Abstract
Background
Sacroiliac joint pain is a challenging condition accounting for approximately 20% of cases of chronic low back pain. Currently, there are no effective long-term treatment options for sacroiliac joint pain.
Methods
A randomized, placebo-controlled study was conducted in 28 patients with injection-diagnosed sacroiliac joint pain. Fourteen patients received L4-5 primary dorsal rami and S1-3 lateral branch radiofrequency denervation using cooling-probe technology following a local anesthetic block, and 14 patients received the local anesthetic block followed by placebo denervation. Patients who failed to respond to placebo injections crossed over and were treated with radiofrequency denervation using conventional technology.
Results
One, 3 and 6-months post-procedure, 11 (79%), 9 (64%) and 8 (57%) of radiofrequency treated patients experienced ≥ 50% pain relief and significant functional improvement. In contrast, only 2 (14%) patients in the placebo group experienced significant improvement at their 1-month follow-up, and none experienced benefit 3-months post-procedure. In the crossover group (n=11), 7 (64%), 6 (55%) and 4 (36%) patients experienced improvement 1, 3 and 6-months post-procedure. One year after treatment, only 2 (14%) patients in the treatment group continued to demonstrate persistent pain relief.
Conclusions
These results provide preliminary evidence that L4 and L5 primary dorsal rami and S1-3 lateral branch radiofrequency denervation may provide intermediate-term pain relief and functional benefit in selected patients with suspected sacroiliac joint pain. Larger studies are needed to confirm our results, and determine the optimal candidates and treatment parameters for this poorly understood disorder.
Introduction
Sacroiliac joint pain is a challenging condition estimated to account for between 15% and 20% of chronic axial low back pain cases.1,2 Presently, there is no reliably effective treatment for sacroiliac pain. In randomized studies evaluating peri- and intra-articular corticosteroid injections in patients suspected of having sacroiliac joint pain, the results are divided as to whether or not they afford any long-term benefit.3–7 Studies evaluating conservative therapies are flawed by the lack of adequate control subjects and inappropriate diagnostic work-ups.1
In the past several years, radiofrequency denervation has emerged as a promising treatment alternative for refractory cases of sacroiliac joint pain.8 The concept of disrupting the nerve supply to pain-generating spinal structures was extrapolated from over 30 years of experience using radiofrequency lesioning for zygapophsial (facet) joint pain.9 In 4 studies evaluating different variants of lower lumbar primary dorsal rami and sacral lateral branch radiofrequency denervation, all reported success rates ranging between 67% and 89%.10–13 However none of these studies were controlled, which raises questions regarding their validity and applicability. In order to determine whether sacroiliac joint denervation is a viable treatment for patients suffering from chronic, intractable, injection-diagnosed sacroiliac joint pain, we conducted a placebo-controlled study evaluating L4 and L5 primary dorsal rami and S1-3 lateral branch radiofrequency lesioning.
Materials and Methods
Permission to conduct this study was granted by the internal review boards at Johns Hopkins Medical Institutions, Baltimore, Maryland, and Walter Reed Army Medical Center, Washington, District of Columbia, and all study participants who provided informed consent. The standardized protocol was performed at both institutions, with recruitment and all procedures occurring between May 2005 and August 2006. A two-tailed power analysis determined a sample size of 14 in each group had 80% power (beta of 0.2) to detect a 2-point difference in the 0–10 numeric rating scale (NRS) between groups with a significance level (alpha) of 0.05.
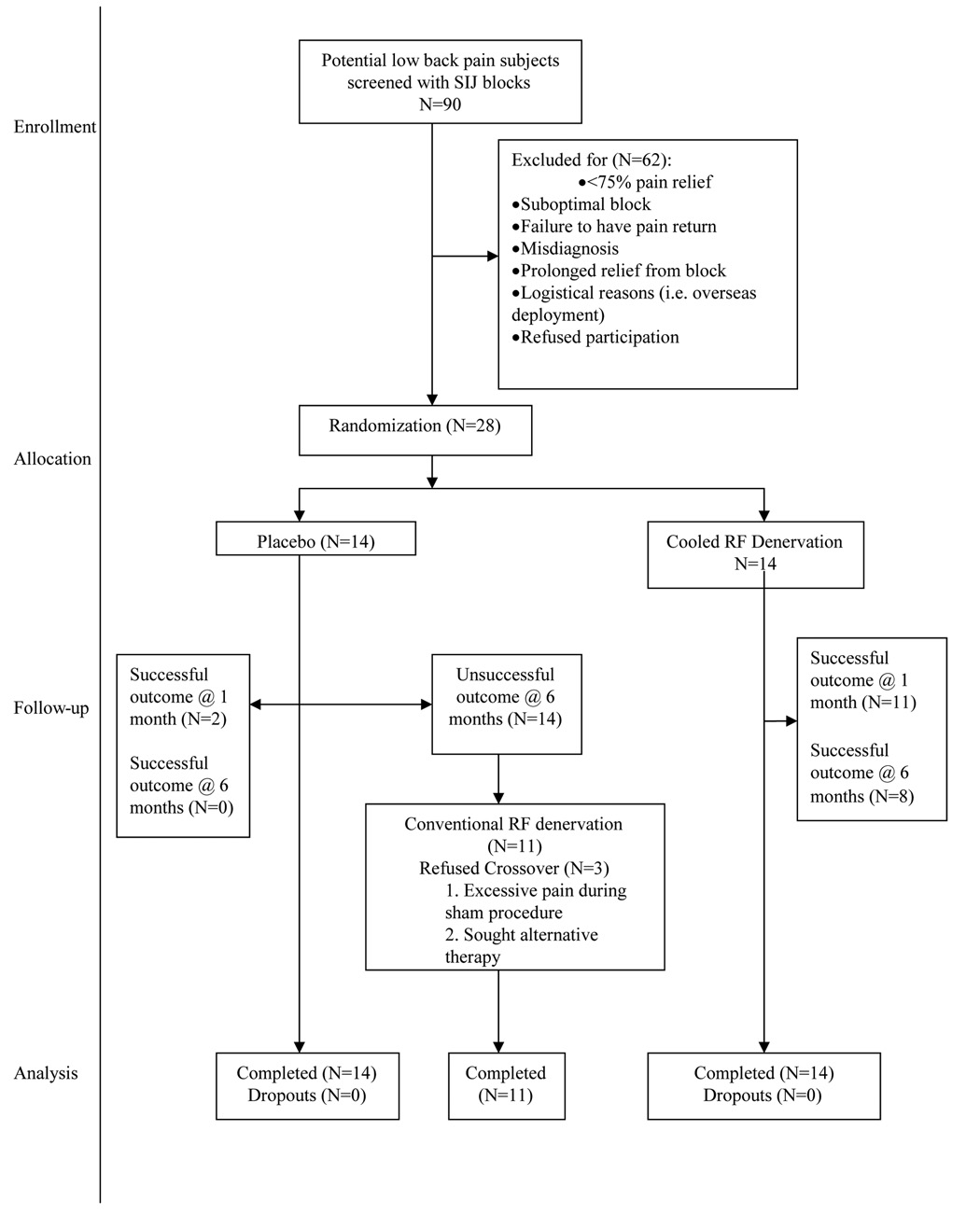
All procedures were done in an outpatient setting using local anesthesia, and for radiofrequency denervation, intravenous sedation. Subjects were recruited from the regular pain clinic populations at the participating institutions. Inclusion criteria included age > 18 years; axial low back or buttock pain ≥ 6 months in duration; tenderness overlying the sacroiliac joint(s); failure to respond to conservative therapy (e.g. physical therapy and pharmacotherapy), including long-term (> 2 months) pain relief with sacroiliac joint corticosteroid injections; and ≥ 75% pain relief as calculated from a 6-hour post-block pain diary following a single diagnostic sacroiliac joint injection. Exclusion criteria were focal neurological signs or symptoms; radiological evidence of a symptomatic herniated disc; spondyloarthropathy; untreated coagulopathy; and unstable medical (e.g. unstable angina) or psychiatric illness (e.g. untreated depression) that might preclude an optimal treatment response. Prior to enrollment, all patients underwent magnetic resonance imaging to rule out other possible sources for their back pain. Six patients underwent previous diagnostic spinal procedures, three in each group. These included four discograms and four medial branch (facet joint nerve) blocks, all of which were negative. Throughout the recruitment phase, 62 patients were excluded for a variety of reasons, of which the most common was failure to achieve ≥ 75% documented pain relief from the diagnostic sacroiliac joint block (n=38; fig. 1).
