Abstract
Context:
Naltrexone hydrochloride treatment for alcohol dependence works for some individuals but not for everyone. Asn40Asp, a functional polymorphism of the μ-opioid receptor gene (OPRM1), might predict naltrexone response.
Objective:
To evaluate whether individuals with alcoholism who are heterozygous (Asp40/Asn40) or homozygous (Asp40/Asp40) for the OPRM1 Asp40 allele respond better to naltrexone.
Design:
Pharmacogenetic analysis conducted between January 1, 2001, and January 31, 2004.
Setting:
Eleven academic sites in the COMBINE Study.
Participants:
Recently abstinent volunteers who met all 3 of the following conditions: (1) DSM-IV criteria for primary alcohol dependence; (2) participation in the COMBINE Study; and (3) availability of DNA.
Interventions:
Alcoholic subjects were treated for 16 weeks with 100 mg of naltrexone hydrochloride (234 Asn40 homozygotes and 67 with at least 1 copy of the Asp40 allele) or placebo (235 Asn40 homozygotes and 68 with at least 1 copy of the Asp40 allele). All participants received medical management (MM) alone or with combined behavioral intervention (CBI).
Main Outcome Measures:
Time trends in percentage of days abstinent, percentage of heavy drinking days, and rates of good clinical outcome.
Results:
Alcoholic subjects with an Asp40 allele receiving MM alone (no CBI) had an increased percentage of days abstinent (P=.07) and a decreased percentage of heavy drinking days (P=.04) if treated with naltrexone vs placebo, while those with the Asn40/Asn40 genotype showed no medication differences. If treated with MM alone and naltrexone, 87.1% of Asp40 carriers had a good clinical outcome, compared with only 54.8% of individuals with the Asn40/Asn40 genotype (odds ratio, 5.75; confidence interval, 1.88-17.54), while, if treated with placebo, 48.6% of Asp40 carriers and 54.0% of individuals with the Asn40/Asn40 genotype had a good clinical outcome (interaction between medication and genotype, P=.005). No gene×medication interactions were observed in those treated with both MM and CBI.
Conclusions:
These results confirm and extend the observation that the functionally significant OPRM1 Asp40 allele predicts naltrexone treatment response in alcoholic individuals. This relationship might be obscured, however, by other efficacious treatments. OPRM1 geno-typing in alcoholic individuals might be useful to assist in selecting treatment options.
While pharmacotherapy for alcohol dependence is limited, several drugs have received Food and Drug Administration approval.1,2 Meta-analyses of many single-site and multisite studies of the opioid antagonist medication naltrexone hydrochloride have suggested that the effect size for response over placebo is in the small to moderate range.3-6 For instance, in the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study,7 naltrexone showed its prime efficacy when used in the context of medical management (MM) (number needed to treat, 6-7) but not when combined with a specialized alcohol counseling approach. On the basis of this extensive experience, it is clear that not all individuals with alcohol dependence respond to naltrexone. Attempts to identify individual factors that would be associated with a positive response have been limited and inconsistent. Therefore, any improvement in the ability to predict naltrexone response would have immense theoretical and clinical value.
It is well known that naltrexone is a specific opioid antagonist targeting endogenous opioid receptors, particularly (but not exclusively) μ-receptors.8,9 It has also been observed that alcohol increases the release of the endogenous opioids β-endorphin and enkephalin in animals10,11 and humans.12 Blocking opioid receptors with naltrexone leads to less alcohol-induced pleasure, high, and intoxication13 and, ultimately, less craving14 and relapse.15 Indeed, there is a high concentration of μ-opioid receptors in human brain areas, such as the nucleus accumbens, amygdala, cingulate gyri, and basal ganglia,16 that have been implicated in the reward pathway associated with alcohol and substance abuse. Occupancy of these receptors is related to alcohol craving.17 It is also known that μ-opioid receptors primarily bind β-endorphin and transduce this binding via G-protein signaling that ultimately alters neuronal firing and leads to neuroadaptive changes. Although not a consistent finding,18 the discovery that a μ-opioid receptor (OPRM1) missense polymorphism, Asn40Asp,19 was capable of changing β-endorphin binding, function, and receptor levels20,21 led to speculation of functional effects in humans and formed the basis for examination of differential naltrexone response.
The asparagine-to-aspartate amino acid substitution at position 40 is caused by an A118G single nucleotide substitution, leading to structural variation in the receptor extracellular domain. This substitution has been reported to increase binding of β-endorphin and increase functional activity in vitro.20 In addition, early reports have suggested that the 15% to 25% of humans22,23 who carry the Asp40 allele show a greater response to alcohol,24,25 greater endocrine response to opioid antagonists,26 and less response to painful stimuli.27 In addition, a similar polymorphism has been reported to reduce alcohol-induced stimulation and alcohol consumption in non-human primates.28 However, as reported when this polymorphism was first described,19 a meta-analysis of human population studies suggested that this OPRM1 polymorphism is not associated with a higher genetic risk of developing alcohol dependence.23
Most germane to this study was a report by Oslin and colleagues29 that suggested, in an exploratory manner, that treatment-seeking alcoholic-dependent individuals with at least 1 copy of the Asp40 allele responded to naltrexone better than did those without the allele. That report, however, combined 3 disparate studies with limited outcome analysis (bivariate abstinence and relapse to heavy drinking). Also, the analytic plan used a number of covariates (such as age, sex, and marital status) that limited the potential generalizability of the finding. Subsequent to that report, a reanalysis of a Veterans Affairs Naltrexone Cooperative Study30 found that individuals who had at least 1 copy of the Asp40 allele showed relapse to heavy drinking rates similar to those of Asn40 homozygotes when treated with naltrexone; however, they did not evaluate other drinking outcome variables.31
The COMBINE Study was a multisite study designed to address whether naltrexone, acamprosate calcium, or specialized counseling, called combined behavioral intervention (CBI), when given in the context of an MM approach, were individually better than placebo or whether combinations of these would be better than any one alone.32 The results of that trial7 indicated that naltrexone was better than placebo on a number of drinking outcome variables, but the naltrexone effect was largely observable only when CBI, ie, alcohol-specialty therapy, was not used concomitantly with MM and naltrexone. Acamprosate was not effective in its own right and did not significantly add to or detract from the response of naltrexone alone.
The goal of this preplanned pharmacogenetic study within the larger COMBINE Study was to examine the role of Asn40Asp as a predictor of naltrexone treatment response.33 We hypothesized that this variant would primarily predict response to naltrexone in the MM-only condition, given that this is the condition in which there was evidence that naltrexone was effective in the primary analyses.
METHODS
OVERVIEW OF STUDY DESIGN
The parent study rationale, design, and methods have been previously detailed34 and the results reported.7 For this pharmacogenetic study, conducted from January 1, 2001, through January 31, 2004, we focused primarily on white subjects receiving naltrexone (with or without acamprosate) or placebo. In brief, after baseline assessment and attainment of 4 days of abstinence, eligible alcohol-dependent individuals were randomized to naltrexone, with or without acamprosate, or to double placebo for 16 weeks of outpatient treatment. All of the groups received MM, a 9-session intervention focused on enhancing medication adherence and abstinence by means of a model that could be adapted to primary care settings. Half of the subjects were randomly assigned to receive a more intensive CBI delivered by alcoholism treatment specialists. An additional group received CBI alone without any pills or MM, but that group's results are not included in this report. Of the 1383 subjects randomized in the main trial, 1013 subjects provided genetic samples; of those, 911 samples (684 whites) were successfully genotyped (Figure 1). Those receiving naltrexone or placebo and MM with or without CBI (n=604) are the subjects of this report.
