Abstract
Src is a non-receptor protein tyrosine kinase that affects proliferation, angiogenesis, differentiation, migration, invasion, and regulation of apoptosis in colorectal cancer cells. Src activation is a frequent early epigenetic event in colorectal cancer, and is progressively increased in metastatic tumors as compared with primary tumors. Src has also been implicated as a component of epidermal growth factor receptor (EGFR) signal transduction. In particular, Src, as a mediator of receptor transactivation, can uniquely activate EGFR in the absence of EGFR ligand, and a Src inhibitor is synergistic with an EGFR monoclonal antibody in vitro in eliciting growth inhibition. Src inhibition is also synergistic in vivo with platinum chemotherapeutics, further increasing the potential of combination regimens with Src inhibitors. The current Src inhibitors in clinical trials are reviewed.
Despite recent advances in the treatment of metastatic colorectal cancer, this disease remains incurable for the vast majority of patients and improved therapies are needed. Targeted therapies including vascular endothelial growth factor (VEGF) receptor and epidermal growth factor receptor (EGFR) antibodies have been used with increasing clinical success. However, the mechanisms of activity and resistance to these agents are areas of active research, and these agents do not greatly prolong survival. One candidate molecule that may modulate the activity of such targeted therapy is the tyrosine kinase Src. Src has been demonstrated to be a relevant signaling kinase in metastatic colorectal cancer, and is involved in the EGFR signaling cascade. This article will review the structure and function of Src and EGFR in colorectal cancer and discuss the rationale for Src inhibition in combination with EGFR targeted therapies.
Src STRUCTURE AND EXPRESSION
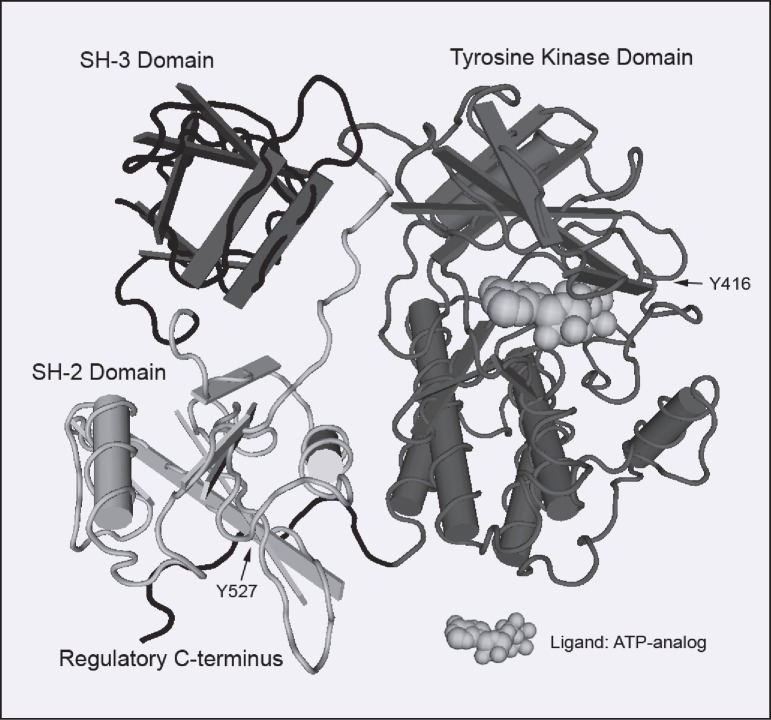
Src, a non-receptor protein tyrosine kinase, affects cellular proliferation, angiogenesis, differentiation, migration, invasion, and regulation of apoptosis. In addition, activation of Src may mediate resistance to chemotherapy in colorectal cancer. The structure of Src is defined by conserved Src homology domains (including SH2, SH3), and a protein tyrosine kinase (SH1) domain (Figure 1). Src is regulated, in part, by phosphorylation of the C-terminal tyrosine by C-terminal Src kinase (CSK), which results in an inactive conformation. CSK has been shown to be a critical regulator of Src activity and to be downregulated early in carcinogenesis.1 In contrast, phosphorylation in the loop of the kinase domain increases Src activity. Src is myristoylated at the N terminus, which is a necessary step for localization to the cellular membrane. In this location, Src has been shown to interact with a number of structural and signaling proteins through its SH2 and SH3 domains.
