Abstract
Background
Cholesterol may have a role in the pathophysiology of depression. Lowering cholesterol levels with statins reduces risks for cardiovascular events, and there is clinical evidence that statins exert neuroprotective properties not fully explained by their effects on serum cholesterol levels. Altered cholesterol levels can affect serotonergic neurotransmission, which might be involved in the clinical efficacy of standard antidepressants.
Methods
We examined interactions between a statin (lovastatin) and a selective serotonin reuptake inhibitor (fluoxetine) using the forced swim test (FST) in rats, a behavioral assay that identifies treatments with antidepressant effects in humans. Specifically, we determined if the addition of lovastatin to the diet would increase the efficacy of a subeffective dose of fluoxetine.
Results
Rats maintained on a lovastatin-enriched diet for 30 days were more sensitive to the antidepressant-like effects of a low (subthreshold) dose of fluoxetine. The behavior of rats treated with this combination resembled that normally seen with higher doses of fluoxetine. No effects were observed in rats maintained on a lovastatin-enriched diet for 3 days.
Conclusions
Lovastatin can augment the antidepressant-like effects of a low dose of fluoxetine in rats, raising the possibility that statins could be used to facilitate the effects of antidepressants in humans.
Keywords: depression, cholesterol, statin, serotonin, model, rat
Cholesterol is an important determinant of the structure and function of cell membranes, and plays an integral role in many neural functions that contribute to mood state and response to antidepressants (Papakostas et al., 2003a; Pucadyil and Chattopadhyay, 2006). These functions include monoaminergic signal transduction, energy metabolism and neuronal growth (Goldstein and Brown, 2001; Maxfield and Tabas, 2005). Although the major source of central nervous system (CNS) cholesterol is de novo synthesis within neurons, accumulating evidence suggests that increased transfer of circulating plasma cholesterol to the brain may be related to the onset of neurodegeneration (Dietschy and Turley, 2001). Hypercholesterolemia is thus suggested to partly mediate age-related brain changes (Yehuda et al., 2002). In this context, cholesterol-lowering drugs, which block systemic cholesterol synthesis, may be a potential treatment option for a range of neurological disorders (Cucchiara and Kasner, 2001; Jick et al., 2000; Rajanikant et al., 2007).
Likewise, a possible link between cholesterol and depression has been suggested in both clinical and preclinical studies. The recently proposed entity of ‘vascular depression’ provides indirect support for hypercholesterolemia as a risk factor in the pathophysiology of depression (Alexopoulos et al., 1997; Thomas et al., 2004). Cardiovascular risk factors, including hypercholesterolemia, have also been related to poor treatment outcomes as well as levels of symptom severity in depression (Alexopoulos et al., 1997; Iosifescu et al., 2005; Thomas et al., 2004).
Concerns about a possible relationship between cholesterol-lowering therapy and suicide/aggressive behaviors have existed since the 1990s (Engelberg, 1992; Papakostas et al., 2004b). Decreased function of the brain 5-HT system, caused by low brain cholesterol-induced alterations in membrane fluidity, has been proposed as a plausible mechanism for relating low cholesterol levels to suicide (Engelberg, 1992; Papakostas et al., 2004b). However, recent large-scale controlled and population-based studies have suggested a positive role of statins or low cholesterol levels on psychological well-being, including a reduced risk for depression (Brown et al., 1994; Downs et al., 1993; Freedman et al., 1995; Wardle et al., 1996; Yang et al., 2003; Young-Xu et al., 2003). Recent clinical trials that have examined the relationship between elevated serum cholesterol levels and antidepressant non-response (Papakostas et al., 2004a; Papakostas et al., 2003b; Sonawalla et al., 2002) also support the potential benefit of reducing cholesterol levels for improved clinical outcomes. Treatment resistance to antidepressants in persons with hypercholesterolemia may be ascribed to a decrease in sensitivity of the 5-HT receptors and/or transporters of the central nervous system (Cohen et al., 1988; Lamping et al., 1999; Shimokawa and Vanhoutte, 1989; Smith, 1997; Stroes et al., 1997).
