Abstract
The ability of adult peripheral sensory neurons to undergo functional and anatomical recovery following nerve injury is due in part to successful activation of transcriptional regulatory pathways. Previous in vitro evidence had suggested that the transcription factor Sox11, a HMG-domain containing protein that is highly expressed in developing sensory neurons, is an important component of this regenerative transcriptional control program. To further test the role of Sox11 in an in vivo system, we developed a new approach to specifically target small interfering RNAs (siRNAs) conjugated to the membrane permeable molecule Penetratin to injured sensory afferents. Injection of Sox11 siRNAs into the mouse saphenous nerve caused a transient knockdown of Sox11 mRNA that transiently inhibited in vivo regeneration. Electron microscopic level analysis of Sox11 RNAi-injected nerves showed that regeneration of myelinated and unmyelinated axons was inhibited. Nearly all neurons in ganglia of crushed nerves that were Sox11 immunopositive showed colabeling for the stress and injury-associated activating transcription factor 3 (ATF3). In addition, treatment with Sox11 siRNAs in vitro and in vivo caused a transcriptional and translational level reduction in ATF3 expression. These anatomical and expression data support an intrinsic role for Sox11 in events that underlie successful regeneration following peripheral nerve injury.
Keywords: Sensory neuron, RNA interference, Axotomy, Cutaneous nerve, Injury, Sry, Penetratin
1. Introduction
The SRY-box containing gene 11 (Sox11) transcription factor is a member of the group C high mobility group transcription factor family, which also includes Sox4 and Sox12 (Azuma et al., 1999; Dy et al., 2008; Hargrave et al., 1997; Wright et al., 1993). Group C proteins are expressed throughout the developing nervous system and comprise one of seven sequence homology defined subgroups in the Sox family (Bergsland et al., 2006; Wegner and Stolt, 2005). Sox gene expression is regulated both spatially and temporally during development (Gubbay et al., 1990; Wilson and Koopman, 2002; Wright et al., 1993) and all appear to have critical roles in embryonic growth (Wegner and Stolt, 2005). Sox factors may activate or repress transcription of target genes and in many cases overlap in expression. For example, in developing mice both Sox11 and Sox4 are required for expression of the pan-neuronal gene Tuj1 (Bergsland et al., 2006). This overlap, coupled with embryonic or perinatal lethality in gene deletion models, has made detailed study of the functional roles of Sox factors challenging (Cheung et al., 2000; Sock et al., 2004).
In addition to their role in development, some Sox proteins have been found to modulate adult injury responses as well. For example, increased Sox18 expression in epithelial cells correlates with capillary sprouting after wounding (Darby et al., 2001). Similarly, Sox15 knockout mice display disrupted muscle regeneration (Meeson et al., 2007) and increased expression of Sox 5, 6 or 9 is important for healing of bone fractures (Uusitalo et al., 2001). Whether Sox11 has a similar role in adult tissues has not been directly tested. Sox11 is expressed at high levels in developing sensory neurons and is hypothesized to regulate neuronal maturation (Hargrave et al., 1997). Its expression is significantly reduced during late phases of gestation and normally remains at low levels in adult neurons. A robust induction occurs however, in adult dorsal root sensory neurons following axotomy (Jankowski et al., 2006; Tanabe et al., 2003), suggesting a regulatory role in nerve regeneration. In support of this possibility, cultured adult DRG neurons treated with Sox11 siRNAs exhibit a significant decrease in regeneration as indicated by reduced neurite length and branching index (Jankowski et al., 2006).
Elements that regulate regeneration in the peripheral nervous system (PNS) following nerve injury are of significant interest because, in contrast to the central nervous system, axon regeneration in the PNS can occur quite successfully (Cajal, 1928; Silver and Miller, 2004). Transcription factors such as c-Jun, a component of the AP-1 transcription factor complex, and activated transcription factor 3 (ATF3), a member of the ATF/cAMP-responsive element binding protein (CREB) family, may underlie part of this dichotomy in regenerative ability. Both genes are normally expressed at low levels in adult DRG neurons and rise significantly following peripheral axotomy (Lindwall et al., 2004; Tsujino et al., 2000; Raivich et al., 2004) or after dissociation and culture (Seijffers et al., 2006). For ATF3, the increase in expression is hypothesized to facilitate expression of survival and axon growth related genes (Lindwall and Kanje, 2005; Seijffers et al., 2006). Indeed, constitutive expression of a Thy-1.2 ATF3 transgene in neurons of transgenic mice enhanced PNS regeneration (Seijffers et al., 2007). Because Sox11 is similarly upregulated following axotomy, we tested its role in vivo using a newly developed RNAi nerve injection delivery system. Results indicate that Sox11 has an important role in axon growth that may involve interaction with ATF3.
2. Results
2.1. Sox11 expression in DRG neurons is increased in response to peripheral but not central nerve injury
DRG neurons that are axotomized or undergo a crush injury express high levels of Sox11 mRNA for up to 2 wks following injury (Jankowski et al., 2006; and this report, Fig. 1). To assess the degree of association between Sox11 expression and regeneration, we compared its expression in DRG following peripheral or central nerve transection. Because injured central axon projections do not successfully regenerate, the prediction was that the rise in Sox11 expression would be substantially less in rhizotomized DRGs.
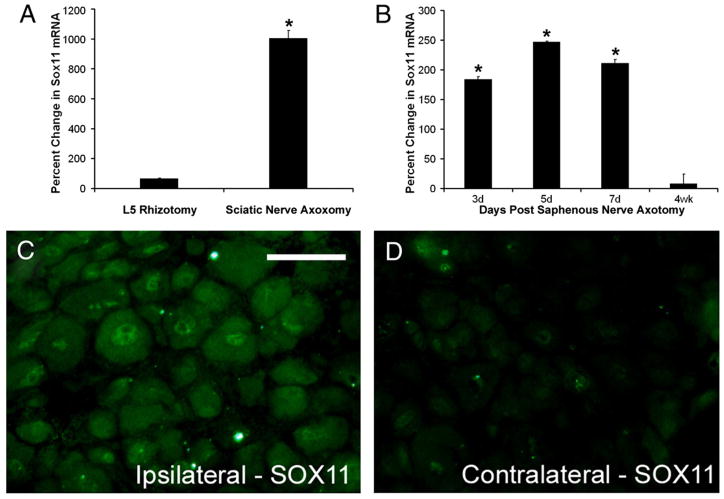
Fig. 1.
Sox11 is expressed in regenerating peripheral neurons. (A) RNA was collected from L5 DRGs following L5 dorsal root transection and from L4 DRGs following sciatic nerve transection (n =4/group). Sciatic nerve transection induced a 1004% increase in Sox11 mRNA in the L4/L5 DRG 3 d after cut compared to the 51% increase in L5 DRG following L5 rhizotomy. (B) Axotomy of the saphenous nerve caused a 184% increase in Sox11 mRNA in L2/L3 ganglia 3 d after nerve cut. Sox11 remains elevated for at least 7 d with return to baseline by 4 wks. (C) Sox11 immunoreactivity is detected in neuronal nuclei in ipsilateral L4 DRG at 7 d after axotomy of the sciatic nerve. Sox11 immunoreactivity is low in contralateral ganglia (D). *p value<0.001 (A) and <0.05 (B); values are relative to contralateral DRGs. Scale bar in C=50 μm.