Figure 1.
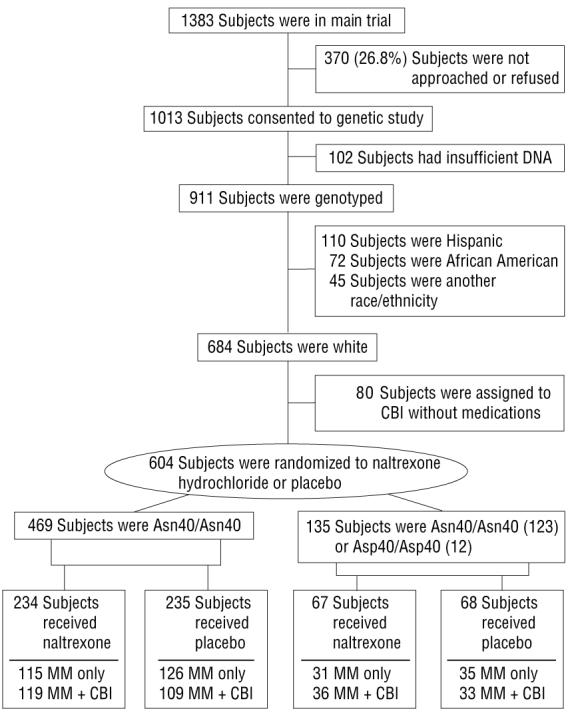
Distribution of subjects in the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study who had blood collected for genetic testing (OPRM1 genotypes). CBI indicates combined behavioral intervention; MM, medical management.
PARTICIPANT RECRUITMENT, ASSESSMENT, AND RANDOMIZATION
At 11 academic sites, participants were recruited by advertisements and from clinical referrals. All participants signed informed consents (accompanied by a certificate of confidentiality issued by the National Institute on Alcohol Abuse and Alcoholism) approved by each site's institutional review board for the main clinical trial. Participants gave consent separately for the pharmacogenetic substudy. Consenting for this substudy began after commencement of randomization for the main trial; therefore, not all participants were available to provide consent for the substudy. Baseline drinking histories, psychosocial data, health screens (including laboratory general health panels), and specific alcohol biomarkers were obtained. Eligibility criteria included (1) alcohol dependence, determined by DSM-IV35 criteria, using the Structured Clinical Interview for DSM-IV36; (2) 4 to 21 days of abstinence; and (3) consumption of more than 14 drinks (women) or 21 drinks (men) per week, with atleast 2 heavy drinking days(defined as ≥4 drinks for women and ≥5 for men) during a consecutive 30-day period within the 90 days before baseline evaluation. Exclusion criteria included (1) history of substance abuse (other than nicotine or cannabis) by DSM-IV criteria in the past 90 days (except 6 months for opioid abuse) or by urine drug screen, (2) psychiatric disorder requiring medication, or (3) unstable medical conditions (eg, serum liver enzyme levels >3 times normal).
In the initial pharmacogenetic analysis of this report, the study sample was restricted to the 604 white participants because (1) previous reports on the OPRM1 allele prediction of naltrexone response were restricted to whites only, (2) the Asp40 allele is reported to be present in less than 5% of African Americans but in 15% to 25% of whites, and (3) white subjects were well represented at all treatment sites and made up the majority of the study subjects providing acceptable OPRM1 allele frequencies for analysis. However, in a secondary analysis, we used all subjects irrespective of reported race/ethnicity (an additional 104 subjects), to check the sensitivity of the findings on the good clinical outcome measure.
ASSESSMENT
Drinking measures obtained from structured interviews at baseline37,38 and during the 16-week treatment period39 served as the basis for evaluating the genetic contribution to naltrexone response. At the 9 MM visits during treatment, research assistants (neither blinded to nor providing psychosocial treatment) assessed alcohol consumption39 and craving.40 At each appointment, adverse medication effects were assessed by a health care professional using the Systematic Assessment of Treatment Emergent Effects interview.41,42 Complete blood cell count and liver and kidney function tests were conducted at baseline and every 4 weeks. The γ-glutamyltransferase level and percentage of carbohydrate-deficient transferrin43,44 were measured at baseline and at weeks 8 and 16. Race and ethnicity were self-designated by participants, using an item allowing open-ended responses. All subjects and study site personnel, including investigators, research staff, evaluators, health care (MM) providers, and CBI therapists, were blind to medication assignment and genotype.
TREATMENT CONDITIONS
Medications
Each participant took up to 8 pills of active medication or placebo daily for 16 weeks. All naltrexone and placebo pills and all acamprosate and placebo pills were identical in appearance. Subjects in each group took the same number of pills per day. Naltrexone or its placebo was given as 2 pills once a day as follows: 1 placebo and 1 containing 25 mg of naltrexone hydrochloride or placebo on days 1 through 4, 1 placebo and 1 containing 50 mg of naltrexone hydrochloride or placebo on days 5 though 7, and two 50-mg pills (100 mg daily) of naltrexone hydrochloride or placebo on days 8 through 112. Acamprosate or its placebo was administered as 2 pills (500 mg each of acamprosate calcium or placebo) 3 times per day (3 g daily). Naltrexone and its placebo differed in appearance from acamprosate and its placebo. On the basis of tolerability, the MM clinician could reduce the acamprosate pills and then reduce the naltrexone pills. Attempts were made to reestablish the full dose. Doses were chosen on the basis of preliminary evidence that doses higher than commonly prescribed could be more efficacious and provide better coverage for missed doses45,46 and were well tolerated.32,47 For this report, we grouped patients according to whether they received active naltrexone or placebo, naltrexone, regardless of whether they received acamprosate or placebo acamprosate.
MM Regimen
The MM48,49 was delivered by a licensed health care professional over 9 sessions (weeks 0, 1, 2, 4, 6, 8, 10, 12, and 16) during which pills were dispensed. The initial visit averaged 45 minutes and began with a review of the alcohol-dependence diagnosis and the negative consequences of drinking. The provider recommended abstinence, provided education about the medications, and developed a medication adherence plan in collaboration with the patient. Attendance at support groups (eg, Alcoholics Anonymous) was encouraged. Subsequent sessions, averaging 20 minutes, included review of drinking, overall functioning, medication adherence, and adverse effects. Patients who resumed drinking were given advice and encouraged to attend support groups. Problems with medication adherence were addressed. Participants who discontinued medication because of intolerance continued in MM sessions to support abstinence.
