Abstract
The larynx sits at the crossroads between gastrointestinal and respiratory tracts. Besides its intrinsic importance in breathing, swallowing and voice production, the larynx is also exposed to unique immunological challenges. Given the propensity of chronic inflammatory conditions such as chronic laryngitis, which affects up to 20% of Western populations, it is perhaps surprising that our understanding of the immunology of this organ remains relatively limited. However, recent work on the immunological architecture of the laryngeal mucosa, and its changes that result from external challenges and inflammatory conditions, provided valuable insight into the fascinating immunology of this organ. The lessons learnt from these investigations may go beyond devising improved therapy for chronic laryngeal inflammation. Establishing whether and how the laryngeal mucosa may be involved in the modulation of wider mucosal responses may provide novel routes to the treatment of other inflammatory diseases of the respiratory and alimentary tracts such as asthma and inflammatory bowel disease.
Introduction
The larynx is a vitally important mucosal organ that orchestrates swallowing, breathing, coughing and, in humans, voice. Loss of a functioning airway is life-threatening, as is loss of airway protection from ingested food and drink. In man, impaired voice production holds significant implications for individual health and wellness, social and occupational function; and societal productivity1, 2. The voice is a primary mode of communication. It is tied to personal identity, and is paramount to the livelihood of millions of individuals. Conservative estimates suggest that between 5–10% of the United States work force have heavy occupational voice demands3, and that approximately 28 million workers in the United States experience voice problems daily4. The societal cost of lost work days and treatment expenses due to voice complaints in teachers alone has been estimated at $2.5 billion annually in the United States5. Given the impact and high incidence of chronic inflammatory conditions affecting the larynx the relative paucity of investigations into the immunology of this organ is striking.
There is evidence supporting a distinct immunological role for the larynx6. The larynx represents the crossroads between the gastrointestinal and respiratory systems, and is the junction between the IgA-dominated upper and IgG-dominated lower airways. It is the narrowest point of the airway. A point of greatest airway turbulence where the deposition of inhaled and sometimes ingested challenges is high. The laryngeal epithelium, like mucosal epithelia elsewhere, comprises two main layers (epithelium, lamina propria) superficial to laryngeal muscle and cartilage. These two layers each have a characteristic immunological architecture. The epithelium of the vocal fold (medial edge) is composed of stratified squamous epithelium, whilst the neighboring ventricular folds and trachea are composed of a mixture of respiratory and stratified squamous epithelium, with the proportion of the latter increasing with age7. The mucosa is covered with a layer of mucus produced by goblet cells and glands. The amount of stored mucus, and expression of various mucin (MUC) genes, is altered in those with chronic airway inflammation8. Within this layer, and within the epithelium itself, resides a diverse bacterial flora9, 10. As a result of its location, the larynx is exposed to environmental challenges on both the airways and upper gastrointestinal tract. This situation could make the larynx a crucial checkpoint in the maintenance of tolerance or activation of appropriate mucosal inflammatory responses in mucosal systems downstream, such as the lung and the GI system.
The Immunological Architecture of Laryngeal Epithelium
Epithelial cells, coated with mucus, present a physical barrier to insults from the laryngeal lumen. However, the expression of antigen presenting molecules on the epithelial cells highlight that they are likely to be more than inert building blocks in a wall between organism and environment.
HLA class I molecules, in humans HLA A, B and C, sample the intracellular environment and present pathogen derived and tumor antigens. Due to this central role in the recognition of infectious agents or malignant transformations practically all nucleated cells in the body express them. Thus, the presence of these molecules on laryngeal epithelial cells is hardly surprising. However, a detailed analysis of their expression indicated that the density of MHC class I molecules decreases towards the lumen. Intriguingly, the levels of β2 microglubulin, a non-polymorphic protein that is essential for the formation of functional MHC class I complexes, showed a much less pronounced reduction in the superficial layers of the epithelium. This discrepancy led to the discovery of the expression of the non-classical MHC class I molecule CD1d on laryngeal epithelial cells. The observed gradual transition between the MHC class Ihi CD1dlo deeper layers to a MHC class Ilo CD1dhi superficial layer is an entirely unique feature of the laryngeal epithelium and suggests an interesting and potentially important compartmentalization of immune responses. While the high density of MHC class I molecules in the deeper layers provides a sensitive detection for viral infections, the role of the superficial CD1d rich layer is more difficult to discern. It is well documented that CD1d presents glycolipid compounds, and potentially other molecules with a lipid side chain, to a subset of lymphocytes. However, the nature of the molecules that interact with CD1d in vivo remains unclear. On one hand, there is evidence for the existence of endogenously derived self antigens – especially in activated dendritic cells (DC) – but the molecular characterization of these is still subject to heated debates. However, the available experimental data strongly suggests that these endogenous ligands play a key role in maintaining self tolerance. On the other hand, a number of bacterially derived CD1d antigens have been chemically identified. Whether these bacterially derived compounds contribute to the initiation of protective immunity against the pathogens that carry them or play a role in regulating self reactivity, remains to be resolved. These facts make the interpretation of the role of the CD1d on laryngeal epithelial cells difficult. Does the superficially placed CD1d expression represent a pathway that is designed to maintain tolerance towards the constant onslaught of inhaled and swallowed antigens? It could be argued that in the absence of such protective mechanism anyone not breathing HEPA filtered air and drinking double distilled water would suffer from a constant laryngeal inflammation. However, it is equally plausible that the presence of CD1d plays a role in monitoring the bacterial content of the larynx and represents an early, relatively non-specific, warning mechanism for the detection of changes in the flora.