Figure 1.
Structure of Src in its active conformation with ATP-analog ligand (rendered in Cn3D based on structure of Xu et al40).
The extended Src family of non-receptor protein tyrosine kinases includes Src, Fyn, Lyn, Yes, Lck, Hck, and Blk. These Src family members have varying degrees of expression in epithelial malignancies.2 The Src oncogene is the archetypal member of the family and was initially discovered early in the 20th century through research on the Rous sarcoma virus. Because of this early identification and subsequent work, Src has been considered the prototypical oncogene and hence has received considerable study for its ability to provide insights into malignant transformation.
Src expression has been shown to be increased in approximately 80% of colorectal cancer specimens compared with normal colonic epithelium.3 Colorectal metastases to lymph nodes and liver demonstrate increased Src activity levels compared to primary colon tumors.4,5 Independent of the stage of disease, increased Src activity levels have also been associated with poor prognosis.6,7 Src activity is increased through association with a variety of upstream components but is not mutated in epithelial cancers.8 Instead, its activation predominantly results from increased transcription, genetic alterations, and activated receptor tyrosine kinases.9
Src FUNCTION
Src is a non-receptor tyrosine kinase that acts as a recruiter of intracellular signaling complexes which bind through the SH2 and SH3 domains. Src is a key element in growth factor receptor signaling transduction and cytoskeleton arrangement, although its effects extend to many tumorigenesis-related processes including metastasis, invasion, adhesion, migration, survival, angiogenesis, and differentiation.10,11
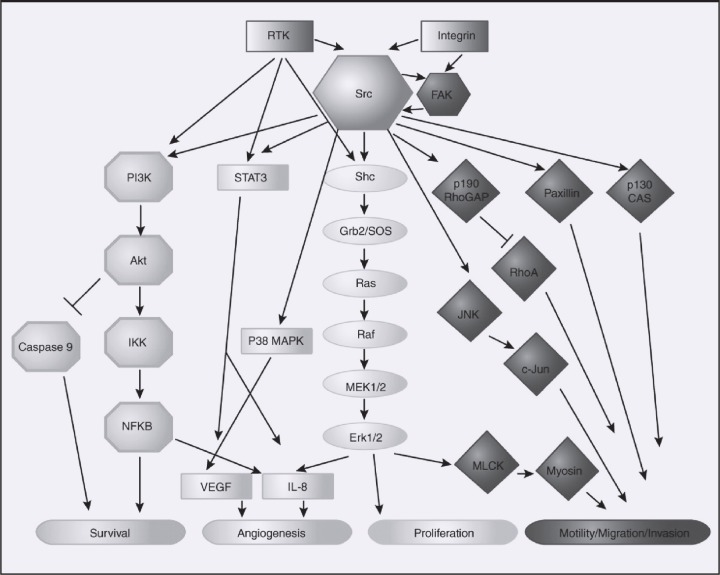
An overview of signaling cascades demonstrates that Src affects survival through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, angiogenesis by VEGF and interleukin 8 (IL-8), and migration and invasion through a variety of pathways involving focal adhesion kinase (FAK), paxillin, and JUN N-terminal kinase (JNK) (Figure 2). Src has also been shown to play a role in mitogen-activated protein kinase (MAPK) signaling.
Figure 2.
Src pathways. Reprinted with permission from Summy et al and the American Association for Cancer Research.11 Abbreviations: FAK = focal adhesion kinase; JNK = JUN N-terminal kinase; MAPK = mitogen-activated protein kinase; RTK = receptor tyrosine kinase (including epidermal growth factor receptor); VEGF = vascular endothelial growth factor.