CBI Regimen
The CBI50,51 was delivered by licensed behavioral health specialists in up to 20 flexible participant need–adjusted 50-minute sessions (an average of 9 sessions were attended). It integrated aspects of cognitive behavioral therapy,52 12-step facilitation,53 motivational interviewing,54 and support system involvement external to the study.55,56
5′ Nuclease Genotyping
Genomic DNA was extracted from peripheral blood mono-nuclear cells. The 5′ nuclease genotyping assay (TaqMan; Applied Biosystems, Foster City, California) is a rapid and accurate method for high-throughput genotyping of single nucleotide polymorphisms, combining polymerase chain reaction amplification and sequence variant detection into a single step. Locus-specific primers and fluorogenic allele-specific probes were designed and manufactured (Applied Biosystems Assays-on-Demand, identification No. C_8950074).
The 5-μL reaction mixture consisted of 2.5 μL of a master mix, 0.125 μL of 20X assay mix, 8μM detection probe for each allele, 36μM forward and reverse primer each (all reagents supplied as TaqMan, Applied Biosystems), and 10 ng of genomic DNA diluted in 2.375 μL of Tris EDTA, pH 8.0 (extracted from serum samples by Quality Biological Inc, Gaithersburg, Maryland). Amplification was performed by PCR (Gene Amp PCR System 9700; Applied Biosystems) using 384-well plates and the following amplification profile: 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 92°C for 15 seconds and 60°C for 1 minute. After amplification, end-point fluorescence intensity was measured directly in the reaction plates (7900 Sequence Detector; Applied Biosystems). Genotypes were determined using proprietary software (Sequence Detection System Software Version 2.0; Applied Biosystems). Four genotyping signal clusters were identified, representing Asn40 and Asp40 homozygotes, Asn40/Asp40 heterozygotes, and no-DNA-template controls. Genotyping accuracy was determined by replicate genotyping of 203 DNA samples. The genotyping error rate was 0%. Genotyping completion rate was 99.6%. No significant deviation from Hardy-Weinberg equilibrium was found (P=.43 for the full sample and .49 for whites only). All genotyping was done at the National Institute on Alcohol Abuse and Alcoholism Laboratory of Neurogenetics (D.G., director), and results were reported blind to treatment assignment and outcome data, which were held separately at the study coordinating center at the University of North Carolina.
OUTCOME MEASURES
The drinking variables evaluated for the gene×medication interactions in each counseling group were time trends in percentage of days abstinent, percentage of heavy drinking days, and endof-study categorical measures of response: the number (and percentage) of subjects with a good clinical outcome. Good clinical outcome was as defined in the main trial, ie, abstinent or moderate drinking without problems, a maximum of 11 (women) or 14 (men) drinks per week, with no more than 2 days on which more than 3 drinks (women) or 4 drinks (men) were consumed, and 3 or fewer alcohol-related problems endorsed on the Drinker Inventory of Consequences scale57 during the last 8 weeks of treatment. A subject with missing values for this variable was deemed to have a poor (not good) clinical outcome.
STATISTICAL METHODS
Baseline characteristics for continuous variables are presented as means and standard deviations. Analysis of variance was used to test equality of means across categories. For each categorical variable, frequencies and percentages are reported, along with the P value for a χ2 test of equality of proportions across the categories.
For the drinking outcomes, percentage of days abstinent and percentage of heavy drinking days, percentages were calculated for each of the four 4-week periods in the 16-week treatment phase of the study. Four separate 4-week percentages were used rather than a single percentage over the 16-week period to allow for examination of trends, to reduce the effect of missing data, and to provide consistency with the analyses in the main trial.7 These variables were analyzed by means of a general linear model, adjusting for clinical center and the relevant baseline drinking variable. An unstructured covariance matrix was used to account for the repeated measures on each individual. Trends over time were investigated by means of a linear term in the 4 periods. A contrast was constructed to test for interactions between time and treatment-genotype combinations without adjustment for multiple tests.
Good clinical outcome, an a priori but arbitrarily defined categorical variable that allows group comparisons of clinical response, was modeled as a dichotomous variable and analyzed as in the main trial report.7 These dichotomous outcomes were modeled using logistic regression, adjusting for clinical center and baseline percentage of days abstinent.
Whether the association of the Asp40 allele to naltrexone response might be exerted through chance association to other nonspecific clinical trial and subject variables was evaluated by examining differences in treatment withdrawal, research completion (full 16-week drinking data reported), medication adherence (number who took at least 80% of prescribed medication), and main adverse events caused by naltrexone (nausea, fatigue, and headache) by means of logistic regression with allele status and medication group as independent and interacting factors.
RESULTS
The demographic characteristics of the pharmacogenetic study sample (whites who received naltrexone or placebo) in Table 1 are broken down by treatment group and by presence or absence of the Asp40 allele. All of the pertinent baseline demographic, drinking, and alcohol severity measures were similar across treatment and genotype groups, with no statistically significant differences. In addition, there were minimal differences in these variables between individuals who did and did not have DNA available.
