Abstract
At the time of diagnosis, approximately 75% of bladder cancers are non-muscle invasive. Appropriate diagnosis and surgical resection at this stage improves prognosis dramatically. However, these lesions, being small and/or flat, are often missed by conventional white-light cystoscopes. Furthermore, it is difficult to assess the surgical margin for negativity using conventional cystoscopes. Resultantly, the recurrence rates in patients with early bladder cancer are very high. This is currently addressed by repeat cystoscopies and biopsies, which can last throughout the life of a patient, increasing cost and patient morbidity. Multiphoton endoscopes offer a potential solution, allowing real time, non-invasive biopsies of the human bladder, as well as an up-close assessment of the resection margin. While miniaturization of the Multiphoton microscope into an endoscopic format is currently in progress, we present results here indicating that Multiphoton imaging (using a bench-top Multiphoton microscope) can indeed identify cancers in fresh, unfixed human bladder biopsies. Multiphoton images are acquired in two channels: (1) broadband autofluorescence from cells, and (2) second harmonic generation (SHG), mostly by tissue collagen. These images are then compared with gold standard hematoxylin/eosin (H&E) stained histopathology slides from the same specimen. Based on a “training set” and a very small “blinded set” of samples, we have found excellent correlation between the Multiphoton and histopathological diagnoses. A larger blinded analysis by two independent uropathologists is currently in progress. We expect that the conclusion of this phase will provide us with diagnostic accuracy estimates, as well as the degree of inter-observer heterogeneity.
Keywords: Multiphoton, human bladder, biopsy, diagnosis, histopathology, autofluorescence, second harmonic generation, optical biopsy
1. INTRODUCTION
1.1 Clinical Background
In the United States, urothelial cell carcinoma of the bladder is the fourth and the eighth most common malignancy among men and women, respectively; with over 50,000 new cases and ~12,000 deaths annually1. At the time of diagnosis, approximately 75% of bladder cancers are superficial, i.e., non-muscle invasive2. If detected and completely resected at this stage, the patients are likely to have excellent prognosis, including the possibility of cure. However, contemporary diagnostic techniques for early bladder cancer are fraught with limitations. Current standard of practice includes two diagnostic tests to rule out urothelial cell carcinoma in patients with microscopic or gross hematuria (presenting symptoms for over 80% of the patients with urothelial cell carcinoma): (1) urine cytology, and (2) white light cystoscopy3. Since the tumor is in direct contact with the urine, tumor cells are frequently shed into urine, and hence a urinary analysis for the presence of malignant cells seems a prudent approach. However, although this technique has high specificity (94% median), the median sensitivity is only 35%4. Conventional white light cystoscopy, while quite efficient at detecting relatively large papillary tumors, performs poorly in identifying flat lesions (particularly Carcinoma in situ or CIS), dysplasia, multifocal growth and microscopic papillary tumors3.
One of the greatest problems with the clinical management of non-muscle invasive bladder cancer is the very high recurrence rate (31–78% at 5 years)3. This high recurrence rate, along with relatively high long term survival, makes bladder cancer the most expensive cancer at the patient level5, with ~60% of the costs attributable to treatment of recurrences6. Interestingly, a study investigating the source of “recurrences” of tumor on repeat cystoscopy found that up to ~50% of the apparent recurrences were in fact pre-existing tumors that were missed during the first cystoscopy7. This is especially a concern if the tumor missed during the initial cystoscopy were a CIS lesion. CIS is a superficial but high-grade lesion, whose presence has been identified as an important prognostic factor for the recurrence and/or progression of bladder cancer8. A recent estimate suggests that conventional white light cystoscopy (trans-urethral bladder endoscopy and biopsy) is apt to miss up to 42% of the CIS9. Thus, in the reality of a clinical practice, the urologist is often faced with a dilemma in the case of suspicious “equivocal” cases: too few biopsies will increase the false negative rate, and too many will increase cost and patient morbidity. Furthermore, small foci of remaining tumor at the edge of resected papillae, which cannot be detected by conventional cystoscopy, may also contribute to occult inadequate resection and thus contribute to disease recurrence.
We propose that Multiphoton microscopy (MPM)10–12, especially when adapted to a Multiphoton endoscope (MPE) format, will be a very powerful diagnostic tool for early bladder cancer. It has the potential to better identify early lesions, including CIS, during initial cystoscopy, and the ability to image the resection margins to confirm negativity.