Signaling through the PI3K/Atk pathway has been demonstrated, as Src family members can directly bind to PI3K through the SH3 domain of Src, leading to PI3K activation.12 This Src-mediated PI3K activation results in both increased cyclin D1 expression, which promotes mitogenesis, and increased Bcl-xL expression, which inhibits apoptosis.13,14 In addition, antiapoptotic effects have been attributed to Src through increased Stat 3 activation, upregulation of the antiapoptotic molecule Bcl-xL, and inhibition of caspase 8.15–17 Inhibition of the MAPK pathway by Src has been demonstrated in multiple models. Treatment with small interfering (si)RNA to Src resulted in decreased phosphorylation of MAPK in vitro and in pancreatic murine models, with reduction in tumor growth.18 In summary, Src has prosurvival effects through several pathways relevant to the malignant phenotype.
Angiogenesis is also affected by Src activity. Establishment of colon cancer cell lines with siRNA to Src demonstrated a decrease in VEGF mRNA expression proportional to the decrease in Src kinase activity.19 Cells treated with Src siRNA had a less than 2-fold increase in VEGF expression under hypoxic conditions compared with a greater than 50-fold increase in VEGF expression in the parental cell line. This effect was also seen in xenograft models with a decrease in tumor-associated vascularity.20 In addition to VEGF, the angiogenic factor IL-8 has been shown to be significantly decreased by inhibition of Src expression, with corresponding inhibition of angiogenesis in in vivo models.21
In addition, Src has been shown to play a role in migration and adhesion. FAK, which is closely associated with Src, has been associated with invasion in several studies.8,22 Src has been shown to be a requirement for the adhesion turnover required for cell migration. VEGF has also been shown to activate Src, resulting in enhanced cellular migration independent of its effect on endothelial cell growth.9
EGFR SIGNAL TRANSDUCTION
EGFR is a member of the ErbB family of receptor tyrosine kinases. The receptor is kept in the inactive form by steric changes in the carboxy terminus. Ligand binding causes a conformational change in EGFR, which induces receptor homodimerization or heterodimerization with other ErbB family members. With dimerization, the receptors can transphosphorylate tyrosine and change to an active conformation of the kinase domain, allowing subsequent downstream phosphorylation of substrates. EGFR is recognized as a relevant pathway in colon cancer. EGFR inhibitors downregulate survival mechanisms by inhibiting both the Ras/MAPK mitogenic pathway and antiapoptotic pathways such as Bcl-2 and NF-kappa B (NF-κB).23
EGFR TRANSACTIVATION BY Src
Importantly, EGFR tyrosine kinase can be activated without the requirement for ligand—a mechanism termed transactivation.24,25 Several additional receptors have been shown to play a role in EGFR transactivation, although the common effector appears to be Src.
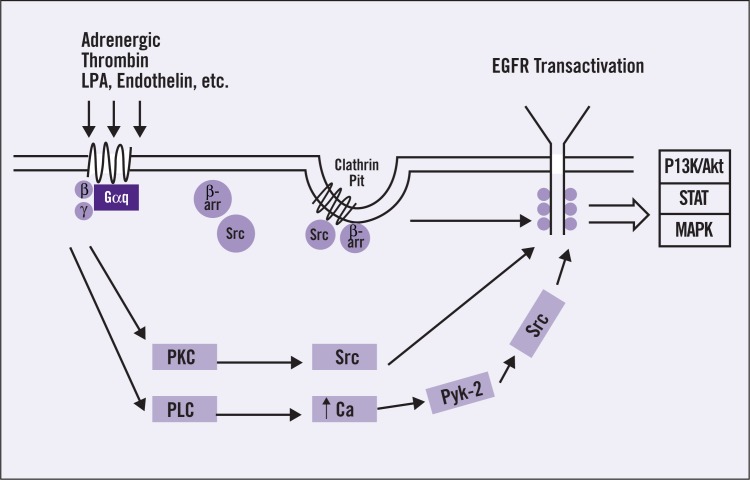
One EGFR transactivation pathway involves integrin. Integrin, by interacting with receptor tyrosine kinases such as EGFR, leads to a clustering and phosphorylation of EGFR. Src is at least one non-receptor tyrosine kinase that seems to be required for this integrin-dependent EGFR phosphorylation.26 G-protein–coupled receptors (GPCR) have also been associated with EGFR transactivation. It has been shown that Src is coupled to nearly all G-protein receptors that lead to EGFR phosphorylation.27 The result is activation of various downstream pathways, including MAPK signaling, in an EGFR-dependent manner.28 Ligands of these GPCRs include acetylcholine, adrenergic, thrombin, and endothelin, which have all been demonstrated to increase EGFR phosphorylation.24 G-protein receptors have been shown to require an intact EGFR to transduce a mitogenic response. Src has clearly been implicated as a signal transduction element between this GPCR and EGFR phosphorylation. The result is a series of signaling pathways that link multiple receptors to EGFR-dependent signaling using Src as an intermediary in EGFR phosphorylation (Figure 3).
