1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. J. Neurosci. 2005;25:11023–11033. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30:810–820. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- 28.Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Fournier GN, Semba K, Rasmusson DD. Modality- and region-specific acetylcholine release in the rat neocortex. Neuroscience. 2004;126:257–262. doi: 10.1016/j.neuroscience.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 30.von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J. Neurosci. 2007;27:5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.