The effect of a central nerve cut on Sox11 expression was assayed using RNA collected from the L5 DRG three days after a rhizotomy of the L5 dorsal root was performed. Rhizotomy increased Sox11 mRNA by 51% using semiquantitative real time PCR (RT-PCR) (Fig. 1A). In comparison, peripheral nerve cut of the sciatic nerve produced a 1004% increase in Sox11 mRNA in L4/L5 DRG at three days (Fig. 1A). Axotomy of the smaller saphenous nerve caused a 184% increase in the corresponding L2/L3 ganglia (Fig. 1B). Thus, Sox11 mRNA level is significantly increased following peripheral nerve injury and only slightly increased in response to central nerve injury.
Sox11 antibody labeling of DRG from transected nerves showed Sox11 protein was also increased following injury. Sciatic nerve axotomy significantly enhanced nuclear and cytoplasmic Sox11 immunoreactivity (Fig. 1C) compared to uninjured, contralateral DRGs where only a low level of staining was evident in a few neurons (Fig. 1D).
2.2. Targeted in vivo knockdown of Sox11 mRNA in DRG neurons
Similar to the in vivo elevation of Sox11 following axotomy, cultured primary DRG neurons also show robust expression of Sox11 mRNA as neurons begin extending processes in the dish (Fig. 2A and Jankowski et al., 2006). This increase in Sox11 mRNA can be detected by 3 h post plating. To determine if Sox11 expression was important for neurite growth we previously used Penetratin-linked Sox11 siRNAs (PenSOX) to achieve a high efficiency of transfection in order to reduce Sox11 expression in cultured DRG neurons (Davidson et al., 2004; Jankowski et al., 2006). The Sox11 specificity and effectiveness of these siRNAs in Neuro2a and DRG neuron cell cultures was previously demonstrated in these studies (Jankowski et al., 2006). Penetratin-mediated delivery of Sox11 siRNAs produced transfection efficiencies of approximately 90% and resulted in a substantial inhibition of neurite growth. In the present study, RNAi-mediated reduction in Sox11 mRNA lasted at least 24 h and returned to control levels (relative to PenCON siRNAs) by 4 d (Fig. 2A). On the protein level, western immunoblots of DRG neurons treated with Sox11 siRNAs also showed a reduced level of Sox11 (Fig. 2B). Knockdown on the protein level also occurred in treated Neuro2a cell cultures (not shown).
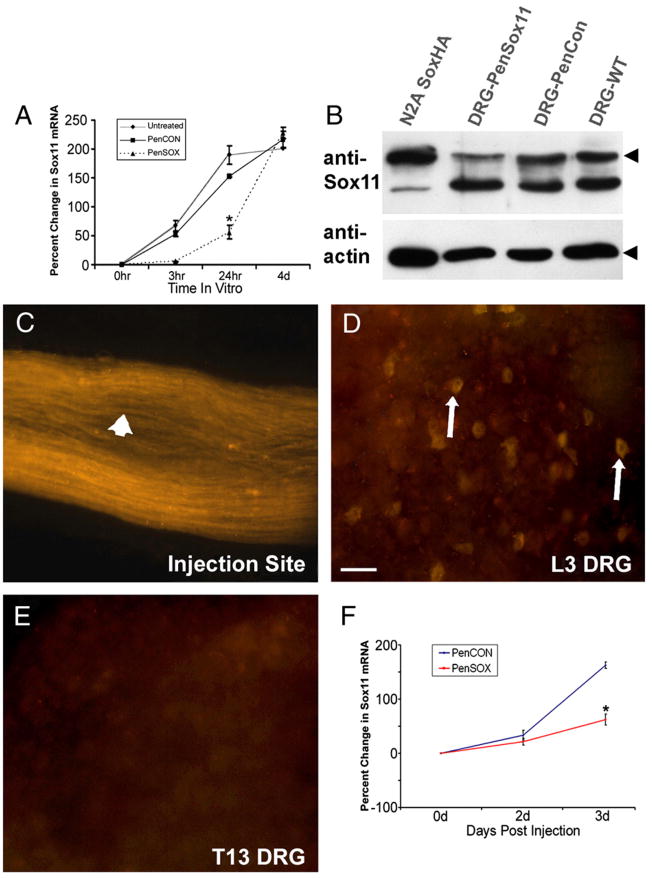
Fig. 2.
Sox11 siRNAs reduce the level of Sox11 mRNA and protein in DRG neurons. (A) Primary DRG neurons treated with Penetratin-linked Sox11 siRNAs (PenSOX) have reduced levels of Sox11 mRNA compared to untreated or nontargeting control siRNA (PenCON) treated cultures. Values are relative to mRNA level at time of dissociation and plating (0 h). N=3–4 per timepoint. (B) Cultured DRG neurons treated with Sox11 siRNAs (lane 2) show reduced Sox11 protein compared to parallel cultures treated with nontargeting siRNA (lane 3) and untreated cultures (lane 4). Sox11 protein from Neuro2a cells transfected with CMV-Sox11 (lane 1) is used to show migration at 44.7 Kd. Arrowhead indicates Sox11; identity of band below Sox11 is unknown. Actin blot indicates sample loading. (C) Injected PenCON Cy3-tagged siRNAs are visible in nerve fibers at injection site (arrowhead). (D) Whole mount image of L3 DRG shows several Cy3-positive somas (arrows) after saphenous nerve injection of PenCON siRNA that are absent in the ipsilateral T13 DRG (E). (F) Fold change in Sox11 mRNA after injection of nontargeting control (PenCON) or Sox11 (PenSOX) siRNAs into the saphenous nerve. *p value<0.05. Scale bar in D=50 μm.
To test if siRNA-mediated reduction in Sox11 affects axon regrowth of DRG neurons in vivo, the feasibility of delivering Penetratin-linked siRNAs to nerve fibers by direct nerve injection was assessed. We used the mouse cutaneous saphenous nerve for these injections because it is anatomically and functionally well-characterized, a simple surgical approach can be used that causes minimal damage to surrounding tissue and a crush injury elicits a detectable change in Sox11 expression (Fig. 1B). Penetratin-linked, CY3 labeled non-targeting control (PenCON) siRNAs or Sox11 targeting (PenSOX) siRNAs were injected into the nerve and siRNA distribution examined using imaging and RNA expression analysis. Injected siRNAs were taken up into nerve fibers (Fig. 2C) and retrogradely transported back to the associated cell bodies within the L2/L3 DRGs (Fig. 2D). Retrograde transport of siRNAs occurred over two days, although knockdown of Sox11 mRNA was not significantly changed (relative to PenCON injected nerves) until 3 d post-injection where a 38% reduction in Sox11 mRNA was measured (Fig. 2F). It should be noted that injection alone of PenCON siRNAs, siRNA buffer or saline into the saphenous nerve increased Sox11 81%–160%, indicating that even the relatively small injury caused by nerve injection is sufficient to induce Sox11 gene expression.
One possible effect of injecting siRNAs in the nerve is that they would be taken up by other cell types (fibroblasts, Schwann cells) in the nerve fiber that express Sox11. This could potentially change their function and affect regeneration. RT-PCR of RNA isolated from ipsilateral and contralateral crushed saphenous nerves (n=3/group) showed however, that although positive for GAPDH, they did not express Sox11 mRNA (not shown).