Figure 3.
Src transactivation. Abbreviations: β-arr = beta-arrestin; EGFR = epidermal growth factor receptor; MAPK = mitogen-activated protein kinase. Adapted from Edwin et al,25 and Luttrell et al.41
In colorectal cancer cell lines, EGFR overexpression is correlated with Src activation.29 Through transactivation, as described above, it appears that the effect of EGFR on tumorigenesis extends beyond ligandmediated effects. Data suggest that transactivation leads to phosphorylation at unique sites of the EGFR, subsequently leading to signal transduction through alternate pathways such as STAT5b pathway.30 Mutation of these Src-specific tyrosine residues on EGFR diminishes EGF-induced mitogenesis.
In summary, these findings imply that Src, as a mediator of other receptor signaling and through constitutive activation, can uniquely activate EGFR in the absence of EGFR ligand. This result therefore implies that activation of the EGFR pathway by Src can occur despite the use of EGFR antibodies such as cetuximab.
EGFR REGULATION AND Src
Degradation of EGFR may also be affected by Src activity. Src inactivates Cbl, a kinase responsible for the ubiquitination and degradation of ligand-activated receptors including EGFR. By promoting destruction of Cbl, Src enables EGFR to evade degradation.31 In addition, EGFR receptor internalization and endosomal signaling is increased by Src phosphorylation of clathrin.32 These changes in EGFR degradation and recycling leads to increased EGFR signaling in the presence of activated Src.
COMBINED EGFR AND Src INHIBITION
Overexpression of Src has been shown to increase DNA synthesis in response to EGF.33 Conversely, EGF-induced DNA synthesis can be inhibited by the overexpression of an inactivated form of Src.34,35 Overexpression of Src and EGFR led to increased EGF-dependent tumor formation in nude mice.36 In summary, EGFR and Src may potentiate receptor-mediated tumorigenesis, suggesting a role for individual or combined inhibition of these targets.
