Abstract
The variable (V) domains of antibodies and T cell receptors (TCRs) share sequence homology and striking structural similarity. Single-chain antibody V domain constructs (scFv) are routinely expressed in a variety of heterologous systems, both for production of soluble protein as well as for in vitro engineering. In contrast, single-chain T cell receptor V domain constructs (scTCR) are prone to aggregation and misfolding and are refractory to display on phage or yeast in their wild-type form. However, through random mutagenesis and yeast display engineering, it has been possible to isolate scTCR mutants that are properly folded and displayed on the yeast surface. These displayed mutants can serve not only as a scaffold for further engineering but also as scTCR variants that exhibit favorable biophysical properties in E. coli expression. Thus, a more comprehensive understanding of the V domain mutations that allowed display would be beneficial. Our goal here was to identify generalizable patterns of important mutations that can be applied to different TCRs. We compared five different scTCRs, four from mice and one from a human, for yeast surface display. Analysis of a collection of mutants revealed four distinct regions of TCR V domains that were most important for enabling surface expression: the Vα-Vβ interface, the HV4 of Vβ, and the region of the Vα and Vβ domains normally apposed against the constant (C) domains. Consistent with the role of the V-C interface in surface display, reconstitution of this interface, by including the constant domains of each chain, allowed V domain display and αβ chain association on the yeast surface, thus providing an alternative TCR scaffold. However, the surface levels of TCR achieved with engineered scTCR mutants were superior to that of the VαCα/VβCβ constructs. Therefore, we describe further optimization of the current strategy for surface display of the single-chain format in order to facilitate yeast display engineering of a broader range of scTCRs.
Keywords: T cell receptor, single-chain, yeast display, directed evolution
Introduction
B cell and T cell receptors are the specific antigen recognition molecules of the adaptive immune system. Both receptors are endowed with diversity surpassing any other known proteins, providing the host with an immune cell repertoire capable of responding to the multitude of pathogens it encounters. Diversity is localized to the antigen-binding variable (V) domains, while the membrane-proximal regions contain constant (C) domains (Davis and Bjorkman, 1988). Each domain adopts an immunoglobulin fold comprising two closely packed anti-parallel beta sheets bridged by a conserved disulfide bond (Wilson and Garcia, 1997). Between each beta strand are intervening loops and, in the V domains of TCRs and Abs, three of these loops are highly diverse and positioned to contact antigen. The mechanism for generating diversity in these hypervariable loops, or complementarity-determining regions (CDRs), is the same during B and T cell development, with CDRs 1 and 2 germline encoded and CDR3 largely non-template encoded and produced by somatic recombination (Davis and Bjorkman, 1988). The antigen-binding site for both TCRs and antibodies is composed of six CDRs, as these receptors heterodimerize to form functional TCR αβ pairs or antibody heavy (H) and light (L) chain pairs.
Although both antibodies and αβ TCRs bind antigen via CDR loops (Garboczi et al., 1996; Garcia et al., 1998; Segal et al., 1974), there are many differences. The antibody repertoire recognizes a broad array of antigen types (e.g. protein, polysaccharide, soluble, membrane-bound), while TCR recognition is largely restricted to 8–20 amino acid antigenic peptide fragments presented at the target cell surface in the context of proteins of the major histocompatibility complex (pMHC) (Bjorkman et al., 1987; Zinkernagel and Doherty, 1974). TCRs bind peptide:MHC (pepMHC) antigen with low affinity (KD ~1–100 μM) compared to many antibody:antigen interactions. Mature T cells bearing high-affinity (KD <100 nM) TCRs have not been found, but antibodies binding antigen with such high affinity are selectively enriched by somatic mutations in B cells responding to antigen, through a process known as affinity maturation (Eisen, 2001; Foote and Eisen, 2000). Finally, the physiological milieu in which TCR and antibody proteins act are quite different: antibodies can be secreted and mediate effector functions in solution, whereas TCRs function exclusively as membrane bound molecules associated with the CD3 signaling subunits (Call et al., 2006; Kuhns and Davis, 2007).
Further differences between TCRs and antibodies are evident from efforts to express these proteins in recombinant forms for biochemical or structural characterization. Antibody Fab (VLCL/VHCH) and scFv (VH-linker-VL) are typically amenable to production in E. coli, and the structures of hundreds of different Ab fragments have been solved. In contrast, since discovery of the αβ TCR over 2 decades ago (Allison et al., 1982; Haskins et al., 1983; Meuer et al., 1983), only about 20 TCR structures have been solved. The difficulty has been largely attributed to low expression yields, aggregation of purified protein, and misfolding (Maynard et al., 2005; Rudolph et al., 2006). Inefficient αβ chain pairing or mis-association has also hampered efforts; which may reflect the low affinity of mouse α and β chains for each other (in one case, estimated to be a KD value of ~ 1 μM) (Pecorari et al., 1999). Many groups have developed strategies to facilitate αβ pairing, including fusion to leucine zipper subunits (Chang et al., 1994), introduction of a non-native disulfide bond (Boulter et al., 2003), or construction of a single-chain format in which TCR V domains are connected by a flexible peptide linker (Novotny et al., 1991; Soo Hoo et al., 1992). Expression of various forms of the TCR has been attempted in mammalian cells, insect cells, yeast, and E. coli. E. coli expression systems are particularly attractive because of their high growth rate and transformation efficiency (e.g., facilitating analysis of panels of mutants), but, despite recently developed strategies that have improved E. coli expression (Maynard et al., 2005), many TCRs remain refractory to expression in E. coli.
The challenges faced in producing soluble TCR in heterologous systems were also encountered in yeast or phage display systems developed for in vitro engineering. Although Ab scFv fragments are again readily expressed on the surface of yeast (Feldhaus and Siegel, 2004) and phage (Hoogenboom, 2005), scTCR display has been problematic. Only one report of scTCR phage display has been published (Weidanz et al., 1998), although two full-length heterodimeric TCRs have been engineered for high affinity using this system (Dunn et al., 2006; Li et al., 2005). Yeast display of scTCR has been achieved, but only through mutagenesis and selection of scTCR mutants capable of being displayed (Kieke et al., 1999; Weber et al., 2005). Nonetheless, the displayed scTCR mutants provided scaffolds for subsequent affinity maturation, allowing for experiments that have yielded insights into how ligand binding affinity influences T cell sensitivity, self-reactivity and cross-reactivity (Donermeyer et al., 2006; Holler et al., 2003; Holler and Kranz, 2003; Weber et al., 2005).
In addition to providing information about fundamental aspects of T cell biology, engineered TCRs are now being pursued in the clinical arena for therapeutics and diagnostics. A recent report of targeting tumor cells with T cells genetically modified to express a second TCR has indicated some promise (Morgan et al., 2006), and using affinity matured TCR variants has been proposed as a strategy to improve the clinical efficacy. Additionally, soluble tumor-specific scTCRs conjugated to immuno-stimulatory cytokines have been tested in mouse studies to enhance anti-tumor responses (Belmont et al., 2006), and such targeted cytokine delivery might be more effective if engineered, high-affinity, cytokine-conjugated scTCR were employed.