2.3. Transient reduction in Sox11 inhibits in vivo nerve regeneration
Based on the in vitro and in vivo data supporting a role for Sox11 in neurite growth, we examined whether RNAi-treatment altered the rate of saphenous nerve regeneration. The saphenous nerve in mouse is comprised of 80% unmyelinated small to medium diameter fibers and 20% myelinated axons (Baron et al., 1988; Stucky et al., 1999). These axons (sensory and sympathetic) innervate hairy skin from the mouse knee to the dorsum of the foot extending to the toes (Kitao et al., 2002; Koltzenburg, 2000). Because it takes 2–4 wks for functional regeneration of the saphenous (McIlwrath et al., 2005), the number of unmyelinated and myelinated axons that regenerated after injury at 3, 7 and 14 days post crush was determined for each experimental group. A nerve crush injury was used for this analysis, rather than a complete axotomy, since crush alone induces a significant rise in Sox11 mRNA, causes degeneration of all nerve fibers and provides consistency in regeneration rate.
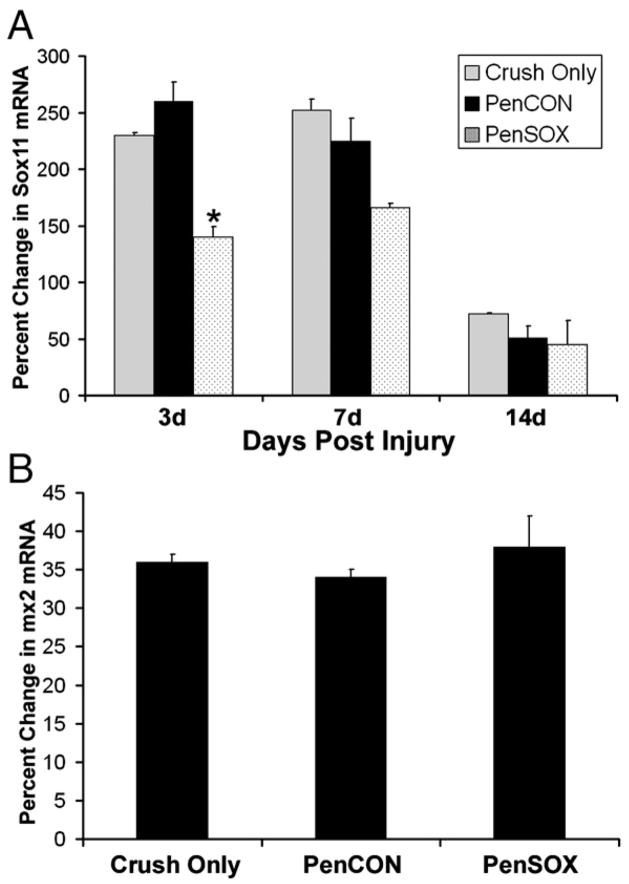
The elevation in Sox11 expression at 3 d and 7 d following saphenous nerve crush was reduced by injection of PenSOX siRNAs, although statistical change was only at 3 d (Fig. 3A). By day 14, Sox11 mRNA level was returned to control levels. To test whether the RNAi treatment related to an activation of the interferon pathway (Alvarez et al., 2006), RT-PCR analysis was used to measure the relative level of the interferon-inducible myxovirus resistance 2 (mx2) transcript (Asano et al., 2003). DRGs of mice that received nerve crush only, PenCON injection and crush or PenSOX injection and crush had the same level of expression following siRNA treatment (Fig. 3B). In addition to the panel of controls previously reported in Jankowski et al. (2006), these data support the specificity of the siRNAs used under these conditions.
Fig. 3.
siRNA injection in crushed saphenous nerve reduces Sox11 mRNA in DRG. (A) At 3 d, nerve crush alone caused a 230% increase in Sox11 in L2/L3 DRGs whereas nerve crush plus PenCON caused a 260% increase. Values are relative to the contralateral DRG where no change in baseline Sox11 mRNA level was measured. PenSOX injection plus nerve crush reduced Sox11 mRNA to 140%. Seven days after crush, a 252% increase in Sox11 was measured while PenCON injected mice had a 225% increase in Sox11. PenSOX siRNA treatment produced a 160% increase in Sox11 that was not statistically different from PenCON values. After 14 days, all experimental conditions had similar levels of Sox11 expression (Sox11: 72% vs 51% vs 45% increase). N=4 per experimental group. (B) Interferon inducible gene mx2 showed no change in expression under all conditions. *p value<0.05.
To determine if RNAi reduction in Sox11 mRNA was sufficient to modulate nerve regeneration, the total number of unmyelinated and myelinated axons in nerves of mice (n=3/group) that received PenCON or PenSOX siRNA injection prior to and at the time of nerve crush was counted at the electron microscopic level. We first analyzed crushed nerves to be able to relate the degree of morphological damage to the time following injury. Analysis of saphenous nerves at the crush site showed significant damage to all fibers at 3 d following injury (Fig. 4B) compared to uninjured nerves (Fig. 4A). At 1 mm distal to the crush site an occasional large myelinated fiber could be found, but most fibers had not regenerated (not shown). By 14 d following nerve crush (Fig. 4C), most myelinated and unmyelinated fibers had organizational features and morphologies similar to those seen in uninjured nerves, which is consistent with prior reports.
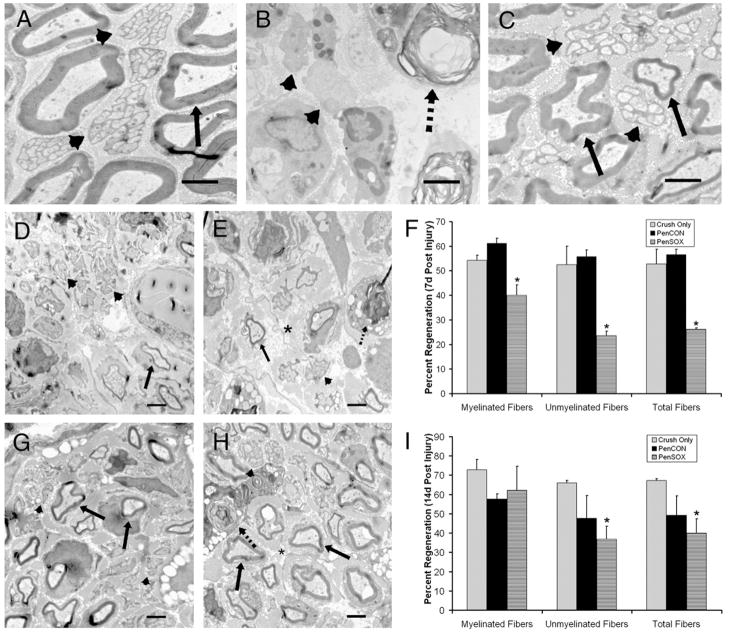
Fig. 4.
