Abstract
The phenotypic progression to esophageal cancer is driven by an ongoing process of genomic instability constituting a number of clonal variants and leading to outgrowth of the “fittest” abnormal cell clones. Factors contributing to this process include exposure to chronic tissue damage, host susceptibilities, and alterations of molecular circuitries implicated in tissue homeostasis. Characterization of the host modifiers and molecular alterations will likely lead to the discovery of biomarkers useful for constructing stratified models defining cancer risk, allowing early detection, prediction of response to primary or secondary intervention, and prognostic evaluation of the disease. In addition, identification of key biologic pathways driving esophageal tumorigenesis will lead to development of new targeted interventions. The advent of increasingly sophisticated “omics” (ie, genomics, transcriptomics, proteomics, kinomics, pharmacogenomics), integration of systems biology, and expansion of biologic platforms bridging developmental physio-biology to cancer pathology constitute the backbone of novel tumor classifications and tailored therapies based on molecular signatures and profiles. Promising molecular targets, particularly those implicated in tissue homeostasis and stem cell maintenance, and their potential use in predictive models will be discussed.
ESOPHAGEAL CANCER: THE NEED FOR MOLECULAR TARGETS
Despite advances in diagnosis and multimodality therapies, esophageal cancer (EC) continues to carry a grim prognosis.1 In the past 2 decades, EC incidence has increased steadily, with a remarkable shift in histology from predominantly squamous to adenocarcinoma, and 5-year overall survival rate has stalled at approximately 14%.2,3 In the United States alone, more than 14,000 new EC cases were diagnosed in 2005; more than 90% of these patients will die of their disease.1 Reasons for such poor outcome include advanced stage at diagnosis, high risk of recurrence after definitive treatment, limited response to preoperative chemoradiation (CRT), and poor survival with palliative therapy.4–8 The natural history of EC can be defined by a biologic triad: aggressiveness, intrinsic resistance to CRT, and high metastatic potential. Thus, there is a critical need to discover molecular targets useful for diagnosis, prognosis, prediction of treatment response, and novel therapeutic interventions.
Several molecular markers, commonly found in other epithelial neoplasias, have been described for EC. These include markers of deregulated growth (ie, epidermal growth factor family such as HER2/- Neu and epidermal growth factor receptor [EGFR], and Ki67), cell cycle checkpoint controls (ie, p16INK4a, p21CIP/WAF1, p53, and cyclin D1), regulation of apoptosis (ie, p53, Blc2, and Bax), inflammation and angiogenesis (ie, cyclooxygenase-related pathways, and vascular endothelial growth factor [VEGF] family), motility and invasion (ie, TIMPS [tissue inhibitor of metalloproteinases] and E-cadherin). Other markers are associated with resistance to chemotherapy, including thymidylate synthetase, p-glycoprotein, glutathione S-transferase, and metallothionein-1. Most of these markers have failed to demonstrate prognostic value or predictivity of response (pathologic complete response [pathCR]) to preoperative CRT. Some of the markers, such as EGFR, HER2/Neu, VEGF, or Cox-2, are potential therapeutic targets for which biologic therapies are in phase I/II clinical trials for EC. Currently, however, clinical development of these biologics has not fulfilled its promise, primarily because molecular fingerprinting that would allow selection of sensitive tumor types is lacking. Future clinical success will require better understanding of the molecular makeup and biologic behavior of tumors, ultimately allowing optimal target choice, and multidrug combinations that also include “older chemotherapeutics.” Development of increasingly sophisticated high-throughput investigative platforms (ie, genomics, transcriptomics, proteomics, kinomics, genome-wide scanning for singlenucleotide polymorphisms or methylation) and expanding knowledge regarding tumor biology are providing novel paradigms for the design of individualized therapeutic programs based on biologic classifiers.
GENOMIC METHYLATION AND TRANSCRIPTIONAL PROFILES DEFINE CANDIDATE MARKERS FOR CRT RESPONSE
Two small, well designed studies have compared molecular signatures in CRTsensitive and -resistant EC. The first study, by Hamilton et al,9 used quantitative methylation-specific polymerase chain reaction (PCR) to investigate the silencing of 11 candidate genes in tumor tissue from 35 treatment-naive EC patients. Study results suggest that the number of methylated genes may discriminate between sensitive and resistant cancers, the latter harboring a significantly higher methylation degree. In addition, methylation occurring at the Reprimo gene, a regulator of the G2-M cell phase arrest through p53, is a promising candidate for integration in multimarker panels for predicting CRT response.