1.2 Multiphoton microscopy
Briefly, MPM involves the illumination of tissue by near-infrared light from a femtosecond pulsed laser, which is used to excite fluorescence from the naturally occurring fluorophores residing at the focal volume. Intrinsic fluorophores abundantly present in most cells include reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotides (FAD). In addition to cellular autofluorescence, MPM allows the identification of non-centrosymmetric structures such as collagen by utilizing a higher order scattering phenomenon called Second Harmonic Generation (SHG).
Using a combination of autofluorescence from cells and SHG signal from the connective tissue rich in collagen, MPM imaging can provide detailed information on tissue architecture and cellular morphology, comparable to that obtained from standard Hematoxylin/eosin (H&E) stained histopathology slides, although intra-nuclear details are unavailable. All this can be done with minimal phototoxicity to cells, and images of adequate quality can be obtained up to depths of several hundred microns in a few seconds.
1.3 Suitability of the human bladder as a first prototype organ for MPM/MPE
The human bladder is an ideal candidate for the standardization of MPM imaging and MPE methodology, since it has a well-defined architecture13, whose perturbation by cancer can be easily detected at cellular and tissue organization levels. The bladder lumen is lined by an ~7-cell layer transitional epithelium or urothelium. The uppermost layer of the urothelium surrounding the lumen is composed of a layer of umbrella cells, with a unique morphology that can be identified by MPM. Perturbation of the urothelial layer is visible by MPM. A layer of connective tissue containing collagen and scattered smooth muscle cells, known as the lamina propria, resides below the urothelium. Beneath this layer, the muscularis propria, a well defined layer of smooth muscle, serves as the functional contractile unit of the bladder. Finally, a layer of perivesical soft tissue, composed of fat, fibrous tissue and blood vessels represents the final layer of the bladder wall.
All bladder carcinomas originate in the urothelium. Early bladder cancers, which are of interest for the current study, may show an increase or decrease in the thickness of the urothelial layer, presence of papillary architecture, and/or a penetration of the urothelial cells into the lamina propria14. Since these changes are limited to the top several hundred microns of the bladder lumen lining, MPM and MPE technologies offer a unique opportunity to image these early lesions.
2. MATERIALS AND METHODS
Fresh bladder biopsy specimens {obtained via trans-urethral resection of bladder tumor (TURBT) or cold cup biopsies} are placed in specimen bottles containing normal saline immediately after excision. The specimen bottles, placed on ice, are then brought directly to the Multiphoton microscope for imaging. In the imaging facility, the biopsy specimen is placed on a small tissue culture dish with a central well, with the urothelium oriented up. If necessary, the specimen is stabilized in optimum orientation using nontoxic modeling clay, and a coverslip (#1 glass) is placed on top of the urothelium to avoid adhesion of the specimen to the water immersion objective due to capillary action.
The specimen, once oriented, is imaged using a custom-built Multiphoton imaging system consisting of an Olympus BX61 upright microscope and a BioRad 1024 scanhead. The specimens are excited using a tunable Ti-Sapphire laser (Mai Tai, Spectra-Physics), tuned to 780 nm. The laser power under the objective is controlled through a Pockel Cell (Conoptics). Images are collected in two channels: (1) SHG signal centered around 390 nm (±35 nm), and (2) broadband autofluorescence at 420–530 nm.
Images are acquired at two magnifications: (1) Low magnification for obtaining overall architectural information (4X, 0.28 NA non-immersion objective). This allows us to image 3 mm2 frames at 6 μm/pixel resolution. (2) High magnification for obtaining detailed cellular and local architectural information (20X, 0.95 NA water-immersion objective). This allows us to image 614 μm2 frames at 1.2 μm/pixel resolution. Higher digital zooms are used to increase magnification, if necessary. When imaging with the 20X objective, a drop of normal saline is placed on the coverslip to achieve water immersion. All images are obtained as stacks of optical sections through the entire depth of the specimen from where reasonable signal can be obtained. Typically, we are able to image up to ~400 μm into the tissue with the 4X objective, and up to ~250 μm with the 20X objective.
For all images presented here, the SHG signal has been color-coded red, and the autofluorescence signal has been coded green. Metamorph (Molecular Dynamics, Inc.) and Adobe Photoshop were used for image color-coding, minimal processing (adjustment of brightness and contrast, placement of scale bars, cropping and presentation).