Electron photomicrographs of representative regions of the saphenous nerve under normal conditions and after siRNA treatment and nerve crush. (A) Uninjured saphenous nerve showing myelineated (arrow) and unmyelinated (arrowheads) fibers. (B) Degeneration of myelinated and unmyelinated fibers occurs 3 d following nerve crush. Extensive myelin unraveling (dashed arrow) and disruption of Remak bundle structure (arrowhead) are evident. (C) Nerve crush site at 7 d post injury. Morphology of myelinated (arrow) and unmyelinated (arrowheads) fibers is almost restored to normal. (D) Regenerating myelinated (arrow) and unmyelinated (arrowheads) axons in PenCON injected nerves 4 mm distal to the crush site at 7 d post injury. (E) PenSOX injected nerves had fewer regenerating myelinated (arrow) and unmyelinated axons (arrowhead). (F) Plot of myelinated, unmyelinated and total axons regenerated at 4 mm distal at 7 d post crush. PenSOX injected nerves had fewer myelinated and unmyelinated regenerated axons compared to nerves injected with PenCON where the percent of myelinated and unmyelinated axons were greater (p values<0.02 and <0.001, respectively). (G, H) At 14 d after injury, 6 mm distal to the nerve crush, the percent of regenerating myelinated fibers (arrows) in PenCON injected nerves (G) was similar to PenSOX injected nerves (H). In PenSOX nerves (H) unmyelinated fiber (arrowheads) regrowth remained inhibited and a few degenerating fibers (dashed arrow) were visible. (I) Plot shows the percentage of regenerated myelinated fibers in crush only, PenCON and PenSOX injected mice is the same at 14 d post injury. Unmyelinated fibers were fewer in PenSOX injected nerves but only when compared to crush only (*p value<0.01 relative to crush only). Total fiber analysis showed PenSOX injected nerves with 40.1% regeneration compared to 67.3% in crush only and 49.3% in PenCON samples (*p value<0.01 relative to crush only). Asterisks in E and H indicate interaxonal space. All scale bars=2 μm.
Given the absence of regenerating fibers at the 3 d time-point, we focused analysis on regenerated fibers visible at 7 d and 14 d post injury. Electron micrographs of areas 1 mm to 2 mm proximal to the injection site and 2 mm, 4 mm and 6 mm distal to the crush site were used to determine the percentage of fiber regeneration (Figs. 4D–F). At the 7 d timepoint, no difference in regenerated fibers was found between PenCON and PenSOX siRNA injected mice 2 mm distal to the crush site (data not shown). At 4 mm distal however, crushed nerves treated with Sox11 siRNA (Fig. 4E) had fewer regenerating myelinated fibers (40%) that were also smaller relative to crush only (54.2%) and crush plus PenCON (61.2%; Fig. 4D). Regeneration of unmyelinated fibers was also affected by Sox11 knockdown. At 4 mm distal to the crush site, unmyelinated fiber number was reduced in PenSOX injected mice (23.6% PenSOX, 55.7% PenCON and 52.5% crush-only), as was their organization into Remak bundles (Figs. 4D–F). At 14 d after nerve crush, when Sox11 mRNA level had returned to baseline levels (see Fig. 3), no difference in the percentage of regenerated myelinated axons between crush-only, crush plus PenCON (Fig. 4G) and crush plus PenSOX (Fig. 4H) groups were measured 6 mm distal to the crush site. However, the percent of unmyelinated axons that had regenerated by 14 d, relative to crush only, was still significantly reduced. Whereas crush-only nerves had 66% of unmyelinated fibers regenerated and crush/PenCON treated nerves had 47.7%, only 36.8% of unmyelinated fibers were regenerated in PenSOX/crush treated mice (Fig. 4I).
2.4. In vivo knockdown of Sox11 reduces expression of ATF3
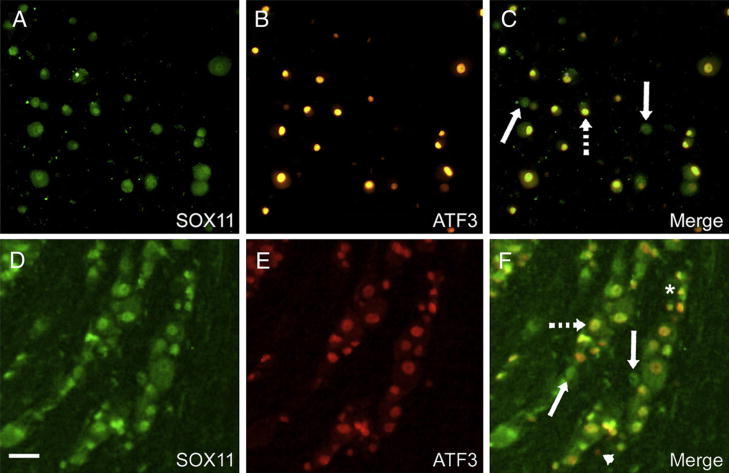
The inhibition in the rate of nerve regeneration by Sox11 siRNAs suggested that Sox11 has an important role in regulating injury-associated transcriptional changes. To further examine Sox11 as a neural injury associated gene, we compared expression of Sox11 with ATF3, a transcription factor known to be elevated following nerve injury (Lindwall et al., 2004; Tsujino et al., 2000). Antibody labeling against ATF3 and Sox11 showed a high degree of overlap in Sox11 and ATF3 expression in cultured DRG neurons. Nearly all neurons were Sox11-immunopositive (Fig. 5A) and, although some Sox11 neurons were ATF3 negative (Figs. 5B and C), all ATF3 neurons expressed Sox11 immunoreactivity. A similar finding was made in DRG ganglia analyzed 3 d after sciatic nerve cut (Figs. 5D, E). Many neurons in the axotomized L3/L4 ganglia were Sox11 immunopositive and nearly all exhibited ATF3 colabeling (Fig. 5F).
Fig. 5.
Sox11 expression colocalizes with and modulates ATF3 expression following nerve injury. (A) Cultured DRG neurons show nuclear localization of Sox11 24 h after plating that colocalizes with ATF3 immunolabeling (B). Dashed arrow in C illustrates one of many overlapping cells. Neurons that were Sox11 positive and ATF3 negative are rare (solid arrows). Following sciatic nerve injury Sox11 expression (D) is found in many neurons of the L3/L4 DRG, many of which overlap (F) with ATF3 immunopositive neurons (E). In F, broken arrow indicates overlap, solid arrows indicate neurons positive for Sox11 alone, arrowhead indicates rare neuron that was only ATF3 positive and asterisk indicates neuron that does not express either Sox11 or ATF3.
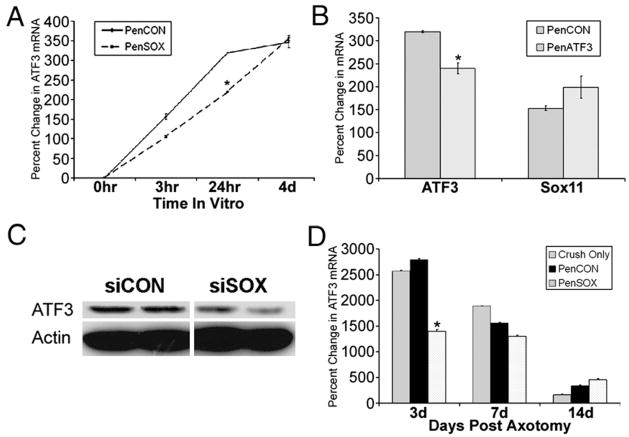
To investigate a possible interaction between Sox11 and ATF3, we compared expression of Sox11 and ATF3 mRNAs in cultured DRG neurons treated with the respective siRNAs. RT-PCR was used to measure ATF3 mRNA in DRG neuron cultures treated with either nontargeting siRNAs or Sox11 siRNAs (Fig. 6A). Similar to Sox11 (Fig. 2), a rise in ATF3 mRNA was seen by 3 h post plating in cultures treated with nontargeting siRNAs. Interestingly, a 24% reduction in ATF3 mRNA was found in Sox11 siRNA treated cultures suggesting the reduced level of Sox11 modulated ATF3 mRNA expression. This was not found for the c-Jun transcription factor which showed no change in mRNA expression following Sox11 siRNA treatment (data not shown).