Mutations that have facilitated scTCR display on yeast also increase the stability and solubility of the purified protein (Shusta et al., 1999), enabling large-scale expression in E. coli with sufficient solubility for crystallization (Colf et al., 2007; Jones et al., 2006; Wang et al., 2007). These favorable features of scTCRs engineered by yeast display led us to explore the V domain framework mutations that enable scTCR yeast display for generalized patterns that might be applied to other TCRs. Given the variability among TCR V domains, not just in CDRs but also framework regions, we engineered three different new scTCRs (2 mouse and 1 human) for display on the surface of yeast for comparison with the two previously engineered scTCRs and a Vβ fragment. By analyzing this collection of mutants isolated from yeast display libraries, we found that certain TCR residues were frequently mutated not just within a particular TCR, but in some cases among multiple TCRs. Analysis of structural features of the positions that appeared critical to scTCR display revealed these mutations clustered to four distinct regions of TCR V domains: the Vα-Vβ interface, the would-be V-C interfaces of α and β chains, and the HV4 Vβ. Notably, these regions differ between TCR and Ab V domains, which could explain the fundamental differences in the ability to express scTCR and scFv fragments. We also generated two-chain heterodimeric TCR constructs (VαCα/VβCβ), which allowed us to examine to what extent reconstituting the V-C interface restored V domain folding and αβ association on yeast. Comparison of the two-chain heterodimer and scTCR formats revealed higher surface display was achieved with the engineered single-chain format, prompting us to develop a further optimized, more broadly applicable strategy for engineering scTCRs for yeast display.
Materials and Methods
Flow cytometry staining reagents
Antibodies and reagents used to detect yeast surface expression include: anti-mouse Vβ8.3, FITC- or PE-conjugated 1B3.3 (BD Pharmingen); anti-mouse Vβ11, RR3-15-PE (BD Pharmingen); anti-mouse Vα3.2, RR3-16-FITC (BD Pharmingen); anti-human Vβ21.3-FITC, clone IG125 (Immunotech/Beckman-Coulter); anti-mouse Vβ8.2, F23.2; anti-2C clonotype, 1B2; anti-human Cβ, 8A3 (Endogen/Pierce); anti-human Cα, 3A8 (Endogen/Pierce); anti-mouse Cβ, H57-957 (BD Pharmingen); anti-HA epitope tag, HA.11 (Covance), chicken anti-c-myc epitope tag (chicken IgY fraction) (Molecular Probes); Cy3 conjugated mouse anti-c-myc Ab, 9E10-Cy3 (Sigma); chicken egg white lysozyme (Acros), which was biotinylated by incubating with NHS-biotin (Sigma); PE- or Alexa488-labeled goat-anti-mouse secondary Ab (Invitrogen); Alexa647- or Alexa488-labeled goat-anti-chicken secondary Ab (Molecular Probes); and biotinylated goat anti-mouse secondary Ab (Rockland, Inc.); streptavidin(SA):488 (Molecular Probes); SA:PE (BD Pharmingen).
Plasmids
scTCRs and the D1.3 scFv were expressed from yeast display plasmid pCT302 (Boder and Wittrup, 2000), which contains a galactose-inducible AGA2 fusion and allows growth in Trp− media. VαCα or VβCβ were expressed from either pCT302-sec, which contains a galactose-inducible secretion construct and allows growth in Trp− media, or p315 (generously provided by Dane Wittrup and colleagues, MIT), which contains a galactose-inducible AGA2 fusion and allows growth in Leu− media.
scTCR and scFv constructs
TCRs used in this study were isolated from mouse CD8+ T cell clones 2C (Kranz et al., 1984), C18 (Ikeda et al., 1997), and mWT1 clone 33 (M. Dossett and P. Greenberg, unpublished data), and human CD8+ T cell clone CE10 (Ho et al., 2006). 2C scTCR constructs used in this study include: 2C-c-myc, in which a c-myc tag (EQKLISEEDL) was added to the C-terminus of the 2C scTCR Vβ-linker(l)-Vα (Vβ8.2, Vα3.1), which was constructed previously; and the 2C-T7 mutant which was engineered previously for increased yeast surface expression (Kieke et al., 1999; Shusta et al., 1999). The C18 scTCR (Vβ8.3, Vα10.1) was subcloned into the pCT302 vector, along with introduced mutations GlyVβ17Glu, HisVβ47Tyr, LeuVβ81Ser, as Vβ-l-Vα. The TCR V domains from mWT1 (Vβ11, Vα3.2) were cloned as Vα-linker-Vβ, Vα-linker-VβCβ, and Vβ-linker-Vα constructs, using SOE PCR (Warrens et al., 1997), and inserted into pCT302. The T cell clone CE10 was generated in vitro from CD8+ cells harvested from a healthy donor as described previously (Ho et al., 2006), and the V domains (Vβ21.3, Vα21) were cloned as scTCRs, Vβ-l-Vα or Vα-l-Vβ using SOE PCR and inserted into pCT302. All scTCRs contained the 25 amino acid peptide linker SSADDAKKDAAKKDDAKKDDAKKDA. The scFv D1.3 VH-(Gly4Ser)3-VL was cloned into pCT302 as described previously (Orr et al., 2003).
To create the VαCα/VβCβ constructs, the α and β chains of the 2C and CE10 TCRs were amplified with the Cys residues that normally participate in the interchain disulfide bond replaced with a stop codon (or c-myc tag); the PCR products were inserted individually into either p315 or pCT302-sec. The C-terminal residues of the 2C VαCα and VβCβ constructs were 87SDVP-c-myc-stop and 123GRADstop127, respectively. The C-terminal residues of the CE10 VαCα and VβCβ constructs were 92PESS-c-myc-stop and 127GRADstop, respectively. One chain was cloned into the p315 plasmid fused in frame to the C terminus of AGA2, and the other chain was cloned into pCT302-sec downstream of a secretion signal.
Single-site mutations Thr48CαCys and Ser57CβCys into 2C and CE10, Cys71CβAla into 2C, and Cys75CβAla into CE10 were introduced using SOE PCR. Single-site mutation LeuVα43Pro was introduced into mWT1-3 using SOE PCR, and hybrid mWT1 scTCR constructs were created using SOE PCR.
Error-prone library construction for display engineering
In several cases, scTCR inserts were amplified under error-prone conditions to introduce random mutations across the entire construct. Four 100 μL PCR reactions were prepared to yield a 0.5% error-rate (as determined in (Daugherty et al., 2000)) as follows: 220 μM dATP, 200 μM dCTP, 340 μM dGTP, 2.4 mM dTTP, 0.3 ng/μL template DNA (scTCR in pCT302 vector), 250 nM Splice 4/L (forward primer), 250 nM T7 (reverse primer), 5 ng/μL Bovine Serum Albumin (BSA), 3.325 mM MgCl2, 0.5 mM MnCl2, 10 μL Taq Polymerase Reaction Buffer (10 μL per 100 μL reaction), and 1 μL Taq Polymerase (Invitrogen). Reactions were then run in a thermocycler using the following program: 94°C for 3 minutes, 30 cycles (of 94°C for 1 minute, 50°C for 2 minutes, 72°C for 3 minutes), and then 72°C for 5 minutes. PCR products and vector backbone were combined at a 6:1 molar ratio insert:vector and electroporated into EBY100, allowing homologous recombination to restore the plasmid. Library sizes were determined by plating aliquots of pooled transformations, counting colonies, and subtracting vector and insert background.
Induction of TCR expression on the yeast cell surface
To induce surface expression of TCR (and scFv) constructs, EBY100 yeast that had been transformed with a yeast display plasmid and cultured to stationary phase in selecting media were pelleted and resuspended in galactose-containing media to drive expression from the galactose-responsive promoter, as described previously (Boder and Wittrup, 2000).