Figure 1.
CONSORT chart showing progression of subjects in study arms.
Footnotes: SIJ- sacroiliac joint, RF- radiofrequency, N- number of patients
Screening Sacroiliac Joint Injections
Sacroiliac joint injections were performed using 22-gauge spinal needles inserted into the bottom one-third of the joint using fluoroscopic guidance in either a slightly oblique or antero-posterior view. Correct placement was ascertained in all cases by a sacroiliac joint arthrogram. Following confirmation of joint penetration, a 3 ml solution containing 2 ml of bupivacaine 0.5% and 1 ml of 40 mg/ml of depo-methylprednisolone (Pharmacia and Upjohn, Kalamazoo, MI) was administered. After the injection, patients were instructed to engage in normal activities and fill out 0–10 numerical rating scale (NRS) pain diaries every half hour over the ensuing 6 hours. Only those patients who experienced ≥ 75% pain relief for at least 3 hours while performing their normal activities of daily living, but whose pain returned to near baseline within 2 months, were eligible for enrollment.
Randomization and Primary Treatment
The treatment of all subjects was done by a physician not involved in randomization. Study patients were randomized in a 1:1 ratio to receive either true or placebo denervation. A research nurse not involved in patient care performed randomization in blocks of 4 via pre-sealed envelopes at each institution. Under sterile conditions with the patient positioned prone, a C-arm intensifier was used to optimize visualization of the target sites. For blockade and lesioning of the L4 and L5 dorsal rami, 22-gauge SMK-C10 (Radionics, Burlington, MA) cannulae with 5-mm active tips were inserted parallel to the course of the nerve until bone was contacted just superior and medial to the junction between the superior border of the transverse and superior articular processes for procedures done at L4, and at the junction of the ala and articular process of the sacrum for L5 procedures, similar to previously published studies.14,15 Since it is not possible to discern electrostimulation between the various branches of the L4 primary dorsal ramus (it is the lateral branch that may innervate the sacroiliac joint), the targeted nerve at this level is referred to as the parent branch. At each level, placement of the electrode in close proximity to the nerve was confirmed using electrostimulation at 50 Hz, with concordant sensation achieved at ≤ 0.5 V. Prior to lesioning, the absence of leg contractions was verified with stimulation at 2 Hz up to 2 V. After satisfactory electrode placement, 0.5 ml of lidocaine 2% was injected through each cannula to reduce thermal pain and ensure blinding. The radiofrequency probe was then reinserted and a 90 second, 80° C lesion was made using a radiofrequency generator set to the lowest audible volume to blend in with ambient noise (Electrothermal 20S Spine System, Smith and Nephew, Andover, MA or Radionics RF Lesion Generator System, Model RFG-3C, Radionics, Valleylab, Boulder, CO).
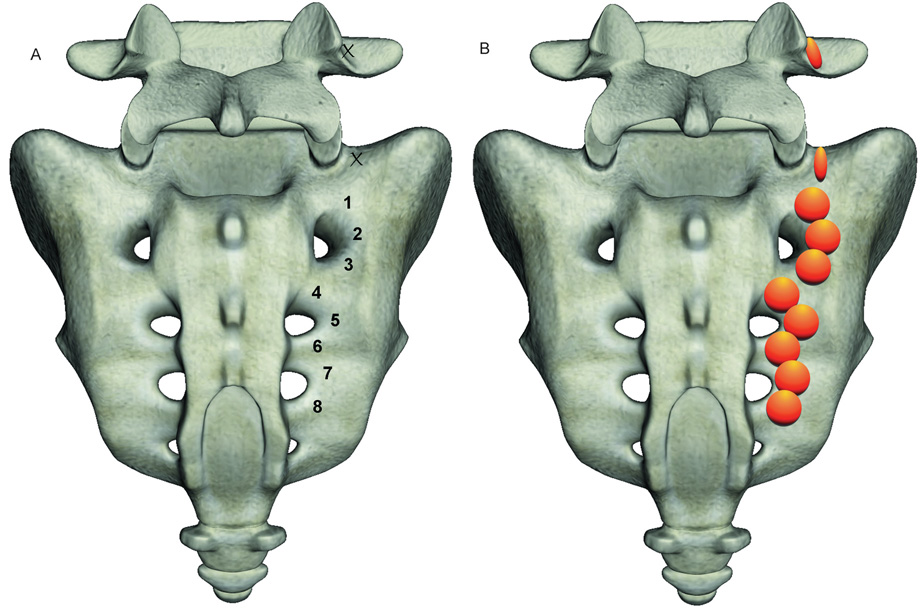
For S1-3 lateral branch procedures, 17-gauge 75-mm cooled electrodes with 4 mm active tips (Baylis Medical, Montreal, Quebec, Canada) were inserted between 3 and 5 mm from the lateral border of the foramina at pre-designated positions. For right-sided S1 and S2 procedures, these approximately corresponded to the 1:00, 3:00 and 5:30 o’clock positions on the face of a clock; on the left, the target sites were at 6:30, 9:00 and 11:00 (fig. 2). At S3, needles were placed at 1:30 and 4:30 on the right side, and 7:30 and 10:30 on the left. In 10 patients in whom the S4 foramen was located level with or just below the inferior portion of the sacroiliac joint, one upper lesion was also done at S4. Sensory stimulation was performed at each level only for the first needle placement, revealing concordant sensation at ≤ 0.5 volts. Prior to lesioning, 0.5 ml of lidocaine 2% was administered per spinal level. In order to ensure that anesthetic spread to adjacent foramina did not impede sensory testing, electrodes were placed and stimulated at contiguous levels before denervation commenced. Once the needles were properly positioned, monopolar electrodes were sequentially inserted into the cannulae and 2.5- minute lesions were made using a water-cooled radiofrequency heating system (Pain Management SInergy System, Baylis Medical) and generator (PMG-115-TD, V2.0A, Baylis Medical). Using cooling-probe technology, the tissue temperature immediately adjacent to the cooled electrode is maintained at 60° C, while the target tissue is heated to 75° C, resulting in a lesion diameter ranging between 8 and 10 mm (fig. 3). For safety reasons, this aggressive lesioning precludes using cooling-probe technology for lumbar primary dorsal rami.