The other tantalizing feature of antigen presentation in the larynx is the presence of HLA class II molecules. Investigations on laryngeal mucosal biopsies taken from the false vocal fold (FVF) of healthy individuals found that laryngeal epithelial cells express MHC Class II antigen presenting molecules distributed uniformly throughout the entire thickness of the laryngeal epithelium in vivo11. These molecules, involved in the presentation of exogenous antigens to CD4+ ‘helper’ T cells, are typically only present on professional antigen presenting cells, such as DC, monocytes/macrophages, although cytokine exposure can induce MHC class II expression in other cells. Normal laryngeal epithelial cells cultured in vitro did not constitutively express MHC class II molecules. However, stimulating these cultures with interferon-γ restored the transcription of these antigen presenting molecules to levels seen in the biopsy specimens12. The fact that the participants in this study did not have any clinical or histological evidence of inflammation raises the question: what is the mechanism that induces the expression of MHC class II molecules in the larynx in vivo? Is this phenomenon due to a basal cytokine secretion elicited by the presence of the bacterial flora or is there a particular cell type in the immune cell compartment present in the epithelial and submucosal layers that constitutively secrete IFNg and/or other cytokines?
As mentioned above, the expression of MHC class II molecules is primarily a “privilege” of antigen presenting cells. On the surface of DC, monocytes and macrophages antigens bound to class II proteins would be accompanied by so called co-stimulatory molecules, such as CD80 and CD86, and the coexistence of these two signals would result in the antigen specific T cell responses. In contrast, MHC class II bound antigens in the absence of co-stimulation results in tolerance induction. Thus, the outcome of this antigen presentation pathway on the surface of laryngeal epithelial cells is to a large extent dependent on the presence of absence of co-stimulatory molecules. Although no data has been reported on this issue in the literature it is tempting to speculate that class II mediated antigen presentation in the absence of CD80/CD86 could be a simple and effective mechanism to induce tolerance towards inhaled, potentially immunogenic, compounds that have the capacity to bind to MHC class II molecules. However, while this assumption is entirely plausible, the fact that the laryngeal epithelium in some species, for example pigs, is devoid of MHC class II molecules may question the importance of this pathway in tolerance induction.
Haemopoietic Cells in the Larynx
Cell types identified in the laryngeal mucosa and associated with changes in challenge states in adult mammals include macrophages, DC, T and B lymophocytes and NK cells, as well as granulocytes and macrophages6, 13–15. Highly organized lymphoid collections, similar to Peyer’s patches (laryngeal associated lymphoid tissue, LALT)16–19 have been identified by post-mortem serial section studies in 80% of persons younger than 20 years of age and 56% of persons older16, 18. These structures are rare below the epiglottis18. However, similarly to the small intestine, more than 90% of immune cell population of the larynx is distributed in diffuse manner throughout the epithelium and lamina propria20. DC, NK cells and T- and B-cells are the most frequent in the subglottis and rarest in the vibratory vocal fold area while granulocytes and macrophages are evenly distributed throughout the whole laryngeal mucosa. In human fetal larynges, DC, T- and B-cells and macrophages are present in the ventricular folds and subglottis, but not the glottis21. γδ-T cells, which are often referred to as ‘intraepithelial lymphocytes’ (IELs) and appear to play an important role in local responses in the skin and bowel, are very rarely observed in any layer in the larynx.
Due to their pivotal role in the initiation of immune responses the DC compartment of the laryngeal tissues has been the subject of multiple studies. In a pig model of laryngeal transplantation, a dense population of DC particularly around the basement membrane has been observed22. Furthermore, the activation status of these cells changed following reperfusion. Dendrites from this area stretch through the epithelium and are likely to contact the superficial mucosal layer that also contains some elements of the bacterial flora6. Similarly to the observations in pigs, a dense population of DC is present in the human laryngeal epithelium11.