Flow cytometry
Yeast surface display of TCRs and the scFv D1.3 was detected using flow cytometry. ~106 induced yeast cells were pelleted, washed, and resuspended in primary Ab. After incubation, cells were washed and resuspended in secondary reagent. All washes, incubations, and dilutions were performed with phosphate-buffered saline containing 0.5% bovine serum albumin (PBS-BSA). Samples were analyzed on a Coulter Epics-XL flow cytometer.
Fluorescence activated cell sorting (FACS)
~108 induced library cells were pelleted, washed and stained as described above, but the cell numbers, staining and washing volume were greater, especially for the first sorts. The most fluorescent cells were selected on a MoFlo high-speed cell sorter (Cytomation), cultured in selective media, and induced for the next sort. To isolate individual clones, an aliquot of cells collected from the last sort in a series was spread onto sorbitol plates. The staining and percent of cells collected for each sort was as described below
For C18, the error-prone library was sorted 5 times, staining alternately with anti-Vβ8.3 and anti-c-myc. Sort 1: stained in 2.67 μg/mL 1B3.3-FITC and top 1% collected; Sort 2: stained in 12 μg/mL 9E10-Cy3 and top 1% collected. Sort 3: stained in 2.67 μg/mL 1B3.3-PE and top 1% collected; Sort 4: stained in 12 μg/mL 9E10-Cy3 and top 1% collected; Sort 5: stained in 2.67 μg/mL 1B3.3-FITC and top 0.6% collected.
The mWT1 error-prone scTCR library was sorted eight times. Sort 1: stained in 3.33 μg/mL anti-Vβ11-PE and top 1% collected; Sort 2: stained in 15 μg/mL 9E10-Cy3 and top 1% collected; Sort 3: stained in 8.33 μg/mL anti-Vα3.2-FITC and top 0.7% collected; Sort 4: stained in 3.33 μg/mL anti-Vβ11-PE and top 0.5% collected; Sort 5: stained in 15 μg/mL 9E10-Cy3 and top 0.5% collected; Sort 6: stained in 8.33 μg/mL anti-Vα3.2-FITC and top 0.2% collected; Sort 7: co-stained in 3.33 μg/mL anti-Vβ11-PE plus 8.33 μg/mL anti-Vα3.2-FITC and top 0.26% collected; Sort 8: co-stained as in sort 7 and top 0.28% collected. For second generation mWT1 sorting, Sort 1: co-stained in 4 μg/mL anti-Vβ11-PE and 10 μg/mL anti-Vα3.2-FITC and top 0.33% collected; Sort 2: co-stained as for sort 1 and top 0.17% collected; Sort 3: co-stained as for Sort 1 and top 0.25% collected.
For CE10 in the Vβ-L-Vα orientation, error prone libraries were sorted as follows. Sort 1: co-stained in 1 μg/mL anti-Vβ21.3-FITC plus 20 μg/mL anti-c-myc followed by Alexa647anti-chicken 1:250 and top 1% collected; Sort 2: stained as for sort 1 and top 0.5% collected; Sort 3: stained as for sort 1 and top 0.3% collected; Sort 4: stained in 5 μg/mL anti-Vβ21.3-FITC plus 10 μg/mL anti-c-myc followed by 1:100 PE-goat anti mouse and 1:250 Alexa647 anti-chicken and top 0.24% collected.
For CE10 in the Vα-L-Vβ orientation, error prone libraries were sorted as follows. Sort 1: Stained in 0.2 μg/mL anti-HA followed by PE goat anti mouse (1:100), collected top 10%; Sort 2: Stained as for sort 1, collected the top 4.0%; Sort 3: Stained with 1 μg/mL anti-Vβ21.3 followed by biotin goat anti-mouse (1:500) and subsequently streptavidin:PE (1:400), collected the top 4.5%. Plasmid DNA from yeast collected after sort 3 was isolated and the plasmid mixture was used as a template for an additional round of error-prone PCR. For sorting a second generation error prone library of CE10 in the Vα-L-Vβ orientation: Sort 4: Stained as for sort 3, collected the top 2% of cells; Sort 5: Stained as sort 4, collected top 10%; Sort 6: yeast were incubated at 50°C for 30 min, and stained on ice in 1 μg/mL Vβ21.3 FITC, collected the top 10%.
Oligonucleotides, plasmid rescue, and DNA sequencing
Oligonucleotide PCR and sequencing primers were synthesized by Integrated DNA Technologies. Yeast display plasmids were rescued from individual yeast cultures using the Zymoprep I Yeast Plasmid Miniprep Kit (Zymo Research). DNA sequencing was performed at the DNA Core sequencing facility at the University of Illinois at Urbana-Champaign.
Buried surface area calculation
The buried surface area between the V and C domains of both chains of the 2C TCR structure (PDB: 1TCR (Garcia et al., 1996)) and D1.3 Fab (PDB: 1FDL (Fischmann et al., 1991)) was calculated in CNS (buried_surface.inp, authors Axel T. Brunger, Paul T. Adams) (Lee and Richards, 1971) using a 1.4 Å radius probe. Domains were defined as: TCR Vα Q1-P116, Cα Y117-C213; Vβ E1-L116a (117 in Figure 5), Cβ E117-C247; and Fab VL D1-R108, CL A109-C214; VHQ1-S118, CH1T119-C218.
Figure 5. TCR V-domain amino acid sequence alignment, highlighting positions frequently mutated in surface displayed variants.
Amino acid sequences of the wild-type V domains of the three tumor-specific TCRs, along with previously described TCRs 2C and 3.L2 and the Vβ fragment hVβ2.1 were aligned based on a ClustalW multiple sequence alignment of the six V domains, IMGT protein display, and known 3-dimensional structural features. Positions mutated in 3 or more unique, independent mutants of at least 1 scTCR, as well as positions shown to contribute to surface display by single-site analysis, are highlighted in red. Positions mutated less frequently— in two independent mutants of the same scTCR, or one mutant of 2 different scTCRs— are highlighted in pink. Conserved immunoglobulin fold residues are highlighted in orange.
Results
Comparison of TCR and Ab variable domains
In contrast to scFv constructs which can routinely be expressed by yeast display, the TCR counterpart in various scTCR formats (e.g. Vβ-linker-Vα) have not been detected on the yeast cell surface, and yeast display has only been achieved through mutagenesis and selection of displayed mutants (Kieke et al., 1999; Weber et al., 2005). To investigate the structural differences between scFv and scTCR that might explain this difference in display, we first directly compared structurally a well characterized “model” scTCR and a “model” scFv.
Yeast transformed with the yeast display plasmid containing the mouse 2C scTCR Vβ-linker-Vα or the mouse D1.3 scFv VH-linker-VL were induced to express the AGA2 fusion, and surface scTCR or scFv levels analyzed by flow cytometry (Figure 1). Cells were stained with high affinity probes for native protein (clonotypic monoclonal Ab 1B2 for 2C and hen egg lysozyme (HEL) which is the ligand for D1.3), which showed that the scFv but not the scTCR was properly folded on the yeast surface. This difference was not simply due to reduced expression of the AGA2 fusion in the scTCR yeast, as N-terminal epitope tags were present at the yeast surface at similar levels for scTCR and scFv. Furthermore, the absence of folded scTCR was not due exclusively to internal proteolytic cleavage since the C-terminal epitope tag was detected for the scTCR, albeit at lower levels than for the scFv, suggesting that full polypeptides are targeted to the surface but they not folded properly. These c-myc profiles are consistent with our observations and those of others (Park et al., 2006) that the yeast protein quality control apparatus does not fully prevent misfolded proteins from being exported through the secretory pathway. Nevertheless, comparison of these flow cytometry profiles reveals an inherent difference between the folding of TCR V domains and Ab V domains on the yeast surface.
