Abstract
In a preliminary screen, Aaptos aaptos showed significant cytotoxic activity towards a panel of cell lines and was thus subjected to bioassay-guided isolation of the bioactive constituents. In addition to the known aaptamine, two new derivatives of the alkaloid were isolated from the bioactive chloroform fraction of the crude methanolic extract. Detailed analysis by NMR and mass spectroscopy enabled their identification to be 3-(phenethylamino)demethyl(oxy)aaptamine and 3-(isopentylamino)demethyl(oxy) aaptamine. The cytotoxic activities of the three alkaloids were further evaluated against CEM-SS cells.
Keywords: Marine sponge, Aaptos aaptos, aaptaminoids, alkaloids, cytotoxicity
Introduction
Marine sponges have received a lot of research attention in the last decade and have been shown to be a prolific source of novel chemicals with promising therapeutic potentials [1–4]. The genus Aaptos in particular have yielded a group of 1H-benzo[d,e][1,6]-naphthyridine alkaloids, known collectively as aaptamines. Aaptamine [5,6] the first isolated alkaloid of this series, is a useful chemotaxonomic marker for sponges of the order Hadromerida [7] although it has also been found to occur in a sponge of the order Haplosclerida [8]. Several members of this class of compounds have been reported to show antitumour properties [9,10], antiHIV [11], mycobacterial [11], cardiac activity [12], and more recently, sortase A inhibitory [13] and antidepressant activities [14].
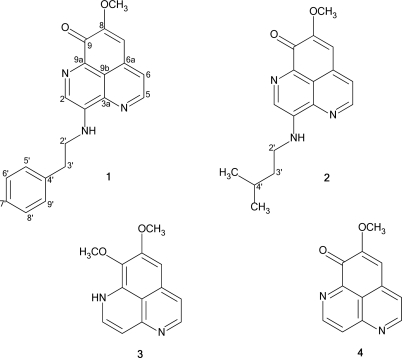
In our search for pharmacologically active substances from marine organisms, we screened a number of marine sponges for cytotoxic activity against a panel of cell lines consisting of HL-60 (promyelocytic leukemia), CEM-SS (T-lymphoblastic leukemia), MCF-7 (breast cancer), HeLa (cervical cancer), HT-29 (colon cancer) and L929 (murine fibrosarcoma from mouse). In our screen, the methanolic extract of Aaptos aaptos showed significant cytotoxic activity towards all the cell lines. Bioassay guided fractionation of the extract led us to the isolation of two new 9H-benzo[d,e][1,6]-naphthyridine alkaloids, 1 and 2, in addition to the known aaptamine (3). Structural elucidation of these alkaloids and their cytotoxic activity against CEM-SS cell line are described herein.
Results and Discussion
The methanolic extract of Aaptos aaptos was cytotoxic against all the cell lines tested, with CD50 values ranging from 3.2 to 24.1 μg/ml (Table 1). Bioassay guided solvent fractionation of the crude methanolic extract of A. aaptos retained a maximum of activity in the chloroform soluble fraction. Chromatographic separation of the active fraction using gel filtration, normal phase chromatography and semi-preparative reverse phase HPLC, yielded small amounts of the two new aaptaminoids, 1 and 2, in addition to aaptamine [5], 3, as the major constituent. The structures of the two alkaloids were elucidated based on NMR and mass spectroscopic methods.
Table 1.
Cytotoxicity of crude methanolic extract and isolated compounds from Aaptos aaptos on cancer cell lines.
| Sample | CD50 (μg/ml)a |
|||||
|---|---|---|---|---|---|---|
| HL-60 | CEM-SS | MCF-7 | HeLa | HT-29 | L929 | |
| Crude methanolic extract | 9.45 ± 0.36 | 5.40 ± 0.16 | 8.00 ± 0.08 | 22.80 ± 0.10 | 24.10 ± 0.90 | 3.20 ± 0.25 |
| Compound 1 | nd | 5.32 ± 0.27 | nd | nd | nd | nd |
| Compound 2 | nd | 6.73 ± 0.35 | nd | nd | nd | nd |
| Aaptamine | nd | 15.03 ± 0.08 | nd | nd | nd | nd |
Results are expressed as IC50 values (μg/ml) ±SD of three experiments; nd: not determined. HL-60 (promyelocytic leukemia), CEM-SS (T-lymphoblastic leukemia), MCF-7 (breast cancer), HeLa (cervical cancer), HT-29 (colon cancer), L929 (murine fibrosarcoma from mouse).