The analysis of various lymphocytes subsets and their relationship to the antigen presenting molecules within the mucosa has seen major advances recently, due to a technical development. Previously, analysis of cellular interactions in situ has relied on the observer examining overlapping cells and assessing whether an interaction was taking place. This is lengthy, subject to inter- and intra-operator variation and gives no indication of whether cells are associating randomly or preferentially as the probability of these “interactions” increases with the number of cells. Analysis by eye is also insufficiently sensitive to utilize all the data generated. A method for automated, pixel based analysis of digital immunofluorescent images has been developed23, 24. A macro written within the freely available image analysis program ImageJ25 counts the number of pixels of each color, and then displays them as proportions of the positive area. Although this analysis does involve some data loss, it provides an objective assessment of the amount of staining and permits statistical analysis of the levels of cellular co-localization. By calculating the expected levels of co-localization within the tissues if cells associate randomly and comparing this to the observed levels of co-localization, it is possible to determine whether overlapping between different cell types is occurring at significantly greater levels than random chance would predict. Increases in co-localization indicate prolonged cell contact; therefore it is possible to gain some information about the dynamic interactions between cells from static tissue sections. This approach was used to evaluate the presence of CD4+ and CD8+ T cells as well as B lymphocytes. The results have shown an increased number of both T and B cells in the lamina propria, compared to the epithelium, and the almost complete absence of B lymphocytes of the epithelial layer. Despite significant inter-individual variability this study demonstrated the preferential localization of CD8+ T lymphocytes to the deeper, MHC class I high layers of the epithelium. As these T cells recognize antigens presented by MHC class I molecules these results appear logical and provide validation for the digital image analysis approach. In the course of this work the accumulation of CD3+ CD161+ lymphocytes was seen in the upper, CD1dhigh layer of the epithelium. The co-expression of these two markers was the first definition of Natural T (NKT) cells, which are sometimes also referred to as Natural Killer T lymphocytes. Although this definition of NKT cells has now been superseded – some CD3+ CD161+ lymphocytes are not true NKT cells while some NKT cells do not express CD161 – the co-localization of these double positive cells with CD1d antigen presenting molecules provides very strong indirect evidence for the presence of NKT cells in the superficial mucosa. NKT cells play a role in the control of some bacterial infections and show direct and indirect cytotoxicity against certain tumor cells. Just as important, in animal models the elimination of this lymphocyte subset resulted in the breakdown of self tolerance. Thus, their presence in the laryngeal epithelium could be consistent with a tolerance inducing function. This makes additional experiments for the confirmation of NKT cell presence paramount. However, currently the lack of suitable reagents limits the use of immunofluorescence for these investigations.
The effects of external challenges on laryngeal mucosal immune architecture
Bacteria
The importance of the bacterial flora in the development of the normal immunological architecture of the gut has been studied rather extensively (for a recent review see reference 11). In the larynx a similar complex interaction between the bacterial flora, the expression of antigen presenting molecules and the lymphocyte/monoyte compartment has been investigated in animal models. In the most extensive study, twenty-three piglets were delivered by Caesarian section into a germ-free ‘bubble’. The laryngeal mucosa from two animals sacrificed on day 0 showed very few CD4+ T cells and no expression of CD16, a marker of activated DC, or MHC class II molecules. As laryngeal epithelial cells in pigs are devoid of class II molecules, the absence of this marker in these animals indicates the lack of monocytes, DC and activated T and B lymphocytes. The remaining animals were divided into three groups. Some were kept germ free, the second group was colonized by a standard bacterial flora (modified Schaedler) and additional bacillus species while the third group received the same standard flora supplemented with staphylococci. At week 3 germ free animals showed little increase in the expression of the above described three markers compared to birth. In contrast, all colonized animals developed a rich CD4+ cell population, which was more extensive in animals also carrying staphylococci. Importantly, animals colonized with the mixture including staphylococci, a normal commensal organism in pigs, were the only group to show an increase in MHC II expression, indicating the presence of a significant monocyte, denritic cell, activated lymphocyte population26. Similarly, in rats the introduction of heat inactivated Moraxella or Bordetella resulted in the recruitment granulocytes, DC and various lymphocyte subsets14. These experiments clearly demonstrated that the development of laryngeal mucosal immune architecture depends on interaction with bacteria, and that the composition of the bacterial flora significantly influences the recruitment of leukocytes to the mucosa. Based on these observations it is reasonable to propose that seemingly minor changes of the commensal flora could significantly alter the immunological environment in the larynx. In turn these changes could play a key role in the development of chronic inflammatory states. Unfortunately, our understanding of the normal bacterial flora in the larynx is currently somewhat limited27. Most studies in this field have concentrated on the presence of Helicobacter pylori, however, the conclusions of this body of work show some contradiction. One group detected no Helicobacter pylori-like organisms in laryngeal samples using Giemsa and haematoxylin-eosin staining28, whereas another study using similar techniques detected organisms resembling Helicobacter, although these bacteria could not be positively identified by immunohistochemistry29. In contrast Fang et al.30, Rubin et al.31, Borkowski et al.32 and Jaspersen et al.,33 all report the presence of helicobacter pylori via urease assays and histology in patients with benign laryngeal lesions or chronic laryngitis.