Table 1.
Demographics of White Participants Who Provided Genetic Samples by Treatment Conditiona
| Medical Management (No CBI) |
CBI + Medical Management |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample |
Placebo |
Naltrexone Hydrochloride |
Placebo |
Naltrexone Hydrochloride |
||||||
|
Characteristic |
Asn40 (n=469) |
Asp40 (n=135) |
Asn40 (n=126) |
Asp40 (n=35) |
Asn40 (n=115) |
Asp40 (n=31) |
Asn40 (n=109) |
Asp40 (n=33) |
Asn40 (n=119) |
Asp40 (n=36) |
| Demographics | ||||||||||
| Age, y, mean (SD) | 45.6 (10.49) | 44.8 (10.51) | 45.2 (10.09) | 46.1 (12.08) | 45.7 (11.17) | 42.5 (10.01) | 45.8 (10.05) | 45.6 (9.53) | 45.7 (10.73) | 44.8 (10.28) |
| Sex, No. (%) M | 332 (70.8) | 91 (67.4) | 91 (72.2) | 23 (65.7) | 77 (67.0) | 21 (67.7) | 81 (74.3) | 24 (72.7) | 83 (69.7) | 23 (63.9) |
| Married, No. (%) | 214 (45.6) | 52 (38.5) | 50 (39.7) | 10 (28.6) | 51 (44.3) | 11 (35.5) | 61 (56.0) | 15 (45.5) | 52 (43.7) | 16 (44.4) |
| Employed, No. (%) | 344 (73.3) | 110 (81.5) | 91 (72.2) | 31 (88.6) | 88 (76.5) | 20 (64.5) | 82 (75.2) | 26 (78.8) | 83 (69.7) | 33 (91.7) |
| Education≤high school, No. (%) | 124 (26.4) | 35 (25.9) | 36 (28.6) | 11 (31.4) | 30 (26.1) | 8 (25.8) | 25 (22.9) | 11 (33.3) | 33 (27.7) | 5 (13.9) |
| Current smoker, No. (%) | 206 (43.9) | 58 (43.0) | 51 (40.5) | 13 (37.1) | 54 (47.0) | 13 (41.9) | 39 (35.8) | 16 (48.5) | 62 (52.1) | 16 (44.4) |
| Alcohol use severity indicators, mean (SD) | ||||||||||
| % of days abstinentb | 23.9 (24.58) | 21.9 (23.91) | 24.5 (25.37) | 19.9 (23.99) | 23.9 (24.38) | 26.9 (27.73) | 19.8 (22.44) | 21.6 (23.38) | 27.1 (25.55) | 19.6 (20.99) |
| Drinks per drinking dayb | 12.6 (7.54) | 11.8 (7.14) | 12.4 (6.69) | 12.3 (8.37) | 12.6 (8.37) | 13.4 (9.14) | 12.4 (7.36) | 11.5 (5.19) | 12.8 (7.81) | 10.2 (5.10) |
| Overall drinks per dayb | 9.3 (6.18) | 9.2 (6.78) | 9.2 (6.17) | 9.5 (7.38) | 9.2 (6.14) | 10.5 (9.48) | 9.7 (5.97) | 8.6 (4.15) | 9.0 (6.47) | 8.3 (5.27) |
| Heavy drinking daysb | 20.0 (8.36) | 20.6 (8.10) | 20.1 (8.40) | 20.4 (7.98) | 19.9 (8.49) | 19.8 (9.10) | 21.1 (7.89) | 21.2 (7.90) | 19.1 (8.58) | 20.9 (7.79) |
| DSM-IV symptoms | 5.5 (1.26) | 5.5 (1.23) | 5.6 (1.29) | 5.4 (1.14) | 5.5 (1.27) | 5.6 (1.36) | 5.6 (1.21) | 5.6 (1.19) | 5.5 (1.27) | 5.3 (1.26) |
| Alcohol dependence scorec | 16.6 (7.26) | 16.9 (7.93) | 17.2 (7.30) | 16.6 (7.45) | 16.2 (7.43) | 18.1 (10.36) | 16.5 (6.99) | 17.7 (7.18) | 16.6 (7.36) | 15.3 (6.58) |
| OCDS score | 26.0 (7.11) | 26.5 (7.24) | 26.7 (7.50) | 24.6 (6.53) | 25.2 (7.33) | 25.8 (7.91) | 26.8 (5.96) | 28.2 (7.95) | 25.4 (7.37) | 27.2 (6.38) |
| Drinking consequences scored | 47.8 (19.91) | 46.5 (20.52) | 49.6 (20.06) | 46.8 (21.31) | 46.2 (20.05) | 45.2 (21.52) | 48.1 (19.78) | 47.0 (20.61) | 47.2 (19.81) | 47.1 (19.58) |
| GGT, IU/L, | 68.8 (112.4) | 61.8 (82.49) | 81.0 (168.7) | 59.3 (63.46) | 62.4 (74.90) | 63.3 (80.15) | 60.3 (53.88) | 64.6 (99.93) | 69.7 (107.4) | 60.5 (86.51) |
| % CDT | 3.5 (2.06) | 3.2 (1.84) | 3.7 (2.55) | 3.3 (2.27) | 3.3 (1.84) | 3.1 (1.59) | 3.5 (1.95) | 3.5 (2.12) | 3.5 (1.77) | 2.9 (1.14) |
| No. (%) | ||||||||||
| GGT>63 IU/L | 137 (29.2) | 33 (24.4) | 36 (28.6) | 9 (25.7) | 32 (27.8) | 8 (25.8) | 34 (31.2) | 10 (30.3) | 35 (29.4) | 6 (16.7) |
| CDT>2.5% | 227 (48.4) | 58 (43.0) | 63 (50.0) | 16 (45.7) | 50 (43.5) | 12 (38.7) | 57 (52.3) | 17 (51.5) | 57 (47.9) | 13 (36.1) |
Abbreviations: CBI, combined behavioral intervention; CDT, carbohydrate-deficient transferrin; GGT, γ-glutamyltransferase; OCDS, obsessive compulsive drinking scale.
SI conversion factor: To convert GGT to microkatals per liter, multiply by 0.01667.
Differences across treatment and genotypes were not significant. For the total sample and within each treatment combination, Asn40 (Asn40/Asn40 homozygote) individuals were compared with Asp40 carriers (Asn40Asp or Asp40Asp). For those receiving CBI plus medical management and naltrexone, “employed” had P=.004. All other P values were greater than .05.
In the 30 days before randomization.
Assessed using the Alcohol Dependence Scale.57
Assessed using the Drinker Inventory of Consequences scale.58
PERCENTAGE OF DAYS ABSTINENT
There were no significant main effects of genotype or medication, and no gene×medication×time interactions, in patients who received CBI in addition to MM (Figure 2A). However, in patients who received MM alone (no CBI), there was an interaction between treatment and genotype for the time trend of percentage of days abstinent (P=.07) among the 4 groups (Figure 2B). In pairwise comparisons, the time trends for naltrexone-treated carriers of Asp40 differed significantly from all other groups (P=.01-.03). Alcoholic patients who had at least 1 copy of the Asp40 allele and who were treated with naltrexone showed an increasing trend in abstinent days over time, while those without the Asp40 allele (Asn40/ Asn40 genotype) responded similarly to naltrexone as to placebo with fewer abstinent days over time. Carriers and noncarriers of the Asp40 allele who received placebo both showed a decreasing trend in abstinence over time.
Figure 2.
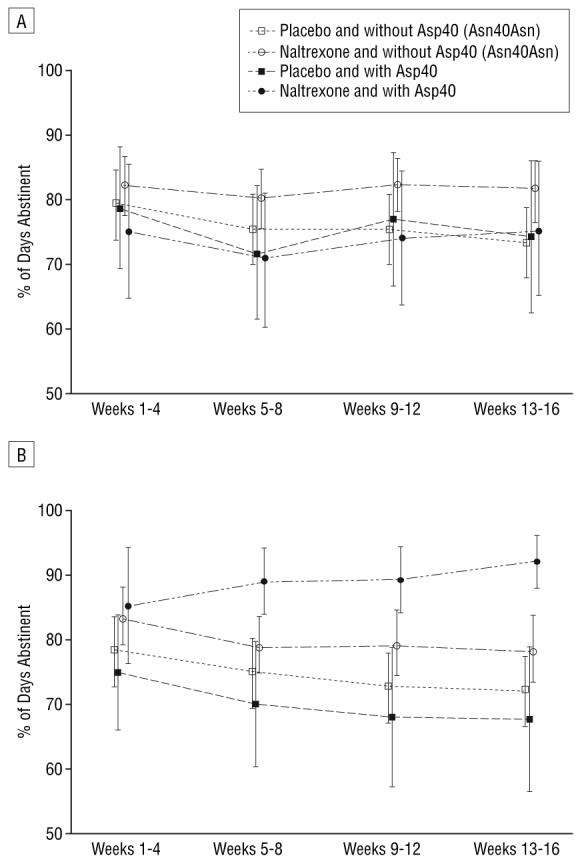
Medication effects on percentage of days abstinent (mean and 95% confidence interval) by OPRM1 genotype. A, Groups assigned to medical management and combined behavioral intervention (CBI) (test of group×time interaction, P=.35). B, Groups assigned to medical management alone (test of group×time interaction, P=.07; pairwise comparison of other groups with naltrexone hydrochloride and with Asp40 group, P=.01-.03).