Immediately after imaging, the biopsies are dropped in standard formalin, and taken to the Pathology department for routine surgical pathology workup and diagnosis. This includes gross morphological assessment of the tissue, as well as microscopic evaluation of H&E stained slides of thin sections prepared from the tissue.
During the initial “training phase”, the uropathologist, the Multiphoton microscopist, and the urologists convened to learn to identify comparable features by a side-by-side assessment of the Multiphoton images and the H&E slides from the specimen. We also used the official surgical Pathology report for each case as a reference.
Very recently, we have begun a blinded trial to assess the diagnostic accuracy of Multiphoton images. Here, the uropathologists have been given sets of de-identified Multiphoton images, each set representing all the images acquired from a given specimen. They have been asked to make a diagnosis based solely on the Multiphoton images, and classify each specimen into one of the following classes: (1) Normal urothelium; (2) Papillary lesion: (a) Benign, (b) Ta, low-grade, (c) Ta, high-grade; (3) CIS; (4) Invasive cancer; (5) Other.
3. RESULTS
Initial side-by-side comparisons of the Multiphoton images and H&E stained histopathology slides from the same specimen showed a high degree of correlation among diagnostic features, as described below. Please note that it is very rare to obtain a complete correspondence between the MPM and the histology images, since for biopsy specimens, there is no control either on the orientation in which the piece is embedded, or on the specific part of the embedded block that is sectioned and stained for histopathologic examination. It is important to remember that a biopsy specimen ranges anywhere from a few mm to up to a cm or more in thickness, whereas each H&E stained section is only 5 – 8 μm thick.
3.1 Biopsy specimen showing normal architecture
We were able to distinguish the urothelium (autofluorescence from cells color-coded green) from the underlying lamina propria (a region rich in collagen, and hence showing SHG signal color-coded red). Please note the flat, uniform, roughly 7-cell layer thick urothelium (with no papillary growth) and a clear boundary between the urothelium and the lamina propria. The uppermost layer of umbrella cells is also visible at high magnification. All these features together indicate the presence of normal histology.
3.2 Low-grade papillary tumor
Once again, we can distinguish the urothelium from the underlying lamina propria. The lamina propria forms “stalks” that extend into proliferating urothelium. A profusion of papillary structures are seen in cross section. Please note that the cells look relatively normal and homogeneneous, indicating a low-grade tumor.
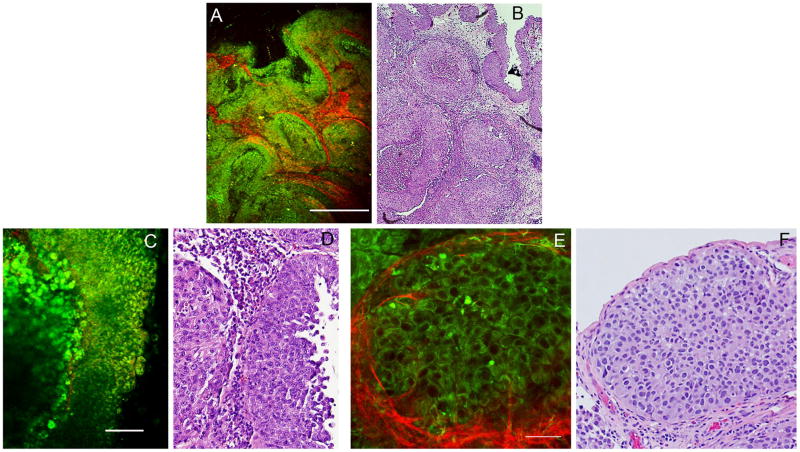
3.3 High-grade, invasive papillary tumor
Here, at low magnification, the cells look highly compacted and arranged in “whorls” (profusion of cells with scant lamina propria around cell clusters). At high magnification, we observe extensive cell heterogeneity (panels C and D), as well as cells with high nuclear to cytoplasmic ratio (panels E and F). Furthermore, panels C and D show cells (green) penetrating into a thin lamina propria (red), indicating that the tumor has invaded at least into the lamina propria (Stage ≥T1). However, due to the limited depth of penetration of this technique, we are unable to distinguish tumor stages T1 (invasive into lamina propria) from T2 (muscle invasive).
Taken together, our results indicate that using a combination of broadband autofluorescence and SHG signatures, we are able to obtain diagnostic quality images, which can be comparable in their information content to the gold standard H&E histopathology. It is important to point out, though, that in MPM images, the nuclei appear dark (identified by the absence of signal). This imaging modality is thus lacking in intra-nuclear details, which is sometimes used in the determination of tumor grade from H&E stained slides.