Fig. 6.
Knockdown of Sox11 modulates ATF3 expression. (A) Cultured DRG neurons transfected with PenSOX targeting siRNA have decreased levels of ATF3 mRNA at 24 h post treatment. (B) Cultured DRG neurons treated with PenCON or PenATF3 siRNAs showed reduction in ATF3 mRNA at 24 h after transfection but no change in Sox11 mRNA. Values are relative to mRNA level at time of dissociation and plating. (C) Transfection of Sox11 siRNAs decreased Sox11 and ATF3 protein in Neuro2a cells. (D) At 3 d after nerve crush ATF3 mRNA in L2/L3 DRGs from untreated and PenCON injected nerves is increased. PenSOX injection plus nerve crush inhibited the rise in ATF3 mRNA. At 7 d and 14 d after injury no difference in ATF3 mRNA level was measured. Values are relative to contralateral side DRG samples. N=3 for all experimental groups. All *p values<0.05. Scale bar in D=40 μm.
Analysis using the Neuro2a cell line showed the reduction in ATF3 was also at the protein level (Fig. 6C). To test if knockdown of ATF3 affected the level of Sox11, cultured DRG neurons were treated with Penetratin conjugated ATF3 siRNAs (Fig. 6B). This treatment reduced ATF3 mRNA although no change in Sox11 mRNA was detected under these conditions.
To test if injury-mediated Sox11 expression affected ATF3 level in vivo, we examined ATF3 expression in ganglia of nerves that had received a nerve crush injury (Fig. 6D). Analysis at the mRNA level showed that in addition to the siRNA mediated knockdown in Sox11 expression measured 3 d following saphenous nerve crush (Fig. 3A), the level of ATF3 mRNA was also decreased (Fig. 6D). The injury-induced elevation of ATF3 mRNA was reduced from 2790% to 1400% (p<0.05) at the 3 d timepoint. Sox11 and ATF3 mRNA levels returned to control levels by day 14. In summary, these data suggest that reduction in the level of Sox11 negatively affects ATF3 expression.
3. Discussion
In this study, the developmentally regulated transcription factor Sox11 was identified as an important component of the transcriptional response that occurs following peripheral nerve injury. A transient RNAi-mediated knockdown of Sox11 expression in injured DRG neurons inhibited axon regeneration following crush injury to the saphenous nerve. Similar to ATF3, Sox11 expression was not restricted to a particular neuronal subtype but was induced in small, medium and large neuron populations. This distribution is also supported by studies of neurons in culture, where large and small neurons showed reduction in neurite growth when transfected with Sox11 siRNAs (Jankowski et al., 2006). In vivo analysis showed regeneration of both myelinated and unmyelinated fibers were slowed by Sox11 knockdown, although this effect was greatest for unmyelinated fibers. This difference could reflect a difference in transfection efficiency, siRNA transport or efficiency in functional knockdown. Another possibility is that unmyelinated and myelinated axon regeneration potential is inherently different and independent of siRNA treatment. This was previously suggested by a study of peripheral nerve regeneration in rat that indicated that a faster, more complete regeneration of myelinated fibers occurs relative to unmyelinated fibers (Lozeron et al., 2004).
To examine the role of Sox11 on regeneration in vivo, we used a novel delivery system in which siRNAs conjugated to the lipid permeable peptide Penetratin are injected into a peripheral nerve. The advantage to this system is that it allowed specific targeting of the knockdown effect to injured afferents. A disadvantage is that the injection itself also evoked a small but measurable injury response. Conjugation of siRNAs to Penetratin was initially shown to enhance transfection efficiency of primary cortical neurons and cultured DRG neurons without inducing cell death and without inhibiting siRNA activity (Davidson et al., 2004; Jankowski et al., 2006). Here we show nerve injection of Penetratin-linked siRNAs to the saphenous cutaneous nerve, a small nerve that innervates the peripheral field of the lower leg and foot. Visualization using CY3 conjugated siRNAs showed efficient uptake by axons and retrograde transport to only a percentage of neuronal somas in the L2/L3 DRG that presumably innervate this restricted target field.
Prior studies using the Neuro2a neuroblastoma cell line suggested Sox11 expression level modulated genes involved in cell survival and regeneration (Jankowski et al., 2006). Whether a similar effect on survival genes operates in vivo is unclear although apoptotic neurons were not detected 3 d following saphenous nerve injury, as measured by immunolabeling for activated caspase 3 in L2/L3 DRGs of PenCON or PenSOX injected and axotomized mice (data not shown). Thus, at least in this short timeframe, the effects of Sox11 siRNA knockdown did not produce the same effect in vivo as in vivo. This difference may reflect a more prolonged time for initiation of cell death as previously reported for injured neurons in vivo (Jiang et al., 2005; Kuo et al., 2005; Thippeswamy et al., 2001) and the more supportive environment of the intact in vivo ganglia.
Expression of Sox11 in DRG appears to be highly sensitive to nerve injury being induced following nerve cut, nerve crush and to a lesser degree, nerve injection. Following crush or cut injury, the steady rise in Sox11 expression coincided with the time in which injured nerves exhibit Wallerian degeneration and subsequent regeneration (Baba et al., 1982; Kury et al., 2001; Mi et al., 2005; Sebille, 1982; Thomas, 1982). For the saphenous nerve, functional regeneration occurs approximately 4 wks after nerve transection (McIlwrath et al., 2005) at which time Sox11 (and ATF3) mRNAs are returned to baseline level. This time course of expression is consistent with a role for Sox11 in functional nerve regeneration that may require interaction with other injury-induced transcriptional regulators such as ATF3. ATF3 has been associated with neurite sprouting (Pearson et al., 2003), is induced in cells upon injury (Campbell et al., 2005; Isacsson et al., 2005; Lindwall and Kanje, 2005; Tsujino et al., 2000) and improves nerve regeneration when constitutively expressed in DRG neurons (Seijffers et al., 2007). Our data suggests an interaction between ATF3 and Sox11 since ATF3 was decreased with Sox11 knockdown. Interestingly, the reverse was not found, i.e., knockdown of ATF3 using ATF3 siRNAs reduced mRNA levels of ATF3, but did not affect Sox11 level. This suggests but does not prove that Sox11 may influence regulation of ATF3 gene expression. Chromatin binding and expression assays to assess interaction of Sox11 with ATF3 gene regulatory regions will be necessary to test this possibility.
Another finding from this study is that, similar to ATF3 and c-Jun (Kenney and Kocsis, 1997; Shortland et al., 2006), L5 rhizotomy does not induce a significant increase in Sox11 in the L5 DRG. Indeed, the modest increase in Sox11 mRNA following rhizotomy could be due to the associated surgical procedure. Given the importance of Sox11 for neurite growth and peripheral nerve regeneration and its potential regulatory influence on ATF3, it is possible that the minimal induction of ATF3 expression in DRGs following central injury is due, in part, to the low level production of Sox11. In this regard, Sox11, as a regulator of gene expression and tissue differentiation, may underlie some of the transcriptional discrepancies that exist in DRG neurons following central and peripheral injuries. A more detailed analysis of Sox11 function and its relationship to other transcriptional regulators active following rhizotomy and axotomy will test this possibility and further reveal the regulatory role of Sox11 in nerve regeneration.
