Abstract
Binding by the drug imipramine to the protein human serum albumin (HSA) was studied by using high-performance affinity chromatography. The association equilibrium constants and number of binding sites for imipramine with HSA were first estimated by utilizing frontal analysis. Imipramine was found to have one major binding site on HSA with an association equilibrium constant of 1.6 × 105 M−1 at pH 7.4 and 37°C, as well as a second group of weaker and non-specific binding regions (8–9 mol/mol HSA) with an average association equilibrium constant of 1.5 × 103 M−1. Competition studies based on zonal elution were performed to identify the location of the major binding site for imipramine on HSA. Imipramine was found to have direct competition with L-tryptophan, which indicated that imipramine was interacting with Sudlow site II, or the indole-benzodiazepine site of HSA. No competition or allosteric effects were noted between imipramine and warfarin, a probe for Sudlow site I or the warfarin-azapropazone site of HSA. The association equilibrium constant found for imipramine at its site of competition with L-tryptophan also agreed with the value that was obtained for the major binding site of imipramine in the frontal analysis studies. These results confirmed that Sudlow site II was the location of the major binding site for imipramine on HSA. These results gave good agreement with previous observations made in the literature and should provide a more detailed description of how imipramine is transported in blood and of how it may interact with other drugs in the body.
Keywords: imipramine, human serum albumin, protein binding, high-performance affinity chromatography, frontal affinity chromatography, competition studies
1. Introduction
The interactions between a drug and protein in blood can influence how the drug functions within the body, including its transport, distribution, metabolism, and excretion [1–4]. This makes the study of these interactions of great importance. One plasma protein that is involved in many of these interactions is human serum albumin (HSA). HSA is the most abundant plasma protein in the body. It is composed of a single polypeptide chain of 585 amino acid residues and has an asymmetric heart-shaped structure with dimensions of approximately 30 Å × 80 Å × 80 Å [3,5]. There are two major binding sites and a number of minor binding regions for drugs and other small solutes on HSA. The two major binding sites for drugs are known as Sudlow sites I and II (or the warfarin-azapropazone and indole-benzodiazepine sites, respectively) [3]. Solutes that bind to Sudlow site I are dicarboxylic acids or heterocyclic compounds with a negative charge, while aromatic carboxylic acids often bind to Sudlow site II [1,3].
One drug that is known to bind to HSA is imipramine (see Figure 1) [6]. Imipramine is a tricyclic antidepressant that relieves depression by increasing the concentrations of chemicals necessary for nerve transmission in the brain [7–9]. There have been reports of a relationship between plasma concentrations and the antidepressant effects for imipramine [8–10]. Previous reports on the binding of imipramine to HSA have made use of techniques such as capillary electrophoresis [11–13], fluorescence spectroscopy [2,6], HPLC and LC/MS [14–17], or equilibrium dialysis [18,19] (see Table 1). Similar methods have been used to examine the interactions of imipramine with bovine serum albumin and α1-acid glycoprotein [20,21]. Early work with equilibrium dialysis suggested that imipramine had only large amount of non-specific interactions with HSA, as described by an overall association equilibrium constant of around 102 M−1 [18,19]. However, experiments based on CE and HPLC have indicated that imipramine has a more specific interaction with HSA with an association equilibrium constant on the order of 103 to 105 M−1 [6,11,13,14]. The number of binding sites for this interaction is still unclear. Models based on non-specific interactions predict up to seven moles of imipramine can bind per mole of HSA [18,19], while the more selective model suggests that one to 2.2 moles of imipramine can bind per mole of HSA [6,13].
Figure 1.
Structure of imipramine (10,11-dihydro-N, N-dimethyl-5H-dibenz[b, f]azepine-5-propanamine).
Table 1.
Previous measurements of imipramine binding to HSA
| Method [Reference] | Conditions | Association equilibrium constant, Ka (M−1) | Number binding sites, n |
|---|---|---|---|
| Spectrofluorimetric titration [6]a | pH 7.4 Phosphate buffer, 20° C | 2.39 × 104 | n = 1.31 |
| Pressure-assisted capillary electrophoresis/frontal analysis [12] | pH 7.4 Phosphate buffered saline, 25°C | 1.8 (± 0.3) × 103 | n = 1b |
| Capillary zone electrophoresis [13] | pH 7.4 Phosphate buffer, 25°C | 1.8 × 105 | n = 2.2 |
| High-performance affinity chromatography [14] | pH 7.4 Phosphate buffer, 37°C | 2.0 × 104 | n = 1b |
| Equilibrium dialysis [18] | pH 7.4 Phosphate buffer, 37°C | 2.3 × 102 | n = 7 |
| Equilibrium dialysis [19]a | Blood versus 0.9% NaCl, 37°C | 4.9 × 102 | n = 7 |
This study will use high-performance affinity chromatography (HPAC) to examine the interactions of imipramine with HSA under physiological conditions. HPAC has been shown to be a useful method to examine the interactions of drugs and other solutes with proteins such as HSA [14,15,17,22–25]. The results of drug binding studies based on HPAC and immobilized HSA have been shown in many studies to give good agreement with data obtained by methods using soluble HSA, such as equilibrium dialysis and ultrafiltration (see Refs. [14,15,22,24]). HPAC will be used in this current report along with the techniques of frontal analysis and competitive binding zonal elution studies [22,24] to more closely examine the interactions of imipramine with HSA. The results of this work should provide a more detailed model of how imipramine binds with HSA. This, in turn, should lead to a better description of how this drug is transported within the body or competes with other drugs and solutes as it binds to HSA in blood.