In a series of in vitro experiments, we have shown that a monoclonal antibody to EGFR combined with a Src tyrosine kinase inhibitor results in synergistic effects in cell growth and colony formation assays. This synergistic effect appears to be mediated through an increase in apoptosis. This is associated with an additive inhibition of the Akt pathways with the combination.37
Src IN COMBINATION WITH CHEMOTHERAPY
Src inhibition may play a therapeutic role in combination with chemotherapy. Silencing RNA to Src reduced total Src levels in vitro and resulted in increased sensitivity to oxaliplatin. In a murine colon cancer model, treatment with oxaliplatin and a Src inhibitor reduced tumor volume and weight more than that achieved with either agent alone. Oxaliplatin treatment also led to a more than 3-fold increase in activated Src levels in these murine tumors. Treatment with a Src inhibitor decreased activated Src, and this low level of Src activation was maintained even after oxaliplatin treatment.9 This effect appears to be specific for oxaliplatin as there was no apparent interaction between Src inhibition and treatment with SN-38, the active form of irinotecan.38
Src has also been shown to mediate effects of cisplatin. In cells that had high Src activity levels, a Src inhibitor sensitized cells to cisplatin-induced apoptosis. The result was a lower level of antiapoptotic Bcl-xL and increased cisplatin sensitivity.15
ONGOING CLINICAL TRIALS
Recognition of the importance of Src in colorectal cancer has led to several trials with Src tyrosine kinase inhibitors. Src tyrosine kinase inhibitors in clinical development or approved for other indications include AZD0530, dasatinib, and SKI-606 (for review, see Ref. 39). Phase I studies have reported the unique toxicities of pleural effusions, and less commonly, pericardial effusions, and rare atypical pulmonary infiltrates. Nevertheless, these therapies are well tolerated in most patients.Table 1 summarizes selected trials of Src inhibitors in gastrointestinal malignances. A phase II trial of AZD0530 as a single agent for second-line therapy in patients with metastatic colorectal cancer is ongoing at M. D. Anderson Cancer Center. A trial of combination therapy using dasatinib, cetuximab (a monoclonal antibody to EGFR), fluorouracil, and oxaliplatin has been initiated at M. D. Anderson Cancer Center in patients with metastatic colorectal cancer. This trial was developed based on the demonstrated in vitro synergy between an EGFR antibody and a Src inhibitor, and additional in vivo data of oxaliplatin combined with a Src inhibitor. The study will include correlative end points in an attempt to optimize the Src inhibitor dose in the regimen.
Table 1.
Selected trials of Src inhibitors in gastrointestinal malignancies.
| Agents | Phase and Setting | Location |
|---|---|---|
| Dasatinib | Phase I, advanced solid tumors | M. D. Anderson Cancer Center, Houston, TX, USA |
| Dasatinib, gemcitabine | Phase I, advanced solid tumors | M. D Anderson Cancer Center, Houston, TX, USA |
| Dasatinib, cetuximab | Phase I, advanced solid tumors | University of Pittsburgh, Pittsburgh, PA, USA |
| Dasatinib, cetuximab, 5-fluorouracil, oxaliplatin | Phase IB, refractory colorectal cancer | M. D. Anderson Cancer Center, Houston, TX, USA |
| Dasatinib | Phase II, refractory colorectal cancer | University of Chicago, Chicago, IL, USA |
| AZD0530 | Phase II, refractory colorectal cancer | M. D. Anderson Cancer Center, Houston, TX, USA |
| AZD0530, gemcitabine | Phase II, locally advanced or metastatic pancreatic cancer | Princess Margaret Hospital, Toronto, ON, Canada |
CONCLUSION
In summary, data indicate that Src and its downstream signaling effects are important in colorectal cancer. In addition, the complicated interactions between EGFR and Src contribute to the tumorigenic phenotype. Finally, Src has a role as a cytotoxic chemotherapy sensitizer. Based on preclinical data and early clinical trial results, ongoing studies are evaluating combined Src and EGFR inhibitors in conjunction with systemic chemotherapy in colorectal cancer.
Footnotes
Disclosures of Potential Conflicts of Interest
Dr. Kopetz has received research support from Bristol-Myers Squibb.