Tobacco smoke
Studies on the immunological consequences of tobacco challenges have repeatedly shown changes in the laryngeal mucosa immune response. In one recent study, the density of immunologically-relevant cells in FVF biopsies were examined in a cohort of sixty-three individuals, both smokers and non-smokers, between the ages of 18 to 80 years, with no history of laryngeal or pharyngeal cancer or other obvious pathology. The presence of lymphocytes34 in the laryngeal mucosa of current cigarette smokers showed increased numbers of CD4+ T cells and there was an association between older age and greater CD4+ T cell numbers in both epithelium and lamina propria. Older age and female gender were associated with decreased lamina propria CD4+ CD45RO+ T cells and an increase in CD4+ CD45RO− T cells, indicating the relative paucity of memory T cells in these particular patients. In a related study of patients with hoarseness, however, DNA microarray analysis of laryngeal polyps did not detect differential expressed transcripts between smokers compared to non-smokers. However, as only seven of the 28 patients recruited were current non-smokers the power of the study was limited, potentially explaining the lack of a recognizable gene expression signature associated with smoking 35. In addition, a considerable number of genes up-regulated in polyps were of immunological relevance including, but not limited to – the HLA class II molecules HLA-DRA and HLA DPB1, RFX5 – a component of the promoter of MHC Class II genes and MMD (monocyte-macrophage differentiation protein). A pilot study measuring antigen-presenting molecule expression in Reinke’s edema (a benign inflammatory bilateral lesion of the membranous vocal folds, common in smokers), demonstrated a dense population of MHC Class II+ and CD68+ cells (Figure 2). Such dual positive cells are likely to be activated macrophages and theoretically cytokine secretion by these, could explain the already mentioned changes in CD4+ T cell numbers in smokers. However, validating this assumption, and elucidating the mechanisms that lead to the appearance of these activated macrophages in the mucosa of smokers will require further work.
Figure 2.
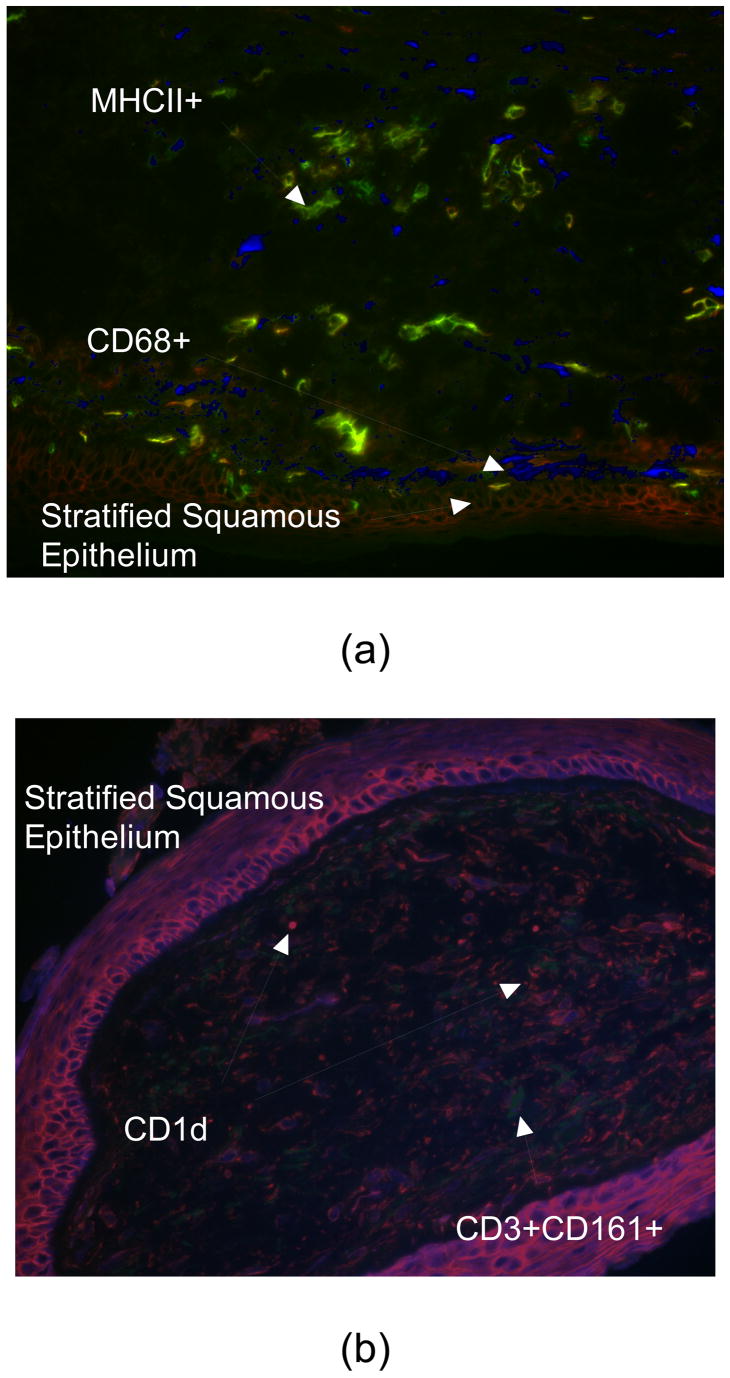
Immuno-fluroescence images of Reinke’s edema. (a) dense population of lamina propria by MHCII+ (green) and CD68+ (blue) cells (b) strong membranous expression of CD1d (red) by epithelial and dendritic cells, and increased CD3+CD161+ (NKT) cells in lamina propria (blue-green).