The second study, conducted by Luthra et al,10 used Affymetrix (Santa Clara, CA) U133A transcriptional profiling coupled with Ingenuity Systems (Redwood City, CA) pathways platform to identify molecular signatures in pretreatment biopsies of CRT-resistant and CRT-sensitive EC. Unsupervised hierarchical cluster analysis segregated cancers into two major molecular subtypes, types 1 and 2. The distinct expression profiles of the two subtypes suggest that one biologic entity of EC is less likely than the other to achieve pathCR. This study also identified more than 400 genes differentially expressed, which thus constitute potential candidates for investigation. Some of these markers will be discussed further.
Chromosome 1q21 Alterations as Potential Biomarkers for CRT Response and EC Progression
The profiling study by Luthra et al identified a significantly lower expression of S100A2 and SPRR3 genes in CRT-resistant cancers.10 Interestingly, both genes are located in an evolutionarily conserved genetic cluster, designated as the epidermal differentiation complex (EDC), at the 1q21 chromosomal band. Further characterization of expression levels of genes mapping within and in close proximity to EDC could define molecular subgroups of EC associated with varying response to CRT. This genetic region, encompassing 2 Mb of genomic DNA, harbors three gene families of approximately 43 genes involved in terminal squamous differentiation of human epidermis. Suppression of transcriptional programs of this chromosomal region has been associated with progression of Barrett’s metaplasia to cancer,11 and with histopathologic grade of squamous cell EC. Moreover, candidate genes in the region, such as esophagin12–14 and c1orf10,15 are being investigated as potential tumor suppressor genes important for EC progression. Thus, alterations of the 1q21 chromosomal region may define the biologic progression and responsiveness of EC to CRT.
Transcription Factor Kappa B (NF-κB) as a Biologic Classifier for CRT Response
Our pilot study comparing molecular signatures in CRT-sensitive and -resistant ECs10 indicated that several genes implicated in the upstream activation (ie, TRAF2, FOXO3) or downstream targets (MMP15, PAFD, ICAM) of NF-κB were differentially expressed toward an NF-κB–activated pathway in CRT nonresponders. Aberrant NF-κB activation is found in inflammatory disorders and cancer.16 NF-κB is a gatekeeper of critical biologic processes and can serve as a survival factor by preventing apoptosis in response to stress or insult.16,17 More important, NF-κB is associated with chemotherapy and radiotherapy resistance.18 NF-κB is activated in response to cytotoxic drugs, including topoisomerase inhibitors, vinca alkaloids, the platinum family, and taxanes. NF-κB is also a potential key signaling molecule in radiation resistance because ionizing radiation up-regulates expression and DNA binding activity of NF-κB.
To validate the preliminary micro-array results, we examined nuclear NF-κB in 80 patients who underwent preoperative CRT. NF-κB status was correlated with pathologic response to therapy (pathCR versus residual cancer [<pathCR]) and survival.19,20 Activated NF-κB prior to any therapy was associated with <pathCR; P = .006. Activated NF-κB in pre- and/or posttherapy cancer specimens was found in 45 (78%) of 58 patients with <pathCR vs. 2 (9%) of 22 patients achieving pathCR (P = .001). PathCR was seen in 43% of 46 patients with NF-κB–negative cancers and only 7% of 29 NF-κB–positive cancers. Most important, pretreatment NF-κB correlated with pathCR, suggesting the usefulness of this marker to assess likelihood of response to CRT. Further investigations are warranted to validate the predictive value of pretreatment NF-κB, and to define its potential use a as biomarker “classifier” for incorporation into clinical decision-making.
NF-κB Independently Predicts Survival and Potential Therapeutic Target
We also examined the effect of NF-κB status on overall (OS) and disease-free (DFS) survivals. At a median follow-up of 32 months, 25 (53%) of 47 patients with NF-κB–activated EC vs. 3 (9%) of 33 patients with NF-κB–negative EC had died. Pretreatment NF-κB activation was the only independent predictor of DFS (P = .01) and OS (P = .007) in a multivariate model. Moreover, NF-κB positivity in the residual resistant EC was also strongly predictive of worse outcome.19,20 These findings underscore the potential to incorporate NF-κB inhibitory strategies into EC management. A growing number of specific NF-κB–axis inhibitors are being developed, including IκB-α super repressor SN50, IKK selective inhibitors or p65 selective NF-κB decay RNAi.21 Future in-depth molecular biology studies will elucidate mechanisms of NF-κB activation in EC and help to characterize exploitable therapeutic targets.