Figure 2.
Schematic diagram illustrating:
A. Target points for right-sided conventional (L4 and L5) and cooled (S1–3) radiofrequency denervation at the junction of the L5 superior articular and transverse processes (L4 primary dorsal ramus), the sacral ala (L5 primary dorsal ramus), and S1-3 foramina (lateral branches).
B. Anticipated lesions at each of the target points.
Footnotes: L4 and L5- 4th and 5th lumbar spinal levels, respectively. S1–3- First, second and third sacral spinal levels, respectively.
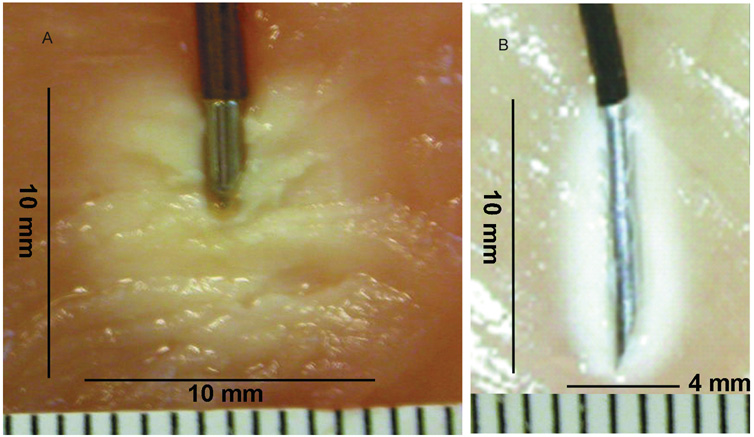
Figure 3.
Adjacent photographs demonstrating the difference in lesion size between cooled (A) and conventional (B) radiofrequency probes in chicken meat. Each small line represents a distance of 1 mm.
In the control group, electrodes were similarly positioned, electrostimulation was performed in an identical manner, and 0.5 ml of lidocaine 2% was administered, but no current was administered. The average time it took to perform the radiofrequency and placebo procedures were comparable (a mean of 61 minutes vs. 54 minutes, respectively, for the first 5 people in each group).
Outcome Measures, Crossover Group Treatment, and Follow-Up
A physician unaware of the patient’s study group assignment obtained all outcome data during scheduled follow-up visits. Between the procedure and first follow-up, no contact was permitted between any patient and investigator(s) except for emergencies. All patients were seen in the treating clinic 1-month post-procedure. If a patient obtained a positive global perceived effect (GPE) and significant (≥ 50%) pain relief obviating the need for further therapy, he or she was re-evaluated 3 and 6-months post-treatment. Abridged follow-up interviews were done by e-mail or telephone every 2 months after the 6-month follow-up in patients who reported persistent relief in order to determine the duration of benefit. Patients who did not obtain adequate symptomatic improvement were unblinded at follow-up. For those who obtained significant relief 1-month post-procedure, unblinding was done 3-months after treatment.
Patients in the initial radiofrequency denervation (cooled electrode) group who failed to obtain a positive outcome were recorded as a treatment failure and offered alternative treatment. All placebo patients who failed to achieve a positive outcome were offered the opportunity to crossover and receive sacroiliac joint denervation using conventional (non-cooled) technology in an open-label parallel arm. The reason for using conventional equipment was based on availability (i.e. cooled equipment was not ordered for patients in whom the outcome and hence treatment plan was not known beforehand). In these patients, 22 gauge SMK-C10 (Radionics) cannulas with 5-mm active tips were placed in an identical fashion to that described for the treatment group. Once concordant sensory stimulation was obtained and 1% lidocaine administered at each level, the monopolar radiofrequency probe was reinserted and a 90 second, 80° C lesion was made using a different generator (Electrothermal 20S Spine System, Smith and Nephew, or Radionics RF Lesion Generator System, Model RFG-3C, Radionics). Data from the crossover group was analyzed separately from that of the initial experimental group.
The primary outcome measure was a 0–10 NRS pain score, which reflected the average pain experienced by the patient for 10 days prior to follow-up. Secondary outcome measures included Oswestry disability index (ODI version 2.0, MODEMS, Des Plaine, IL, reflecting the 10 days prior to follow-up) score, reduction in analgesic medications (defined as a 20% reduction in opioid use or complete cessation of a non-opioid analgesic),16 GPE, and a composite successful outcome. A positive GPE was defined as an affirmative response to the following 3 questions:
My pain has improved/ worsened/ stayed the same since my last visit;
The treatment I received improved/ did not improve my ability to perform daily activities;
I am satisfied/ not satisfied with the treatment I received and would recommend it to others.
The composite binary variable “successful outcome” was predefined prior to initiation of the study as a ≥ 50% reduction in numerical pain score, a positive GPE, and either a 10-point decrease in ODI or a 4-point decrease coupled with a reduction in medication usage.17,18
Statistical Measures
Statistical analyses were performed using STATA version 10.0 (Statcorp, College Station, TX). The Shapiro-Wilk W test for normal data was performed on continuous outcome measures. The distribution of categorical variables in each group was compared using Fisher exact test. Continuous variables are reported as mean and standard deviation or median and interquartile range. Categorical data are reported by number of subjects and percentage. Comparisons between the initial radiofrequency treatment group and the placebo group were made with unpaired t-tests or Mann-Whitney U test. Since the continuous data in each group had a normal distribution, comparisons between and within the initial radiofrequency and crossover treatment groups were made with two-way analysis of variance (ANOVA). For multiple significance testing, post-hoc Bonferroni correction was used. Because baseline ODI differences were a potential confounding factor, an adjusted multiple linear and logistic regression analysis was performed for each continuous and categorical outcome measure, respectively.