PERCENTAGE OF HEAVY DRINKING DAYS
Alcoholic patients who received CBI plus MM did not show significant genotype, medication, or genotype×medication×time differences in the percentage of heavy drinking days (Figure 3A). However, in alcoholic patients receiving MM alone (no CBI), there was a significant (P=.04) difference in the time trend for percentage of heavy drinking days across the 4 genotype×medication groups (Figure 3B), again attributable to the combined effect of naltrexone and the Asp40 allele. In pairwise comparisons, alcoholic patients who were treated with naltrexone and who had at least 1 copy of Asp40 differed in time trend of heavy drinking days from all other groups, the pairwise P values ranging from .01 to .03. As Figure 3B shows, alcoholic patients with at least 1 copy of the Asp40 allele and who were treated with naltrexone had fewer heavy drinking days over time than did Asp40 carriers who were treated with placebo and Asp40 noncarriers (Asn40 homozygotes) who were treated with either naltrexone or placebo.
Figure 3.
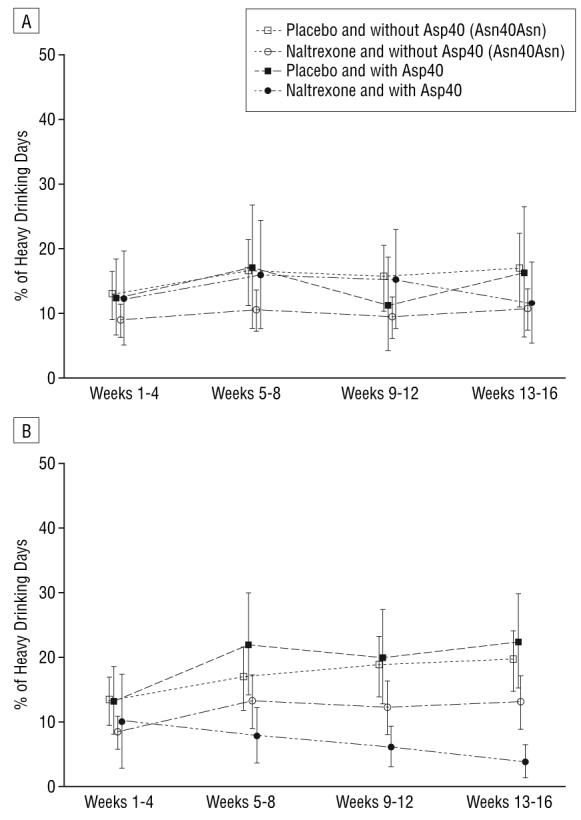
Medication effects on percentage of heavy drinking days (mean and 95% confidence interval) by OPRM1 genotype. A, Groups assigned to medical management and combined behavioral intervention (CBI) (test of group×time interaction, P=.35). B, Groups assigned to medical management alone (test of group×time interaction, P=.04; pairwise comparison of other groups with naltrexone hydrochloride and with Asp40 group, P=.01-.03).
CLINICAL OUTCOME
Figure 4 shows a significant genotype×medication interaction (P=.005) in those treated with MM only (without CBI). Naltrexone-treated patients who carried the Asp40 allele had the best outcome (87.1% good outcome), while patients with the Asp40 allele but receiving placebo did worse (48.6% good outcome). Noncarriers of the Asp40 allele (Asn40 homozygotes) had similar rates of good clinical outcome regardless of whether they received naltrexone (54.8% good outcome) or placebo (54.0% good outcome), and both were lower than the rate of Asp40 carriers receiving naltrexone. Comparing Asp40 carriers with noncarriers, the odds ratio of having a good clinical outcome after naltrexone treatment was 5.75 (95% confidence interval, 1.88-17.54). This analysis was also repeated to include subjects of all races (Asp40 allele frequencies: African American, 7%; Hispanic, 34%; other, 35%), leading to essentially the same result, with a significant medication×allele interaction (P=.03) and a genotype odds ratio of 3.33 (95% confidence interval, 1.49-7.47). In addition, adding sex as a covariate to the white-only analysis did not materially change the findings.
Figure 4.
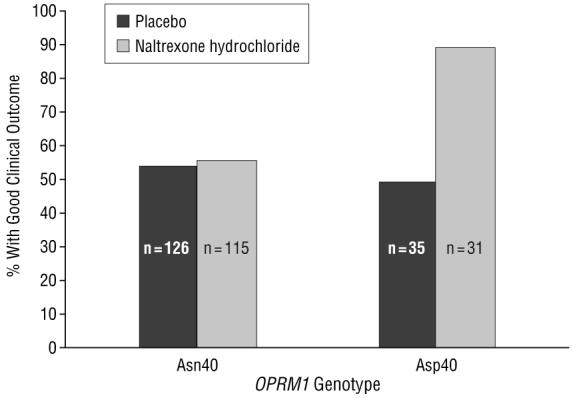
Good clinical outcome based on OPRM1 and medication group in those receiving medical management alone (no combined behavioral intervention) (test of genotype×medication interaction, P=.005). All subjects with missing values were considered not to have a good response. Asn40 includes subjects who were Asn40/Asn40 homozygotes. Asp40 includes those with either Asn40/Asp40 or Asp40/Asp40 genotypes.
EFFECT OF GENOTYPE AND MEDICATION GROUP ON STUDY COMPLETION, MEDICATION ADHERENCE, AND ADVERSE EVENTS
There were no significant gene×treatment group differences in study completion rates, drinking data obtained, medication adherence, or reported adverse events (Table 2). The lack of influence of these salient intervening variables supports the specificity of the naltrexone×OPRM1 genotype treatment interaction.
Table 2.
Retention, Medication Adherence, and Adverse Events by Genotype and Medication Group for Those Receiving Medical Management Alone (No Combined Behavioral Intervention)a
| No. (%) |
|||||
|---|---|---|---|---|---|
| Placebo |
Naltrexone Hydrochloride |
||||
| Asn40 (n=126) |
Asp40 (n=35) |
Asn40 (n=115) |
Asp40 (n=31) |
P Value |
|
| Withdrew | 16 (12.7) | 7 (20.0) | 20 (17.4) | 4 (12.9) | .62 |
| Full drinking data obtained | 119 (94.4) | 34 (97.1) | 111 (96.5) | 30 (96.8) | .92 |
| 80% of pills taken | 113 (89.7) | 30 (85.7) | 96 (83.5) | 25 (80.6) | .37 |
| Adverse events | |||||
| Headache | 34 (27.0) | 7 (20.0) | 43 (37.4) | 11 (35.5) | .14 |
| Fatigue | 46 (36.5) | 10 (28.6) | 37 (32.2) | 12 (38.7) | .73 |
| Nausea | 67 (53.2) | 16 (45.7) | 50 (43.5) | 12 (38.7) | .34 |
Asn40 includes subjects who were Asn40/Asn40 homozygotes. Asp40 includes those with either Asn40/Asp40 or Asp40/Asp40 genotype.