4. Experimental procedures
4.1. Neuro2a cell culture and siRNA treatment
The mouse neuroblastoma cell line Neuro2a (ATCC clone number CCL-131, Manassas, VA) (Olmsted et al., 1970) was maintained as described in Jankowski et al. (2006). Cells were plated into 6-well plates at a concentration of 50,000 cells/well, grown to 50% confluence (18–24 h) and then treated with siRNAs. Two hours prior to siRNA transfection fresh medium was added to cultures and transfection was carried out of 10 nM Sox11 siRNAs (sense strand 5′GGU CCA AGA UCG AGC GCA GUU-3′), ATF-3 siRNAs (5′GUG GUG ACC UAC UGC AUU GUU-3′) or 10 nM non-targeting siRNAs, all purchased from Dharmacon (Lafayette, CO). The non-targeting siRNA sense sequence was 5′-Th-UAG CGA CUA AAC ACA UCA A-dT-dT-3′ and the antisense sequence is 5′-DY547-UUG AUG UGU UUA GUC GCUA-dT-dT-3′. TRANSIT-TKO transfection reagent (Mirus Corporation) (4 μl) and serum-free MEM (100 μl) were incubated at room temperature (RT) for 5–20 min and then the appropriate volume of 1 μm siRNAs was added to the TKO/MEM mixture to obtain a final concentration of 10 nM siRNA per well. Solutions were mixed, incubated at RT for 5–20 min and then added dropwise to the cultures. Cells were allowed to incubate for 24 h prior to protein isolation.
4.2. Primary neuron culture
Male C57/Bl6 (Jackson Laboratories, Bar Harbor, ME) mice approximately 2–3 months of age were deeply anesthetized with 2.5% avertin and intracardially perfused with 35 mL of Hank’s balanced salt solution (HBSS; Gibco). DRGs were isolated and grown as described in Malin et al. (2007).
4.3. Penetratin-1/siRNA linkage and transfection
siRNAs were conjugated to Penetratin-1 (Q-Biogene) peptide as previously described (Davidson et al., 2004; Jankowski et al., 2006). Penetratin-1 is a patented 16-amino acid peptide (NH2-RQIKIWFQNRRMKWKK-COOH) corresponding to the third helix of the homeodomain of Antennapedia protein that is able to translocate across biological membranes and convey oligonucleotides to the cytoplasm of many cell types (Derossi et al., 1998). siRNAs were synthesized with a 5′ thiol modification on the sense strand to allow Penetratin conjugation. The non-targeting siRNA duplex also contained a 5′ CY3 molecule conjugated to the antisense strand. Equimolar concentrations of activated Penetratin-1, reconstituted in sterile water, were added to each of the siRNAs dissolved in 1× siRNA buffer (Dharmacon), incubated 15 min at 65 °C and then 1 h at 37 °C. Stock siRNAs were brought to 400 nM NaCl to enhance solubility of Penetratin linked siRNAs (Pen-siRNAs) and kept at −80 °C until used. Pen-siRNAs were heated to 65 °C for 15 min prior to DRG neuron transfections. 500 μL of media with NGF (50 ng/ml) was removed from DRG cultures and Penetratin-1 linked control (non-targeting; PenCON) and/or Penetratin-1 linked Sox11 (targeting; PenSox) or Penetratin linked ATF3 (targeting; PenATF3) siRNAs were added. Media containing Pen-siRNAs was gently mixed and added back to cultures as a 2× solution to give a final concentration of 80 nM siRNAs per well. Cells were then incubated at 37 °C/5% CO2 for appropriate times.
4.4. RNA isolation and analysis
RNA isolation from cells and tissues was performed using Qiagen RNeasy mini kits using the supplied protocol. 1 μg of total RNA was treated with DNase I (Invitrogen), annealed to random primers, reverse transcribed using Superscript II reverse transcriptase (Invitrogen) and stored at −80 °C until used in SYRB green labeled real time PCR (RT-PCR) reactions. For RT-PCR, 20 ng of cDNA were added to SYBR Green MasterMix (Applied Biosystems), run in triplicate on an Applied Biosystems Imager and values normalized to GAPDH or neuronal specific enolase (NSE). Both of these internal control genes were unchanged following axotomy or cell culture, respectively (not shown). Changes in expression are reported as a ΔΔCt value that is calculated by subtracting the gene expression by the GAPDH or NSE control for each sample. Fold change is described as 2ΔΔCt (Applied Biosystems) and 2-fold change equals 100% change.
4.5. Western blot
Primary DRG neurons from 8 C57/Bl6 mice were isolated, pooled, plated into separate wells and then transfected with either control or Sox11 targeting siRNAs at 1 h and 25 h after plating. At 48 h post plating, neurons from similarly treated wells were pooled and homogenized in lysis buffer containing 20 mM, HEPES pH 7.9, 10 mM KCl2, 5 mM MgCl2, 5 mM EDTA pH 8, 10% glycerol, 2 mM DTT, 1% Triton X-100, 300 mM NaCl, 50 mM NaF, 1 mM Na3VO4 and protease inhibitors (1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml aprotinin; Sigma Biochemicals). Samples were sonicated, incubated on a rotator at 4 °C for 20 min, centrifuged, boiled 10 min in a denaturing buffer containing β-mercaptoethanol and SDS, separated on a 10% polyacrylamide SDS-PAGE gel and transferred to nitro-cellulose membrane (BioRad) that was blocked and then incubated with primary antibodies overnight at 4 °C (Sox11, 1:1000, sc-20096, Santa Cruz; ATF3, 1:1000; actin, 1:5000). Antibody binding was visualized using horseradish peroxidase-conjugated goat anti-rabbit or donkey anti-goat secondary antibodies (1:5000) and chemiluminescent detection (Pierce Biochemical). Molecular weight of Sox11 was estimated by gel interpolation.
Transfected Neuro2a cells were dissolved in lysis buffer containing 1% sodium dodecyl sulfate (SDS), 10 mM Tris–HCl (pH 7.4) and protease inhibitors (1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM sodium orthovanadate and 100 μg/ml phenylmethylsulfonyl fluoride). Samples (25 μg) were centrifuged, boiled 10 min in denaturing buffer containing β-mercaptoethanol and SDS, separated on a 10% polyacrylamide SDS-PAGE gel and transferred to a nitrocellulose membrane (BioRad) that was blocked and then incubated with primary antibodies overnight at 4 °C (Sox11, 1:100, sc; ATF3, 1:1000; actin, 1:10,000). Antibody binding was visualized as described above using chemiluminescent detection.