Results
Demographics
Data were analyzed on 28 patients. Demographic (including active duty status) and clinical characteristics were balanced between the radiofrequency denervation treatment and control group. Two patients, one each in the control and treatment groups, received bilateral procedures. Thirteen patients were taking opioids, and 24 were on non-opioid analgesics. There were no differences with regard to treatment location with the exception of military duty status, which was not present in those subjects treated at Johns Hopkins. Pre-procedure NRS scores did not appear to differ between the radiofrequency treatment and the placebo group (6.5 ± 1.9 and 6.1 ± 1.8, respectively). However, preprocedural ODI scores did differ between the treatment and placebo arms (37.1 ± 10.6 and 47.9 ± 9.3, respectively, table 1).
Table 1.
Demographic and Clinical Characteristics of Study Patients
| Placebo (n=14) | Lateral Branch Denervation (n=14) | |
|---|---|---|
| Sex | ||
| Male (n=11) | 6 (43%) | 5 (36%) |
| Female (n=17) | 8 (57%) | 9 (64%) |
| Age (SD, range) | 51.8 (13.1; 31–74) | 51.9 (13.6; 27–75) |
| Active Duty (n=6) | 3 (21%) | 3 (21%) |
| Opioid Use (n=13) and Dosage in Morphine | 7 (50%) | 6 (43%) |
| Equivalents per Day (mean, SD, range) | 46.4 (43.1, 7.5–130) | 60 (50.0, 7.5–150) |
| Worker’s Compensation, Disability, or Military Medical Board Claim (n=9) | 3 (21%) | 6 (43%)* |
| Failed Back Surgery Syndrome (n=6)** | 4 (29%) | 2 (14%) |
| Baseline Numerical Rating Scale Score (SD, | 6.5 (1.9; 3.5–10) | 6.1 (1.8; 3–8) |
| range) Median (interquartile range) | 6 (5.5–7) | 6 (5–8) |
| Baseline Oswestry Disability Index (SD, | 47.9 (9.3; 28–58) | 37.1 (10.6; 18–49) |
| range) | 50.5 (44–56) | 41 (26–46) |
| Median (interquartile range) | ||
Continuous data listed as the mean and (standard deviation, range) and median and (interquartile range, 25%–75%), categorical data as number and (percentage).
Includes 3 active duty soldiers undergoing a medical board.
Includes 5 patients with spinal fusion and 1 status post-laminectomy.
Three patients in the placebo group declined to crossover to the radiofrequency denervation treatment group. Among these 3 patients, one elected not to receive the true procedure because the placebo treatment was “too painful”, and 2 sought alternative care. Based on the demographic and clinical characteristics, there appeared to be no difference between these patients and those who elected to cross-over.
Primary Outcome Measure
A significant difference in the primary outcome, NRS pain score, was detected between the treatment and placebo groups at follow-up (table 2). One month after the procedure, the treatment group had significantly lower NRS scores than the placebo group (2.4 ± 2.0; range 0–8 vs. 6.3 ± 2.4; range 2–10, p<0.001, respectively). In the placebo group, only 2 patients at 1 month and no patients at 3 months reported a positive outcome. The primary outcome remained significantly different between the two groups when baseline ODI scores were analyzed as a covariate (coefficient of variation −3.8, 95% CL −5.8 to −1.8; p<0.001). At 3 and 6-month follow-up, 8 and 4 patients in the treatment group, respectively, reported NRS pain scores ≤ 2.
Table 2.
Numerical Rating Pain Scores Stratified by Treatment Group and Time Point
| Time point | Placebo (n=14) | Lateral Branch Denervation (n=14) | Lateral Branch Denervation Crossover (n=11) |
|---|---|---|---|
| Baseline | |||
| Mean (SD, range) | 6.5 (1.9, 3.5–10) | 6.1 (1.8, 3–8) | 6.3 (2.4, 2–10) |
| Median (interquartile range) | 6 (5.5–7) | 6 (5–8) | 6 (4–7) |
| One month | *, ** | *, ** | |
| Mean (SD, range) | 6.3 (2.4, 2–10) | 2.4 (2.0, 0–8) | 3.6 (2.6, 0–10) |
| Median (interquartile range) | 7 (4–7) | 2 (1–3) | 3 (2–5) |
| Three months | (n=2) | ** | ** |
| Mean (SD, range) | 6 (0, 6–6) | 2.4 (2.3, 0–7) | 2.1 (2.4, 0–7) |
| Median (interquartile range) | 6 (6–6) | 1.5 (1–4.5) | 1.5 (0.5–3) |
| Six months | ** | ** | |
| Mean (SD, range) | (no data) | 2.6 (2.2, 0–7) | 3.1 (2.1, 0–6) |
| Median (interquartile range) | 2 (1.5–2.5) | 3.5 (1.5–4) | |
P<0.05 as compared to placebo group
P<0.05 as compared to baseline of the respective group
In a within-groups analysis, subjects who received radiofrequency treatment reported significantly lower NRS scores at 1, 3 and 6 months post-procedure compared to baseline scores (p<0.001). Patients’ pain scores were reduced by 60%, 60%, 57% at 1, 3 and 6 months, respectively. In contrast, the 1-month NRS scores of subjects who received the placebo treatment were unchanged from baseline (6.4 ± 1.9 and 6.3 ± 2.4, respectively; p>0.9). No further within groups analysis was performed because of insufficient patients remaining in the placebo group at 3 (n=2) and 6-month (n=0) time points.
Eleven subjects in the placebo arm crossed over to the radiofrequency treatment; 9 crossed over at 1-month and 2 at 3-months. In the crossover phase of this trial, the placebo group’s NRS scores after conventional radiofrequency treatment did not significantly differ from those of the initial radiofrequency group (3.6 ± 2.6 vs. 2.4 ± 2.0, respectively). Similar to the initial treatment group, the placebo crossover group experienced a significant decrease in NRS scores 1 (44%), 3 (67%) and 6 (52%) months after denervation when compared to baseline (p<0.001, table 2).
Secondary Outcome Measures
Oswestry Disability Index
A significant difference in ODI was detected between treatment and placebo groups. One month after the procedure, the treatment group had lower ODI scores than the placebo group (20.9 ± 10.9; range 4–38 vs. 43.6 ± 14.0; range 16–70, respectively; p<0.03). In a within group analysis, subjects who received radiofrequency treatment reported significantly lower ODI scores at 1, 3 and 6 months when compared to their baseline scores (p<0.001, table 3). Subjects’ ODI scores were reduced by 44%, 50%, 39% at 1, 3 and 6 months, respectively. In contrast, the mean 1 month ODI score of subjects who received the placebo treatment was unchanged from baseline (43.6 vs. 47.9 ± 9.3; range 28–59, respectively).