COMMENT
These data, from the large and well-controlled multisite COMBINE Study, support and extend evidence that the reportedly functional OPRM1 Asn40Asp substitution, altering β-endorphin binding to the receptor, influences treatment response to naltrexone among alcoholic patients. In the main COMBINE Study trial,7 naltrexone showed efficacy compared with placebo only in patients not receiving CBI therapy. Therefore, we had hypothesized that the MM-alone (no CBI) group would most likely show a gene × medication interaction. In the COMBINE Study, CBI, a more intensive and specific alcohol intervention, may have compensated for the placebo effect, thereby suppressing the chances of observing a main effect of naltrexone or a genetic interaction. The data presented herein are consistent with this thinking. A gene×medication interaction may be observable only in patients who can show obvious benefit from the medication over placebo. External influences, such as alcoholism severity, psychosocial instability, other illnesses, and the use of an effective adjunctive treatment (in this case, CBI), might all obscure meaningful biological effects of genes on which a specific medication can act. In our case, the MM-alone (no CBI) condition enabled enough interindividual variability in clinical response for both the naltrexone7 and the naltrexone×gene interaction to emerge.
Oslin and colleagues29 previously reported in a retrospective analysis of several disparate studies that naltrexone reduced relapse drinking after controlling for therapy, age (>55 years), sex, and marital status, and including only patients who received at least 5 weeks of treatment. The analysis presented in that report suggested a naltrexone×gene interaction on 1 outcome variable (relapse to a day of heavy drinking) in 141 subjects (42 with an Asp40 allele). Nevertheless, using somewhat different and more complex drinking and outcome variables, we have replicated and extended their initial observations. More recently, Gelernter and colleagues,31 in a reanalysis of the Veterans Affairs Naltrexone Cooperative Study,30 did not find a significant gene×naltrexone interaction on relapse drinking. Interestingly, they did report a main effect of naltrexone, which was not observed in the initial intent-to-treat analysis. The reasons for these discrepancies are not obvious, but the authors speculate that individuals who provided genetic samples may have been more motivated, more adherent to the medication regimen, and, perhaps, more socially stable than those who did not, leading to a more favorable naltrexone response in those subjects. The lack of an observable naltrexone×gene interaction in their hands is difficult to reconcile with the favorable data of Oslin et al29 and the data reported herein. However, the COMBINE Study used a naltrexone hydrochloride dosage of 100 mg/d, as did 2 of the 3 studies in the report by Oslin et al,29 while the Veterans Affairs Naltrexone Cooperative Study30 used a standard 50-mg/d dosage. It is unknown whether this could alter the treatment interaction with the Asp40 allele. It is also important that we observed that the gene×naltrexone interaction emerges over time, consistent with the antireinforcement effects of naltrexone. This is particularly evident in a reduction in heavy drinking days over time, consistent with previous observations on naltrexone's action.59 Therefore, various outcome variables and time effects may need to be considered to completely appreciate gene×medication interactions in the treatment of alcohol dependence.
Despite several reports suggesting that Asp40 carriers may respond differently to alcohol, prestudy drinking and alcoholism severity in our study did not differ between alcoholic subjects with and without Asp40. This is in contrast to a Korean study in which homozygous Asp40 carriers had higher levels of pretreatment drinking.60 Finally, although we did not perform a case-control study, the Asp40 and Asn40 allele frequencies observed in our alcoholic subjects are similar to allele frequencies in population studies, suggesting no major effect of the Asp40 allele on the development of alcoholism or its severity.
Our data speak most clearly to the interaction of the OPRM1 genotype and naltrexone. While the strength of this interaction leading to differential treatment efficacy might be surprising in view of the clinical diversity, different neurobiologic origins of vulnerability, and genetic heterogeneity of alcoholism, naltrexone is a unique medication engineered for specificity of action at the μ-opioid receptor, although it also binds to δ- and κ-opioid receptors. Therefore, either a direct effect on μ-opioid receptor function or a shift in balance between μ-opioid and δ- and κ-opioid receptor function caused by the OPRM1 genetic variant might form the basis of naltrexone's therapeutic action. This may not be as clear for medications that lack such specificity of action.
The increased naltrexone response in Asp40 carriers could have immense clinical importance, especially because, in our population, noncarriers (Asn40 homozygotes) responded no better than if given placebo. In our hands, and in the context of MM, the odds of a good clinical outcome with naltrexone were 5 times better in Asp40 carriers than noncarriers. Because almost 25% of the treatment-seeking population carries the Asp40 allele, genetic testing of individuals before naltrexone treatment might be worth the cost and effort, especially if structured behavioral treatment were not being considered. Given that alternative treatments such as CBI,7 acamprosate,1,2,6 and topiramate61 can be offered, one could make the case that naltrexone should be used first, or primarily, in OPRM1 Asp40 allele carriers. Naltrexone is relatively easy to administer and free of serious adverse effects and, as we observed in the Asp40 carriers we studied, it appears to be highly effective.
Future reports from this study will evaluate whether other more extensive genotyping (other single-nucleotide polymorphisms and haplotypes) of the OPRM1 gene might lead to improved prediction of naltrexone response, adverse events, or other salient clinical information.
There are a number of limitations of this study. Although genetic samples were obtained to examine this and other treatment-related issues, subjects were not randomized by OPRM1 genotype. It is possible that consent for the substudy differed by genotype because some subjects (about 27%) either completed or dropped out of the study before genetic consent and an additional 10% of the remaining had nonusable DNA. Although key variables seem to be equally distributed between patients with and without the Asp40 allele, it is possible that some other genetic/nongenetic influence biased responding. Also, the numbers of Asp40 carriers were somewhat limited, especially in the MM-only cells. However, confluence of effect across drinking variables, along with the consistency of the observation of naltrexone response in the subgroup that also showed a naltrexone effect in the main trial, argues against a spurious result. Nevertheless, the foregoing caveats suggest that these results be replicated in a prospective trial where individuals are initially randomized by OPRM1 Asp40 status.
In addition, because the numbers of different racial/ethnic minorities were relatively low, we could not directly assess the effect of the allele differences in these subgroups. However, adding individuals of African American and Hispanic origin into the analysis did not change the directionality of the main finding that naltrexone improved clinical global outcome primarily in Asp40 carriers. Future studies might want to consider this issue more thoroughly. In addition, we did not separately explore the effects of acamprosate. However, acamprosate did not demonstrate efficacy or added benefits to naltrexone in the main COMBINE Study trial.7 Also, acamprosate is thought to be a glutamate modulator and would not be expected to show differential efficacy based on changes in the μ-opioid receptor.
In summary, in alcoholic individuals who received naltrexone in the context of MM (the most purely pharmacologic condition studied), there was a greater response and improvement if a person had at least 1 copy of the OPRM1 Asp40 allele. This finding could have considerable theoretical importance for drug development (such as studying only Asp40 carriers during opioid antagonist development) and also for selectively targeting μopioid antagonist medications and other treatments for alcohol-dependent individuals.
Acknowledgments
Funding/Support: This study was supported by National Institute on Alcohol Abuse and Alcoholism cooperative agreements U10AA11715, 11716, 11721, 11727, 11756, 11768, 11773, 11776, 11777, 11783, 11787, 11799, and 11773 and the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program (Dr Goldman). Lipha Pharmaceuticals supplied the medication for this study.