Considering the high rate of partial or non-response to antidepressants such as serotonin selective reuptake inhibitors (SSRI) (Fava and Davidson, 1996), frequently used first-line agents for the treatment of depression, cholesterol-lowering drugs as adjuncts to SSRIs may facilitate serotonergic function and thereby improve treatment outcomes (Cucchiara and Kasner, 2001). Statins are the most widely used cholesterol-lowering agents, acting as inhibitors of 3-hydroxy 3-methylglutaryl coenzyme A reductase, which catalyzes the rate-limiting step in cholesterol biosynthesis (Armitage, 2007). In addition to their effects on serum cholesterol levels, statins also appear to affect brain cellular mechanisms of lipid and non-lipid systems in a variety of ways and have been shown to improve endothelial function, decrease inflammatory response, maintain plaque stability, and prevent thrombus formation (Cucchiara and Kasner, 2001). To examine the potential role of cholesterol lowering therapy in modulating the response to SSRI treatment of major depressive disorder (MDD), we utilized the Forced Swim Test (FST) to study the behavior of rats treated with subtherapeutic doses of fluoxetine, with or without lovastatin. The FST is well known for its ability to identify in rats treatments with antidepressant efficacy in humans (Willner et al., 1984). Lovastatin is lipophilic, facilitating its ability to penetrate the blood-brain barrier efficiently and influence brain levels of cholesterol (Tsuji et al., 1993). We hypothesized that lovastatin augmentation of a sub-effective dose of fluoxetine would reduce depressive-like behaviors in the FST, which would indicate that lovastatin can increase the efficacy of fluoxetine and produce behavior similar to that produced when rodents are treated with standard doses of antidepressant drugs prior to the FST.
MATERIALS AND METHODS
Animals
Sixty-two male Sprague-Dawley rats (Charles River Laboratories, Boston MA) were used in these studies. The rats were housed in groups of 3–4 and weighed 330–370 gm at the time of behavioral testing. Rats were maintained on a 12 h light (0700–1900 h)-12 h dark cycle with free access to food and water except during testing. Experiments were conducted in accordance with the 1996 Guide for the Care and Use of Laboratory Animals (NIH) and McLean Hospital policies.
Drugs
Fluoxetine HCl (FLX) was obtained from Sigma (St. Louis MO) and administered in a distilled water vehicle (VEH) at a volume of 1 cc/kg. Lovastatin (Spectrum Chemicals, Gardena CA) was administered as a dietary supplement in a laboratory chow at 0.01% w/w (Research Diets Inc., New Brunswick NJ). Rats ate an average of 20 gm of food each day, regardless of whether they were assigned standard chow (STD) or lovastatin-enriched chow (LOV); this amount of the LOV diet contained a total of 2.0 mg lovastatin (dosage per day). This amount of LOV is within a dose range known to safely reduce brain concentrations of cholesterol with chronic treatment (Vecka et al., 2004). The diets were equivalent in overall fat, protein, carbohydrate and caloric content.
Forced Swim Test (FST)
The FST studies were conducted as described previously (Carlezon et al., 2005) with minor modifications. The FST is a two-day procedure in which rats swim under conditions in which escape is not possible. On the first day, rats are placed in clear, 65 cm tall-25 cm diameter cylinders filled to 48 cm with 25°C water. The rats initially struggle to escape from the water, but eventually they adopt a posture of immobility in which they make only the movements necessary to keep their heads above water.
After 15 min of forced swimming, the rats are removed from the water, dried with towels, and placed in a warmed enclosure for 30 min. The cylinders are emptied and cleaned between rats. When the rats are re-tested 24 hours later under identical conditions in 5 min sessions, immobility is increased. Treatment with standard antidepressant drugs within the 24 hr period between the first exposure to forced swimming and re-testing can attenuate facilitated immobility, an effect correlated with antidepressant efficacy in humans (Carlezon et al., 2002; Detke et al., 1995; Porsolt et al., 1977a; Porsolt et al., 1977b).
Rats tested with FLX (5 mg/kg) received 3 separate intraperitoneal (IP) injections of drug (or VEH), at 1 hr, 19 hr, and 23 hr after the first exposure to forced swimming. This commonly used dosing regimen identifies the antidepressant-like effects of many standard agents (Carlezon et al., 2002; Carlezon et al., 2005; Detke et al., 1995; Porsolt et al., 1977a; Porsolt et al., 1977b). We chose 5 mg/kg FLX because this dose is sub-effective in the FST (Carlezon et al., 2002; Carlezon et al., 2005), which would enable us to detect an enhanced response in LOV-treated rats. Rats tested with LOV (or STD) received the special diet for 3 or 30 days prior to the start of the swim test, and received VEH or FLX injections (IP) at 1, 19, and 23 hr after the forced swim. There were 7–8 rats per treatment condition, and separate rats were used for each treatment (3- or 30-day) regimen. We used this duration of LOV treatment because previous work has demonstrated our ability to detect antidepressant effects of an omega-3 fatty acid-enriched diet given for 30 but not 3 or 10 days (Carlezon et al., 2005).