Table 3.
Oswestry Disability Index Score (%) Stratified by Treatment Group and Time Point
| Time point | Placebo | Lateral Branch Denervation (n=14) | Lateral Branch Denervation Crossover (n=11) |
|---|---|---|---|
| Baseline | (n=14) | ||
| Mean (SD, range) | 47.9 (9.3, 28–59) | 37.1 (10.6, 18–49) | 43.6 (14, 16–70) |
| Median (interquartile range) | 50.5 (44–56) | 41, (26–46) | 41 (34–56) |
| One month | (n=14) | *, **, *** | |
| Mean (SD, range) | 43.6 (14, 16–70) | 20.9 (10.9, 4–38) | 34.3 (16.2, 4–58) |
| Median (interquartile range) | 41 (34–56) | 19 (14–29) | 33 (24–46) |
| Three months | (n=2) | *** | *** |
| Mean (SD, range) | 24 (8.5, 18–30) | 18.5 (11.6, 0–36) | 19.4 (18.1, 0–44) |
| Median (interquartile range) | 24 (18–30) | 20 (9.5–27) | 16 (4–44) |
| Six months | *** | *** | |
| Mean (SD, range) | No data | 22.6 (10.6, 7–40) | 24.3 (21.0, 0–56) |
| Median (interquartile range) | 20 (16–24) | 20 (8–42) | |
P<0.05 as compared to placebo group
P<0.05 as compared to lateral branch denervation crossover group
P<0.05 as compared to baseline of the respective group
In the crossover phase, the placebo group’s ODI scores after radiofrequency treatment were reduced by 28%, 59% and 49% at 1-, 3- and 6-months post-procedure, respectively. ODI scores in the crossover group did not significantly differ from those of the initial treatment group 3 or 6 months after the procedure. However, the initial radiofrequency treatment group had significantly lower ODI scores at 1 month compared to the placebo/crossover group (20.9 vs. 34.3 ± 16.3; range 4–58, respectively; p<0.03). The difference between baseline and post-procedure ODI scores in the crossover group was statistically significant 3 and 6-months after the conventional radiofrequency procedure (p<0.02), but not 1-month following denervation.
Global Perceived Effect (GPE)
Subjects who received radiofrequency treatment reported a significantly higher proportion of positive GPE responses at 1 month compared to subjects who received placebo treatment (93% vs. 21%, respectively; p<0.001). The percentage of subjects in the treatment group (n=14) with a positive GPE was 93% (n=13), 71% (n=10) and 50% (n=7) at 1, 3 and 6 months, respectively (table 4). In the crossover phase, the percentage of subjects who underwent conventional denervation (n=11) with a positive GPE was 72% (n=8), 64% (n=7) and 46% (n=5) at 1, 3 and 6-months post-procedure, respectively. The crossover group’s GPE proportion after treatment did not significantly differ from those of the initial radiofrequency group.
Table 4.
Percent Positive Global Perceived Effect Stratified by Treatment Group and Time Point
| Time point | Placebo | Lateral Branch Denervation (n=14) | Lateral Branch Denervation Crossover (n=11) |
|---|---|---|---|
| One month | (n=14) | * | |
| Percent (95% Cl) | 21 (2–45) | 93 (78–100) | 72 (41–100) |
| Three months | (n=2) | ||
| Percent (95% Cl) | 0 | 83 (59–100) | 86 (51–100) |
| Six months | |||
| Percent (95% Cl) | No data | 89 (63–100) | 89 (63–100) |
P<0.05 as compared to placebo group
Medication Reduction
The radiofrequency treatment group had a significantly higher proportion of patients able to reduce their analgesic medications following the procedure at 1 month compared to subjects who received placebo treatment (77%; 10/14 vs. 8%; 1/11, respectively; p<0.001). The percentage of subjects in the radiofrequency group who were able to reduce their analgesic intake was 77% (n=10), 64% (n=9) and 36% (n=5) at 1, 3 and 6 months, respectively (table 5). In the crossover phase (n=11), the percentage of subjects who reported a decrease in medication requirements were 73% (n=8), 46% (n=5) and 27% (n=3) at 1, 3 and 6 months, respectively. The reduction in analgesic intake between the original and crossover radiofrequency groups was not statistically different.
Table 5.
Positive Percent Medication Reduction Stratified by Treatment Group and Time Point
| Time point | Placebo | Lateral Branch Denervation (n=14) | Lateral Branch Denervation Crossover (n=11) |
|---|---|---|---|
| One month | (n=14) | * | * |
| Percent (95% Cl) | 8 (0–25) | 77 (52–100) | 78 (44–100) |
| Three months | (n=2) | ||
| Percent (95% Cl) | 0 | 82 (55–100) | 82 (55–100) |
| Six months | |||
| Percent (95% Cl) | No data | 67 (28–100) | 60 (0–100) |
P<0.05 as compared to placebo group
Percent Successful Treatment
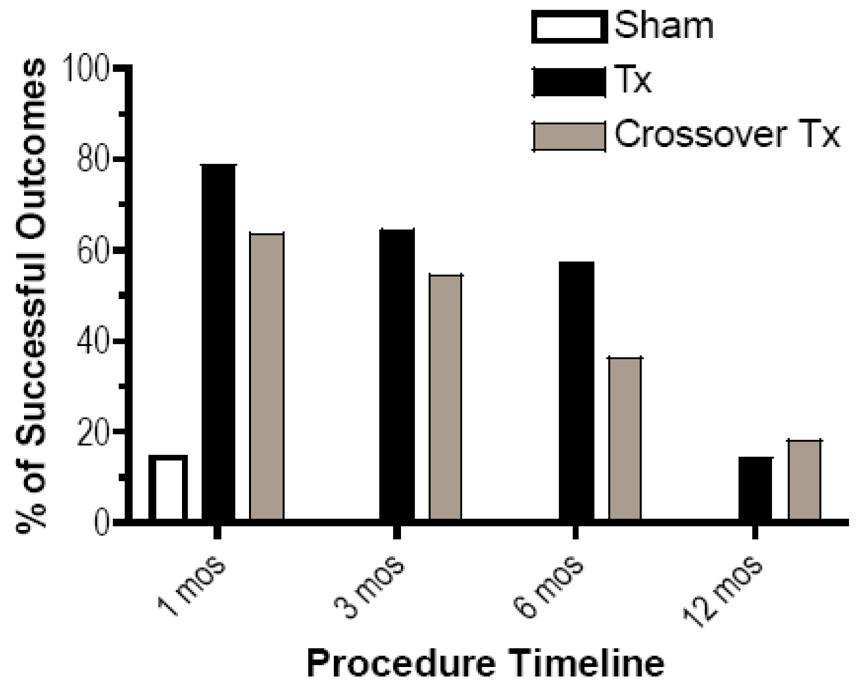
The proportion of subjects who experienced a “positive outcome” was significantly higher in the denervation group than control group (p<0.001). This success rate persisted at 3- and 6-month follow-up visits (fig. 4). In contrast, only 2 (14.3%) subjects in the placebo group experienced a positive composite outcome 1-month post-procedure, and none obtained relief exceeding 3 months.