Also, in our experience, we can obtain images of adequate quality in human bladders up to a maximum depth of ~400 μm using our 4X objective (NA 0.28, lateral resolution 6 μm/pixel), and up to ~250 μm using our 20X objective (NA 0.95, lateral resolution 1.2 μm/pixel). As a result, as discussed in section 3.3, we are unable to distinguish tumor stages T1 (invasive into lamina propria) from T2 (muscle invasive). However, the information obtained is sufficient for surgical decision making, i.e., whether T1 or T2, the tumor will be resected and further analyzed by H&E histopathology.
4. DISCUSSION
4.1 What is wrong with the current standard of care for bladder cancer patients?
As discussed in the introduction, bladder cancer predominantly presents as non-muscle invasive cancer, which, if detected and completely resected, considerably improves prognosis and is likely to result in cure. However, the current diagnostic techniques, including urine cytology and white light cystoscopy, have very high miss rates for early bladder cancer such as flat lesions (particularly CIS), dysplasia, multifocal growth and microscopic papillary tumors3.
In the current standard of practice, if tumor is detected by urine cytology and/or surveillance cystoscopy, the patient is then subjected to TURBT (trans-urethral resection of bladder tumor) procedure. This involves white light cystoscopy along with resection of the tumor, and possible biopsy of additional suspicious regions. All excised tissue is then assessed by H&E histopathology to determine stage (degree of invasiveness) and grade (degree of transformation of the cells). Whereas surveillance cystoscopy is routinely done as an outpatient procedure, TURBT procedures require anesthesia, and often an overnight hospital stay. Taking a segment of the bladder endoscopically can be associated with significant complications and morbidity for the patient.
In terms of patient management, if the biopsies or TURBTs reveal only localized (non-muscle invasive tumor), then the patient may or may not be treated pharmacologically (depending of exact stage and grade), but is routinely monitored for recurrence, most often via repeat cystoscopies. If the patient is found to have muscle invasive bladder tumor upon TURBT, the standard of care is radical cystectomy.
As discussed in the introduction, one of the major concerns regarding management of patients with early bladder cancer is the rather high recurrence rates3, and the source of up to half of the apparent recurrences has been traced to pre-existing tumors that were missed during the initial cystoscopy7. This high recurrence rate, along with relatively high long-term survival, makes bladder cancer the most expensive cancer at the patient level5, with ~60% of the costs attributable to treatment of recurrences6.
Thus, the availability of a non-invasive diagnostic tool that could obtain histopathologic analysis comparable to gold standard histology would be of extraordinary benefit to the medical community.
4.2 What can be done to rectify some of the problems with the standard of care?
As experience accumulates from other surgical specialties where “multimodal imaging” has become more common (e.g., colorectal cancer, or Barrett’s Esophagus), it is becoming clear that at least two types of endoscopic techniques need to be used in conjunction. In general, these can be classified into “primary detection” techniques and “targeted imaging” techniques15. The primary detection is the localization of tumor in an organ. A good primary detection technique will be able to detect tumors with high specificity and sensitivity. White light endoscopy is the conventional modality, and is being currently supplemented with several newer “red flag” techniques, which are designed to draw the attention of the endoscopist to regions that might contain early tumors or dysplasia. Such techniques include chromoendoscopy (using contrast agents such as methylene blue or indigo carmine) and narrow band imaging (NBI). NBI involves the illumination of the mucosal surface with narrow bands of blue and green light, which, due to their short wavelength, penetrate only the superficial layers. Furthermore, the blue light is preferentially absorbed by the hemoglobin in the blood vessels, which makes them appear dark. Together, they produce increased contrast, allowing better visualization of mucosal and vascular patterns16.
One such red flag technique, which has been shown to be especially useful in improving detection of early bladder cancer, is Fluorescence cystoscopy (also referred to as Photodynamic detection or PDD)17. The most commonly used agent for this approach is 5-aminolevulinic acid (ALA). ALA is a normal component of the heme biosynthetic pathway. One of the intermediates in the conversion of ALA to heme is Protoporphyrin IX, which, when excited by blue light, gives a bright red fluorescence. When treated with ALA, tumor cells preferentially accumulate Protoporphyrin IX, and thus appear brighter when illuminated by blue light. The mechanism of this preferential accumulation is not clear, and is possibly a result of multiple causes. One interesting observation is that many tumor cells have a reduced activity of the enzyme ferrochelatase, which normally converts Protoporphyin IX to heme, possibly due to a diminished supply of iron. For detection of early bladder cancers during cystoscopy, ALA is instilled intravesically (by filling the bladder with an ALA containing buffer for several hours), thereby minimizing many side effects of ALA such as photosensitivity.