Swim tests were videotaped from the side of the cylinders, and later scored by raters unaware of the treatment conditions. Behavior during the re-test (day 2) of the FST was rated at 5 sec intervals throughout the duration of the forced swimming session. At each 5 sec interval, the predominant behavior was assigned to one of 3 categories: immobility, swimming, or climbing (Detke et al., 1995). A rat was judged to be immobile if it was making only movements necessary to keep its head above water, climbing if it was making forceful thrashing movements with its forelimbs directed against the walls of the cylinder, swimming if it was actively making swimming movements that caused it to move within the center of the cylinder. Diving beneath the water is not reported because it rarely occurred. The behavioral sampling method differentiates classes of antidepressant drugs: for example, norepinephrine reuptake inhibitors decrease immobility and increase climbing without affecting swimming, whereas SSRIs decrease immobility and increase swimming without affecting climbing. We used this scoring procedure to be maximally sensitive to the antidepressant effects of FLX, which are sometimes difficult to detect using other scoring procedures (Detke et al., 1995), and to determine if the combination of LOV and FLX retains an SSRI-like behavioral profile in this test.
Statistical Analyses
The number of occurrences of each category of behavior (immobility, swimming, climbing) was analyzed using one-way (treatment) analyses of variance (ANOVAs). Likewise, the weights of the rats immediately prior to testing were analyzed using one-way ANOVAs. Significant effects were analyzed further using post hoc Newman-Keuls tests. Data from the 3-day and 30-day LOV treatment regimens were analyzed separately.
RESULTS
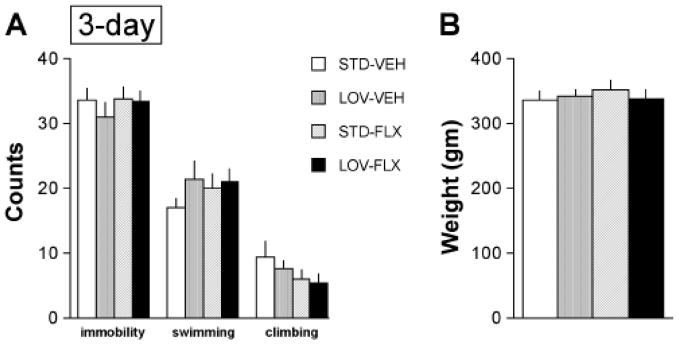
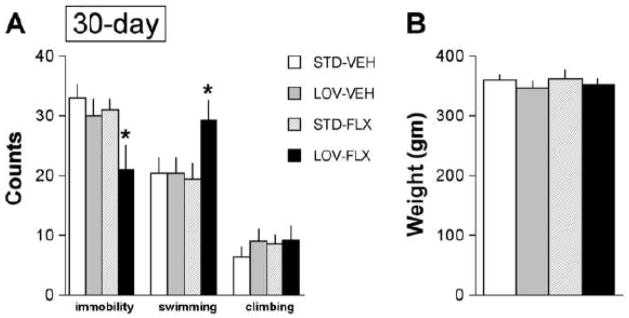
No effects were observed in rats given a 3-day LOV dietary treatment regimen (Figure 1A). Neither LOV alone, FLX alone, nor the combination of LOV plus FLX affected occurrences of immobility (F(3,27)=0.52, not significant (NS)), swimming (F(3,27)=1.33, NS), or climbing (F(3,27)=1.30, NS). The weights of the rats did not differ among groups at the time of the re-test (Figure 1B), which is important because weight can influence swimming behaviors(Pliakas et al., 2001). In cont rast, a 30-day LOV dietary treatment regimen affected behavior in the FST (Figure 2A). There was a main effect of treatment on occurrences of immobility (F(3,27)=3.69, p<0.05). Post hoc analysis revealed that the combination of the LOV-enriched diet and FLX treatment significantly reduced the occurrences of immobility (p<0.05, Newman-Keuls tests). There was also a main effect of treatment on occurrences of swimming (F(3,27)=3.09, p<0.05), due to increased occurrences of swimming in the rats treated with the combination of the LOV-containing diet and FLX (p<0.05, Newman-Keuls tests). However, there was no effect of treatment on occurrences of climbing (F(3,27)=0.53, NS). This pattern of results—reduced immobility behavior and increased swimming behavior without changes in climbing behavior—is similar to that typically seen with standard SSRIs (Carlezon et al., 2005; Carlezon et al., 2002; Detke et al., 1995). The weights of the rats did not differ among groups at the time of the re-test (Figure 2B).