Figure 4.
Bar graph demonstrating the percentage of patients with a successful treatment outcome at various time points. A positive outcome is defined as a ≥ 50% reduction in numerical pain score, a positive global perceived effect, and either a 10-point decrease in Oswestry disability index score or a 4-point decrease coupled with a reduction in medication usage.
Footnote: TX- treatment
Placebo subjects who crossed over to conventional radiofrequency treatment (n=11) experienced slightly lower success rates than the original treatment group (7 (64%) versus 11 (79%) at 1 month, 6 (55%) versus 9 (64%) at 3 months, and 4 (36%) versus 8 (57%) at 6 months, respectively). However, the proportion of successful procedures in the crossover group was not statistically different than in the initial radiofrequency treatment group.
Duration of Pain Relief
Subjects in the treatment group had a mean duration of pain relief of 5.8 (± 4.2; range 0–12) months vs. 0.7 (±1.6; range 0–1) months in the placebo group. The mean duration of relief in the radiofrequency crossover group did not significantly differ from that of the initial treatment group (4.6 ± 4.6; range 0–12 vs. 5.8 ± 4.2 months, respectively). Two patients each in the cooled and conventional radiofrequency groups continued to experience significant pain relief 1 year after treatment. Among patients with a successful outcome at any time point, the mean duration of pain relief was 7.9 ± 4.7 months.
Adequacy of Blinding
A disinterested observer querying patients before discharge from their procedure assessed the adequacy of blinding. In the 14 patients in the radiofrequency group, 9 thought they received denervation, 2 thought they received placebo treatment and 3 were unable to guess which group they were randomized to despite prodding. In the 14 placebo patients, 8 believed they received radiofrequency denervation, 3 felt they received the placebo treatment and 3 were unsure which group they were randomized. In the radiofrequency group, the 11 successful outcomes at 1-month were comprised of 8 patients who thought they received denervation, one who felt he received placebo treatment, and 2 patients who were unsure which group they were allocated to. Both successful outcomes in the placebo-arm at 1-month thought they received denervation.
Complications
A majority of patients reported temporary worsening pain typically lasting between 5 and 10 days after the procedure, which was attributed to both procedure-related pain and/or temporary neuritis, the latter which may be attenuated by preemptive corticosteroid administration.19 However, there were no serious complications reported for either the 14 placebo or 25 radiofrequency treatments. In the radiofrequency treatment group, one patient reported transient non-painful buttock paresthesias that resolved without therapy.
Discussion
The results of this placebo-controlled study provide preliminary evidence that radiofrequency denervation of the L4 and L5 primary dorsal rami and S1-3 lateral branches may provide significant pain relief and functional improvement in carefully selected patients with suspected sacroiliac joint pain. At 1-, 3- and 6-months post-procedure, 79%, 64% and 57% of patients, respectively, obtained ≥ 50% pain relief and clinically relevant functional improvement.
The high success rate in this study may be partially explained by the combination of stringent inclusion criteria employed and several innovations over previously described sacroiliac joint denervation techniques. First, rather than targeting individual nerves, this technique endeavored to lesion a continuous volume of tissue lateral to the S1-3 foramina. The rationale for this approach is based on a recent cadaveric study demonstrating a complex arcade of small nerve fibers anastamosing with multiple primary dorsal rami around each foramina.12 While individual branch location was shown to vary from level to level and specimen to specimen, they all course through a finite volume of tissue between the lateral edge of the foramen and joint. By placing electrodes strategically around the foramen, this finite volume of tissue can be heated to neuroablative temperatures, thus severing all nociceptive input converging on the primary dorsal ramus. If single lesions had been used as in previously published studies,10–12 some of the afferent input from the sacroiliac joint would likely have remained intact. Creating strip lesions has been previously advocated for sacroiliac joint lesioning,13 but were described using smaller electrodes. Since there is a direct correlation between lesion size and electrode diameter,20 the use of small electrodes increases the likelihood of inadvertently sparing neural input. The probability of 3 geometrically-configured lesions failing to coalesce was further reduced by the use of a water-cooled electrode. Internal cooling enhances lesion size by removing the constraint of high temperature charring in tissue adjacent to the electrode, thus allowing effective ionic heating at a greater distance.21
This study was not powered or designed to detect a difference between outcomes or duration of benefit in patients who underwent denervation with the 17-gauge water-cooled system and those who were treated with the conventional 22-gauge needles, but the slightly higher success rate in the former group (albeit in a non-randomized comparison) despite a lower inferred placebo response is consistent with pre-clinical and clinical data supporting larger lesions for radiofrequency denervation.22 Typically, reported success rates in open-label studies tend to be higher than in controlled studies using similar techniques. This issue needs to be examined in a subsequent randomized trial to determine whether lesion size is an important factor in the success after radiofrequency denervation.
Since we elected for ethical reasons to treat our placebo-controlled patients with conventional denervation at their 1-month follow-up, one can only speculate about any long-term differences between the treatment and placebo arms. The rationale for this decision was based on pilot data examined before embarking on this study that determined the chances of someone obtaining long-term benefit if none was experienced 1-month postprocedure to be exceedingly low.
Finally, our main inclusion criterion of ≥ 75% pain relief after a single diagnostic SI joint injection was stricter than that used in some prior studies.10–13 This relatively high inclusion threshold may have contributed to our high success rate. Thus, caution must be heeded when extrapolating these results to conditions wherein less rigorous selection criteria are employed. In a prevalence study conducted in 43 patients with low back pain below L5-S1, Schwarzer et al.23 found that 30% obtained ≥ 75% pain relief following low-volume sacroiliac joint infiltration. Since the intent of this trial was to examine the therapeutic benefit of this technique, the use of strict inclusion criteria was deemed justified in order to limit the number of patients who did not have true sacroiliac joint-related pain (i.e. “false-positives”), thereby enhancing the internal validity of the trial. Once the beneficial effects of treatment are established, subsequent trials can be conducted under less rigorous conditions in order to better assess external validity.