Additional Contributions: We acknowledge the work of all of the COMBINE Study investigators and staff whose names are listed on the COMBINE Study Web site (http://www.cscc.unc.edu/combine) and Barbara Bozarth, MS, who provided editorial and manuscript preparation assistance. We dedicate this report to the memory of James D. Hosking, PhD, who, before his untimely death, served as principal investigator of the COMBINE Study Coordinating Center (University of North Carolina).
Footnotes
Financial Disclosure: Dr Anton has reported receiving consultation fees and honoraria from Forest Laboratories and Alkermes Inc (the maker of long-acting injectable naltrexone); consultation fees and grants from Bristol-Myers Squibb and Hythiam; consultation fees, honoraria, and grants from Contral Pharma/Biotie Pharmaceuticals and Johnson & Johnson/Ortho-McNeil; consultation fees and grant funding from Pfizer Inc; and consultation fees from AstraZeneca, Axis Shield, Cephalon, Drug Abuse Sciences, and Sanofi-Aventis. In the near future, he anticipates receiving consultation fees from Solvay Pharmaceuticals and a grant from Eli Lilly. Dr O'Malley has reported receiving research support (grant support or clinical supplies) from Alkermes Inc, DuPont, GlaxoSmithKline, Forest Laboratories, Lipha Pharmaceuticals, Ortho-McNeil, Bristol-Myers Squibb, Pfizer Inc, Sanofi-Aventis, and Mallinckrodt; serving as a consultant to Alkermes Inc, Forest Laboratories, GlaxoSmithKline, Ortho-McNeil, Pfizer Inc, and Johnson & Johnson; and receiving travel reimbursement from Alkermes Inc; she is an inventor on patents held by Yale University entitled “Smoking Cessation Treatments Using Naltrexone and Related Compounds.” Dr Swift has reported receiving grant funding from Ortho-McNeil and Pfizer Inc; and serving as a consultant to or on the advisory board or speakers bureau of Alkermes Inc, Cephalon, Ortho-McNeil, Pfizer Inc, Transoral Pharmaceuticals, and Forest Laboratories. Dr Pettinati has reported receiving research support from Alkermes Inc, AstraZeneca, Bristol-Myers Squibb, Cephalon, Forest Laboratories, Lipha Pharmaceuticals and Merck KGaA, and Ortho-McNeil, and serving as a consultant to or on the advisory board or speakers bureau of Alkermes Inc, AstraZeneca, Cephalon, and Forest Laboratories.
Previous Presentation: This study was presented in part at the American College of Neuropsychopharmacology Meeting; December 5, 2006; Fort Lauderdale, Florida.
Trial Registration: clinicaltrials.gov Identifier: NCT00006206
REFERENCES
- 1.Anton RF, Swift RM. Current pharmacotherapies of alcoholism: a U.S. perspective. Am J Addict. 2003;12(suppl 1):S52–S68. doi: 10.1111/j.1521-0391.2003.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 2.Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. J Psychiatr Res. 2006;40(5):383–393. doi: 10.1016/j.jpsychires.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8(2):267–280. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- 4.Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001;36(6):544–552. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- 5.Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25(9):1335–1341. [PubMed] [Google Scholar]
- 6.Bouza C, Angeles M, Munoz A, Amate J. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99(7):811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [published correction appears in Addiction. 2005;100(4):573] [DOI] [PubMed] [Google Scholar]
- 7.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gast-friend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 8.Verebey K, Mulé SJ. Naltrexone pharmacology, pharmacokinetics, and metabolism: current status. Am J Drug Alcohol Abuse. 1975;2(34):357–363. doi: 10.3109/00952997509005661. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt WK, Tam SW, Shotzberger GS, Smith DH, Jr, Clark R, Bernier VG. Nalbuphine. Drug Alcohol Depend. 1985;14(34):339–362. doi: 10.1016/0376-8716(85)90066-3. [DOI] [PubMed] [Google Scholar]
- 10.Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neuro-transmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21(23):RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marinelli PW, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of Metenkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2005;29(10):1821–1828. doi: 10.1097/01.alc.0000183008.62955.2e. [DOI] [PubMed] [Google Scholar]
- 12.Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary β-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry. 1996;53(3):250–257. doi: 10.1001/archpsyc.1996.01830030072011. [published correction appears in Arch Gen Psychiatry. 1996;53(6):555] [DOI] [PubMed] [Google Scholar]
- 13.O'Malley SS, Jaffe AJ, Rode S, Rounsaville BJ. Experience of a “slip” among alcoholics treated with naltrexone and placebo. Am J Psychiatry. 1996;153(2):281–283. doi: 10.1176/ajp.153.2.281. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JS, Anton RF, Latham PK, Moak DH. Factor structure and predictive validity of the Obsessive Compulsive Drinking Scale. Alcohol Clin Exp Res. 1999;23(9):1484–1491. [PubMed] [Google Scholar]
- 15.Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156(11):1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- 16.Zubieta J-K, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156(6):842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 17.Bencherif B, Wand GS, McCaul ME, Kim YK, Ilgin N, Dannals RF, Frost JJ. Muopioid receptor binding measured by [11C]carfentanil positron emission tomography is related to craving and mood in alcohol dependence. Biol Psychiatry. 2004;55(3):255–262. doi: 10.1016/j.biopsych.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280(38):32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 19.Bergen AW, Kokoszka J, Peterson R, Long LC, Virkkunen M, Linnoila M, Goldman D. Mu opioid receptor gene variants: lack of association with alcohol dependence. Mol Psychiatry. 1997;2(6):490–494. doi: 10.1038/sj.mp.4000331. [DOI] [PubMed] [Google Scholar]
- 20.Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beyer A, Koch T, Schröder H, Schulz S, Höllt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem. 2004;89(3):553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- 22.Gelernter J, Kranzler H, Cubells J. Genetics of two μ opioid receptor gene (OPRM1) exon I polymorphism: population studies, and allele frequencies in alcohol- and drug-dependent subjects. Mol Psychiatry. 1999;4(5):476–483. doi: 10.1038/sj.mp.4000556. [DOI] [PubMed] [Google Scholar]
- 23.Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the μ-opioid receptor gene with substance dependence: a meta-analysis. Drug Alcohol Depend. 2006;83(3):262–268. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Ray LA, Hutchinson KE. A polymorphism of the μ-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28(12):1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- 25.van den Wildenberg E, Wiers RW, Dessers J, Janssen RGJH, Lambrichs EH, Smeets HJM, van Breukelen GJP. A functional polymorphism of the μ-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcohol Clin Exp Res. 2007;31(1):1–10. doi: 10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 26.Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26(1):106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 27.Lötsch J, Stuck B, Hummel T. The human μ-opioid receptor gene polymorphism 118A > G decreases cortical activation in response to specific nociceptive stimulation. Behav Neurosci. 2006;120(6):1218–1224. doi: 10.1037/0735-7044.120.6.1218. [DOI] [PubMed] [Google Scholar]