2. Theory
The use of HPAC to examine the binding of drugs with HSA has been reviewed previously [22,24]. The following model is often used in such work to describe the binding between a drug or solute (A) and a single type of site on an immobilized ligand (L) such as HSA.
| (1) |
| (2) |
In these equations, Ka is the association equilibrium constant for the binding of A to L, while [A-L], [A], and [L] represent the molar concentrations of A, L and their complex (A-L) at equilibrium. Although these particular expressions assume that only one type of binding site is present on L for A, similar equations can be written for multi-site systems.
Frontal analysis is one tool that is commonly used in HPAC (giving a method known as frontal affinity chromatography) to determine association equilibrium constants for solute-ligand interactions. In this method, a known concentration of the solute or drug of interest is continuously applied to a column containing an immobilized ligand (e.g., HSA), while the amount of solute that exits from the column is monitored. As the binding sites on the immobilized ligand become saturated with the solute, a characteristic breakthrough curve is formed, as shown in Figure 2. If relatively fast association and dissociation kinetics are present in this system, it is possible to relate the mean position of this breakthrough curve to the total moles of binding sites for the solute and the association equilibrium constant for these sites. For the binding of a solute at a single type of ligand site, the following equations can be used to describe the expected response [22,24],
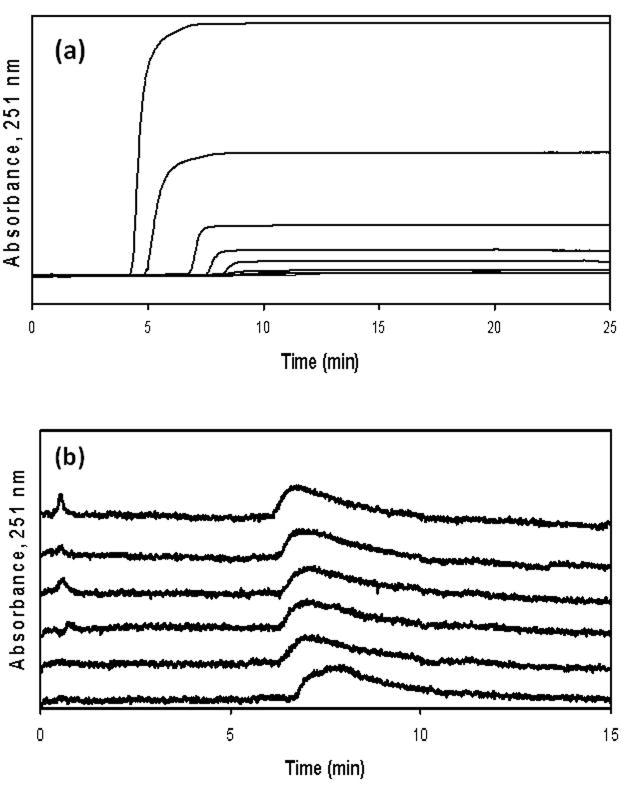
Figure 2.
(a) Frontal analysis curves obtained for imipramine on an HSA column and (b) competition studies on an HSA column in which imipramine is the injected analyte and L-tryptophan is used as a mobile phase additive. The concentrations of applied imipramine in (a) (from top-to-bottom) were 100, 50, 20, 10, 5, 2, 1.5, 1, and 0.75 μM, respectively. In (b) the injected sample contained 20 μM imipramine and the concentrations of L-tryptophan in the mobile phase (from top-to-bottom) were 300, 150, 100, 50, 20, and 5 μM, respectively. The dashed line in (b) is included as a reference point and shows the mean position of the peak for imipramine in the presence of 0 μM L-tryptophan. All of these experiments were conducted at pH 7.4 and 37°C. Other experimental conditions are given in the text.
| (3) |
| (4) |
where mLapp is the apparent moles of analyte required to saturate the column, [A] is the concentration of applied analyte, and mL is the moles of binding sites in the column. Eqn. (3) predicts that a plot of 1/mLapp versus 1/[A] will give a linear response with a slope equal to 1/(Ka mL) and an intercept of 1/mL for a system following a one-site binding model. This feature makes it possible to calculate the association equilibrium constant for this interaction and the total moles of binding sites in the column.