Five of 14 (36%) patients in the treatment arm and 5 of 11 (45%) in the open-label crossover group failed to obtain significant improvement 3-months after the procedure. There are several explanations for this including a short-lived placebo-response to the diagnostic block but not the definitive treatment, the high false-positive rate associated with single sacroiliac joint blocks,24 and the fact that the L4 thru S3 primary dorsal rami do not supply all the innervation to the sacroiliac joint.8 In the first two scenarios, the use of double confirmatory diagnostic sacroiliac blocks done with 2 different local anesthetics might reduce the failure rate. In the latter case, performing prognostic lateral branch blocks might screen out those patients whose pain emanates from a part(s) of the sacroiliac joint not innervated by the targeted dorsal rami branches. In the two studies whereby both sacroiliac joint and lateral branch blocks were used to screen radiofrequency treatment candidates, the authors reported identical 9-month success rates of 89%.10,13
One disappointing finding is that the high success rate and more aggressive lesion size realized with cooled radiofrequency did not translate into a longer duration of pain relief. Similar to studies conducted with conventional radiofrequency technology,1,9,25 the duration of benefit seems to be constrained by nerve regeneration to between 6 months and one year. Future studies should address whether refinements in technique (e.g. creating bipolar lesions), and/or selection criteria (e.g. examining pain referral patterns, and the use of controlled sacroiliac joint or prognostic lateral branch blocks) can influence the success rate or duration of pain relief, and what the long-term consequences of repeat denervation(s) are.
One criticism that might be made levied against this study is our decision to target five levels for lesioning. The innervation of the sacroiliac joint is a subject of great contention. Whereas some experts have cited contributions to the superior aspect of the joint from as high as L4,1,26 other investigators have failed to confirm these findings.27 Branches derived from the L4 and L5 dorsal rami may ostensibly innervate not only the sacroiliac joint and surrounding ligaments, but also paraspinal muscles, the L5-S1 zygapophysial joint, and the inferior pole of the L4–5 zygapophysial joints as well.9 Although screening sacroiliac joint blocks were performed on all our patients, the specificity of diagnostic spinal injections is inherently low.28,29 In particular, uncontrolled sacroiliac joint blocks are associated with a high false-positive rate.24,30 Whether a less aggressive lesioning scheme targeting fewer levels would yield similar results is something that should be addressed in future clinical trials.
There are several flaws in this study that need to be addressed. First, although the power analysis designed to detect significant differences between the groups was borne out by positive results, the small number of patients enrolled in this study had the unintended consequence of creating two groups of patients with potentially clinically significant differences in several variables known to influence outcome, including baseline functionality (i.e. ODI scores), prior spine surgery, and disability or worker’s compensation cases.15,31,32 Recruiting more patients in a bi-center pilot study to redress these inequities when a beneficial effect for the studied treatment has purportedly been proved would undermine the goodwill of subjects who were paid nothing for their participation. Large multi-center studies, which are needed to confirm our preliminary results, should be adequately powered to address these issues.
The small numbers of patients enrolled also leaves unresolved questions regarding the safety of cooled radiofrequency. Fourteen patients is an insufficient number to detect the small but clinically significant risk of a neurological complication, which may be magnified by the more ambitious lesioning scheme used here. Caution should thus be heeded until large numbers of patients are safely treated by multiple clinicians.
A second flaw revolves around our testing of blinding adequacy. The effectiveness of blinding in this study was evaluated shortly after the conclusion of the procedure, when the effects of the local anesthetic were still active. A more valid indicator of the adequacy of blinding might have been to query patients several days after the procedure, when the cues of actual treatment (e.g. procedure-related pain) were more manifest.
In summary, the results of this placebo-controlled study provide preliminary support for the use of radiofrequency denervation to treat presumptive sacroiliac joint pain. Larger, multi-center studies with long-term follow-up and comprehensive outcome measures are needed to confirm our findings, further establish safety, and determine how best to identify candidates for this treatment.
Acknowledgments
Funded in part by the John P. Murtha Neuroscience and Pain Institute, Johnstown, Pennsylvania and the Army Regional Anesthesia & Pain Medicine Initiative, Washington, DC (SPC and CCB), and National Institutes of Health grant # MH075884 (RWH), Bethesda, Maryland.
Footnotes
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Dept. of the Army or the Dept. of Defense.
Conflict of Interest: Steven Cohen lectured on sacroiliac joint pain at 2 symposiums sponsored by Baylis Medical: American Society of International Pain Physicians 9th Annual Meeting, June 25, 2007, Washington, DC; and International Spinal Interventional Society 15th Annual Meeting, July 20, 2007, Baltimore, Maryland. Disposable equipment (e.g. RF tubing and needles) supplied by Baylis Medical, Montreal, Quebec, Canada.
Trial Registration: clinicaltrials.gov identifier: NCT00373724
Summary Statement: This randomized controlled study evaluating sacroiliac joint radiofrequency denervation provides preliminary evidence that the procedure may provide intermediate-term pain relief and functional improvement in carefully selected patients.