CANCER STEM CELL-LIKE CELLS AND IMPLICATIONS FOR THERAPY
In recent years, the concept that cancer arises from and contains altered stem cells has gained acceptance.22–25 Studies in hematologic malignancies have demonstrated the existence of cancer stem cell-like cell (CSCLC) entities and have characterized several biologic markers for their detection.26 CSCLC share with normal stem cells the capacity to self-renew and can differentiate to give rise to phenotypically diverse cancer progenies.26 CSCLC are thought to be relatively quiescent, thus resistant to drugs and toxins. In the field of solid tumors, the origins and phenotypes of CSCLC remain mostly unknown and are actively debated, mainly because specific markers identifying these entities are lacking. However, the discovery that certain embryonic signaling pathways involved in adult stem cell maintenance (ie, Bmi-1, Notch, Wnt, Sonic Hedgehog) are aberrantly re-activated in solid tumors supports the hypothesis that deregulated CSCLC self-renewal and proliferation may be critical for solid tumor growth and maintenance.
The intrinsic biologic characteristics and functions of CSCLC are likely to affect the success of anti-neoplastic therapy. While CRT targets proliferating cells, it fails to eliminate the small population of CSCLC that can repopulate the tumor. Like normal stem cells that maintain tissue homeostasis, CSCLC exit cell cycle as the tumor volume enlarges and can escape cytotoxic effects of chemotherapy and radiation. Although CSCLC are relatively quiescent prior to treatment, they begin to proliferate when tumor volume decreases, a phenomenon known as “accelerated repopulation.” The number of CSCLC may represent an important rate-limiting factor for therapeutic efficacy. As the burden of CSCLC increases, (1) tumor burden increases along with continuous waves of accelerated repopulation, and (2) only a fraction of CSCLC participates in repopulation processes and is sensitive to therapy while the majority of SCLC remains quiescent in a state of intrinsic resistance to CRT.
ACTIVATION OF EMBRYONIC SONIC HEDGEHOG SIGNALING PATHWAY IN EC: THERAPEUTIC IMPLICATIONS
The Hedgehog (HH) signaling pathway is critical for growth and differentiation during embryonic development.27 Secreted hedgehog molecules (Sonic, Desert, and Indian) bind to and inhibit the cell surface receptor patched (PTCH). This physical inhibition relieves PTCH-mediated suppression of the transmembrane protein smoothened (SMO), leading to a cascade of intracellular events that cause activation (ie, proteolytic processing) and nuclear translocation of the Gli family of transcription factors (Gli-1, 2, and 3). Gli-1 is a strong positive regulator of HH pathway targets and is itself a transcriptional target of the mammalian HH pathway. Transcriptional targets of the oncoprotein Gli-1 include genes implicated in cell cycle control (ie, N-Myc, D- and E-type cyclins, or phosphatase CDC25B), cell adhesion, signal transduction, vascularization (ie, VEGF, platelet-derived growth factor [PDGF]) apoptosis (ie, Bcl2) and PTCH itself.28 Like many pathways implicated in embryonic development, the HH pathway is switched off during adulthood. After birth HH protein expression decreases and becomes restricted to specific areas in skin, blood, prostate, nervous system, and digestive tissues where it is involved in maintenance of the stem cell population and production of the progeny that will further differentiate in specialized cell lineages. Interestingly, the different HH ligand proteins, Sonic (SHH), Indian (IHH), and Desert (DHH), appear to control independent stem cells pools. Convincing evidence from studies in diverse cellular lineages shows that HH signaling promotes cellular proliferation by opposing signals to physiologic growth arrest. The association between the abnormally activated HH pathway and cancer was established by identification of heterozygote germline mutations affecting the membrane receptor PTCH, and resulting in abnormal activation of the HH pathway in basal cell carcinoma, rhabdomyosarcoma, and neural tumors.29 Recently, several studies have shown constitutive, liganddependent activation of HH signaling pathway in digestive tract cancers. In vitro studies of esophageal, biliary tract, stomach and pancreas malignancies show that cancer cell growth is mediated by ligand-dependent stimulation of HH signaling, by demonstrating growth inhibitory activity of HHneutralizing antibodies and growth-stimulatory activity of exogenously added SHH. Furthermore, treatment of adenocarcinoma xenografts with the SMO-inhibitory compound cyclopamine reduces proliferation rates, indicating that HH pathway activation may be essential for tumor growth maintenance. The majority of gastrointestinal (GI) tumors lack mutations in HH pathway genes, suggesting that the mechanism of pathway activation in GI tumors is distinct from that in neural and skin tumors, and further, it may represent a conserved mechanism for establishing niche-independent stem cancer cell proliferation.