Frontal analysis can also be used to examine systems that involve multi-site interactions. As an example, Eqns. (5) or (6) give the response that would be expected for a two-site model, where the analyte (A) binds at two classes of independent sites (L1 and L2) [22,24].
| (5) |
| (6) |
In these equations, Ka1 is the association equilibrium constant for the binding site with the highest affinity for the analyte, and Ka2 is the association equilibrium constant for the site with weaker binding, where 0 < Ka2 < Ka1. The terms mL1 and mL2 represent the moles present for each of these sites, mLtot is the total moles of all binding sites for the analyte (where mLtot = mL1 + mL2 for a two-site case), and α1 is the fraction of all binding regions for the analyte that belong to the first group of sites (where α1 =mL1,tot/mLtot). The term β2 is the ratio of the association equilibrium constants for the low affinity binding sites versus the high affinity sites (where β2=Ka2/Ka1). Similar expressions can be written for systems with more than two classes of binding sites for an analyte [25].
Zonal elution involves injecting a known concentration of analyte onto a column while monitoring its elution. Zonal elution can be used to determine association equilibrium constants for solute-ligand interactions and the location of binding sites for these interactions through competition studies. This type of competition can be described by using both the reaction in Eqn. (1) and following reaction for the binding of a competing agent (I) to a site on ligand L,
| (7) |
where KI is the association equilibrium constant for I at the site of competition with A. Eqn. (8) can be used with this model to describe the effect of I on the retention of A [22,24],
| (8) |
where k is the retention factor of the injected analyte, VM is the void volume of the column, [I] is the molar concentration of competing agent in the mobile phase, mL is the moles of binding sites involved in the competition between I and A, and Ka is now the association equilibrium constant for A at its site of competition with I. Eqn. (8) predicts that a plot of 1/k versus [I] will give a linear response for a system with 1:1 competition between A and I and in which A has no other binding sites in the column. The above relationship also predicts this line will have a slope equal to (VM KI)/(Ka mL) and an intercept of VM/(Ka mL). This information makes it possible to calculate the association equilibrium constants for the analyte and competing agent at their site of competition on the ligand [22,24]. This relationship will be used in this study with competition studies and site-selective probes to identify the binding site(s) for impramine on HSA and to measure the association equilibrium constant(s) for these interactions.
3. Experimental
3.1 Reagents
Human serum albumin (Cohn fraction V, essentially fatty acid free, ≥ 96% pure), imipramine hydrochloride (≥ 98%), L-tryptophan (> 98%), and racemic warfarin (98%) were from Sigma (St. Louis, MO, USA). Nucleosil Si-300 silica (300 Å pore size, 5 μm particle size) was from Macherey Nagel (Düren, Germany). Reagents for the bicinchoninic acid (BCA) protein assay were from Pierce (Rockford, IL, USA). All buffers and aqueous solutions were prepared using water from a Nanopure system (Barnstead, Dubuque, IA, USA) and filtered using Osmonics 0.22 μm nylon filters from Fisher (Pittsburgh, PA, USA).
3.2 Apparatus
The chromatographic system consisted of an isocratic Shimadzu LC-10AD pump, a Dynamax solvent delivery system (Rainin, Woburn, MA, USA), a six-port Lab Pro valve (Rheodyne, Cotati, CA, USA), and a UV-2075 detector (Jasco, Easton, MD, USA). An Alltech water jacket (Deerfield, IL, USA) and a circulating water bath from Fisher (Pittsburgh, PA, USA) were used to maintain a temperature of 37 (± 0.1)°C for the chromatographic system during all experiments described in this report. The columns were packed using an Alltech slurry packer. The diol content of the silica was measured using an MDQ capillary electrophoresis system (Beckman, Fullerton, CA, USA). Chromatographic data were collected and processed using in-house programs written in LabView 5.1 (National Instruments, Austin, TX, USA).
3.3 Methods
The stationary phase used in these studies consisted of silica particles containing immobilized HSA. To make this material, Nucleosil Si-300 silica was converted into a diol-bonded form, as described previously [26]. The final diol coverage of silica prior to activation was measured in triplicate by an iodometric capillary electrophoresis assay [27], giving a value of 260 (± 40) μmol diol per gram of silica. HSA was immobilized onto the diol silica by using the Schiff base method [28,29]. In this method, 1 g of diol silica was combined with 2 g periodic acid in 100 mL of a 90% acetic acid solution in water, with this mixture then being allowed to react for 3 h at room temperature with shaking. The resulting aldehyde-activated silica was washed six times with water and two times with 0.10 M, pH 6.0 potassium phosphate buffer.
The aldehyde-activated silica was next combined with a 10 mL solution of 0.10 M, pH 6.0 potassium phosphate buffer containing 0.3 g HSA and 0.15 g sodium cyanoborohydride. This mixture was allowed to shake on a rotary mixer at 4°C for 9 days. The HSA silica that this reaction produced was washed four times with 0.10 M, pH 8.0 potassium phosphate buffer and slowly combined with three portions of 0.030 g sodium borohydride, added over the course of 90 min while the mixture was allowed to react at room temperature. This last step was used to convert any excess aldehyde groups on the support into alcohols. This slurry was washed three times with 0.10 M, pH 8.0 potassium phosphate buffer supplemented with sodium chloride and washed two times with 0.067 M, pH 7.4 potassium phosphate buffer. The final HSA silica was stored in 0.067 M, pH 7.4 potassium phosphate buffer at 4°C until use. The control support for this material was prepared in the same method but with no HSA being added during the immobilization step.