References
- 1.Cohen SP. Sacroiliac joint pain: A comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101:1440–1453. doi: 10.1213/01.ANE.0000180831.60169.EA. [DOI] [PubMed] [Google Scholar]
- 2.Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12:255–265. doi: 10.5435/00124635-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Luukkainen R, Nissila M, Asikainen E, Sanila M, Lehtinen K, Alanaatu A, Kautiainen H. Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondyloarthropathy. Clin Exp Rheumatol. 1999;17:88–90. [PubMed] [Google Scholar]
- 4.Luukkainen R, Wennerstrand PV, Kautiainen HH, Sanila MT, Asikainen EL. Efficacy of periarticular corticosteroid treatment of the sacroiliac joint in non-spondyloarthropathic patients with chronic low back pain in the region of the sacroiliac joint. Clin Exp Rheumatol. 2002;20:52–54. [PubMed] [Google Scholar]
- 5.Maugars Y, Mathis C, Vilon P, Prost A. Corticosteroid injection of the sacroiliac joint in patients with seronegative spondylarthropathy. Arthritis Rheum. 1992;35:564–568. doi: 10.1002/art.1780350512. [DOI] [PubMed] [Google Scholar]
- 6.Hanly JG, Mitchell M, MacMillan L, Mosher D, Sutton E. Efficacy of sacroiliac corticosteroid injections in patients with inflammatory spondyloarthropathy: results of a 6 month controlled study. J Rheumatol. 2000;27:719–722. [PubMed] [Google Scholar]
- 7.Fischer T, Biedermann T, Hermann KG, Diekmann F, Braun J, Hamm B, Bollow M. Sacroiliitis in children with spondyloarthropathy: therapeutic effect of CT-guided intra-articular corticosteroid injection. Rofo. 2003;175:814–821. doi: 10.1055/s-2003-39925. (in German) [DOI] [PubMed] [Google Scholar]
- 8.Cohen SP. Epidemics, evolution and sacroiliac joint pain. Reg Anesth Pain Med. 2007;32:3–6. doi: 10.1016/j.rapm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SP, Raja SN. Pathogenesis, diagnosis and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology. 2007;106:591–614. doi: 10.1097/00000542-200703000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Cohen SP, Abdi S. Lateral branch blocks as a treatment for sacroiliac joint pain: a pilot study. Reg Anesth Pain Med. 2003;28:113–119. doi: 10.1053/rapm.2003.50029. [DOI] [PubMed] [Google Scholar]
- 11.Buijs EJ, Kamphuis ET, Groen GJ. Radiofrequency treatment of sacroiliac joint-related pain aimed at the first three sacral dorsal rami: a minimal approach. Pain Clinic. 2004;16:139–146. [Google Scholar]
- 12.Yin W, Willard F, Carreiro J, Dreyfuss P. Sensory stimulation-guided sacroiliac joint radiofrequency neurotomy: technique based on neuroanatomy of the dorsal sacral plexus. Spine. 2003;28:2419–2425. doi: 10.1097/01.BRS.0000085360.03758.C3. [DOI] [PubMed] [Google Scholar]
- 13.Burnham RS, Yasui Y. An alternate method of radiofrequency neurotomy of the sacroiliac joint: A pilot study of the effect on pain, function and satisfaction. Reg Anesth Pain Med. 2007;32:12–19. doi: 10.1016/j.rapm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 14.van Kleef M, Barendse GA, Kessels A, Voets HM, Weber WE, de Lange S. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine. 1999;24:1937–1942. doi: 10.1097/00007632-199909150-00013. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SP, Hurley RW, Christo PJ, Winkley J, Mohiuddin MM, Stojanovic MP. Clinical predictors of success and failure for lumbar facet radiofrequency denervation. Clin J Pain. 2007;23:45–52. doi: 10.1097/01.ajp.0000210941.04182.ea. [DOI] [PubMed] [Google Scholar]
- 16.Cohen SP, Wenzell D, Hurley RW, Kurihara C, Buckenmaier CC, 3rd, Griffith S, Larkin TM, Dahl E, Morlando BJ. Intradiscal etanercept as a treatment for discogenic low back pain and sciatica: a double-blind, placebo-controlled, dose-response pilot study. Anesthesiology. 2007;107:99–105. doi: 10.1097/01.anes.0000267518.20363.0d. [DOI] [PubMed] [Google Scholar]
- 17.Hagg O, Fritzell P, Nordwall A. Swedish Lumbar Spine Study Group: The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12:12–20. doi: 10.1007/s00586-002-0464-0. [DOI] [PubMed] [Google Scholar]
- 18.Meade TW, Dyer S, Browne W, Townsend J, Frank AO. Low back pain of mechanical origin: randomised comparison of chiropractic and hospital outpatient treatment. BMJ. 1990;300:1431–1437. doi: 10.1136/bmj.300.6737.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobrogowski J, Wrzosek A, Wordliczek J. Radiofrequency denervation with or without addition of pentoxifylline or methylprednisolone for chronic lumbar zygapophysial joint pain. Pharmacol Rep. 2005;57:475–480. [PubMed] [Google Scholar]
- 20.Bogduk N, Macintosh J, Marsland A. Technical limitations to the efficacy of radiofrequency neurotomy for spinal pain. Neurosurgery. 1987;20:529–534. doi: 10.1227/00006123-198704000-00004. [DOI] [PubMed] [Google Scholar]
- 21.de Baere T, Denys A, Wood BJ, Lassau N, Kardache M, Vilgrain V, Menu Y, Roche A. Radiofrequency liver ablation: experimental comparative study of water-cooled versus expandable systems. AJR Am J Roentgenol. 2001;176:1213–1215. doi: 10.2214/ajr.176.1.1760187. [DOI] [PubMed] [Google Scholar]
- 22.Walach H, Sadaghiani C, Dehm C, Bierman D. The therapeutic effect of clinical trials: understanding placebo response rates in clinical trials--a secondary analysis. BMC Med Res Methodol. 2005;5:26. doi: 10.1186/1471-2288-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20:31–37. doi: 10.1097/00007632-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21:1889–1892. doi: 10.1097/00007632-199608150-00012. [DOI] [PubMed] [Google Scholar]
- 25.Lord SM, Barnsley L, Wallis BJ, McDonald GJ, Bogduk N. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335:1721–1726. doi: 10.1056/NEJM199612053352302. [DOI] [PubMed] [Google Scholar]
- 26.Bernard TN, Cassidy JD. The sacroiliac syndrome. In: Frymoyer JW, editor. Pathophysiology, diagnosis and management, The Adult Spine: Principles and Practice. New York: Raven; 1991. pp. 2107–2130. [Google Scholar]
- 27.Grob KR, Neuhuber WL, Kissling RO. Innervation of the sacroiliac joint in humans. Zeitschrift fur Rheumatologie. 1995;54:117–122. (in German) [PubMed] [Google Scholar]
- 28.North RB, Kidd DH, Zahurak M, Piantadosi S. Specificity of diagnostic nerve blocks: a prospective, randomized study of sciatica due to lumbosacral spine disease. Pain. 1996;65:77–85. doi: 10.1016/0304-3959(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 29.Cohen SP, Hurley RW. The ability of diagnostic spinal injections to predict surgical outcomes. Anesth Analg. 2007;105:1756–1775. doi: 10.1213/01.ane.0000287637.30163.a2. [DOI] [PubMed] [Google Scholar]
- 30.Irwin RW, Watson T, Minick RP, Ambrosius WT. Age, body mass index, and gender differences in sacroiliac joint pathology. Am J Phys Med Rehabil. 2007;86:37–44. doi: 10.1097/phm.0b013e31802b8554. [DOI] [PubMed] [Google Scholar]
- 31.Underwood MR, Morton V, Farrin A. UK BEAM Trial Team: Do baseline characteristics predict response to treatment for low back pain? Secondary analysis of the UK BEAM dataset. Rheumatology. 2007;46:1297–1302. doi: 10.1093/rheumatology/kem113. [DOI] [PubMed] [Google Scholar]
- 32.Vaccaro AR, Ring D, Scuderi G, Cohen DS, Garfin SR. Predictors of outcome in patients with chronic back pain and low-grade spondylolisthesis. Spine. 1997;22:2030–2034. doi: 10.1097/00007632-199709010-00018. [DOI] [PubMed] [Google Scholar]