In our pilot study of expressional profiling in EC,10 expression of the SHH ligand was significantly increased in pretreatment EC specimens of patients failing to achieve pathCR after preoperative CRT (unpublished observation). To determine if SHH signaling pathway activation was associated with CRT resistance, we measured post-treatment protein expression levels of cytoplasmic SHH and nuclear Gli-1 in EC specimens from 43 patients who received preoperative CRT.30 Thirty-six (83.7%) of the 43 resistant cancers had sustained activation of the SHH pathway, defined by presence of nuclear Gli-1 protein. Immunostaining was used for spatial localization of SHH and Gli-1 within the tumor. SHH staining was usually clustered in small patches surrounded by larger areas of Gli-1 expression, suggesting that regional activation of the HH signaling pathway follows a paracrine dynamic. Further, results showed that: (1) in EC xenografts, the temporal kinetics of SHH signaling preceded increases in proliferation biomarker expression and tumor size during tumor re-growth30; and (2) in EC cell lines, SHH pathway activity influences proliferation rates through upregulation of the G1-cyclin- Rb axis.30 In addition, blocking SHH signaling enhanced radiation cytotoxicity of EC cells. These results suggest that HH pathway activation may promote tumor repopulation after CRT and contribute to treatment resistance in EC. Ongoing studies are investigating SHH pathway alterations in pre-treatment EC specimens and their affect on CRT response and clinical outcome.
FUTURE PERSPECTIVES: MULTINETWORK MODELING TO IDENTIFY MOLECULAR CLASSIFIERS
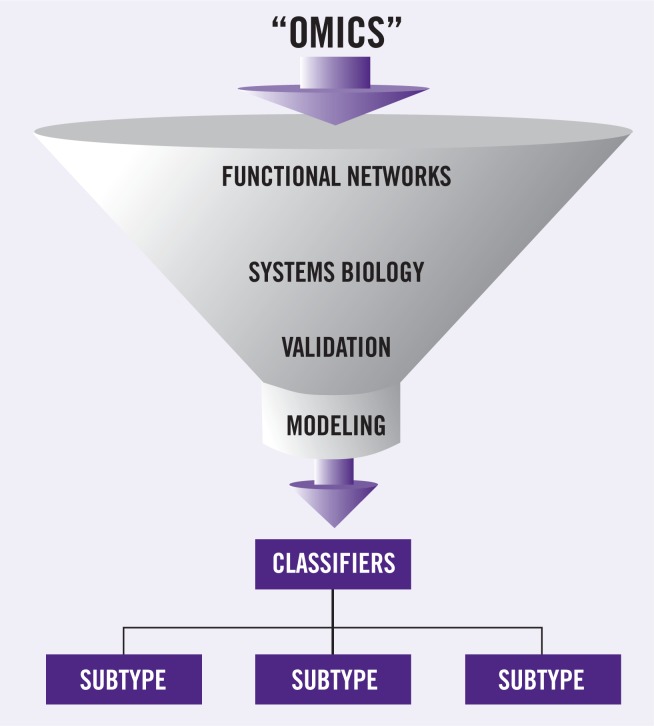
Close coordination of clinical, translational, and basic research avenues will be required to achieve individualized therapeutic programs as a standard of care for EC. Important steps in this process include the construction of functional and regulatory networks fed by the “omics” platforms, followed by integration into systems biologybased circuitries, which would then define candidate networks for drug design and nodal points potentially useful as biologic classifiers (Figure 1). Most of the challenges are still ahead, perhaps embedded in technical issues and our limited knowledge; however, to quote Albert Einstein: “Learn from yesterday, live for today, hope for tomorrow. The important thing is not to stop questioning”; and, to paraphrase another of his statements, not to let our technology exceed our humanity.
Figure 1.
The era of molecular oncology. Integration of high-throughput technologies, and functional biologic networks, followed by systematic validation of candidate markers, “funnels” into multi-parameters models of prediction and prognosis based on molecular classification of patients.
Acknowledgments
Supported in part by NIH-NCI RO1 DE13157-04, EDRN CA 86390, CCSG-GI Cancer Program CA 16672 and the University of Texas M. D. Anderson Esophageal Multidisciplinary Research Project Grant, Riverkreek Foundation, and the Dallas, Cantu, Smith, and Park Families.