Extra-Esophageal Reflux
Extra-esophageal reflux (also referred to as posterior laryngitis or laryngo-pharyngeal reflux [LPR]) is a very common condition, affecting an estimated 20% of the adult US population36. This disease is costly in terms of both health care expenditure and quality of life. Over 200 million is spent annually in the US on proton pump inhibitors37, the most common medical management for LPR. The main clinical features are chronic mucosal inflammation, including edema and erythema. Globus pharyngeus and chronic cough are symptoms frequently associated with the condition. In patients suffering from LPR, the larynx is repeatedly exposed to gastric contents. This chronic challenge could conceivably alter the immunological responses. In a study by Rees et al38, twelve patients with florid LPR were compared to 11 control individuals who did not suffer from the condition. Diagnosis was based on validated clinical scoring39, dual-probe pH studies, transnasal esophagoscopy and manometry. Multiple color immunofluorescence studies of biopsies taken from the posterior (non-vibratory) vocal fold detected a strongly statistically significant increase in CD8+ T cells numbers in the laryngeal epithelium of LPR sufferers. It is noteworthy that, despite the change in the numbers of the cytotoxic T cells – which recognize MHC class I restricted antigens, there was no detectable change in the density of the antigen presenting molecules themselves on the epithelial cells38. The expression of MHC class II molecules was also unchanged in LPR patients. However, there was a marked increase in the presence of the non-classical MHC molecule CD1d in the patient group and this coincided with an elevated number of T cells co-localizing with the CD1d expressing epithelial cells. As discussed before, currently the lack of reagents hinders the positive identification of NKT cells that recognize antigens presented via CD1d. However, by definition NKT cells specifically recognize antigens via this pathway, and currently there is no evidence for other lymphocyte subsets binding to this molecule. Thus, based on the available data, it seems safe to conclude that in LPR elevated CD1d levels lead to increased NKT cell contact with the superficial layers of the laryngeal epithelium. This situation raises three questions: (1) what is the mechanism that leads to the up-regulation of CD1d expression; (2) what antigens are being presented by CD1d in LPR and (3) is the observed up-regulation protective, or detrimental in terms of the inflammatory response seen in the disease? Obviously, in the absence of experimental data we can only speculation on the answers. However, recent work has identified a number of structural features that determine whether CD1d restricted antigens are recognizable by the T cell receptors on NKT cells or not40. This work highlights two interesting facts. Firstly, the likelihood of bacterial compounds binding to CD1d seems higher than previously appreciated. Thus, relatively subtle changes in the laryngeal flora may have a significant effect on CD1d mediated antigen presentation. Perhaps even more interesting, it appears that the chemical modification of the sugar components of CD1d bound antigens profoundly influence their ability to interact with the T cell receptor. It is tempting to speculate that the presence of the acidic refluxate in LPR may result in such chemical changes. These alterations could potentially “inactivate” previously tolerance inducing CD1d antigens, leading to the loss of their “immunosuppressive” properties. Alternatively, the chemical modification may enhance the NKT cell-mediated recognition of otherwise innocuous CD1d binding bacterial compounds, resulting in a productive immune response. Obviously, either of these mechanisms could explain the development of the chronic inflammations seen in the disease.
Does the laryngeal mucosa influence lower airway immune responses?
The larynx is positioned at an immunological and anatomical cross-road. It represents the junction between the sterile, IgG-dominated lower airway and the bacteria-rich, IgA-dominated upper airway. Everything we inhale passes through its turbulent, narrow aperture in ‘breathing mode’ and everything we ingest passes over its surface in ‘sphincter mode’ (Figure 3). Teleologically, it seems necessary to place a tolerance inducing mechanism here that would protect against sensitization to inhaled and ingested compounds, which could otherwise act as antigens. Furthermore, the effective functioning of this tolerance induction could protect both the downstream respiratory tract and the GI system from potentially harmful, and debilitating sensitization. Thus, the larynx would be an ideal point to place a mucosal immune checkpoint. However, despite the innate attractiveness of this paradigm, presently there is only tenuous circumstantial evidence to support it. As presented, the larynx possesses a sophisticated immune architecture which alters significantly in response to a wide variety of incident challenges. Cell type and co-localization studies suggest that laryngeal mucosa is equipped with antigen presentation and cellular pathways that could fulfill the proposed protective role. It is widely observed, although poorly quantified, that patients who have had a total laryngectomy suffer increased chest and other infections41, also suggesting a wider immune role for this organ. It could be argued that inhaled steroids and other medications for asthma exert their therapeutic effect via the larynx, since particles are likely to be deposited in much higher densities on the larynx than more distally in the respiratory tree. Studies to establish the exact place of the larynx fits in the hierarchy of respiratory mucosal immunity, are hampered by the lack of a suitable animal model, since it is almost impossible to separate immunological challenges of the upper and lower airways in small animals and appropriate conditional and transgenic mice are lacking. Nonetheless, this question remains the most pressing in the field of laryngeal biology, and demands detailed investigation. The possible implications of a better understanding of laryngeal immunology for the way we treat respiratory disease could be far reaching.
Figure 3.
The laryngeal crossroads represents the junction between the sterile, IgG-dominated lower airway and the bacteria-rich, IgA-dominated upper airway. Things inhaled pass through a turbulent, narrow aperture in ‘breathing mode’ and everything ingested passes over the larynx’s surface in ‘sphincter mode’.