Figure 1. Yeast surface display of scTCR and scFv, comparing surface levels of N- and C-terminal expression tags as well as levels of native protein.
Yeast cells transformed with yeast display plasmid containing either the 2C Vβ-linker-Vα scTCR or the D1.3 VH-linker-VL constructs were induced to express AGA2 fusions. Induced cells (filled histograms) and uninduced cells (grey outline) were incubated with primary antibodies anti-HA (N-terminal epitope tag), anti-c-myc (C-terminal epitope tag), 1B2 conformation-specific antibody for 2C scTCR or ~100 μg/mL biotinylated HEL ligand for D1.3 scFv. Cells were washed and resuspended in respective secondary reagents Alexa488 goat anti-mouse for anti-HA and 1B2, Alexa488 anti-chicken for c-myc, and streptavidin:Alexa488 for biotin-HEL. Aliquots of induced cells were incubated with secondary antibody alone (black outline). Cells were then assayed by flow cytometry. Note: The negative population in each histogram is consistently observed in the yeast display system and serves as an internal control.
We then asked whether there are obvious structural features of V domains in TCRs and antibodies that might account for the differences in ability to be displayed (i.e. properly folded and exported to the surface). When the first crystal structures of the TCR β chain (Bentley et al., 1995) and αβ heterodimer (Garcia et al., 1996) were solved, one of the more salient differences noted between the TCR and Fabs was the more extensive contact between the V and C domains in TCRs, particularly in the β chain (Wilson and Garcia, 1997). Indeed, a direct comparison of the buried surface area in the V-C interfaces of the 2C TCR and D1.3 Fab revealed the that 2C TCR buries nearly 1.5 times the area than D1.3 Fab at the V-C interfaces (1503 Å2 compared to 992 Å2), with the Vβ domain burying 1.5 times as many atoms as the VL domain and nearly twice as many as the VH domain (Figure 2). TCR residues determined to be buried in this analysis were VαS13, E14, W82, L115, P116, CαY117, I118, S148, Q149, V167, D169, K176; VβN10, K11, V12, V14, T15, P84, R113, S115, L116a(117), CβE117, D118, L119, V122, F153, P154, D155, H156, V157, E158, H217, G218, L219, D223; and buried Fab residues were VLQ40, K103, E105, I106, R108, CLA109, A111, Y140, D165, Q166, D170, S171, T172, Y173; VHL11, T113, S115, S116, A117, S118, CH1T119, T120, F149, P150, D176 and Y178. Two TCR residues (L219 and D223) in the Cβ F-G loop, a unique feature of TCR Cβ and Cγ domains (Rudolph et al., 2006), also contribute to the Vβ-Cβ buried surface area in 2C. Solvent exposure of normally buried regions of the V domains in the single-chain constructs could contribute to misfolding (e.g. by promoting aggregation), and, since the TCR V domains contain more atoms that would normally be buried, this effect may be more pronounced in scTCRs than in scFv.
Figure 2. Comparison of surface area buried between the variable and constant domains in crystal structures of the 2C TCR and D1.3 Fab.
Buried surface area between the V and C domains of both chains of the 2C TCR structure (PDB: 1TCR) and D1.3 (PDB: 1FDL) was generated in CNS (buried_surface.inp) using a 1.4 Å radius probe. Buried residues are shown as magenta spheres. Figure was constructed in PyMol (DeLano Scientific). Total V-C buried surface for each protein is indicated.
Engineering three tumor-specific scTCRs for yeast surface expression
Two scTCRs have been engineered whereby various mutations in the V regions enabled display on the surface of yeast (Kieke et al., 1999; Weber et al., 2005). To further elucidate the features of such mutations that enable display, three additional scTCRs were cloned into the yeast display vector. These three TCRs, isolated from CTLs specific for tumor-associated antigens, include: 1) mouse C18 TCR, which recognizes a mutated MAP kinase peptide bound to Kd (Ikeda et al., 1997), 2) mouse mWT1 TCR which recognizes a peptide fragment of the WT-1 tumor antigen bound to Kb (M. Dossett and P. Greenberg, unpublished data) and 3) human CE10 TCR which recognizes a peptide fragment of the WT1 tumor antigen bound to HLA-A2 (Ho et al., 2006). All three scTCRs were cloned into the yeast display plasmid flanked by N-terminal (HA) and C-terminal (c-myc) epitope tags. The C18 V domains were cloned as a Vβ-linker-Vα orientation, whereas the mWT1 and CE10 scTCRs were cloned as both Vβ-linker-Vα and Vα-linker-Vβ orientations. Surface scTCR expression was analyzed by flow cytometry using commercially available anti-Vβ domain antibodies and an anti-Vα Ab (for mWT1 only). Surface Vβ was not detected with anti-TCR antibodies for any of these three scTCRs, despite the presence of N- and C-terminal epitope tags (Figure 3). As with the 2C scTCR (Figure 1), these results suggest that expression of the AGA2-fused polypeptide is induced, but the TCR V domains are not properly folded.
Figure 3. Three different tumor-specific scTCRs stain positive for N- and C-terminal epitope tags, but not Vβ, by flow cytometry.
Yeast transformed with a yeast display plasmid encoding either of three tumor-specific scTCRs (mouse C18 scTCR Vβ-linker-Vα, mWT1-Vα-linker-Vβ, or human CE10 Vβ-linker-Vα) were induced to express the AGA2 fusion. Induced cells were washed and incubated with primary antibody to HA (N-terminal epitope tag), anti-c-myc (C-terminal epitope tag), as well as antibodies to the respective Vβ domains for each single-chain (C18, mouse Vβ8.3; mWT1, mouse Vβ11; CE10, human Vβ21.3) (filled histograms). The Vβ stains were as follows: 4 μg/mL PE-conjugated anti-mouse Vβ8.3 antibody 1B3.3 for the C18 scTCR, 4 μg/mL PE-conjugated anti-mouse Vβ11 antibody RR3-15 for the mWT1 scTCR, and 5 μg/mL anti-Vβ21.3, followed by biotin goat anti-mouse and SA:PE for the CE10 scTCR. Samples of induced cells were incubated with secondary antibody alone (grey outline).
To isolate surface displayed mutants of the scTCRs using directed evolution, mutations were introduced randomly into the C18 Vβ-linker-Vα scTCR, the mWT1 Vα-linker-Vβ scTCR, and the CE10 Vα-linker-Vβ and Vβ-linker-Vα scTCRs by PCR amplification using error-prone conditions. PCR products were introduced, along with the linearized pCT302 yeast display vector, into EBY100 where homologous recombination generates the recombinant plasmid with scTCR inserts. C18 and mWT1 yeast libraries consisted of 107 independent transformants and the CE10 libraries consisted of 5 × 106 independent transformants for each orientation. The libraries were sorted using anti-TCR Vβ antibodies (and anti-Vα for mWT1), as well as anti-c-myc antibodies, through several cycles of selection, propagation, induction and re-selection, as described below and in detail in Materials and Methods.