Small portions of the HSA silica and control support were washed several times with water and dried under vacuum at room temperature. These dried samples were analyzed in triplicate using a BCA protein assay [30], using HSA as the standard and the control support as the blank. The final protein content measured for the HSA silica by this approach was 44 (± 3) mg HSA per gram of silica. The remaining portions of the original HSA silica and control support were downward slurry packed at 3500 psi (24 MPa) into separate 2.1 mm I.D. × 3.5 mm long stainless steel columns using 0.067 M, pH 7.4 potassium phosphate buffer as the packing solution. Each column was placed in a water jacket and connected to a circulating water bath for temperature control. These columns were stored at 4°C in pH 7.4, 0.067 M phosphate buffer when not in use. These columns were used within a period of 6 months and were typically stable for up to one year under the storage and experimental conditions utilized in this study.
Imipramine is known to be light sensitive [31], but aqueous solutions of this drug were found in this study to be stable for several weeks when stored in the dark at 4°C. Solutions of warfarin in a pH 7.4, 0.067 M phosphate buffer are also known to be stable for several days when stored at 4°C [32], but solutions of L-tryptophan were prepared daily due to the limited stability of this reagent under such conditions [33,34]. All solutions used in this report were made in pH 7.4, 0.067 M potassium phosphate buffer and stored at 4°C. All mobile phases for the chromatographic studies were prepared from this same buffer and were degassed for 25 min prior to use.
Two methods were employed for examining the binding of imipramine with HSA. Frontal analysis was conducted by using two pumps: the first pump was used to apply only buffer (i.e., pH 7.4, 0.067 M potassium phosphate buffer) to the column, while the other pump was used to apply a solution containing a known concentration of imipramine in this buffer. After a breakthrough curve had been obtained, only pH 7.4, 0.067 M potassium phosphate buffer was applied to the column to elute the retained drug. Identical studies were carried out on a control column to correct for the void time of the system and for any secondary interactions between imipramine and the support. In the frontal analysis studies, the concentrations of imipramine that were used ranged from 0.75–400 μM. These solutions were applied at a flow rate of 0.3 mL/min. The wavelength of detection was 251 nm for all concentrations except for 200–400 μM, for which a wavelength of 300 nm was employed. These two sets of wavelengths were used to ensure that all measured absorbance values were within the linear region of response for the UV/Vis absorbance detector. The mean breakthrough point of each frontal analysis curve was determined by using the equal area method [22,24].
The probe compounds used in the competition studies to identify imipramine’s binding sites on HSA were racemic warfarin and L-tryptophan. Racemic warfarin is known to bind to Sudlow site I, and L-tryptophan is known to bind to Sudlow site II [3]. The detection wavelength was 251 nm when imipramine was used as the injected solute and 280 nm when L-tryptophan was the injected probe (note: warfarin was used only as a mobile phase additive). Zonal elution using imipramine as the competing agent was done by injecting a 5 μL sample of 20 μM imipramine at 0.5 mL/min in the presence of mobile phases containing various known concentrations of racemic warfarin (2–20 μM) or L-tryptophan (5–500 μM). A 20 μM imipramine sample was chosen for this work because no noticeable change was noted in the measured retention of this analyte when using small concentrations, thus indicating that linear elution conditions were present, as is assumed in Eqn. (8). The void time of the system was determined by injecting 5 μL of 25 μM sodium nitrate in 0.067 M, pH 7.4 potassium phosphate buffer at 205 nm, with 0.067 M, pH 7.4 potassium phosphate buffer also being used as the mobile phase. The central moment of each peak was determined using Peakfit 4.12 software (Systat Software, San Jose, CA, USA).
4. Results and Discussion
4.1 Frontal analysis studies of imipramine/HSA binding
Frontal analysis was performed to determine the extent of the overall interaction between imipramine and HSA. Figure 3 shows the results when the data were plotted according to Eqn. (3). This plot showed non-linear behavior, which indicated that at least two different types of binding regions were involved in the interactions of imipramine with HSA, as predicted by Eqn. (5) for a two-site system. However, the response seen for imipramine did approach a linear response at low concentrations (or high values of 1/[Imipramine]), as is expected for even a multisite system [22,25].
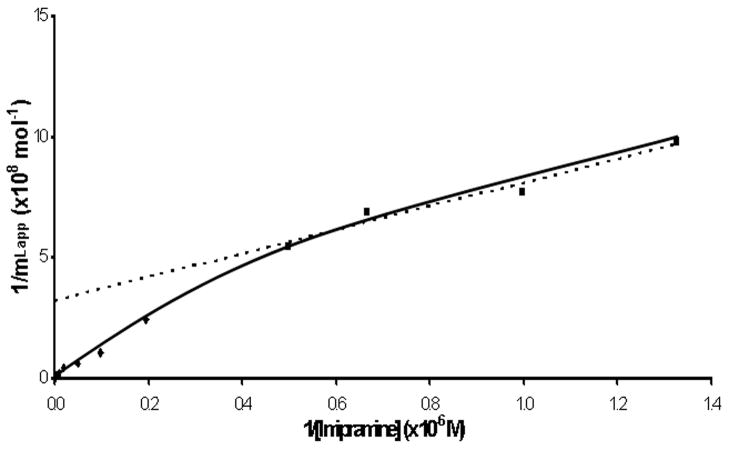
Figure 3.