The ESI-MS (positive ion mode) of 1 exhibited [M+Na]+ and [M+H]+ pseudomolecular ion peaks at m/z 354.20 and 332.07, respectively. The molecular formula was determined by HR-ESIMS data (m/z 332.1291), to be C20H17N3O2 (calc. 332.1321). In the 1H NMR spectra, the signals observed at δH 8.76 (d, J 4.5), 7.47 (d, J 4.5), 6.63 (s) and 8.42 (s) were characteristic of the coupled protons H-5 and H-6, the lone proton H-7, and H-2 of the aaptamines [8], suggesting a benzo[de][1,6]naphthyridine skeleton. Unlike the other aaptaminoids which have been thus far reported, H-2 appeared as a singlet, thus indicating that C-3 was substituted. The 1H NMR spectra also exhibited a signal for only one methoxyl group, observed at δ 3.99 (s), which could be assigned to either position 8 or 9. However, the 13C NMR also exhibited a carbonyl signal at δc 176.3 which is consistent with the characteristic C-9 carbonyl observed in demethyl(oxy)aaptamine (4), previously isolated from the Okinawan Aaptos aaptos [6]. Careful analysis of the 1H-1H COSY, HSQC and HMBC correlations, as summarized in Table 2 further confirmed the placement of the carbonyl carbon on position 9 and substitution on C-3.
Table 2.
NMR data for compounds 1 and 2 recorded in CDCl3 (500MHz)a.
| Position | Compound 1
|
Compound 2
|
||||||
|---|---|---|---|---|---|---|---|---|
| δH | δC (mult) | HMBC
|
δH | δC (mult) | HMBC
|
|||
| 2J | 3J | 2J | 3J | |||||
| 2 | 8.42 s | 129.8 (CH) | 3 | 3a, 9a | 8.40 s | 129.8 (CH) | 3 | 3a, 9a |
| 3 | - | 144.1 (C) | - | - | - | 144.4 (C) | - | - |
| 3a | - | 136.5 (C) | - | - | - | 136.5 (C) | - | - |
| 5 | 8.76 d (4.5) | 151.0 (CH) | 6 | 3a | 8.78 d (4.5) | 151.0 (CH) | 6 | 3a |
| 6 | 7.47 d (4.5) | 121.8 (CH) | 5 | 9b, 7 | 7.48 d (4.5) | 121.8 (CH) | 5 | 9b, 7 |
| 6a | - | nd | - | - | - | nd | - | - |
| 7 | 6.63 s | 106.5 (CH) | 8 | 9, 6, 9b | 6.63 s | 106.3 (CH) | 8 | 9, 6, 9b |
| 8 | - | 158.1 (CH) | - | - | - | 158.2 (CH) | - | - |
| 8-OCH3 | 3.99 s | 56.2 (CH3) | - | 8 | 3.99 s | 56.2 (CH3) | - | 8 |
| 9 | - | 176.3 (C) | - | - | - | 176.2 (C) | - | - |
| 9a | - | 134.9 (C) | - | - | 8.40 s | 134.6 (C) | - | - |
| 9b | - | 118.1 (C) | - | - | - | 118.1 (C) | - | - |
| 1’ | 7.03 bt (5.6) | - | - | - | 6.93 t | - | - | - |
| 2’ | 3.85 q (6.8) | 44.3 (CH2) | 3’ | 4’, 3 | 3.59 q | 41.2 (CH2) | 3’ | 4’, 3 |
| 3’ | 3.15 t (6.8) | 35.5 (CH2) | 4’, 2’ | 5’/9’ | 1.77 q | 38.1 (CH2) | 2’, 4’ | 5’/6’ |
| 4’ | - | 138.1 (C) | - | - | 1.87 m | 26.0 (C) | 5’/6’ | - |
| 5’/9’ | 7.31 m | 129.1* (CH) | - | 3’,7’ | - | - | - | - |
| 6’/8’ | 7.39 dd (7.8, 7.6) | 129.0* (CH) | - | 4’, 5’/9’ | - | - | - | - |
| 7’ | 7.31m | 127.2 (CH) | - | - | - | - | - | - |
| #CH3 | 1.05 d (7.0) | 22.6 (5’, 6’) (CH3) | 4’ | 3’, 5’/6’ | ||||
J values are in parentheses and reported in Hz; chemical shifts are given in ppm; *,** are interchangeable; # Carbons 5’& 6’ are equivalent; nd: δc not determined since no H-C correlations could be seen in the HMBC spectrum.