Diagnosis of early bladder cancer, and especially CIS, is dramatically improved with ALA treatment followed by blue light illumination. Several recent clinical trials comparing ALA-mediated diagnosis with conventional white light cystoscopic diagnosis found a mean increase in sensitivity of ~20%, with sensitivities for ALA-mediated diagnosis being >95%3. Indeed, an outcomes study looking at an 8-year recurrence free survival showed 71% positive response in the ALA-mediated diagnosis group, compared to only 45% in the conventional white light cystoscopy group.
However, the specificity of this method is relatively low and quite variable17–19. This high false positive rate appears to be associated with changes in Protoporphyrin IX accumulation in various types of non-neoplastic cells, chief among them being sites of inflammation18. This also results in much higher false positive rates in patients with a recent history of cystoscopic or pharmacological treatment for bladder cancer20.
Newer fluorescence agents based on the ALA model are currently being tested21. One of these is the hexyl ester of ALA (HAL), which, being more hydrophobic, is taken up by cells more readily, thus requiring lower concentrations and shorter instillation periods. Hypericin, extracted from Hypericum perforatum (St. John’s wort) is being investigated as another potential alternative. Early studies indicate that this agent may label tumor cells with high sensitivity as well as specificity.
“Targeted imaging” techniques, on the other hand, allow a more detailed evaluation of the sites picked up as “of potential interest” by the red flag techniques described above3. These techniques have often been referred to in the literature as “optical biopsies.” Primary among these techniques is confocal microendoscopy22, 23, which can provide subcellular details of the superficial mucosal layers. Confocal microendoscopy is typically conducted in the fluorescence mode, although confocal reflectance endoscopy is also being investigated as a potential option. Other optical biopsy techniques currently under investigation include optical coherence tomography and Raman spectroscopy3, 22, 23.
4.3 Multiphoton endoscopy: a new frontier in optical biopsy?
Confocal fluorescence endoscopy, although it can produce images of resolution comparable to gold standard histology, has several limitations, the most important one being the necessity of using an exogenous contrast agent such as Fluorescein for optimal imaging. Also, the relatively short wavelength of excitation significantly limits light penetration into the tissue, allowing the imaging of only the superficial layers of cells. There are also concerns about photodamage, since although signal is collected from only one focal plane at a time, the illuminating light can bleach and potentially photodamage cells in the entire illumination cone. Optical coherence tomography, while eliminating some of these concerns, has a much lower resolution than confocal imaging.
Our preliminary results presented here strongly suggest that images of adequate resolution and contrast can be obtained from fresh, unstained human tissue using MPM (using a combination of autofluorescence and SHG signals). When compared with H&E stained histopathology slides prepared from the same specimen, we obtain strong correlation among the diagnostic features between the MPM and the H&E images. Of course, the ongoing blinded studies will reveal whether the current imaging approach (two signals: SHG and broad band fluorescence) will provide adequate diagnostic accuracy to make reliable diagnoses in real time in the eventual endoscopic format.
Several factors must be considered for the extension of MPM imaging technology from ex vivo biopsies to endoscopic imaging in real patients. One consideration that is common to all optical biopsy techniques is that these techniques, because of the limited field of view and relatively slow image acquisition speeds, must be combined with another high specificity primary detection technique. It is plausible that ALA-mediated imaging will be an optimal system for this purpose, primarily because it has been demonstrated to detect early tumors in bladder with high sensitivity. The lower specificity of ALA detection may be rectified during cystoscopy by switching to MPM imaging mode, to more closely scrutinize the suspicious regions. However, ALA treatment requires instillation of an exogenous reagent, thus increasing total treatment time and potential complications. Also, to the best of our knowledge, no thorough investigation has been carried out to test whether the presence of ALA or Protoporphyrin IX in cells affect their autofluorescence signatures in MPM. If these limitations present significant challenges in clinical adaptation, other red flag techniques such as NBI or confocal reflectance may be investigated as better options for pairing with Multiphoton endoscopy.