Double-reciprocal frontal analysis plot prepared according to Eqn. (3) for the application of imipramine to an HSA column for imipramine concentrations that ranged from 0.75 to 400 μM. The dashed line shows the best-fit linear response to the four points to the right of this plot, which represent imipramine concentrations of 0.75 of 20 μM. The equation for this best-fit line was y = 486 (± 65) x + 3.2 (± 0.6) × 108 and had a correlation coefficient of 0.9825.
It is known from previous work that this linear range can be used with Eqn. (5) to obtain a preliminary estimate of the association equilibrium constant for the highest affinity site in the system [22,25]. This was done by using a best-fit line for the upper values in Figure 3 (i.e., as represented by the dashed line and imipramine concentrations of 0.75–20 μM), which had a correlation coefficient of 0.9825 (n = 4). The estimated association equilibrium constant for the high affinity site that was obtained through this approach was 6.6 (± 3.9) × 105 M−1 at pH 7.4 and 37°C.
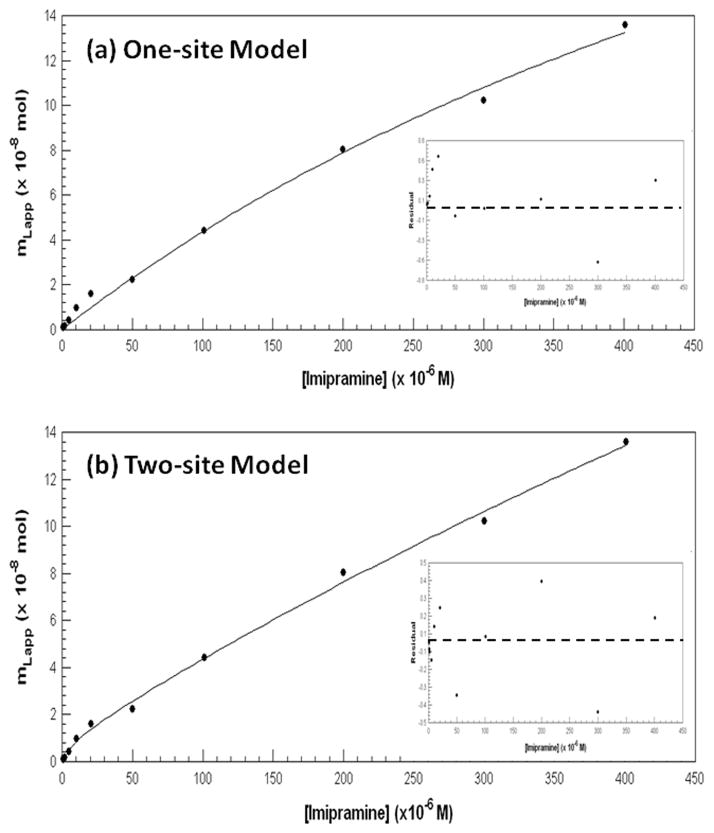
Results from the frontal analysis studies were examined more closely by also plotting the data according to Eqns. (4) and (6). Figures 4(a) and 4(b) show the results that were obtained when the entire data set (n = 12 over a 500-fold range of imipramine concentrations) was fit to either a one-site or two-site model. Both models gave high correlation coefficients (one-site model, 0.9976; two-site model, 0.9988). However, a comparison of the overall fit of these models and the resulting residual plots (see insets for Figure 4) indicated that the two-site model clearly gave a better fit to the results from these studies.
Figure 4.
Examination of frontal analysis data for imipramine on an HSA column using (a) a one-site model based on Eqn. (4) or (b) a two-site model based on Eqn. (6). The association equilibrium constants and binding capacities that were found using the two-site model are given in the text. The insets to these figures show the residual plots for the two fits, where the residual values on the y-axis are the difference in the actual and best-fit values at a given concentration of imipramine.
The association equilibrium constants for the higher affinity site in the fit to the two-site model was estimated from Figure 4(b) to be 3 (± 7) × 105 M−1, which agreed with the previous value obtained from Figure 3 (note: a more precise estimate for this value will be provided later in this study). The association equilibrium constant for the second and weaker group of binding sites in this model was found to be 1.6 (± 0.4) × 103 M−1. The size of these binding constants suggested that the high affinity interaction involves a specific region on HSA, while the lower affinity sites involve more general and non-specific interactions. Similar binding behavior has recently been noted in the binding of other drugs and solutes to HSA [34,35].
For the sake of comparison, the apparent association equilibrium constant that was found when using a one-site model was also determined by using the results in Figure 4. This gave an apparent Ka value of 2.0 (± 0.3) × 103 M−1 for the overall binding of imipramine with HSA. It is interesting to note that this apparent value is statistically identical to the association equilibrium constant that was reported in Ref. [12] for the same system when binding at a single site was assumed to be present.