The remaining aromatic protons at δH 7.39 (t, 2H) and 7.31 (d, 3H, J 7.5) were those of a monosubstituted benzene ring, which must thus be part of the substituent on C-3. This was supported by LC-MS/MSn experiments, where the MS/MS fragmentation of both the [M+Na]+ and the [M+H]+ ions, respectively, gave daughter ions at m/z 263 and 241 for the loss of 91 amu, indicative of the loss of a tropilium ion C7H7+. Another spin system, made up of a deshielded 1H broad triplet at δ 7.03, a 2H quartet at δ 3.85 and a 2H triplet at δ 3.15, was also evident from the 1H NMR spectra. The latter two signals were due to a pair of methylene groups, adjacent to each other and directly attached to the carbons at δc 44.3 and 35.5, respectively, based on an HSQC experiment. However, the 1H triplet at δ 7.03 was not directly attached to any carbon and based on its highly deshielded nature, the proton was deduced to be an NH proton, giving the spin system –NH-CH2-CH2- which was also evident from the 1H-1H COSY spectrum. From the COSY correlations the protons could be assigned to H-1’ (δ 7.03) correlated to H2-2’ (δ 3.85) which was in turn correlated to H2-3’ (δ 3.15). Careful analysis of the correlations observed in the HSQC and HMBC spectra connected the ethylamino spin system to the monosubstituted benzene ring. A 3J correlation from H2-2’ to the quaternary carbon at δc 144.1 (C-3) connected the 2-phenethylamino substituent to the main benzo[de][1,6]naphthyridine moiety. Based on these data, the structure of compound 1 was assigned as 3-(phenethylamino)demethyl(oxy)aaptamine.
The ESI-MS (positive ion mode) of 2 exhibited an [M+Na]+ and [M+H]+ pseudomolecular ion peaks at m/z 320.13 and 298.20, respectively. Again, HRESIMS (m/z 298.1445) gave the molecular formula C17H19N3O2 (calc. 298.1477) for 2. Meanwhile, the 1H NMR spectra of 2, closely resembled that of 1, showing the same C-3 substituted benzo[de][1,6]naphthyridine moiety, as well as the –NH-CH2-CH2- spin system of the C-3 substituent (Table 2). The difference was the absence of the phenyl protons of the C-3 substituent, which were replaced by an isopropyl group as deduced from the two equivalent methyl doublets at δ 1.05 (6H, J = 7.0 Hz, δc 22.6) and a methine proton at δ 1.87. The 2J and 3J HMBC correlations between H2-3’ and H2-2’, respectively, to this methine carbon (δc 26.0), assigned as C-4’, were the key correlations that connected the isopropyl group to the –NH-CH2-CH2- spin system. Again, a 3J correlation observed from H2-2’ to the quaternary carbon C-3 (δc 144.4) connected the isopentylamino substituent to the main benzo[de][1,6]naphthyridine moiety, leading us to assign 2 as 3-(isopentylamino)demethyl(oxy)aaptamine. All the three isolated alkaloids exhibited significant cytotoxic activity against CEM-SS cells with CD50 values of 5.3 (1), 6.7 (2) and 15.0 (aaptamine) μg/ml, respectively.