Fig. 1.
Effects of 3-day dietary supplementation with lovastatin (LOV) (2.0 mg/kg/day) on behaviors in the FST. (A) Neither LOV (3 days) alone, fluoxetine (FLX) (5 mg/kg) alone, nor the combination of LOV plus FLX affected occurrences of immobility, swimming, or climbing in the FST. (B) The treatments did not affect the weights of the rats. 7–8 rats per group.
Fig. 2.
Effects of 30-day dietary supplementation with LOV (2.0 mg/kg/day) on behaviors in the FST. (A) Whereas neither LOV alone nor FLX alone affected behavior, the combination of LOV plus FLX reduced immobility and increased swimming, a pattern of behaviors similar to that seen with SSRIs at standard doses. (B) The treatments did not affect the weights of the rats. 7–8 rats per group. *p<0.05, Newman-Keuls post hoc tests, 7–8 rats per group.
DISCUSSION
The present studies establish that dietary supplementation with LOV increases the antidepressant efficacy of FLX in laboratory animals. We observed that this combination produced antidepressant-like effects to a greater extent than FLX alone, as evidenced by reduced immobility and increased swimming in rats. Effects on the swimming measures, rather than climbing, in the FST suggest that the antidepressant-like effects of LOV augmentation may be attributed to the alteration of serotonergic function (Carlezon et al., 2002; Carlezon et al., 2005; Detke et al., 1995). All major classes of antidepressant treatments—including NRIs, SSRIs, monoamine oxidase inhibitors, and electroconvulsive shock therapy (Borsini and Meli, 1988; Detke et al., 1995; Porsolt et al., 1977a; Porsolt et al., 1977b)—effectively reduce indicators of immobility in the FST. Indeed, the main strength of the FST is its ability to identify in rats treatments with antidepressant efficacy in people (Willner, 1984).
Although SSRIs are generally effective and safe as first-line agents in treating clinical depression, these medications take weeks to achieve full efficacy. During this lag period, patients are exposed to an elevated risk for suicide and continue to be functionally impaired (Jick et al., 2000). High partial- or non-response rates constitute other major challenges in the treatment of MDD. Approximately 30 to 50% of patients treated with antidepressants do not achieve remission (Fava and Davidson, 1996). As such, augmenting antidepressants with other agents that have mechanisms facilitating the recovery of pre-existing monoamine depletion may be useful to bolster the efficacy of antidepressant effects. The present studies, however, do not address the question of whether the onset of antidepressant efficacy would be shortened; indeed, there are currently no animal screening methods that reliably model the lag in efficacy in humans that characterize all current antidepressant treatments. The effects of statins in humans are not immediate, and maximal effect may take four to six weeks to achieve. Consistent with this observation, we found that the ability of LOV to potentiate FLX effects required chronic (30 days) of treatment, whereas a shorter regimen (3 days) was without effect. It is important to emphasize that statins can occasionally cause adverse effects, particularly with long-term exposure. One percent of patients treated suffer from conditions including myopathy and liver toxicity (Farmer & Torre-Amione, 2000). More research is needed to determine if interactions between statins and antidepressants would enable the use of dosages far below those with the potential to cause side effects.