- 28.Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a functional polymorphism in the μ-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Arch Gen Psychiatry. 2007;64(3):369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- 29.Oslin DW, Berrettini W, Kranzler HR, Pettinati HM, Gelernter J, Volpicelli JR, O'Brien CP. A functional polymorphism on the μ-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28(8):1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- 30.Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- 31.Gelernter J, Gueorguieva R, Kranzler HR, Zhang H, Cramer J, Rosenheck R, Krystal JH. VA Cooperative Study #425 Study Group. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin Exp Res. 2007;31(4):555–563. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 32.COMBINE Study Research Group Testing combined pharmacotherapies and behavioral interventions for alcohol dependence (the COMBINE Study): a pilot feasibility study. Alcohol Clin Exp Res. 2003;27(7):1123–1131. doi: 10.1097/01.ALC.0000078020.92938.0B. [DOI] [PubMed] [Google Scholar]
- 33.Goldman D, Oroszi G, O'Malley S, Anton R. COMBINE genetics study: the pharmacogenetics of alcoholism treatment response: genes and mechanisms. J Stud Alcohol. 2005 July;66(suppl 15):56–64. doi: 10.15288/jsas.2005.s15.56. [DOI] [PubMed] [Google Scholar]
- 34.COMBINE Study Research Group Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: rationale and methods. Alcohol Clin Exp Res. 2003;27(7):1107–1122. doi: 10.1097/00000374-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 35.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P). Version 2.0. New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- 37.Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: an instrument for assessing alcohol treatment outcome. J Stud Alcohol. 1997;58(4):358–364. doi: 10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]
- 38.Miller WR. Form 90: A Structured Assessment Interview for Drinking and Related Behaviors. Vol. 5. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1996. (Project MATCH Monograph Series. NIH publication 96-4044). [Google Scholar]
- 39.Sobell LC, Sobell MB. Timeline followback: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- 40.Anton RF, Moak DH, Latham PK. The Obsessive Compulsive Drinking Scale (OCDS): a new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53(3):225–231. doi: 10.1001/archpsyc.1996.01830030047008. [Arch Gen Psychiatry. 1996;53(7):576] [DOI] [PubMed] [Google Scholar]
- 41.Levine J, Schooler N. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22(2):343–381. [PubMed] [Google Scholar]
- 42.Johnson BA, Ait-Daoud N, Roache JD. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J Stud Alcohol. 2005;66(2suppl 15):157–167. doi: 10.15288/jsas.2005.s15.157. [DOI] [PubMed] [Google Scholar]
- 43.Anton RF, Dominick C, Bigelow M, Westby C. CDTect Research Group. Comparison of Bio-Rad %CDT TIA and CDTect as laboratory markers of heavy alcohol use and their relationships with γ-glutamyltransferase. Clin Chem. 2001;47(10):1769–1775. [PubMed] [Google Scholar]
- 44.Anton RF, Lieber C, Tabakoff B. CDTect Study Group. Carbohydrate deficient transferrin and γ-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol Clin Exp Res. 2002;26(8):1215–1222. doi: 10.1097/01.ALC.0000023986.42254.F5. [DOI] [PubMed] [Google Scholar]
- 45.Mason BJ. Rationale for combining acamprosate and naltrexone for treating alcohol dependence. J Stud Alcohol. 2005;66(suppl 15):148–156. doi: 10.15288/jsas.2005.s15.148. [DOI] [PubMed] [Google Scholar]
- 46.Swift R, Pettinati HM. Choosing pharmacotherapies for the COMBINE study—process and procedures: an investigational approach to combination pharmacotherapy for the treatment of alcohol dependence. J Stud Alcohol. 2005;66(suppl 15):141–147. doi: 10.15288/jsas.2005.s15.141. [DOI] [PubMed] [Google Scholar]
- 47.Johnson BA, O'Malley SS, Ciraulo DA, Roache JD, Chambers RA, Sarid-Segal O, Couper D. Dose-ranging kinetics and behavioral pharmacology of naltrexone and acamprosate, both alone and combined, in alcohol-dependent subjects. J Clin Psychopharmacol. 2003;23(3):281–293. doi: 10.1097/01.jcp.0000084029.22282.bb. [DOI] [PubMed] [Google Scholar]
- 48.Pettinati HM, Weiss RD, Dundon W, Miller WR, Donovan D, Ernst DB, Rounsaville BJ. A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. J Stud Alcohol. 2005;66(suppl 15):170–178. doi: 10.15288/jsas.2005.s15.170. [DOI] [PubMed] [Google Scholar]
- 49.Pettinati HM, Weiss RD, Miller WR, Donovan D, Ernst DB, Rounsaville BJ. Medical Management (MM) Treatment Manual. Vol. 2. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2004. (COMBINE Monograph Series. NIH publication 04-5289). [Google Scholar]
- 50.Miller WR. Combined Behavioral Intervention Manual. Vol. 1. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2004. (COMBINE Monograph Series; NIH publication 04-5288). [Google Scholar]
- 51.Longabaugh R, Zweben A, Locastro JS, Miller WR. Origins, issues and options in the development of the combined behavioral intervention. J Stud Alcohol. 2005 July;66(suppl 15):179–187. doi: 10.15288/jsas.2005.s15.179. [DOI] [PubMed] [Google Scholar]
- 52.Kadden RP, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R. Cognitive-Behavioral Coping Skills Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals With Alcohol Abuse and Dependence. Vol. 3. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1995. (Project MATCH Monograph Series. NIH publication 94-3724). [Google Scholar]
- 53.Nowinski J, Baker S, Carroll K. Twelve-Step Facilitation Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals With Alcohol Abuse and Dependence. Vol. 1. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1995. (Project MATCH Monograph Series. NIH publication 94-3722). [Google Scholar]
- 54.Miller WR, Zweben A, DiClimente C, Rychtarik R. Motivational Enhancement Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals With Alcohol Abuse and Dependence. Vol. 2. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1994. (Project MATCH Monograph Series. NIH publication 94-3723). [Google Scholar]
- 55.Azrin NH, Sisson RW, Meyers R, Godley M. Alcoholism treatment by disulfiram and community reinforcement therapy. J Behav Ther Exp Psychiatry. 1982;13(2):105–112. doi: 10.1016/0005-7916(82)90050-7. [DOI] [PubMed] [Google Scholar]
- 56.Meyers RJ, Smith JE. Clinical Guide to Alcohol Treatment: The Community Reinforcement Approach. Guilford Press; New York, NY: 1995. [Google Scholar]
- 57.Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91(3):199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- 58.Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse (Test Manual) US Dept of Health and Human Services, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1995. (DHHS publication No. 95-3911). [Google Scholar]
- 59.Anton RF, Moak DH, Latham PK, Waid LR, Malcolm RJ, Dias JK, Roberts JS. Posttreatment results of combining naltrexone with cognitive-behavior therapy for the treatment of alcoholism. J Clin Psychopharmacol. 2001;21(1):72–77. doi: 10.1097/00004714-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Kim S-G, Kim C-M, Kang D-H, Kim Y-J, Byun W-T, Kim S-Y, Park J-M, Kim M-J, Oslin DW. Association of functional opioid receptor genotypes with alcohol dependence in Koreans. Alcohol Clin Exp Res. 2004;28(7):986–990. doi: 10.1097/01.alc.0000130803.62768.ab. [DOI] [PubMed] [Google Scholar]
- 61.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O'Malley SS, Swift RM. Topiramate for Alcoholism Advisory Board and the Topiramate for Alcoholism Study Group. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]