We have isolated two new aaptamines, 8-methoxy-2-(phenethylamino)-9H-benzo- [de][1,6]naphthyridin-9-one (1) and 2-(isopentylamino)-8-methoxy-9H-benzo[de][1,6]naphthyridin-9-one (2) from the tropical marine sponge Aaptos aaptos. To the best of our knowledge, this is the first report of naturally occurring C-3 substituted aaptamines. SAR studies carried out on analogs of aaptamine and isoaaptamine on several cell lines including against murine P-388 lymphocytic leukemia [9, 15–17] suggested that hydroxylation at C-9 is important for cytotoxicity, and para-substituted phenyl substituents on one or both of the nitrogens are important for increased activity. It is interesting to note that in this study, the cytotoxicity of the C-3 substituted aaptamines, 1 and 2, on CEM-SS human T-lymphoblastic leukaemia cells were also observably higher than that shown by aaptamine. This suggests that C-3 substitution may also influence the cytotoxity of this class of compounds, towards this type of cell line.
Experimental
General Exprimental Procedures
UV and IR spectra were recorded on CARY 100 Conc UV-Vis (Varian) and Perkin-Elmer RXI FTIR spectrometers, respectively. Mass spectra were recorded on Polaris Q Mass Spectrometers (Thermo Finnigan San Jose CA), with ionization being induced by electron impact at 70eV. HRESIMS were measured using Finnigan MAT95XL-T spectrometers. LCMS/MSn were performed on a ThermoFinnigan model LCQDeca (San Jose, CA). 1H, gCOSY, gHSQC and gHMBC NMR spectra for 1 and 2 were recorded on Varian Unity INOVA 500 Spectrometer, acquired using a gHX nanoprobe.
Adsorbent used for vacuum liquid chromatography (VLC) and column chromatography (CC) was Merck Kieselgel 60 (230–400 mesh). Gel filtrations were carried out using LH-20 (Sephadex 17-0090-01 Pharmacia Biotech). Fractions were monitored by analytical TLC, using aluminium precoated sheets (Si gel 60 F254, 0.25 mm thick) with visualization under UV (254 and 366 nm), as well as with 25% H2SO4 or Dragendorff spray reagents. Analytical reversed-phase HPLC (Inertsil ODS-3 column, 7.6 x 250 mm, isocratic MeOH/H2O 7:3) were performed with a JASCO pump (PU-2080) equipped with a UV-Vis detector model UV-1578/1575 linked by JASCO BORWIN version 1.5 software.
Animal material
Aaptos aaptos was collected from the coastal waters of Terengganu, on the eastern part of Peninsular Malaysia. A specimen (registry No. P03.015) has been deposited at the Department of Biological Science, Faculty of Science and Technology, Universiti Malaysia Terengganu.
Extraction and isolation
Fresh samples of Aaptos aaptos (250 g) were cut and macerated in a high-speed blender with methanol at room temperature. The methanolic extract (5.5 g) was filtered, evaporated in vacuo, and lyophilized before subjected to cytotoxity assay [18] against a panel of cancer cell lines. The crude methanolic extract was further partitioned into n-hexane (1 g), chloroform (0.6 g), ethyl acetate (1.1 g) and aqueous (2.3 g) fractions. All the fractions were assayed against CEM-SS, where the cytotoxity (CD50=2.4 μg/ml) was found to be concentrated in the chloroform fraction. Further isolation was thus focused on this bioactive fraction. The extract was chromatographed on a silica gel column eluting with chloroform with increasing amounts of MeOH as eluent. The fraction eluted with 20% MeOH yielded large amounts of aaptamine (50 mg). The fraction eluted with 10% MeOH was further subjected to silica gel column, eluted with dichloromethane with increasing amounts of acetone. The subfraction eluted with 30% acetone was further subjected to reversed-phase HPLC using MeOH/H2O 7:3 as eluent (isocratic, flow rate 3 ml/min, wavelength 366 nm) to yield 1.0 mg each of compound 1 and 2.