A model for imipramine-HSA binding that was based on a high affinity, specific binding region and a second group of general, weaker binding sites was supported by the binding capacities that were determined from the two-site fit in Figure 4(b). A binding capacity of 3.8 (± 3.7) nmol imipramine was measured for the high affinity site and 310 (± 50) nmol imipramine was measured for the weaker binding regions. The total content of HSA was determined through a protein assay to be 36 (± 3) nmol. The resulting specific activity for imipramine at its high affinity site was 0.11 (± 0.10) mol/mol HSA, which is a value similar to that seen in other studies dealing with drugs that bind to specific sites on immobilized HSA [36,37]. On the other hand, the binding of imipramine to the second group of lower affinity sites gave a specific activity of 8.6 (± 1.6) mol imipramine/mol HSA, which clearly indicates the presence of multiple and probably non-specific binding regions for this particular interaction [34,35].
The relatively large number of non-specific binding regions that were found to be present in this analysis (i.e., accounting for up to 8–9 mol imipramine/mol HSA) is consistent with the results that were reported in Refs. [18,19]. The large number of these weak binding regions, plus the relatively large amounts of imipramine that were used in these other studies (i.e., up to a 5–20 fold mole excess of imipramine versus HSA) [18,19] further explains why a higher affinity site for imipramine on HSA was not observed in these previous reports.
4.2 Competition of imipramine with warfarin
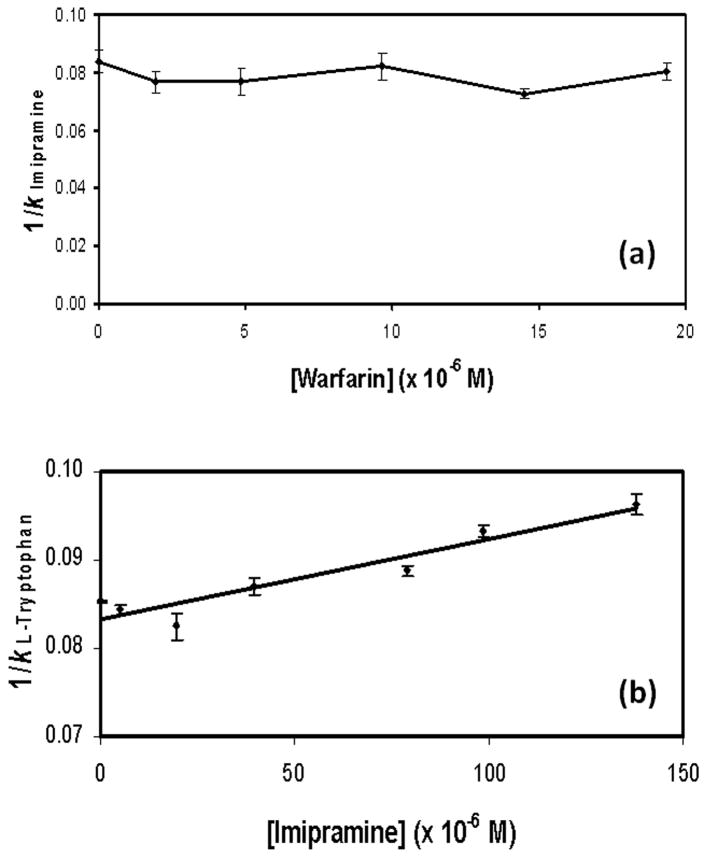
The next series of experiments were designed to identify the high affinity site for imipramine on HSA and to better define the association equilibrium constant for imipramine at this site. This was accomplished by using zonal elution to look at the competition of imipramine with several probe compounds that had known binding sites on HSA. The first probe used in this work was warfarin, which is known to bind to Sudlow site I [23]. In these studies, a small amount of imipramine was injected onto the HSA column and control column as varying concentrations of racemic warfarin were placed into the mobile phase (i.e., 0 to 20 μM). The retention factor for imipramine was then measured and compared at the various concentrations of warfarin that were examined. The results are shown in Figure 5(a).
Figure 5.
Competition of (a) imipramine with warfarin as mobile phase additive or (b) L-tryptophan with imipramine as a mobile phase additive. Both of these studies were conducted at pH 7.4 and 37°C. Other experimental conditions are given in the text. The error bars represent a range of ±1 S.D. The equation for the best-fit line in (b) was y = 87 (± 10) x + 0.084 (± 0.001), with a correlation coefficient of 0.9668 (n = 7).
According to Eqn. (8), a plot of 1/kImipramine versus [Warfarin] would be expected to give a linear relationship if simple one-site competition was present between warfarin and imipramine as they undergo binding to HSA. However, what was observed was only random variations in the retention of imipramine, which indicated that no interactions were present between these two solutes [22]. This result demonstrated that imipramine did not bind to Sudlow site I, the site at which warfarin is known to interact with HSA. This data also indicated that imipramine did not have any allosteric interactions with warfarin. This second piece of information further suggested that the tamoxifen site of HSA was also not the location of the high affinity site of imipramine, since solutes that bind at this site tend to have an allosteric interaction with warfarin at Sudlow site I [38–40].