REFERENCES
- 1.Kunte DP, Wali RK, Koetsier JL, et al. Down-regulation of the tumor suppressor gene C-terminal Src kinase: an early event during premalignant colonic epithelial hyperproliferation. FEBS Lett. 2005;579:3497–3502. doi: 10.1016/j.febslet.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch CL, Smith-Windsor EL, Bonham K. Src family kinase members have a common response to histone deacetylase inhibitors in human colon cancer cells. Int J Cancer. 2006;118:547–554. doi: 10.1002/ijc.21383. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Bolen JB, Veillette A, Schwartz AM, et al. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci U S A. 1987;84:2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Termuhlen PM, Curley SA, Talamonti MS, et al. Site-specific differences in pp60c-src activity in human colorectal metastases. J Surg Res. 1993;54:293–298. doi: 10.1006/jsre.1993.1046. [DOI] [PubMed] [Google Scholar]
- 6.Dehm SM, Bonham K. SRC gene expression in human cancer: the role of transcriptional activation. Biochem Cell Biol. 2004;82:263–274. doi: 10.1139/o03-077. [DOI] [PubMed] [Google Scholar]
- 7.Aligayer H, Boyd DD, Heiss MM, et al. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344–351. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- 8.Lee F, Lombardo L, Camuso A, et al. BMS-354825 potently inhibits multiple selected oncogenic tyrosine kinases and possesses broad-spectrum antitumor activities in vitro and in vivo. 2005 AACR Annual Meeting; Anaheim, CA. Apr 16–20, 2005. (abstr 159) [Google Scholar]
- 9.Lesslie DP, III, Parikh NU, Shah A, et al. Combined activity of dasatinib (BMS-354825) and oxaliplatin in an orthotopic model of metastatic colorectal carcinoma. 2006 AACR Annual Meeting; Washington, DC. Apr 1–5, 2006. (abstr 1114) [Google Scholar]
- 10.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 11.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12:1398–1401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 12.Pleiman CM, Hertz WM, Cambier JC. Activation of phosphatidylinositol-3’ kinase by Src-family kinase SH3 binding to the p85 subunit. Science. 1994;263:1609–1612. doi: 10.1126/science.8128248. [DOI] [PubMed] [Google Scholar]
- 13.Castoria G, Migliaccio A, Bilancio A, et al. PI3-kinase in concert with Src promotes the Sphase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 2001;20:6050–6059. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chellaiah M, Fitzgerald C, Alvarez U, et al. c-Src is required for stimulation of gelsolin-associated phosphatidylinositol 3-kinase. J Biol Chem. 1998;273:11908–11916. doi: 10.1074/jbc.273.19.11908. [DOI] [PubMed] [Google Scholar]
- 15.Karni R, Levitzki A. pp60(cSrc) is a caspase-3 substrate and is essential for the transformed phenotype of A431 cells. Mol Cell Biol Res Commun. 2000;3:98–104. doi: 10.1006/mcbr.2000.0197. [DOI] [PubMed] [Google Scholar]
- 16.Bromberg JF, Horvath CM, Besser D, et al. Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cursi S, Rufini A, Stagni V, et al. Src kinase phosphorylates Caspase-8 on Tyr380: a novel mechanism of apoptosis suppression. EMBO J. 2006;25:1895–1905. doi: 10.1038/sj.emboj.7601085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summy JM, Trevino JG, Lesslie DP, et al. Inhibition of c-Src expression and kinase activity inhibit progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. 2006 AACR Annual Meeting; Washington, DC. Apr 1–5, 2006. (abstr 1198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summy JM, Trevino JG, Baker CH, et al. c-Src regulates constitutive and EGF-mediated VEGF expression in pancreatic tumor cells through activation of phosphatidyl inositol-3 kinase and p38 MAPK. Pancreas. 2005;31:263–274. doi: 10.1097/01.mpa.0000178280.50534.0c. [DOI] [PubMed] [Google Scholar]
- 20.Ellis LM, Staley CA, Liu W, et al. Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-src. J Biol Chem. 1998;273:1052–1057. doi: 10.1074/jbc.273.2.1052. [DOI] [PubMed] [Google Scholar]
- 21.Trevino JG, Summy JM, Gray MJ, et al. Expression and activity of Src regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res. 2005;65:7214–7222. doi: 10.1158/0008-5472.CAN-04-3858. [DOI] [PubMed] [Google Scholar]