Compound 1. 3-(phenethylamino)demethyl(oxy)aaptamine or 8-methoxy-3-(phenethylamino)-9H-benzo[de][1,6]naphthyridin-9-one. Orange gum. 1H and 13C NMR data recorded in CDCl3 see Table 2. HRESIMS [M+H]+ found at m/z 332.1291, calc. 332.1321 for C20H17N3O2.
Compound 2. 3-(isopentylamino)demethyl(oxy)aaptamine or 3-(isopentylamino)-8-methoxy-9H-benzo[de][1,6]naphthyridin-9-one. Orange gum. 1H and 13C NMR data recorded in CDCl3, see Table 2. HRESIMS [M+H]+ found at m/z 298.1445, calc. 298.1477 for C17H19N3O2.
Evaluation of Cytotoxicity
Cytotoxic activity was measured against a panel of cell lines as described previously [18].
Acknowledgments
This work was partly supported by the Ministry of Science, Technology and Innovation (MOSTI) under the e-science funding mechanism. Mr. Jasnizat Saidin of Universiti Malaysia Terengganu and Prof. Eduardo Hadju of the National Muzeum, Department of Invertebrates, Federal University of Rio De Jeneiro, Brazil, are respectively acknowledged for their kind assistance in sample collection and species identification.
Footnotes
Samples Availability: Not available.
References
- 1.Mayer AMS, Gustafson KR. Marine pharmacology in 2001-2: Antitumour and cytotoxic compounds. European Journal of Cancer. 2004;40:2676–2704. doi: 10.1016/j.ejca.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Mayer AMS, Hamann MT. Marine pharmacology in 2001–2: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comparative Biochemistry and Physiology – Part C. 2005;140:265–286. doi: 10.1016/j.cca.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer AMS, Gustafson KR. Marine pharmacology in 2003–4: Antitumour and cytotoxic compounds. European Journal of Cancer. 2006;42:2241–2270. doi: 10.1016/j.ejca.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Mayer AMS, Rodriguez AD, Berlinck RGS, Hamann MT. Marine pharmacology in 2003–4: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comparative Biochemistry and Physiology – Part C. 2007;145:553–581. doi: 10.1016/j.cbpc.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura H, Kobayashi J, Ohizumi Y, Hirata Y. Isolation and structure of aaptamine, a novel heteroaromatic substance possessing α-blocking activity from the sea sponge Aaptos aaptos. Tetrahedron Letters. 1982;23:5555–5558. [Google Scholar]
- 6.Nakamura H, Kobayashi J, Ohizumi Y, Hirata Y. Aaptamines. Novel benzo[de][1.6]naphthyridines from the Okinawan marine sponge, Aaptos aaptos. Journal of the Chemical Society, Perkin Transactions. 1987;1:173–176. [Google Scholar]
- 7.Bergquist PR, Cambie RC, Kernan MR. Aaptamine, a taxonomic marker for sponges of the order Hadromerida. Biochemical Systematics and Ecology. 1991;19:289–290. [Google Scholar]
- 8.Calcul L, Longeon A, Mourabit AA, Guyot M, Bourguet-Kondracki M-L. Novel alkaloids of the aaptamine class from an Indonesian marine sponge of the genus Xetospongia. Tetrahedron. 2003;59:6539–6544. [Google Scholar]
- 9.Shen YC, Lin TT, Sheu JH, Duh CY. structures and cytotoxicity relationship of isoaaptamine and aaptamine derivatives. Journal of Natural Products. 1999;62:1264–1267. doi: 10.1021/np990156g. [DOI] [PubMed] [Google Scholar]