The C18 library was sorted alternately on the Vβ8.3 and the C-terminal c-myc epitopes to avoid isolation of truncated Vα− mutants, as has been observed when sorting the 2C Vβ-linker-Vα scTCR using only anti-Vβ Ab (Kieke et al., 2001). After the fourth sort, a distinct Vβ8.3+ population of yeast had been enriched (Figure 4A, C18Sorted). The top 0.6 % Vβ8.3+ of these were collected and plated to isolate individual colonies. All 8 colonies that were screened were positive for Vβ8.3, HA, and c-myc (clone C18-6 in Figure 4B, and data not shown). DNA sequencing of the plasmids rescued from these eight colonies revealed that seven were unique clones. Six of these contained a Thr to Ala substitution at Vβ position 13, and five contained a Thr to Ile substitution at Vβ position 68 (Supplementary Figure 1).
Figure 4. Isolation of surface-displayed scTCR mutants.
Error-prone yeast libraries of scTCRs C18, mWT1, and CE10 were incubated with fluorescently labeled anti-TCR antibodies and sorted by FACS as described in detail in Materials and Methods. Several rounds of sorting enriched the populations for yeast expressing higher surface levels of the scTCR, as evidenced by positive flow cytometric staining with anti-TCR Vβ antibodies (A). Selected scTCR mutants stained with antibody to the N-terminal epitope tag, TCR Vβ (and Vα for mWT1-B7), and c-myc. (B) Staining was performed as indicated for Figure 3, with secondary antibody alone control histograms shown as grey outlines. Inset: mWT1 Vα-l-Vβ wild-type (hatchmarks) and B7 mutant (black fill) stained with FITC-conjugated anti-Vα3.2 antibody RR3-16
The selection scheme for isolating surface displayed mWT1 mutants from the mWT1 Vα-linker-Vβ error-prone library was facilitated by the mWT1 expressing one of the few mouse Vα gene products for which there is an anti-Vα Ab. Thus, this library was sorted based on surface expression of three epitopes: Vβ, Vα, and c-myc. The library was sorted eight times, alternately staining for the first six sorts, then co-staining with anti-Vα and anti-Vβ for the last two sorts. This approach yielded only one unique mutant, mWT1-3, that exhibited modest improvement in surface scTCR levels based on Vα, Vβ, and c-myc staining (data not shown). mWT1-3 contained four Vα framework mutations HisVα81Arg, TrpVα85Arg, SerVα93Gly, and AspVα108Asn, one Vβ framework mutation PheVβ33Tyr, and one CDR3β mutation ThrVβ97Ile (Supplementary Figure 1). Noting the similarity between the mWT1-3 HisVα81Arg mutation and the TrpVα82Arg mutation that improved surface display of the 2C TCR, and given the potential for improvement in the surface levels of mWT1-3, the amino acid sequence was scanned for other candidate residues to mutate based on similarity to the 2C Vα. LeuVα43, present in mWT1 Vα3.2 and 2C Vα3.1, was changed to proline in the surface displayed mutants of 2C scTCR, which, as discussed in Kieke et al., likely enhances Vα-Vβ association (Kieke et al., 1999). Indeed, LeuVα43Pro significantly increased surface levels of the mWT1-3 Vα and Vβ (data not shown). To explore the possibility of further increasing mWT1 surface levels, the mWT1-3 LeuVα43Pro was used as a template upon which to construct a second error-prone library, which was sorted in three rounds of FACS based on dual-labeling with PE-conjugated anti-Vβ11 and FITC-conjugated anti-Vα3.2. Following the second of these sorts, the population had been enriched for yeast expressing much higher levels of Vβ (Figure 4A, mWT1Sorted) and Vα (data not shown). The top 0.25% Vα and Vβ-positive of these cells were selected and plated for isolation of single colonies. Of the 10 colonies picked, 8 were unique mutants (named mWT1-B1 through B7 and B9), all of which exhibited enhanced surface scTCR levels compared to the mWT1-3 LeuVα43Pro template as detected by flow cytometric analysis of Vβ and Vα levels (Figure 4B, mWT1-B7, and data not shown). All mutants contained the mWT1-3 template mutations, and, although no conserved framework mutations were detected, multiple mutations were present at codons for Vβ40, Vα42, and CDR3α residues (Supplementary Figure 1).
Error-prone libraries of the CE10 scTCR were constructed in both the Vβ-linker-Vα and the Vα-linker-Vβ orientations. Each library was induced separately and pooled 1:1 before staining for the first sort. Following three sorts based on co-staining with anti-Vβ21.3 and antic-myc and a fourth sort with Vβ21.3 only, the population was enriched for mutants positive for both epitopes (Figure 4A CE10Sorted and data not shown). After plating to isolate single colonies, 19 colonies were analyzed, with 13 staining positive for anti-Vβ21.3, and 5/13 representing unique clones. Notably, all 5 were orientated Vβ-linker-Vα. Two of the 5 unique sequences were truncations at the start of the Vα and thus stained negative for c-myc. The remaining three, CE10-2, CE10-3, and CE10-11, had in-frame scTCR sequences and were positive for both Vβ and c-myc, at similar levels (Figure 4B and data not shown). CE10-2, -3, and -11 each contained two Vβ mutations: ArgVβ16Gly and LeuVβ91Phe. Interestingly, the Vβ domains present in the truncated Vβ+ clones also contained the ArgVβ16Gly mutation but were wild-type at Vβ91. One mutant contained in-frame domains and was c-myc+ but anti-Vβ-negative. This mutant was wild-type at Vβ16, providing further evidence of the importance of the ArgVβ16Gly mutation. While CE10-11 contained no other Vβ or Vα mutations, CE10-2 and CE10-3 were mutated elsewhere (Supplementary Figure 1). Despite the presence of additional mutations in CE10-2 and CE10-3, Ab staining was similar to the CE10-11 clone, suggesting the additional mutations contributed little to display and stability. However, the importance of ArgVβ16Gly and LeuVβ91Phe was evident, and single-amino acid substitutions indicated that both were needed to recapitulate the Vβ21.3 staining of CE10-11 (data not shown).
V domain mutations that facilitate scTCR yeast display
To identify the TCR positions most important for surface display, we aligned the Vβ and Vα domains of the three tumor-specific scTCR engineered here, as well as the 2C and 3.L2 scTCRs, and the previously displayed individual hVβ2.1 wild-type sequence (Figure 5), highlighting those residue positions where mutations have been observed after selections for yeast surface display (this report, Supplementary Figure 1, and (Buonpane et al., 2005; Kieke et al., 1999; Shusta et al., 2000; Weber et al., 2005)). Residues mutated most frequently, or directly implicated in improving surface display via single-site analysis, were deemed most important and highlighted in red (Figure 5). Less frequently mutated residues were highlighted in pink. This analysis implicated 11 Vβ domain framework residues and 3 Vα framework residues in allowing yeast surface display. Highlighting the positions of the most important (red) residues in the crystal structure of the 2C V domains (PDB 1TCR (Garcia et al., 1996)) revealed clustering of these positions at four regions of the TCR: the would be Vα-Cα and Vβ-Cβ interfaces, the Vα-Vβ interface, and flanking residues of the HV4 loop of Vβ (Figure 6A).
Figure 6. Frequently mutated positions cluster to 4 distinct regions of the TCR V domains.