Footnotes
Disclosures of Potential Conflicts of Interest
Dr. Izzo reports no potential conflicts of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Pickens A, Orringer MB. Geographical distribution and racial disparity in esophageal cancer. Ann Thorac Surg. 2003;76:S1367–S1369. doi: 10.1016/s0003-4975(03)01202-5. [DOI] [PubMed] [Google Scholar]
- 3.Pera M, Manterola C, Vidal O, et al. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92:151–159. doi: 10.1002/jso.20357. [DOI] [PubMed] [Google Scholar]
- 4.Hofstetter W, Swisher SG, Correa AM, et al. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236:376–384. doi: 10.1097/00000658-200209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol. 1993;11:1118–1123. doi: 10.1200/JCO.1993.11.6.1118. [DOI] [PubMed] [Google Scholar]
- 6.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 7.Kelsen DP. Multimodality therapy of esophageal cancer: an update. Cancer J. 2000;6(suppl 2):S177–S181. [PubMed] [Google Scholar]
- 8.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton JP, Sato F, Greenwald BD, et al. Promoter methylation and response to chemotherapy and radiation in esophageal cancer. Clin Gastroenterol Hepatol. 2006;4:701–708. doi: 10.1016/j.cgh.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Luthra R, Wu TT, Luthra MG, et al. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J Clin Oncol. 2006;24:259–267. doi: 10.1200/JCO.2005.03.3688. [DOI] [PubMed] [Google Scholar]
- 11.Kimchi ET, Posner MC, Park JO, et al. Progression of Barrett's metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res. 2005;65:3146–3154. doi: 10.1158/0008-5472.CAN-04-2490. [DOI] [PubMed] [Google Scholar]
- 12.Smolinski KN, Abraham JM, Souza RF, et al. Activation of the esophagin promoter during esophageal epithelial cell differentiation. Genomics. 2002;79:875–880. doi: 10.1006/geno.2002.6775. [DOI] [PubMed] [Google Scholar]
- 13.Kimos MC, Wang S, Borkowski A, et al. Esophagin and proliferating cell nuclear antigen (PCNA) are biomarkers of human esophageal neoplastic progression. Int J Cancer. 2004;111:415–417. doi: 10.1002/ijc.20267. [DOI] [PubMed] [Google Scholar]
- 14.Abraham JM, Wang S, Suzuki H, et al. Esophagin cDNA cloning and characterization: a tissue-specific member of the small prolinerich protein family that is not expressed in esophageal tumors. Cell Growth Differ. 1996;7:855– 860. [PubMed] [Google Scholar]
- 15.Xu Z, Wang MR, Xu X, et al. Novel human esophagus-specific gene c1orf10: cDNA cloning, gene structure, and frequent loss of expression in esophageal cancer. Genomics. 2000;69:322–330. doi: 10.1006/geno.2000.6344. [DOI] [PubMed] [Google Scholar]
- 16.Karin M, Cao Y, Greten FR, et al. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 19.Izzo JG, Malhotra U, Wu TT, et al. Association of activated transcription factor nuclear factor kappaB with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol. 2006;24:748–754. doi: 10.1200/JCO.2005.03.8810. [DOI] [PubMed] [Google Scholar]
- 20.Izzo JG, Correa AM, Wu TT, et al. Pretherapy nuclear factor-kappaB status, chemoradiation resistance, and metastatic progression in esophageal carcinoma. Mol Cancer Ther. 2006;5:2844–2850. doi: 10.1158/1535-7163.MCT-06-0351. [DOI] [PubMed] [Google Scholar]
- 21.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 22.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 23.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 24.Houghton J, Morozov A, Smirnova I, et al. Stem cells and cancer. Semin Cancer Biol. 2007;17(3):191–203. doi: 10.1016/j.semcancer.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 26.Lotem J, Sachs L. Epigenetics and the plasticity of differentiation in normal and cancer stem cells. Oncogene. 2006;25:7663–7672. doi: 10.1038/sj.onc.1209816. [DOI] [PubMed] [Google Scholar]
- 27.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 28.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 29.Pasca DM, Hebrok M. Hedgehog signaling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 30.Sims-Mourtada, Izzo JG, Apisarnthanarax S, et al. Hedgehog: an attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation response. Clin Cancer Res. 2006;12:6565–6572. doi: 10.1158/1078-0432.CCR-06-0176. [DOI] [PubMed] [Google Scholar]