Indeed, this is a common trend in other cancer diagnosis scenarios as well. Attempts are currently underway to further increase the range of techniques at the disposal of an endoscopist. For example, a recent international, multi-center feasibility study involved tri-modal imaging options (high-resolution video endoscopy, autofluorescence imaging and NBI) within a single endoscopic device, for diagnosis of early neoplasia in Barrett’s esophagus24. The results indicated that combined high-resolution endoscopy and autofluorescence imaging had very high sensitivity, but poor specificity. The false positive rate of this combination was reduced by a detailed inspection of the Barrett’s mucosa with NBI.
Another issue that needs further investigation is the maximum tolerated light dosage – both to avoid frank tissue burning, as well as to ensure that no insidious negative effects, such as increased mutation rates, are caused as a result of illumination with a femtosecond pulsed laser source, which would be necessary for this type of imaging.
However, assuming that Multiphoton endoscopy, along with a suitable primary detection method, can be translated to clinical use, it can tremendously impact the current practice by allowing: (1) selective resection of malignant tissue (high specificity), (2) identification and resection of early lesions, and (3) assessment of resection margins to ensure negativity. Also, since routine surveillance cystoscopy (cystoscopy without biopsy) is an outpatient procedure, it is likely to reduce the overall cost of management of bladder cancer survivors. TURBTs will then only be scheduled in follow-up cases when surveillance Multiphoton endoscopy confirms the presence of new tumors.
Figure 1.
Multiphoton images and Hematoxylin/eosin (H&E) stained histopathology slides showing comparable architecture in biopsy with normal histology.
A: Multiphoton image at low magnification, acquired with a 4X (NA 0.28) dry objective. Color-coding: Green = broadband autofluorescence; Red: Second Harmonic Generation (SHG) signal. Scale bar = 500 μm.
B: Image acquired from a corresponding area of an H&E stained slide prepared from the same specimen.
C: Multiphoton image at high magnification, acquired with a 20X (NA 0.95) water immersion objective. Color-coding: Green = broadband autofluorescence; Red: Second Harmonic Generation (SHG) signal. Scale bar = 100 μm.
D: Image acquired from a corresponding area of an H&E stained slide prepared from the same specimen.
Figure 2.
Multiphoton images and Hematoxylin/eosin (H&E) stained histopathology slides showing comparable architecture in a low-grade papillary lesion.
A: Multiphoton image at low magnification, acquired with a 4X (NA 0.28) dry objective. Color-coding: Green = broadband autofluorescence; Red: Second Harmonic Generation (SHG) signal. Scale bar = 500 μm.
B: Image acquired from a corresponding area of an H&E stained slide prepared from the same specimen.
C: Multiphoton image at high magnification, acquired with a 20X (NA 0.95) water immersion objective. Color-coding: Green = broadband autofluorescence; Red: Second Harmonic Generation (SHG) signal. Scale bar = 100 μm.
D: Image acquired from a corresponding area of an H&E stained slide prepared from the same specimen.
E: Multiphoton image at high magnification at a second location, acquired with a 20X (NA 0.95) water immersion objective. Color-coding: Green = broadband autofluorescence; Red: Second Harmonic Generation (SHG) signal. Scale bar = 100 μm.
F: Image acquired from a corresponding area of an H&E stained slide prepared from the same specimen.
Figure 3.
Multiphoton images and Hematoxylin/eosin (H&E) stained histopathology slides showing comparable architecture in a high-grade papillary lesion.
A: Multiphoton image at low magnification, acquired with a 4X (NA 0.28) dry objective. Color-coding: Green = broadband autofluorescence; Red: Second Harmonic Generation (SHG) signal. Scale bar = 500 μm.
B: Image acquired from a corresponding area of an H&E stained slide prepared from the same specimen.
C: Multiphoton image at high magnification, acquired with a 20X (NA 0.95) water immersion objective. Color-coding: Green = broadband autofluorescence; Red: Second Harmonic Generation (SHG) signal. Scale bar = 100 μm.
D: Image acquired from a corresponding area of an H&E stained slide prepared from the same specimen.
E: Multiphoton image at high magnification at a second location, acquired with a 20X (NA 0.95) water immersion objective. Color-coding: Green = broadband autofluorescence; Red: Second Harmonic Generation (SHG) signal. Scale bar = 50 μm (a digital zoom of 2 was used).
F: Image acquired from a corresponding area of an H&E stained slide prepared from the same specimen.