4.3 Competition of imipramine with L-tryptophan
The second major binding site of HSA is Sudlow site II [3]. L-Tryptophan is known to bind to this region and is often used as a site-selective probe for this site. Experiments were first conducted by injecting a sample of imipramine in the presence of various concentrations of L-tryptophan in the mobile phase. The chromatograms that were obtained, as shown in Figure 2(b), gave a shift in the retention of imipramine as the mobile phase concentration of L-tryptophan was increased. Such behavior indicated that either direct competition or negative allosteric interactions were occurring between L-tryptophan and imipramine on the HSA column.
The nature of this interaction was examined more closely by performing the reverse study in which small amounts of L-tryptophan were injected in the presence of imipramine as a mobile phase additive. Because L-tryptophan is known to bind to only a single well-defined site on HSA, this experiment made it possible to determine whether direct competition or allosteric effects were occurring between this compound and imipramine. Figure 5(b) shows the results that were obtained when the data were analyzed according to Eqn. (8). A linear relationship was noted for a plot of 1/kL-Tryptophan versus [Imipramine], which is consistent with a model in which direct competition is occurring between imipramine and L-tryptophan at a single site on HSA (i.e., Sudlow site II).
Eqn. (8) was used along with the slope and intercept of Figure 5(b) to determine the association equilibrium constant for imipramine at Sudlow site II. This gave a value of 1.6 (± 1.0) × 105 M−1, which was consistent with the estimate that was obtained for the high affinity site of imipramine on HSA in the frontal analysis studies. This more precise estimate of Ka for the high affinity site of imipramine was then used with a two-site model to obtain an improved estimate for the association equilibrium constant of the weaker affinity sites. The association equilibrium constant that was found for the weaker binding regions by this approach was 1.5 (± 0.4) × 103 M−1. The corresponding binding capacities and specific activities obtained in this revised fit were as follows: 4.8 (± 2.4) nmol or 0.13 (± 0.6) mol imipramine/mol HSA for the high affinity site; and 320 (± 50) nmol or 8.9 (± 1.4) mol imipramine/mol HSA for the weaker binding regions.
5. Conclusions
This report investigated the binding of imipramine to HSA by using HPAC. Based on frontal analysis studies, imipramine was found to have one high affinity site on HSA. The best estimate for the association equilibrium constant of imipramine at this site was 1.6 (± 1.0) × 105 M−1 at pH 7.4 and 37°C. A second group of weaker, non-specific binding regions were also identified (8–9 mol/mol HSA) with an average association equilibrium constant of 1.5 (± 0.4) × 103 M−1 at pH 7.4 and 37°C. Competition studies with L-tryptophan indicated that imipramine was binding to Sudlow site II, which was also identified as the high affinity site for imipramine on HSA. No competition or allosteric interactions were noted in similar competition studies conducted between imipramine and warfarin, which indicated that imipramine was not interacting with Sudlow site I or the tamoxifen site of HSA. The results of this study are consistent with previous observations that have been made in the literature. For instance, this study indicated that there is a specific site for imipramine on HSA, as has been reported in Refs. [6,12,13], as well as a large group of weak affinity regions, as noted in Refs. [18,19]. These new results should provide a more detailed description of how imipramine is transported in blood and of how it may interact with other drugs in the circulatory system.
Acknowledgments
This work was supported by the National Institutes of Health under grants R01 NS052484 and R01 GM044931 was conducted in facilities that were renovated under NIH grant RR015468.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Otagiri M. Drug Metab Pharmacokinet. 2005;20:309. doi: 10.2133/dmpk.20.309. [DOI] [PubMed] [Google Scholar]
- 2.Kragh-Hansen U. Pharmacol Rev. 1981;33:17. [PubMed] [Google Scholar]
- 3.Peters T., Jr . All About Albumin: Biochemistry, Genetics, and Medical Applications. Academic Press; San Diego, CA: 1996. [Google Scholar]