Concluding Remarks
In summary, the laryngeal epithelium has a rich immunological architecture, with molecular and cellular pathways that could control unwanted immune responses against harmless everyday challenges. Whether such responses actually take place, and mechanism involved in them, remain currently unknown. Investigations of the immunological basis of chronic inflammation of the larynx are of fundamental importance to making progress in our understanding, and thereby treatment, of the millions of people with laryngeal symptoms each year. In addition, clarification of the size and type of contribution of the larynx to respiratory and systemic immunity is required. It may very well be that the larynx, the caretaker of the anatomical cross-roads, also plays a similar central role in the aerodigestive mucosal immunity. Improving our understanding of the immunological responses of the laryngeal musoca may have far reaching implications for the future management of diseases as diverse as asthma, food allergy, pseudocroup, chronic cough and esophagitis.
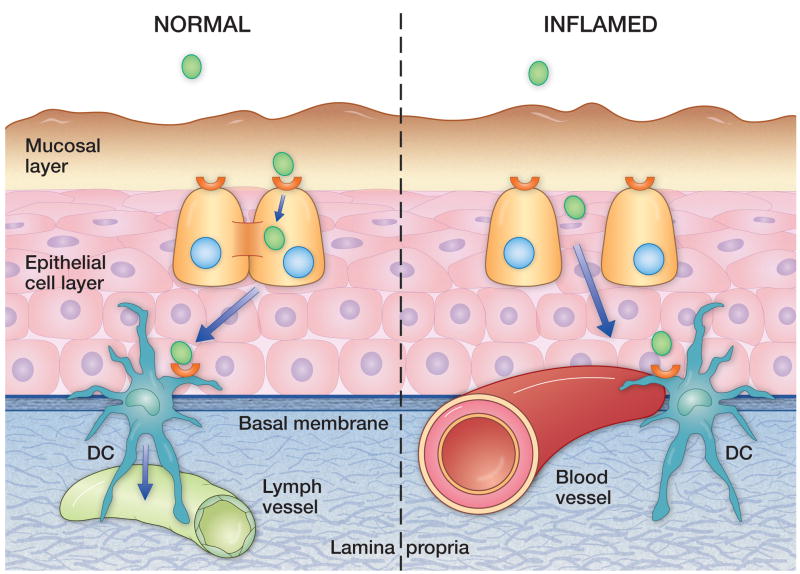
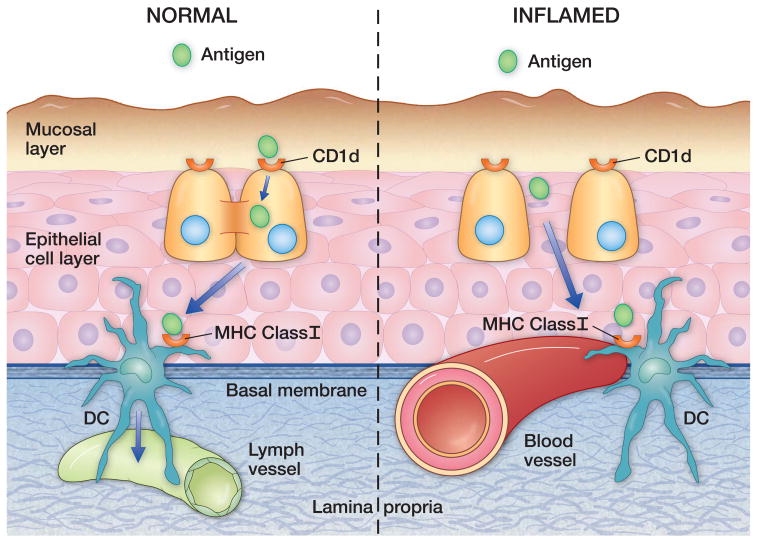
Figure 1.
The laryngeal mucosa is divided into two layers in terms of expression of antigen presenting molecules by epithelial cells: a superior, CD1d-rich layer and and deep, MHC Class1 -rich layer. We hypothesize that challenges which break down intercellular adhesion permit the presentation of incident antigens directly to the more ‘pro-inflammatory’ deeper layer, resulting in chronicity. Dendritic cells straddle the basement membrane and may orchestrate this response.
Contributor Information
Susan Thibeault, Assistant Professor, Division of Otolaryngology – Head and Neck Surgery, Department of Surgery, University of Wisconsin Madison, 5107 WIMR, 1111 Highland Ave, Madison, WI 53705- 2275, Email: thibeault@surgery.wisc.edu, T: 608 263 6751, Fax: 608-252-0939.
Louisa Rees, Research Associate, School of Clinical Veterinary Science, Division of Veterinary Pathology, Infection and Immunity, University of Bristol, Churchill Building, Langford, Bristol BS40 5DU t: 0117 9289289, e: L.E.Rees@bristol.ac.uk.
Laszlo Pazmany, School of Clinical Sciences, University of Liverpool, 3.48 Clinical Sciences Building, University Hospital Aintree, Liverpool, Email: L.pasmany@liverpool.ac.uk, T: +44 (0) 1515295896, F01515295222.