Alteration in cholesterol and phospholipids of brain cell membranes may influence membrane fluidity, consequently affecting various catecholamine neurotransmitter systems, including 5-HT and noradrenaline (Heron et al., 1980). Preclinical studies have demonstrated that low cholesterol levels may lead to decreased 5-HT function in the brain through reduced numbers and/or function of postsynaptic 5-HT receptors (Hawton and Morgan, 1993; Muldoon et al., 1990). In contrast, mechanisms by which cholesterol depletion may favorably affect the 5-HT system have also been proposed. These include an inverse correlation between platelet 5-HT concentrations and serum cholesterol levels in patients with hyperlipidemia or renal disease; an association between cholesterol lowering treatment and normalization of initially low intraplatelet 5-HT; and a directly adverse impact of elevated cholesterol levels on 5-HT transporter or receptor function (Barradas et al., 1992; Delva et al., 1996; Papakostas et al., 2003a; Ringo et al., 1994).
Recent theoretical mechanisms for depression pertaining to immune-mediated alterations in serotonin function (Muller and Schwarz, 2007) would be another plausible explanation for the ability of LOV to potentiate FLX effects. Current immune-mediated concepts on the etiology of depression include increased proinflammatory cytokines, and final activation of tryptophan- and serotonin degrading enzyme indoleamine 2, 3-deoxygenase, which may cause a reduction of serotonergic neurotransmission in MDD (Muller and Schwarz, 2007). Considering the anti-inflammatory and immunomodulatory properties of statins (Cucchiara and Kasner, 2001; Rajanikant et al., 2007), adjunctive use of statins with SSRIs may block or reverse a cascade of immune-mediated serotonin depletion in depression. The present findings that suggest the antidepressant efficacy of LOV may occur through serotonergic (rather than noradrenergic) pathways support our proposal that statins increase serotonergic function. Other possible mechanisms include antioxidant effects, vascular effects, such as anti-platelet and anti-thrombotic effects, and the modulation of cholesterol distribution within brain cell membranes (Cucchiara Kasner, 2001; Kirsch et al., 2003; Rajanikant et al., 2007).
Due to the relatively high concentration of cholesterol in the brain, the detection of changes specific to membrane cholesterol is difficult. Although we were unable to measure changes in membrane cholesterol levels, LOV is known to cross the blood-brain barrier (Guillot et al., 1993), and rats treated with LOV (0.1 mg/day for six weeks) have previously been reported to have reductions in their brain cholesterol levels (Vecka et al., 2004). Measurement of blood levels of cholesterol as a proxy would be of limited utility because brain levels are much more tightly regulated (Waelsh et al., 1941; Pardridge and Mietus, 1980). Our behavioral sampling data suggest preferential effects of LOV on the serotonergic system, but additional work (e.g., in vivo microdialysis) is needed to examine the effects of statins on brain catecholamines. Regardless, this is the first report of the ability of a statin drug to potentiate key behavioral effects of an SSRI.
Acknowledgments
This study was supported by grants from the National Institute of Mental Health (MH58681 to PFR and MH063266 to WC), and the Stanley Medical Research Institute (to BMC).
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
Dr. Renshaw is a consultant for Novartis, Kyowa Hakko, and Roche and has received research support from GSK and Eli Lilly.
Mr. Parsegian has neither financial interests nor conflicts of interest.
Mr. Yang has neither financial interests nor conflicts of interest.
Ms. Novero has neither financial interests nor conflicts of interest.
Dr. Yoon has neither financial interests nor conflicts of interest.
Dr. Lyoo has received research support from AstraZeneca, Eli Lilly and Organon.
Drs. Cohen, Renshaw, and Carlezon are on a patent application regarding cholesterol lowering agents to augment the effects of antidepressants.