- 4.Bertucci C, Domenici E. Curr Med Chem. 2002;9:1463. doi: 10.2174/0929867023369673. [DOI] [PubMed] [Google Scholar]
- 5.He XM, Carter DC. Nature. 1992;358:209. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- 6.Sharples D. J Pharm Pharmac. 1975;27:379. doi: 10.1111/j.2042-7158.1975.tb09463.x. [DOI] [PubMed] [Google Scholar]
- 7.Lader M. In: Handbook of Experimental Pharmacology. SH Preskorn, CY Stanga, JP Feighner, R Ross., editors. Vol. 157. Springer-Verlag; Berlin: 2004. pp. 185–209. [Google Scholar]
- 8.Preskorn SH, Dorey RC, Jerkovich GS. Clin Chem. 1988;34:822. [PubMed] [Google Scholar]
- 9.Samanidou VF, Nika MK, Papadoyannis IN. J Sep Sci. 2007;30:2391. doi: 10.1002/jssc.200700142. [DOI] [PubMed] [Google Scholar]
- 10.Brasfield KH. Nursing Clin N Am. 1991;26:651. [PubMed] [Google Scholar]
- 11.Sowell J, Mason JC, Strekowski L, Patonay G. Electrophoresis. 2001;22:2512. doi: 10.1002/1522-2683(200107)22:12<2512::AID-ELPS2512>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Jia Z, Ramstad T, Zhong M. J Pharm Biomed Anal. 2002;30:405. doi: 10.1016/s0731-7085(02)00223-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhou D, Li F. Sepu. 2003;21:143. [Google Scholar]
- 14.Kim HS, Wainer IW. J Chromatogr B. 2008;870:22. doi: 10.1016/j.jchromb.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollosy F, Valko K, Hersey A, Nunhuck S, Keri G, Bevan C. J Med Chem. 2006;49:6958. doi: 10.1021/jm050957i. [DOI] [PubMed] [Google Scholar]
- 16.Hanai T, Koseki A, Yoshikawa R, Ueno M, Kinoshita T, Homma H. Anal Chim Acta. 2002;454:101. [Google Scholar]
- 17.Buchholz L, Cai CH, Andress L, Cleton A, Brodfuehrer J, Cohen L. Eur J Pharm Sci. 2002;15:209. doi: 10.1016/s0928-0987(01)00219-6. [DOI] [PubMed] [Google Scholar]
- 18.Javaid JI, Hendricks K, Davis JM. Biochem Pharmacol. 1983;32:1149. doi: 10.1016/0006-2952(83)90263-0. [DOI] [PubMed] [Google Scholar]
- 19.Bickel MH. J Pharm Pharmacol. 1975;27:733. doi: 10.1111/j.2042-7158.1975.tb09392.x. [DOI] [PubMed] [Google Scholar]
- 20.Omran A, El-Sayed AY, Shehata AM, El-Erian MA. J Appl Sci Res. 2007;3:1730. [Google Scholar]
- 21.Mathias U, Jung M. Anal Bioanal Chem. 2007;388:1147. doi: 10.1007/s00216-007-1351-7. [DOI] [PubMed] [Google Scholar]
- 22.Hage DS. J Chromatogr B. 2002;768:3. doi: 10.1016/s0378-4347(01)00482-0. [DOI] [PubMed] [Google Scholar]
- 23.Loun B, Hage DS. Anal Chem. 1994;66:3814. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 24.Hage DS, Chen J. In: Handbook of Affinity Chromatography. Hage DS, editor. CRC Press/Taylor & Francis; New York: 2006. Chapter 22. [Google Scholar]
- 25.Tweed SA, Loun B, Hage DS. Anal Chem. 1997;69:4790. doi: 10.1021/ac970565m. [DOI] [PubMed] [Google Scholar]
- 26.Ruhn PF, Garver S, Hage DS. J Chromatogr A. 1994;669:9. doi: 10.1016/0021-9673(94)80332-3. [DOI] [PubMed] [Google Scholar]
- 27.Chattopadhyay A, Hage DS. J Chromatogr. 1997;758:255. doi: 10.1016/s0021-9673(96)00742-x. [DOI] [PubMed] [Google Scholar]
- 28.Larson PO. Methods Enzymol. 1984;104:212. doi: 10.1016/s0076-6879(84)04091-x. [DOI] [PubMed] [Google Scholar]
- 29.Loun B, Hage DS. J Chromatogr. 1992;579:225. [PubMed] [Google Scholar]
- 30.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Anal Biochem. 1985;150:76. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 31.Syed AA, Silwadi MF, Khatoon BA. J Pharmaceut Biomed Anal. 2002;28:501. doi: 10.1016/s0731-7085(01)00665-3. [DOI] [PubMed] [Google Scholar]
- 32.Moser AC, Kingsbury C, Hage DS. J Pharmaceut Biomed Anal. 2006;41:1101. doi: 10.1016/j.jpba.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Lee MG, Rogers CM. J Parenteral Sci Technol. 1988;42:20. [PubMed] [Google Scholar]
- 34.Conrad M. MS Thesis. University of Nebraska-Lincoln; Lincoln, NE: 2008. [Google Scholar]
- 35.KS Joseph, AC Moser, S Basiaga, JE Schiel, DS Hage. J Chromatogr A. in press. [Google Scholar]
- 36.Yang J, Hage DS. J Chromatogr. 1993;645:241. doi: 10.1016/0021-9673(93)83383-4. [DOI] [PubMed] [Google Scholar]
- 37.Loun B, Hage DS. Anal Chem. 1994;66:3814. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta A, Hage DS. Anal Chem. 1999;71:3821. doi: 10.1021/ac9903499. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Hage DS. Anal Chem. 2006;78:2672. doi: 10.1021/ac052017b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjoholm I, Ekman B, Kober A, Ljungstedt-Pahlman I, Seiving B, Sjodin T. Mol Pharmacol. 1979;16:767. [PubMed] [Google Scholar]