Acknowledgments
SM acknowledges support from a K12 award (1 KL2 RR024997-01) from the Clinical and Translational Science Center (CTSC) of the Weill Cornell Medical College. WWW acknowledges support from DHHS-NIH-NIBIB award number 1 R01 EB006736-01, Development of Medical Multiphoton Microscopic Microscopy.
References
- 1.“American Cancer Society: Cancer Facts and Figures”. (1995).
- 2.Nieder AM, Soloway MS. Eliminate the term “superficial” bladder cancer. Journal of Urology. 2006;175(2):417–8. doi: 10.1016/S0022-5347(05)00290-9. [DOI] [PubMed] [Google Scholar]
- 3.Lee CSD, Yoon CY, Witjes JA. The past, present and future of cystoscopy: the fusion of cystoscopy and novel imaging technology. BJU International. 2008;102:1228–33. doi: 10.1111/j.1464-410X.2008.07964.x. [DOI] [PubMed] [Google Scholar]
- 4.Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. European Urology. 2005;47:736–48. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Botteman MF, et al. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 6.Avritscher EB, et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68(3):549–53. doi: 10.1016/j.urology.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 7.Brausi M, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. European Urology. 2002;41(5):523–31. doi: 10.1016/s0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 8.Sylvester RJ, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European Urology. 2006;49(3):466–5. doi: 10.1016/j.eururo.2005.12.031. discussion 475–7. [DOI] [PubMed] [Google Scholar]
- 9.Schmidbauer J, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. Journal of Urology. 2004;171(1):135–8. doi: 10.1097/01.ju.0000100480.70769.0e. [DOI] [PubMed] [Google Scholar]
- 10.Williams RM, Zipfel WR, Webb WW. Multiphoton microscopy in biological research. Current Opinion in Chemical Biology. 2001;5(5):603–8. doi: 10.1016/s1367-5931(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 11.Zipfel WR, et al. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(12):7075–80. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nature Biotechnology. 2003;21(11):1369–77. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 13.Ross MH, Pawlina W. Histology: A Text and Atlas. 5. Baltimore: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 14.Eble JN, editor. World Health Organization Classification of Tumours. Pathology & Genetics: Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 15.Curvers WL, Bergman JJ. Multimodality imaging in Barrett’s esophagus: looking longer, seeing better, and recognizing more.[comment] Gastroenterology. 2008;135(1):297–9. doi: 10.1053/j.gastro.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Kara MA, et al. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett’s esophagus by using narrow band imaging. Gastrointestinal Endoscopy. 2006;64(2):155–66. doi: 10.1016/j.gie.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 17.Joudi FN, Konety BR. Fluorescence cystoscopy and bladder surveillance. Current Opinion in Urology. 2004;14(5):265–70. doi: 10.1097/00042307-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 18.D’Hallewin MA, Vanherzeele H, Baert L. Fluorescence detection of flat transitional cell carcinoma after intravesical instillation of aminolevulinic acid. American Journal of Clinical Oncology. 1998;21(3):223–5. doi: 10.1097/00000421-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Kriegmair M, et al. Detection of early bladder cancer by 5-aminolevulinic acid induced porphyrin fluorescence.[see comment] Journal of Urology. 1996;155(1):105–9. discussion 109–10. [PubMed] [Google Scholar]
- 20.Filbeck T, et al. 5-aminolevulinic acid-induced fluorescence endoscopy applied at secondary transurethral resection after conventional resection of primary superficial bladder tumors. Urology. 1999;53(1):77–81. doi: 10.1016/s0090-4295(98)00430-0. [DOI] [PubMed] [Google Scholar]
- 21.Schmidbauer J, Marberger M. Recent developments in fluorescence cystoscopy: do novel agents bring a benefit? Current Opinion in Urology. 2007;17(5):347–51. doi: 10.1097/MOU.0b013e3282c8c73f. [DOI] [PubMed] [Google Scholar]
- 22.Kiesslich R, Goetz M, Neurath MF. Confocal laser endomicroscopy for gastrointestinal diseases. Gastrointestinal Endoscopy Clinics of North America. 2008;18(3):451–66. doi: 10.1016/j.giec.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Pierce MC, Javier DJ, Richards-Kortum R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. International Journal of Cancer. 2008;123(9):1979–90. doi: 10.1002/ijc.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curvers WL, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57(2):167–72. doi: 10.1136/gut.2007.134213. [DOI] [PubMed] [Google Scholar]