Dr. Carlezon has a patent (US 6,528,518; Assignee: McLean Hospital) related to the use of kappa-opioid antagonists for the treatment of depressive disorders, and is part of a larger group (Assignees: McLean Hospital and Temple University) that has applied for a patent related to the use of compounds derived from a naturally occurring kappa-opioid agonist in the treatment of psychiatric and addictive disorders. Within the last 3 years he has received compensation as a consultant from HUYA Bioscience International, Infinity Pharmaceuticals, and Psychogenics, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- Barradas MA, Mikhailidis DP, Winder AF. Low serum cholesterol and suicide. Lancet. 1992;339:1168. [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Brown SL, Salive ME, Harris TB, Simonsick EM, Guralnik JM, Kohout FJ. Low cholesterol concentrations and severe depressive symptoms in elderly people. BMJ. 1994;308:1328–1332. doi: 10.1136/bmj.308.6940.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byington RP, Davis BR, Plehn JF, White JB, Cobbe SM, Shepherd J. Reduction of stroke events with pravastatin. Circulation. 2001;103:387–392. doi: 10.1161/01.cir.103.3.387. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Pliakas AM, Parow AM, Detke MJ, Cohen BM, Renshaw PF. Antidepressant-like effects of cytidine in the forced swim test in rats. Biol Psychiatry. 2002;51:882–889. doi: 10.1016/s0006-3223(01)01344-0. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Zitnay KM, Haudenschild CC, Cunningham LD. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ Res. 1988;63:903–910. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- Cucchiara B, Kasner SE. Use of statins in CNS disorders. J Neurol Sc. 2001;187:81–89. doi: 10.1016/s0022-510x(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Delva NJ, Matthews DR, Cowen PJ. Brain serotonin (5-HT) neuroendocrine function in patients taking cholesterol-lowering drugs. Biol Psychiatry. 1996;39:100–106. doi: 10.1016/0006-3223(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Downs JR, Oster G, Santanello NC. HMG CoA reductase inhibitors and quality of life. JAMA. 1993;269:3107–3108. doi: 10.1001/jama.269.24.3107. [DOI] [PubMed] [Google Scholar]
- Engelberg H. Low serum cholesterol and suicide. Lancet. 1992;339:727–729. doi: 10.1016/0140-6736(92)90609-7. [DOI] [PubMed] [Google Scholar]
- Farmer JA, Torre-Amione G. Comparative tolerability of the HMG-CoA reductase inhibitors. Drug Saf. 2000;23:197–213. doi: 10.2165/00002018-200023030-00003. [DOI] [PubMed] [Google Scholar]
- Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Byers T, Barrett DH, Stroup NE, Eaker E, Monroe-Blum H. Plasma lipid levels and psychologic characteristics in men. Am J Epidemiol. 1995;141:507–517. doi: 10.1093/oxfordjournals.aje.a117465. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Molecular medicine. The cholesterol quartet Science. 2001;292:1310–1312. doi: 10.1126/science.1061815. [DOI] [PubMed] [Google Scholar]
- Guillot F, Misslin P, Lemaire M. Comparison of fluvastatin and lovastatin blood-brain barrier transfer using in vitro and in vivo methods. J Cardiovasc Pharmacol. 1993;21:339–346. doi: 10.1097/00005344-199302000-00022. [DOI] [PubMed] [Google Scholar]
- Hawton K, Morgan HG. Suicide prevention by general practitioners. Br J Psychiatry. 1993;162:422. doi: 10.1192/bjp.162.3.422a. [DOI] [PubMed] [Google Scholar]
- Heron DS, Shinitzky M, Hershkowitz M, Samuel D. Lipid fluidity markedly modulates the binding of serotonin to mouse brain membranes. Proc Natl Acad Sci U S A. 1980;77:7463–7467. doi: 10.1073/pnas.77.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosifescu DV, Clementi-Craven N, Fraguas R, Papakostas GI, Petersen T, Alpert JE, et al. Cardiovascular risk factors may moderate pharmacological treatment effects in major depressive disorder. Psychosom Med. 2005;67:703–706. doi: 10.1097/01.psy.0000170338.75346.d0. [DOI] [PubMed] [Google Scholar]
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- Kirsch C, Eckert GP, Mueller WE. Statin effects on cholesterol micro-domains in brain plasma membranes. Biochem Pharmacol. 2003;65:843–856. doi: 10.1016/s0006-2952(02)01654-4. [DOI] [PubMed] [Google Scholar]