(A) Positions of residues most frequently mutated in scTCR mutants selected for yeast surface display (highlighted in red in figure 5) shown here as red spheres in the V domains from the crystal structure of the 2C TCR (PDB: 1TCR). These residues cluster at the would-be V-C interfaces, the Vα-Vβ interface, and at the HV4Vβ. (B) An overlay of the crystal structure of the stabilized scTCR mutant 2C-T7 (pale green) (PDB: 2IO9) and the V domains of the wild-type 2C (grey) (PDB: 2CKB). Side chains of the stabilizing framework mutations are displayed in bright green. Wild type counterparts are displayed in magenta for comparison. Figure was constructed in PyMol (DeLano Scientific).
The recently solved crystal structure of the 2C scTCR (Colf et al., 2007) containing previously identified stabilizing mutations affords a unique opportunity to compare structural aspects of the wild-type V domains with the yeast displayed “stabilized” V domains. Accordingly, the crystal structures of the stabilized 2C scTCR, called 2C-T7 (Colf et al., 2007) and the V domains from the full-length 2C TCR (Garcia et al., 1998) were superimposed (Figure 6B), revealing a close backbone alignment (root mean square deviation=1.0 Å). The areas of greatest difference were found in the CDR loops (which could be attributed to the two different pepMHC ligands in the two complexes), and perhaps most interestingly in the loops formed at the base of each V domain at the Vα:Vβ interface. Not surprisingly, the side chains of some framework stabilizing mutations (green, Figure 6B), and the wild-type counterparts (magenta, Figure 6B) also showed changes in positioning. While some mutations replaced a surface exposed hydrophobic residue with an exposed hydrophilic residue (e.g. TrpVα82Arg), other mutations provided additional contacts with other framework residues (e.g. GlyVβ17Glu), perhaps providing enhanced local stability of that region of the protein.
A better understanding of the structural features critical to improving stability, especially interactions between mutated residues and other framework residues (for which structural data is particularly informative), will likely aid in efforts to predict stabilizing mutations. Therefore, a detailed analysis of each of these 2C mutations, and other mutations identified among the collection of different TCRs (red in Figures 5 and 6A), is presented in the discussion section.
Reconstitution of the V-C interface facilitates V domain folding and yeast surface display
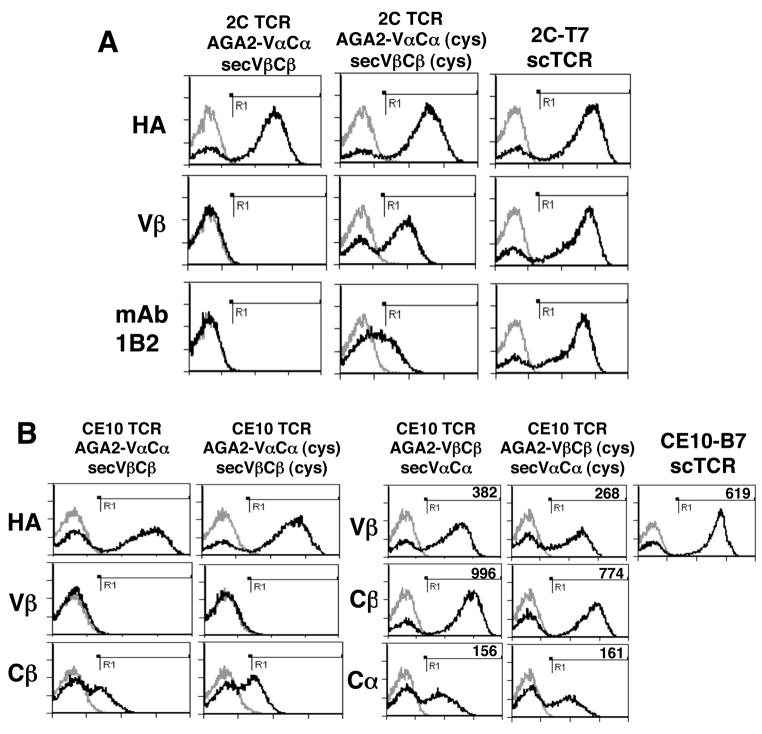
Since the regions at the V-C interfaces emerged as two of the “hot spots” for V-domain mutations in displayed scTCR mutants, we examined the effects of including the C-domains, thereby restoring the V-C interface in the yeast display constructs. The hypothesis is that the presence of the C domains in the form of VαCα and VβCβ constructs would stabilize the V-C interface, thereby improving surface levels of the V domains. In addition, the inclusion of the C domains may provide additional energy for the association of α and β chains via the Cα:Cβ association. However, given that V-C interfaces were not the only regions implicated in scTCR display, it was uncertain whether the full-length constructs would be able to form properly folded and associated αβ heterodimers on the yeast surface, and if so to what extent.
To examine these issues, full-length VαCα and VβCβ constructs of the 2C TCR were cloned as an AGA2 fusion and secreted form, respectively, into two different yeast plasmids, allowing for selection of yeast co-transformants. The advantage of the 2C TCR system is that the high-affinity probe, mAb 1B2, for the properly folded Vα and Vβ domains is available. The Cα and Cβ domains normally participate in a membrane-proximal, inter-chain disulfide bond. Although this bond likely stabilizes the αβ heterodimer on the surface of T cells, the presence of these cysteines has been shown to negatively effect TCR expression in E. coli (Pecorari et al., 1999) and is frequently not included in TCR expression constructs (Boulter et al., 2003). Therefore, the yeast display VαCα and VβCβ constructs were terminated just prior to the cysteines involved in the native disulfide bond. Flow cytometric analysis of the 2C full-length wild-type co-transformants using antibodies to HA, Vβ8.2, and mAb 1B2 indicated that, despite secretion of properly folded VβCβ evidenced by ELISA analysis (data not shown), the α and β chains did not associate efficiently (Figure 7A, left). However, some level of association could be achieved upon introduction of a C-domain non-native disulfide bond (Boulter et al., 2003)(Figure 7A, middle), as evidenced by staining with anti-Vβ Ab and mAb 1B2. While surface displayed full length TCR was detected with mAb 1B2, the lower staining intensity compared to anti-Vβ mAb suggests only a fraction of the fully reconstituted αβ heterodimer is in the native conformation (i.e. the Vα domain may be misfolded in some heterodimers). TCR expression levels in the 2C full-length heterodimer format were compared to those of the engineered single-chain mutant 2C-T7 (Figure 7A, right). The staining with anti-Vβ and mAb 1B2 revealed that about ten-fold higher levels of properly folded TCR were achieved in the scTCR mutant 2C-T7 compared to the two-chain, disulfide-stabilized format.
Figure 7. Reconstituting the V-C interface can restore Vβ folding and allow for yeast surface display of 2-chain VαCα/VβCβ construct.
(A)Yeast co-transformed with plasmid p315-2C VαCα targeting the 2C α chain to the cell surface and pCT302-sec 2C VβCβ directing secretion of the 2C β chain were induced to express both constructs. Wild-type (left column) and C-domain Cys mutants (middle column), as well as previously engineered mutant 2C-T7 (right column) were incubated with primary antibody to HA (N-terminal epitope tag), anti-mouse Vβ8.2 antibody F23.2, and anti-2C TCR clonotype 1B2, followed by biotin goat anti-mouse and SA:PE, and analyzed by flow cytometry (B) Yeast co-transformed with p315-CE10 VαCα and pCT302-sec CE10 VβCβ (top) or p315-CE10 VβCβ and pCT302-sec CE10 VαCα were induced to express both constructs. Wild-type (left column) and C-domain Cys mutants (middle column), as well as yeast expressing engineered CE10 scTCR mutant CE10-B7 (right column), were incubated with primary antibody to HA (N-terminal epitope tag), humanVβ21.3, human Cβ and human Cα, followed by biotin goat-anti-mouse and SA:PE, and analyzed by flow cytometry. Histograms from samples stained with only secondary reagents are shown with grey outlines.