- 22.Brunton VG, Ozanne BW, Paraskeva C, et al. A role for epidermal growth factor receptor, c-Src and focal adhesion kinase in an in vitro model for the progression of colon cancer. Oncogene. 1997;14:283–293. doi: 10.1038/sj.onc.1200827. [DOI] [PubMed] [Google Scholar]
- 23.Camp ER, Summy J, Bauer TW, et al. Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clin Cancer Res. 2005;11:397–405. [PubMed] [Google Scholar]
- 24.Carpenter G. Employment of the epidermal growth factor receptor in growth factor-independent signaling pathways. J Cell Biol. 1999;146:697–702. doi: 10.1083/jcb.146.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwin F, Wiepz GJ, Singh R, et al. A historical perspective of the EGF receptor and related systems. Methods Mol Biol. 2006;327:1–24. doi: 10.1385/1-59745-012-x:1. [DOI] [PubMed] [Google Scholar]
- 26.Moroni M, Veronese S, Benvenuti S, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6:279–286. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]
- 27.Barber TD, Vogelstein B, Kinzler KW, et al. Somatic mutations of EGFR in colorectal cancers and glioblastomas. N Engl J Med. 2004;351:2883. doi: 10.1056/NEJM200412303512724. [DOI] [PubMed] [Google Scholar]
- 28.Daub H, Weiss FU, Wallasch C, et al. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 29.Osherov N, Levitzki A. Epidermal-growth-factordependent activation of the src-family kinases. Eur J Biochem. 1994;225:1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- 30.Kloth MT, Laughlin KK, Biscardi JS, et al. STAT5b, a mediator of synergism between c-Src and the epidermal growth factor receptor. J Biol Chem. 2003;278:1671–1679. doi: 10.1074/jbc.M207289200. [DOI] [PubMed] [Google Scholar]
- 31.Bao J, Gur G, Yarden Y. Src promotes destruction of c-Cbl: implications for oncogenic synergy between Src and growth factor receptors. Proc Natl Acad Sci U S A. 2003;100:2438–2443. doi: 10.1073/pnas.0437945100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn S, Lucaveche CL, Reedy MC, et al. Srcdependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J Biol Chem. 2002;277:26642–26651. doi: 10.1074/jbc.M201499200. [DOI] [PubMed] [Google Scholar]
- 33.Twamley-Stein GM, Pepperkok R, Ansorge W, et al. The Src family tyrosine kinases are required for platelet-derived growth factormediated signal transduction in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1993;90:7696–7700. doi: 10.1073/pnas.90.16.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson LK, Luttrell DK, Parsons JT, et al. pp60csrc tyrosine kinase, myristylation, and modulatory domains are required for enhanced mitogenic responsiveness to epidermal growth factor seen in cells overexpressing c-src. Mol Cell Biol. 1989;9:1536–1544. doi: 10.1128/mcb.9.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roche S, Koegl M, Barone MV, et al. DNA synthesis induced by some but not all growth factors requires Src family protein tyrosine kinases. Mol Cell Biol. 1995;15:1102–1109. doi: 10.1128/mcb.15.2.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maa MC, Leu TH, McCarley DJ, et al. Potentiation of epidermal growth factor receptormediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci U S A. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kopetz S, Wu J, Johnson F, et al. Anti-tumor effects of combination therapy with anti-EGFR and anti-Src therapy in colorectal cancer. 2006 AACR Molecular Targets Meeting; Washington DC. Sep, 2006. (abstr B51) [Google Scholar]
- 38.Griffiths GJ, Koh MY, Brunton VG, et al. Expression of kinase-defective mutants of c-Src in human metastatic colon cancer cells decreases Bcl-xL and increases oxaliplatin- and Fas-induced apoptosis. J Biol Chem. 2004;279:46113–46121. doi: 10.1074/jbc.M408550200. [DOI] [PubMed] [Google Scholar]
- 39.Lee D, Gautschi O. Clinical development of SRC tyrosine kinase inhibitors in lung cancer. Clin Lung Cancer. 2006;7:381–384. doi: 10.3816/clc.2006.n.020. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Yu Y, Marciniak D, et al. Epidermal growth factor receptor (EGFR)-related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol Cancer Ther. 2005;4:435–442. doi: 10.1158/1535-7163.MCT-04-0280. [DOI] [PubMed] [Google Scholar]
- 41.Luttrell LM, Ferguson SS, Daaka Y, et al. Betaarrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]