- Lamping KG, Nuno DW, Chappell DA, Faraci FM. Agonist-specific impairment of coronary vascular function in genetically altered, hyperlipidemic mice. Am J Physiol. 1999;276:R1023–1029. doi: 10.1152/ajpregu.1999.276.4.R1023. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301:309–314. doi: 10.1136/bmj.301.6747.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Iosifescu DV, Petersen T, Hamill SK, Alpert JE, Nierenberg AA, et al. Serum cholesterol in the continuation phase of pharmacotherapy with fluoxetine in remitted major depressive disorder. J Clin Psychopharmacol. 2004a;24:467–469. doi: 10.1097/01.jcp.0000132343.58584.1d. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Ongur D, Iosifescu DV, Mischoulon D, Fava M. Cholesterol in mood and anxiety disorders: review of the literature and new hypotheses. Eur Neuropsychopharmacol. 2004b;14:135–142. doi: 10.1016/S0924-977X(03)00099-3. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Mischoulon D, Hughes ME, Alpert JE, Nierenberg AA, et al. Serum cholesterol and serotonergic function in major depressive disorder. Psychiatry Res. 2003a;118:137–145. doi: 10.1016/s0165-1781(03)00066-0. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Sonawalla SB, Merens W, Iosifescu DV, Alpert JE, et al. Serum cholesterol in treatment-resistant depression. Neuropsychobiology. 2003b;47:146–151. doi: 10.1159/000070584. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Mietus LJ. Palmitate and cholesterol transport through the blood-brain barrier. J Neurochem. 1980;34:463–466. doi: 10.1111/j.1471-4159.1980.tb06621.x. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977a;229:327–336. [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977b;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Chattopadhyay A. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog Lipid Res. 2006;45:295–333. doi: 10.1016/j.plipres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Rajanikant GK, Zemke D, Kassab M, Majid A. The therapeutic potential of statins in neurological disorders. Curr Med Chem. 2007;14:103–112. doi: 10.2174/092986707779313462. [DOI] [PubMed] [Google Scholar]
- Ringo DL, Lindley SE, Faull KF, Faustman WO. Cholesterol and serotonin: seeking a possible link between blood cholesterol and CSF 5-HIAA. Biol Psychiatry. 1994;35:957–959. doi: 10.1016/0006-3223(94)91242-4. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Vanhoutte PM. Hypercholesterolemia causes generalized impairment of endothelium-dependent relaxation to aggregating platelets in porcine arteries. J Am Coll Cardiol. 1989;13:1402–1408. doi: 10.1016/0735-1097(89)90318-5. [DOI] [PubMed] [Google Scholar]
- Smith GD. Serum cholesterol concentration and postpartum depression. BMJ. 1997;314:144. [PMC free article] [PubMed] [Google Scholar]
- Sonawalla SB, Papakostas GI, Petersen TJ, Yeung AS, Smith MM, Sickinger AH, et al. Elevated cholesterol levels associated with nonresponse to fluoxetine treatment in major depressive disorder. Psychosomatics. 2002;43:310–316. doi: 10.1176/appi.psy.43.4.310. [DOI] [PubMed] [Google Scholar]
- Stroes E, de Bruin T, de Valk H, Erkelens W, Banga JD, van Rijn H, et al. NO activity in familial combined hyperlipidemia: potential role of cholesterol remnants. Cardiovasc Res. 1997;36:445–452. doi: 10.1016/s0008-6363(97)00199-5. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Kalaria RN, O’Brien JT. Depression and vascular disease: what is the relationship? J Affect Disord. 2004;79:81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- Tsuji A, Saheki A, Tamai I, Terasaki T. Transport mechanism of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors at the blood-brain barrier. J Pharmacol Exp Ther. 1993;267:1085–1090. [PubMed] [Google Scholar]
- Vecka M, Tvrzicka E, Stankova B, Novak F, Novakova O, Zak A. Hypolipidemic drugs can change the composition of rat brain lipids. Tohoku J Exp Med. 2004;204:299–308. doi: 10.1620/tjem.204.299. [DOI] [PubMed] [Google Scholar]
- Waelsch H, Sperry WM, Stoyanoff VA. The influence of growth and myelination on the deposition and metabolism of lipids in the brain. J Biol Chem. 1941;140:885–897. [Google Scholar]
- Wardle J, Armitage J, Collins R, Wallendszus K, Keech A, Lawson A. Randomised placebo controlled trial of effect on mood of lowering cholesterol concentration. Oxford Cholesterol Study Group BMJ. 1996;313:75–78. doi: 10.1136/bmj.313.7049.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology (Berl) 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163:1926–1932. doi: 10.1001/archinte.163.16.1926. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging. 2002;23:843–853. doi: 10.1016/s0197-4580(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Young-Xu Y, Chan KA, Liao JK, Ravid S, Blatt CM. Long-term statin use and psychological well-being. J Am Coll Cardiol. 2003;42:690–697. doi: 10.1016/S0735-1097(03)00785-X. [DOI] [PMC free article] [PubMed] [Google Scholar]