Martin A. Birchall, John Farndon Professor of Surgery and Professor of Laryngology, Laryngeal Research Group, Clinical Sciences at South Bristol, University of Bristol, Churchill Building, Langford House, Bristol BS40 5DU, Email: Btinternet.com, T: (0044)-117-33-19060, F: (+44)-117-9289282.
References
- 1.Jacobson BH, Johnson A, Grywalski C. The voice handicap index (VHI): Development and validation. American Journal of Speech Language Pathology. 1997;6:66–70. [Google Scholar]
- 2.Ma EP, Yiu EM. Voice activity and participation profile: assessing the impact of voice disorders on daily activities. Journal of Speech Language and Hearing Research. 2001;44:511–524. doi: 10.1044/1092-4388(2001/040). [DOI] [PubMed] [Google Scholar]
- 3.Roy N, et al. Three treatments for teachers with voice disorders: a randomized clinical trial. Journal of Speech Language and Hearing Research. 2003;46:670–688. doi: 10.1044/1092-4388(2003/053). [DOI] [PubMed] [Google Scholar]
- 4.Verdolini K, Ramig L. Review: Occupational risks for voice problems. Logopedics Phoniatrics Vocology. 2001;26:37–46. [PubMed] [Google Scholar]
- 5.Coyle SM, Weinrich BD, Stemple JC. Shifts in relative prevalence of laryngeal pathology in a treatment-seeking population. Journal of Voice. 2001;15:424–40. doi: 10.1016/S0892-1997(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 6.Barker E, Haverson K, Stokes CR, Birchall M, Bailey M. The larynx as an immunological organ: immunological architecture in the pig as a large animal model. Clinical and Experimental Immunology. 2006;143:6–14. doi: 10.1111/j.1365-2249.2005.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stell PM, Stell IM, Watt J. Age changes in the epithelial lining of the human larynx. Gerontology. 1982;28:208–214. doi: 10.1159/000212533. [DOI] [PubMed] [Google Scholar]
- 8.Innes AL, et al. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130:1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- 9.Gillet S, et al. Molecular identification of the bacterial flora of the larynx. Clinical Otolaryngology. (In press.) [Google Scholar]
- 10.Gillet S, Rees LE, Coogan T, Birchall M, Bailey M. Characterization of the bacterial flora of the larynx. Clinics in Otolaryngology. 2006;31:583. [Google Scholar]
- 11.Rees LE, Ayoub O, Birchall MA, Haverson K, Bailey M. Quantiatitive immunofluorescence to mesure MHC Class II expression on laryngeal epithelium. Clinical and experimental immunology. 2003;134:497–502. doi: 10.1111/j.1365-2249.2003.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rees LE, Gunasekaran S, Sipaul F, Birchall MA, Bailey M. The isolation and characterisation of primary human laryngeal epithelial cells. Molecular Immunology. 2006;43:725–730. doi: 10.1016/j.molimm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Jecker P, Ptok M, Pabst R, Westermann J. Distribution of immunocompetent cells in various areas in the normal laryngeal mucosa of the rat. European Archives of Otorhinolaryngology. 1996;253:142–146. doi: 10.1007/BF00615111. [DOI] [PubMed] [Google Scholar]
- 14.Jecker P, et al. Acute laryngitis in the rat induced by Moraxella catarrhalis and Bordetella pertussis: number of neutrophils, dendritic cells, and T and B lymphocytes accumulating during infection in the laryngeal mucosa strongly differs in adjacent locations. Pediatric Research. 1999;46:760–766. doi: 10.1203/00006450-199912000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Jecker P, Mann WJ, McWilliam AS, Holt PG. Dendritic cell influx differs between the subglottic and glottic mucosae during acute laryngotracheitis induced by a broad spectrum of stimuli. Annals of Otology, Rhinology and Laryngology. 2002;111:567–572. doi: 10.1177/000348940211100701. [DOI] [PubMed] [Google Scholar]
- 16.Debertin AS, et al. Coincidence of different structures of mucosa-associated lymphoid tissue (MALT) in the respiratory tract of children: no indications for enhanced mucosal immunostimulation in sudden infant death syndrome (SIDS) Clinical and Experimental Immunology. 2006;146:54–59. doi: 10.1111/j.1365-2249.2006.03190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kracke A, et al. Larynx-associated lymphoid tissue (LALT) in young children. Anatomical Record. 1997;248:413–420. doi: 10.1002/(SICI)1097-0185(199707)248:3<413::AID-AR14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Hiller AS, Tschernig T, Kleemann WJ, Pabst R. Bronchus-associated lymphoid tissue (BALT) and larynx-associated lymphoid tissue (LALT) are found at different frequencies in children, adolescents and adults. Scandavian Journal of Immunology. 1998;47:159–162. doi: 10.1046/j.1365-3083.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- 19.Kutta H, Steven P, Tillmann BN, Tsokos M, Paulsen FP. Region-specific immunological response of the different laryngeal compartments: significance of larynx-associated lymphoid tissue. Cell and Tissue Research. 2003;311:365–371. doi: 10.1007/s00441-002-0692-y. [DOI] [PubMed] [Google Scholar]
- 20.Rees LE, et al. Lifestyle factors influence immunology architecture of the human larynx. Clinical immunology. 2006;118:324–7. doi: 10.1016/j.clim.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich C, Jecker P, Tschernig T, Mann WJ. Presence of dendritic cells, T lymphocytes, macrophages, B lymphocytes and glandular tissue in the human fetal larynx. Acta Otolaryngologica. 2004;124:833–838. doi: 10.1080/00016480410018269. [DOI] [PubMed] [Google Scholar]
- 22.Barker E, et al. Early immunological changes associated with laryngeal transplantation in a major histocompatibility complex-matched pig model. Clinical and Experimental Immunology. 2006;146:503–508. doi: 10.1111/j.1365-2249.2006.03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inman CF, et al. Validation of computer-assisted, pixel-based analysis of multiple-colour immunofluorescence histology. Journal of immunological Methods. 2005;302:156–167. doi: 10.1016/j.jim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Inman C, Bailey JC, Cook S, Bailey M. Interations between immune cells and their microenvironment. Veterinary Immunology and Immunopathology. doi: 10.1016/j.vetimm.2007.07.007. (In press) [DOI] [PubMed] [Google Scholar]
- 25.http://rsb.info.nih.gov/ij/.