To explore if the full-length format could be applied to a different TCR, the VαCα and VβCβ chain genes from the human TCR CE10 were cloned in two different formats, one with the α chain fused to AGA2 and the β chain secreted, the other with the β chain fused to AGA2 and α chain secreted, with incorporation of the stabilizing C region cysteines tested in both formats. In the AGA2-α chain format, Cβ was detected on the yeast surface with and without the introduced Cys residues (Figure 7B). However, somewhat unexpectedly, Vβ was not detected in either WT and Cys constructs, despite detection of properly folded VβCβ in the media by sandwich ELISA (data not shown). It is unclear why folded Vβ is detectable in the media but not on the yeast cell surface. One possible explanation is the ELISA has sufficient sensitivity to detect levels of the Vβ21.3 that are lower than levels detectable by flow cytometry. The modest Cβ staining and the disparity between surface Cβ and Vβ staining is not a general feature of human TCRs in the two-chain format, since a different human TCR showed that both Cβ and Vβ were detected at high levels on the yeast surface with the introduced Cys (data not shown). Moreover, in the alternative format (AGA2-β), both CE10 Vβ and Cβ were folded, and αβ association was detected by positive staining with anti-human Cα (Figure 7B). Furthermore, this format achieved αβ association with or without introduced Cys mutations, as indicated by flow cytometric detection of surface displayed Cα using the anti-Cα Ab. Thus, the AGA2-fused β chain and secreted α chain, is the preferred arrangement for the CE10 TCR, and the expression did not benefit from a potential C region disulfide. We presume both the fusion format and need for a stabilizing disulfide in C regions are influenced directly by the V regions used by a TCR. Finally, we noted that like 2C scTCR, the surface level of Vβ on the stabilized scTCR of CE10, as well as Vβ staining of a second generation scTCR (see below), were higher than the full-length construct (Figure 7B, right).
Comparison of TCR expression platforms and further optimization of scTCR surface display using an Ab to a single V domain
Given the potential to engineer higher yeast surface levels of a scTCR, which also have correlated with large-scale production in E. coli for biochemical and structural studies, strategies to select stable scTCR mutants would be useful. However, for most TCRs an Ab to only one domain, the Vβ, is available. The lack of a Vα domain probe complicates engineering of scTCRs because the folded state of the Vα is uncertain. Thus, a strategy to optimize single-chain TCR engineering using an Ab to only the Vβ domain was investigated. The approach we explored was based on the notion that the position of the Vβ domain at the N- or C-terminus of the single-chain format may influence whether this domain is folded (e.g. location of Vβ at the N-terminus may allow it to fold independently of the C-terminal Vα domain, whereas location of Vβ at the C-terminus may require that Vα be folded in order for Vβ to fold).
To explore this possibility, we used the mWT1 scTCR and the engineered surface displayed mutant mWT1-B7 (Figure 4B), as this system has mAb probes for both the Vα and Vβ domains. We constructed two hybrid Vα-linker-Vβ scTCRs, one with the Vα from B7 (containing 7 Vα mutations) linked with the wild type Vβ (Vα(B7)-linker-Vβ(WT)), and one with the wild-type Vα linked to the Vβ from B7 (containing 2 Vβ mutations) (Vα(WT)-linker-Vβ(B7)). We then tested the yeast surface levels of each domain in the hybrid constructs, compared to the wild-type Vα-linker-Vβ and the original engineered mWT1-B7 mutant (Vα-linker-Vβ). Neither of the wild type Vα or Vβ domains was detected on the yeast surface, in either the fully wild type construct or the hybrid constructs (Figure 8A). While the mutated Vα from B7 was folded in the presence of the wild-type Vβ in the Vα(B7)-linker-Vβ(WT) hybrid, the mutated Vβ from B7 in the Vα(WT)-linker-Vβ(B7) was not detected on the yeast surface. These results suggest that the presence of the wild-type Vα domain at the N-terminus prevented the Vβ(B7) domain from folding. Indeed, the influence of the stability of one V domain on the folding of another V domain has been demonstrated in the analagous scFv system (Hoyer et al., 2002).
Figure 8. Interdomain influence in yeast surface expression of scTCR.
(A) Yeast expressing either mWT1 Vα-l-Vβ WT (grey fill), the yeast displayed mutant mWT1-B7 (black outline), or hybrid single-chains containing the Vα from B7 and the wild-type Vβ (green outline), or inversely, the WT Vα and the Vβ from B7 (blue outline) were assayed for surface TCR display by flow cytometry. Cells were incubated with antibodies to mouse Vα3.2 (left) or mouse Vβ11 (right). (B) Yeast expressing either wild-type CE10 Vβ-l-Vα (grey fill), mutant CE10-3 Vβ-l-Vα engineered for yeast surface display (black outline), or the re-oriented version of CE10-3, CE10-Vα-l-Vβ (blue outline) (left) or engineered Vα-l-Vβ mutant CE10-B7, were assayed for yeast surface display by flow cytometry. Cells were incubated with primary anti-humanVβ21.3 antibody, followed by biotin goat anti-mouse and SA:PE. Histograms from samples stained with only secondary reagents are shown in hatch marks (left) and black outline (right).
The finding that the engineered C-terminal mWT1 Vβ(B7) domain was rendered misfolded (i.e. unrecognizable by the anti-Vβ11 Ab) by an N-terminal wild-type Vα domain prompted us to examine the CE10 TCR. Accordingly, we reversed the orientation of the engineered surface display mutant CE10-3 from Vβ-linker-Vα to Vα-linker-Vβ, keeping all point mutations intact. This reoriented construct exhibited much reduced Vβ staining (Figure 8, top). This decreased Vβ staining in the Vα-linker-Vβ orientation suggested the Vα is not optimally folded in CE10-3, and that it may be possible to detect enhanced Vα folding by isolating further Vα-linker-Vβ mutants in which Vβ surface expression levels are restored. To improve Vα folding, the Vα domain in the reoriented Vα-linker-Vβ construct was subjected to random mutagenesis by error-prone PCR and a yeast library generated. The library was sorted for Vβ expression, and several mutants with substantially improved Vβ staining were isolated. To further improve scTCR display (e.g. to achieve levels comparable to that of 2C-T7), plasmids rescued from these mutants were pooled and subjected to another round of mutagenesis and selection. Selected mutants exhibited Vβ levels significantly improved not only above the reoriented template CE10-3 Vα-linker-Vβ, but also the original CE10-3 Vβ-linker-Vα mutant (see mutant CE10-B7, Figure 8B, bottom). Several Vα framework mutations were identified in these selected mutants (Supplemental Figure). For example, PheVα45 was mutated in all three selected mutants, to Tyr once and Ser twice, and LeuVα75 was mutated to Gln in two mutants. However, single-site mutagenesis will be required to verify which mutations contribute to higher Vβ levels on the yeast surface. Without an Ab to the Vα it also remains uncertain if the Vα domain is properly folded in these mutants.