- 10.Longley RE, McConnell OJ, Essich E, Harmody D. Evaluation of marine sponge metabolites for cytotoxicity and signal transduction activity. Journal of Natural Products. 1993;56:915–920. doi: 10.1021/np50096a015. [DOI] [PubMed] [Google Scholar]
- 11.Gul W, Hammond NL, Yousaf M, Bowling JJ, Schinazi RF, Wirtz SS, de Castro Andrews G, Cuevas C, Hamann MT. Modification at the C9 position of the marine natural product isoaaptamine and the impact on HIV-1, mycobacterial, and tumour cell activity. Bioorganic & Medicinal Chemistry. 2006;14:8495–8505. doi: 10.1016/j.bmc.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohizumi Y, Kajiwara A, Nakamura H, Kobayashi J. α-adrenoceptor blocking action of aaptamine, a novel marine natural product in vascular smooth muscle. Journal of Pharmacy and Pharmacology. 1984;36:785–786. doi: 10.1111/j.2042-7158.1984.tb04876.x. [DOI] [PubMed] [Google Scholar]
- 13.Jang KH, Chung S-C, Shin J, Lee S-H, Kim T-I, Lee H-S, Oh K-B. Aaptamines as sortase A inhibitors from the tropical sponge Aaptos aaptos. Bioorganic & Medicinal Chemistry Letters. 2007;17:5366–5369. doi: 10.1016/j.bmcl.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Diers JA, Ivey KD, El-Alfy A, Shaikh J, Wang J, Kochanowska AJ, Stoker JF, Hamann MT, Matsumoto RR. Identification of antidepressant drug leads through the evaluation of marine natural products with neuropsychiatric pharmacophores. Pharmacology Biochemistry and Behavior. 2008;89:46–53. doi: 10.1016/j.pbb.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettit GR, Hoffmann H, Herald DL, Blumberg PM, Hamel E, Schmidt JM, Chang Y, Pettit RK, Lewin NE, Pearce LV. Antineoplastic Agents 499. Synthesis of hystatin 2 and related 1H-benzo[de]-1,6-naphthyridinium salts from aaptamine. Journal of Medicinal Chemistry. 2004;47:1775–1782. doi: 10.1021/jm030070r. [DOI] [PubMed] [Google Scholar]
- 16.Pettit GR, Hoffmann H, Herald DL, McNulty J, Murphy A, Higgs KC, Hamel E, Lewin NE, Pearce LV, Blumberg PM, Pettit RK, Knight JC. Antineoplastic agents 491. Synthetic conversion of aaptamine to isoaaptamine, 9-demethylaaptamine, and 4-methylaaptamine. Journal of Organic Chemistry. 2004;69:2251–2256. doi: 10.1021/jo0300486. [DOI] [PubMed] [Google Scholar]
- 17.Pettit GR, Hoffmann H, McNulty J, Higgs KC, Murphy A, Molloy DJ, Herald DL, Williams MD, Pettit RK, Doubek DL, Hooper JNA, Albright L, Schmidt JM, Chapuis JC, Tackettt LP. Antineoplastic agents 380. Isolation and crystal structure determination of isoaaptamine from the Replublic of Singapore Hymeniacidon sp. And conversion to the phosphate prodrug hystatin-1. Journal of Natural Products. 2004;67:506–509. doi: 10.1021/np0204592. [DOI] [PubMed] [Google Scholar]
- 18.Mackeen MM, Ali AM, Lajis NH, Kawazu K, Hassan Z, Amran M, Habsah M, Mooi LY, Mohamed SM. Antimicrobial, antioxidant, antitumour-promoting and cytotoxic activities of different plant part of Garcinia atroviridis Griff ex T. Anders. J Etnopharmacol. 2000;72:395–402. doi: 10.1016/s0378-8741(00)00245-2. [DOI] [PubMed] [Google Scholar]