- 26.Birchall M, et al. The development of upper airway mucosal immune architecture depends on peri-natal bacterial colonisation. Clinical Otolaryngology. 2008;33:299. [Google Scholar]
- 27.Tanaka I, Suzuki K, Tanaka E, Baba S. Investigation of normal bacterial flora in the upper respiratory tract. Acta Otolaryngologica, Supplement. 1996;525:44–50. [PubMed] [Google Scholar]
- 28.Kizilay A, et al. Histopathologic examination for Helicobacter pylori as a possible etiopathogenic factor in laryngeal carcinoma. Chemotherapy. 2006;52:80–82. doi: 10.1159/000091727. [DOI] [PubMed] [Google Scholar]
- 29.Akbayir N, Basak T, Seven H, Sungun A, Erdem L. Investigation of Helicobacter pylori colonization in laryngeal neoplasia. European Archives of Otorhinolaryngology. 2005;262:170–172. doi: 10.1007/s00405-004-0794-0. [DOI] [PubMed] [Google Scholar]
- 30.Fang TJ, Lee LA, Li HY, Yang C, Huang CG. Helicobacter pylori colonization in the larynges of patients with hoarseness. Laryngoscope. 2008;118:389–393. doi: 10.1097/MLG.0b013e31815d8e2d. [DOI] [PubMed] [Google Scholar]
- 31.Rubin JS, Benjamin E, Prior A, Lavy J, Ratcliffe P. The prevalence of Helicobacter pylori infection in benign laryngeal disorders. Journal of Voice. 2002;16:87–91. doi: 10.1016/s0892-1997(02)00076-0. [DOI] [PubMed] [Google Scholar]
- 32.Borkowski G, et al. A possible role of Helicobacter pylori infection in the etiology of chronic laryngitis. European Archives of Otorhinolaryngology. 1997;254:481–482. doi: 10.1007/BF02439987. [DOI] [PubMed] [Google Scholar]
- 33.Jaspersen D, et al. Is chronic laryngitis associated with Helicobacter pylori? Results of a prospective study. Z Gastroenterology. 1998;36:369–372. [PubMed] [Google Scholar]
- 34.Rees LE, et al. Smoking influences the immunological architecture of the human larynx. Clinical Immunology. 2006;118:342–347. doi: 10.1016/j.clim.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Duflo S, et al. Differential gene expression profiling of vocal fold polyps and Reinke’s edema by cDNA microarray. Annals of Otology, Rhinology and Laryngology. 2006;115:703–14. doi: 10.1177/000348940611500910. [DOI] [PubMed] [Google Scholar]
- 36.Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngology Head Neck Surgery. 2000;123:385–388. doi: 10.1067/mhn.2000.109935. [DOI] [PubMed] [Google Scholar]
- 37.Karkos PD, Wilson JA. Empiric treatment of laryngopharyngeal reflux with proton pump inhibitors: a systematic review. Laryngoscope. 2006;116:144–148. doi: 10.1097/01.mlg.0000191463.67692.36. [DOI] [PubMed] [Google Scholar]
- 38.Rees LE, et al. The mucosal immune response to laryngopharyngeal reflux. American journal of respiratory and critical care medicine. doi: 10.1164/rccm.200706-895OC. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belafsky PC, Postma GN, Amin MR, Koufman JA. Symptoms and findings of laryngopharyngeal reflux. Ear Nose Throat Journal. 2002;81:10–13. [PubMed] [Google Scholar]
- 40.Tonti E, et al. NKT cell help to B lymphocytes can occur independently of cognate interaction. Blood. 2008 doi: 10.1182/blood-2008-06-166249. [DOI] [PubMed] [Google Scholar]
- 41.Fontana GA, et al. Coughing in laryngectomized patients. American Journal of Respiratory and Critical Care Medicine. 1999;160:1578–1584. doi: 10.1164/ajrccm.160.5.9901093. [DOI] [PubMed] [Google Scholar]