4.6. Nerve injury and siRNA injection
For saphenous nerve injuries and analysis of regeneration, male Swiss Webster mice were used. DRG neurons cultured from Swiss Webster mice show a similar level of Sox11 induction as found for the Blk6/C3 H strain suggesting parallel mechanisms of Sox11 actions. Mice 4–6 wks of age (Hilltop Lab Animals, Scottdale, PA) were anesthetized by intramuscular injection of a mixture of ketamine and xylazine (90 mg/kg and 10 mg/kg, respectively). A small incision made in the mid-thigh region exposed the saphenous nerve that was transected using fine irredectomy scissors. Wounds were closed using 7.0 silk sutures. Nerve crush was performed using the same procedure except the exposed nerve was crushed using number 5 forceps (Fine Science Tools, Forester City, CA) held together for 4–5 s. For L5 rhizotomy, an incision was made just proximal to the iliac crest, a laminectomy was performed to expose the L5 dorsal root, which was loosened from the spinal cord and transected. Sciatic nerve axotomy was done on mice anesthetized with isofluorane. The nerve was tightly ligated with 6.0 silk sutures, transected distal to the suture and the muscles sutured and wound closed with microclips (Roboz, Gaithersburg, MD). In animals that received siRNA injection, 0.1–0.2 μL of 90 μM Penetratin-1 linked control (PenCON) or Sox11 targeting (Pen-SOX) siRNAs were pressure injected into the saphenous nerve using a glass microelectrode connected to a pico-spritzer. This amount was chosen based on dose response/efficiency of knockdown analysis previously done in cultured neurons (Jankowski et al., 2006). Injections were done the day before and at the time of nerve crush. Nerve cuts/crushes were made 1–2 mm distal to the injection site without cleaning the surrounding area or damage to vasculature. Nerves were collected 2–28 d after nerve injury for immuno-cytochemical, electron microscopic and RT-PCR analysis. Animals were cared for and used in accordance with guidelines of the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals and following institutional AAALAC approved practices.
4.7. Immunocytochemistry
Ganglia were removed from animals perfused with 4% paraformaldehyde (PF), postfixed in 4% PF for 1 h, washed 3×s in phosphate buffered saline (PBS) and embedded in 10% gelatin in 0.1 M phosphate buffer (PB). Cultured DRG neurons were washed in 0.1 M PBS and fixed 5 min in 4% PF. DRG sections were cut at 30 or 50 μm and collected in 0.1 M PBS. Cells and/or sections were washed in PBS and incubated overnight in goat Sox11 antibody (1:50; Santa Cruz) or rabbit ATF3 antibody (1:500, Santa Cruz) diluted in PBS containing 0.25% triton X-100. Binding was visualized using CY2, CY3 or CY5 conjugated secondary antibodies (1:1000; Jackson Labs). Sections were washed in PBS, mounted on Superfrost slides (VWR) and images captured using a Leica fluorescent microscope.
4.8. Electron microscopy
Myelinated and unmyelinated axons in the saphenous nerve were counted at the electron microscopic level using photographic montages taken across the entire diameter of the saphenous nerve. Nerves were collected from deeply anesthesized animals intracardially perfused with cold saline following a 5 min fixation in situ with 2% gluteraldehyde/4% paraformaldehyde. Nerves were post fixed in 2% gluteraldehyde/4% PF for 2 h, cut into 1 mm blocks, post-fixed in 2% osmium tetroxide in 0.1 M PB for 1 h and dehydrated in an increasing series of ethanols and propylene oxide. Blocks were embedded in EMBed 812 (Electron Microscopy Sciences), cut on an ultramicrotome at 80–90 nm and collected on formvar/carbon coated copper slot grids. Grids were counter stained in uranyl acetate and lead citrate and analyzed on a Morgagni electron microscope. Images were taken at 4400× of the entire nerve using AMT digital imaging software and compiled into montages using Adobe Photoshop. Axon numbers were counted across montaged photographs taken of sections at 7 d and 14 d and then averaged across animals (n = 3 per condition). Axons of comparable morphology to uninjured nerves and those not meeting the criteria for electron-dense degeneration according to Peters et al. (1991) and Mugnaini and Friedrich (1981) were used to establish the criteria for degenerating axons. These criteria do not meet those established for filamentous, flocculent or watery degeneration (Mugnaini and Friedrich, 1981). Degenerating axons were determined to be electron dense with swollen mitochondria (when present) and associated with surrounding glial cells. The number of non-degenerating axons at −1 mm to −2 mm, 2 mm, 4 mm and 6 mm distal to the site of the nerve crush at various times after injury were recorded and presented as an average difference in the percent of regenerating fibers between PenCON and PenSOX injected mice normalized to the number of axons present proximal (−1 to −2 mm) to the injection site. Data were segregated into myelinated and unmyelinated groups. Statistical significance was set at p<0.05 for all evaluations.
Acknowledgments
These studies were supported by grants from the NINDS (NS33730, K.M.A.; NS23725, H.R.K and T32NS007433, M.P.J.).
References
- Alvarez VA, Ridenour DA, Sabatini BL. Retraction of synapses and dendritic spines induced by off-target effects of RNA interference. J Neurosci. 2006;26:7820–7825. doi: 10.1523/JNEUROSCI.1957-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano A, Jin HK, Watanabe T. Mouse Mx2 gene: organization, mRNA expression and the role of the interferon-response promoter in its regulation. Gene. 2003;306:105–113. doi: 10.1016/s0378-1119(03)00428-1. [DOI] [PubMed] [Google Scholar]
- Azuma T, Ao S, Saito Y, Yano K, Seki N, Wakao H, Masuho Y, Muramatsu M. Human SOX11, an upregulated gene during the neural differentiation, has a long 3′ untranslated region. DNA Res. 1999;6:357–360. doi: 10.1093/dnares/6.5.357. [DOI] [PubMed] [Google Scholar]
- Baba M, Fowler CJ, Jacobs JM, Gilliatt RW. Changes in peripheral nerve fibres distal to a constriction. J Neurol Sci. 1982;54:197–208. doi: 10.1016/0022-510x(82)90182-4. [DOI] [PubMed] [Google Scholar]
- Baron R, Janig W, Kollmann W. Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical organization of the lumbar sympathetic nervous system of the rat. J Comp Neurol. 1988;275:460–468. doi: 10.1002/cne.902750310. [DOI] [PubMed] [Google Scholar]
- Bergsland M, Werme M, Malewicz M, Perlmann T, Muhr J. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev. 2006;20:3475–3486. doi: 10.1101/gad.403406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal R. Degeneration and Regeneration of the Nervous System. Oxford Univ. Press; London: 1928. [Google Scholar]
- Campbell G, Hutchins K, Winterbottom J, Grenningloh G, Lieberman AR, Anderson PN. Upregulation of activating transcription factor 3 (ATF3) by intrinsic CNS neurons regenerating axons into peripheral nerve grafts. Exp Neurol. 2005;192:340–347. doi: 10.1016/j.expneurol.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Cheung M, Abu-Elmagd M, Clevers H, Scotting PJ. Roles of Sox4 in central nervous system development. Brain Res Mol Brain Res. 2000;79:180–191. doi: 10.1016/s0169-328x(00)00109-1. [DOI] [PubMed] [Google Scholar]
- Darby IA, Bisucci T, Raghoenath S, Olsson J, Muscat GE, Koopman P. Sox18 is transiently expressed during angiogenesis in granulation tissue of skin wounds with an identical expression pattern to Flk-1 mRNA. Lab Invest. 2001;81:937–943. doi: 10.1038/labinvest.3780304. [DOI] [PubMed] [Google Scholar]
- Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, Troy CM. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004;24:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- Dy P, Penzo-Mendez A, Wang H, Pedraza CE, Macklin WB, Lefebvre V. The three SoxC proteins–Sox4, Sox11 and Sox12–exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res. 2008;36:3101–3117. doi: 10.1093/nar/gkn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Hargrave M, Wright E, Kun J, Emery J, Cooper L, Koopman P. Expression of the Sox11 gene in mouse embryos suggests roles in neuronal maturation and epithelio-mesenchymal induction. Dev Dyn. 1997;210:79–86. doi: 10.1002/(SICI)1097-0177(199710)210:2<79::AID-AJA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Isacsson A, Kanje M, Dahlin LB. Induction of activating transcription factor 3 (ATF3) by peripheral nerve compression. Scand J Plast Reconstr Surg Hand Surg. 2005;39:65–72. doi: 10.1080/02844310410004892. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Cornuet PK, McIlwrath S, Koerber HR, Albers KM. SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience. 2006;143:501–514. doi: 10.1016/j.neuroscience.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zhang JS, Jakobsen J. Differential effect of p75 neurotrophin receptor on expression of pro-apoptotic proteins c-jun, p38 and caspase-3 in dorsal root ganglion cells after axotomy in experimental diabetes. Neuroscience. 2005;132:1083–1092. doi: 10.1016/j.neuroscience.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Kocsis JD. Temporal variability of jun family transcription factor levels in peripherally or centrally transected adult rat dorsal root ganglia. Brain Res Mol Brain Res. 1997;52:53–61. doi: 10.1016/s0169-328x(97)00211-8. [DOI] [PubMed] [Google Scholar]
- Kitao Y, Robertson B, Kudo M, Grant G. Proliferation patterns of dorsal root ganglion neurons of cutaneous, muscle and visceral nerves in the rat. J Neurocytol. 2002;31:765–776. doi: 10.1023/a:1025760116189. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M. Neural mechanisms of cutaneous nociceptive pain. Clin J Pain. 2000;16:S131–S138. doi: 10.1097/00002508-200009001-00004. [DOI] [PubMed] [Google Scholar]
- Kuo LT, Simpson A, Schanzer A, Tse J, An SF, Scaravilli F, Groves MJ. Effects of systemically administered NT-3 on sensory neuron loss and nestin expression following axotomy. J Comp Neurol. 2005;482:320–332. doi: 10.1002/cne.20400. [DOI] [PubMed] [Google Scholar]
- Kury P, Stoll G, Muller HW. Molecular mechanisms of cellular interactions in peripheral nerve regeneration. Curr Opin Neurol. 2001;14:635–639. doi: 10.1097/00019052-200110000-00013. [DOI] [PubMed] [Google Scholar]
- Lindwall C, Kanje M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol Cell Neurosci. 2005;29:269–282. doi: 10.1016/j.mcn.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Lindwall C, Dahlin L, Lundborg G, Kanje M. Inhibition of c-Jun phosphorylation reduces axonal outgrowth of adult rat nodose ganglia and dorsal root ganglia sensory neurons. Mol Cell Neurosci. 2004;27:267–279. doi: 10.1016/j.mcn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Lozeron P, Krarup C, Schmalbruch H. Regeneration of unmyelinated and myelinated sensory nerve fibres studied by a retrograde tracer method. J Neurosci Methods. 2004;138:225–232. doi: 10.1016/j.jneumeth.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Natl Protoc Dir. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- McIlwrath S, Lawson JJ, Anderson CE, Koerber HR. Sensitization of cutaneous nociceptors after nerve cut and regeneration in mouse. Soc Neurosci Abst. 2005 doi: 10.1523/JNEUROSCI.3474-08.2009. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeson AP, Shi X, Alexander MS, Williams RS, Allen RE, Jiang N, Adham IM, Goetsch SC, Hammer RE, Garry DJ. Sox15 and Fhl3 transcriptionally coactivate Foxk1 and regulate myogenic progenitor cells. EMBO J. 2007;26:1902–1912. doi: 10.1038/sj.emboj.7601635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Beirowski B, Gillingwater TH, Adalbert R, Wagner D, Grumme D, Osaka H, Conforti L, Arnhold S, Addicks K, Wada K, Ribchester RR, Coleman MP. The slow Wallerian degeneration gene, WldS, inhibits axonal spheroid pathology in gracile axonal dystrophy mice. Brain. 2005;128:405–416. doi: 10.1093/brain/awh368. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Friedrich VL., Jr . Electron microscopy: identification and study of normal and degenerating neural elements by electron microscopy. In: Heimer L, Robards MJ, editors. Neuroanatomical Tract-Tracing Methods. Plenum Press; New York: 1981. pp. 377–406. [Google Scholar]
- Olmsted JB, Carlson K, Klebe R, Ruddle F, Rosenbaum J. Isolation of microtubule protein from cultured mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1970;65:129–136. doi: 10.1073/pnas.65.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson AG, Gray CW, Pearson JF, Greenwood JM, During MJ, Dragunow M. ATF3 enhances c-Jun-mediated neurite sprouting. Brain Res Mol Brain Res. 2003;120:38–45. doi: 10.1016/j.molbrainres.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. Neurons and Their Supporting Cells. Oxford; New York: 1991. The Fine Structure of the Nervous System. [Google Scholar]
- Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, Lipp HP, Aguzzi A, Wagner EF, Behrens A. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Sebille A. [Peripheral neuropathies. Current data concerning nerve regeneration (author’s transl)] Nouv Presse Med. 1982;11:1206–1215. [PubMed] [Google Scholar]
- Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006;32:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortland PJ, Baytug B, Krzyzanowska A, McMahon SB, Priestley JV, Averill S. ATF3 expression in L4 dorsal root ganglion neurons after L5 spinal nerve transection. Eur J Neurosci. 2006;23:365–373. doi: 10.1111/j.1460-9568.2005.04568.x. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sock E, Rettig SD, Enderich J, Bosl MR, Tamm ER, Wegner M. Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol Cell Biol. 2004;24:6635–6644. doi: 10.1128/MCB.24.15.6635-6644.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Koltzenburg M, Schneider M, Engle MG, Albers KM, Davis BM. Overexpression of nerve growth factor in skin selectively affects the survival and functional properties of nociceptors. J Neurosci. 1999;19:8509–8516. doi: 10.1523/JNEUROSCI.19-19-08509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Bonilla I, Winkles JA, Strittmatter SM. Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci. 2003;23:9675–9686. doi: 10.1523/JNEUROSCI.23-29-09675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thippeswamy T, Jain RK, Mumtaz N, Morris R. Inhibition of neuronal nitric oxide synthase results in neurodegenerative changes in the axotomised dorsal root ganglion neurons: evidence for a neuroprotective role of nitric oxide in vivo. Neurosci Res. 2001;40:37–44. doi: 10.1016/s0168-0102(01)00205-x. [DOI] [PubMed] [Google Scholar]
- Thomas PK. Selective vulnerability of the centrifugal and centripetal axons of primary sensory neurons. Muscle Nerve. 1982;5:S117–121. [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- Uusitalo H, Salminen H, Vuorio E. Activation of chondrogenesis in response to injury in normal and transgenic mice with cartilage collagen mutations. Osteoarthr Cartil. 2001;9(Suppl A):S174–S179. [PubMed] [Google Scholar]
- Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Wilson M, Koopman P. Matching SOX: partner proteins and co-factors of the SOX family of transcriptional regulators. Curr Opin Genet Dev. 2002;12:441–446. doi: 10.1016/s0959-437x(02)00323-4. [DOI] [PubMed] [Google Scholar]
- Wright EM, Snopek B, Koopman P. Seven new members of the Sox gene family expressed during mouse development. Nucleic Acids Res. 1993;21:744. doi: 10.1093/nar/21.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]