Discussion
Analysis of a collection of scTCR mutants, engineered for yeast surface display by mutagenesis and selection, has pointed to four regions of the V domains that appear to be important in limiting the proper folding and/or stability of the scTCR on yeast. Notably, three of the four V domain regions differ in a characteristic way between TCR V domains and Ab V domains. Mutations at these regions in TCR V domains can overcome these limitations, allowing for expression of scTCR comparable to that of scFv, thereby creating a suitable scaffold not only for subsequent ligand affinity maturation (Holler et al., 2000), but also for large-scale scTCR expression in E. coli (Buonpane et al., 2005; Colf et al., 2007; Wang et al., 2007). These framework mutations, highlighted in Figure 6A, will now be discussed in detail, with respect to the three-dimensional structures of TCRs and a comparison of the primary amino acid sequences of all V regions, in an effort to reveal general principles that may facilitate the engineering of TCRs in the future.
Mutations at V-C interfaces
Residue Vβ13: Position Vβ13 was mutated in four different TCRs: C18 (mVβ8.3), mWT1 (mVβ11), an individual β chain fragment, hVβ2.1 (Buonpane et al., 2005), and 2C (mVβ8.2) (Kieke et al., 1999). In all four of these Vβs, the mutations introduced a more hydrophobic side chain (threonine to alanine or isoleucine, and alanine to valine). TCR crystal structures show that the Vβ13 side chain is pointed into the core of the Vβ domain, largely buried by other core residues. Threonine is the most common amino acid at this position in human Vβ and the third most common in mouse Vβ (behind lysine and alanine). The single structure in which a threonine is present at Vβ13, ((Tynan et al., 2007) PDB: 2NW2)) is quite revealing, as it shows the threonine side chain more solvent exposed than other Vβ13 side chains, angling slightly away from the rest of the Vβ. In this position, the threonine γ oxygen is only 3.04 Å from a δ oxygen on Asp224, which is on the Cβ F-G loop, possibly engaging in a hydrogen bond. This contact would of course be absent in a scTCR, and an unsatisfied H-bond would presumably destabilize the Vβ (Ewert et al., 2003). mVβ8.3 and mVβ11 encode a threonine at Vβ13, and mutations from threonine to valine or leucine would lack the unsatisfied H-bond, and could be replaced with more stabilizing buried contacts. However, such a mutation requires two nucleotide changes within the codon and would be highly unlikely to be isolated from an error-prone library. A threonine to isoleucine substitution, on the other hand, is possible through a single base mutation, as occurred in one of the mWT1 mutants. The reason for a substitution of threonine for alanine at this position in the C18 scTCR, as opposed to isoleucine, is not clear but the strong preference for an alanine to valine substitution in hVβ2.1 (Buonpane et al., 2005) suggests that the additional hydrophobicity stabilized the domain, perhaps by providing additional buried contacts. Thus, it is reasonable to predict that mutation of a less hydrophobic residue (threonine, alanine) to a more hydrophobic residue (valine, isoleucine, or leucine) would improve the stability of scTCRs. Also, the proximity of Vβ13 to Vβ12, which was buried at the Cβ interface in the 2C TCR, may also influence the optimal Vβ13 residue. Finally, it is worth noting that position 13 in all VH and VL (with the exception of the few mouse Vλ genes, which surprisingly all encode a threonine), is most commonly a valine, alanine, or leucine, and never a threonine. Therefore, introducing valine or alanine at Vβ13 endowed the TCR Vβ with a more Ab-like feature, likely contributing to the improved surface display.
Residue Vβ16: The majority of TCR α and β chains and Ab H and L chains contain a glycine at position 16. However, CE10 expresses one of the rare genes encoding a non-glycine residue, arginine, at position 16. Our data indicate that reversion of Vβ16 from arginine to the consensus glycine was necessary for yeast surface display of the CE10 scTCR (and isolated Vβ truncations). The detrimental effect of a residue other than glycine at position 16 of Ab V domains on folding and stability has been attributed to the necessary positive phi angle at this position, which is allowed by the flexible glycine (Ewert et al., 2003). Our data suggest that the same pressure to maintain the positive phi angle is present in TCR Vα and Vβ domains. It is worth noting that the arginine is tolerated in the yeast displayed VβCβ, as evidenced by yeast surface detection of CE10 VβCβ (Figure 7B), and the VβCβ ArgVβ16Gly mutant exhibited only modestly improved Vβ staining over wild-type CE10 VβCβ chain (data not shown), suggesting the presence of the Cβ can compensate for instability caused by residues other than glycine at Vβ16. Notwithstanding the detailed structural aspects of this position, the fact that a non-glycine residue is extremely rare at position 16 suggests another lesson from Ab V domain analysis that could be applied toward the improvement of TCR V domains– rare amino acids found at certain positions tend to be unfavorable. In this regard, mutation of rare amino acids to the consensus improves Ab V domain stability with a 60% success rate (Steipe et al., 1994). These findings suggest that any non-glycine residue at position 16 should be reverted to glycine to improve scTCR biophysical behavior.
Residue Vβ17: This solvent-exposed Vβ position is most frequently a glutamine in mouse and human TCR Vβ domains, but is a glycine in the 2C TCR Vβ. Mutation of glycine to glutamic acid (glycine to glutamine would have required more than one nucleotide substitution) was sufficient to allow moderate levels of yeast surface display of properly folded 2C TCR (Kieke et al., 1999). In the crystal structure of the 2C-T7 scTCR (Colf et al., 2007), glutamic acid at Vβ17 is solvent-exposed (Figure 6B), like the glutamine in this position in other TCR crystal structures. In addition, Vβ17Glu also contacts Vβ14, a valine residue buried at the Cβ interface in the 2C full-length TCR. These additional contacts may stabilize this region of the V domains in the scTCR format. These findings suggest a glycine at position 17 should be mutated to a glutamic acid and/or glutamine, especially when position 14 is hydrophobic, to improve scTCR expression levels on yeast or E. coli.
Residues Vβ87 and Vβ88: These residues lie close to the beta strand known as the f strand, following the short α-helix at the VβCβ interface. Although neither residue is buried at the VβCβ interface, they contact numerous surrounding Vβ residues, including those buried in the Cβ or involved in Vα contacts. These residues, therefore, seem to behave as linchpins in the full-length TCR. Vβ87 is most commonly a serine in human and mouse Vβ genes. The second most common amino acid at this position, threonine, can contact Vβ positions 84, 114, and 116. Whereas a ThrVβ87Ser mutation was important in surface expression of the 3.L2 scTCR, threonine was tolerated in the C18 and 2C scTCRs, suggesting that the influence on yeast surface display of position 87 depends on its context and may differ among TCRs. The adjacent residue, Vβ88, is usually an alanine in TCR Vα and Vβ and Ab VH and VL genes. In contrast to Vβ87, which is angled toward the Cβ, the Vβ88 side chain is typically pointed toward the core of the Vβ. In this position, the side chain frequently contacts the main string of Vβ residues involved in the Vβ-Vα interface (e.g. Vβ37Gln), as well as other Vβ residues, such as those closer to the Vβ-Cβ interface. Mutation of Vβ88Ser to glycine was necessary for yeast surface expression of the individual hVβ2.1 domain (Buonpane et al., 2005), potentially by permitting restructuring of the Vβ to compensate for the lack of Vα.
Residue Vα82: Single-site mutational analysis revealed the importance of a TrpVα82Arg mutation in yeast surface display of the 2C TCR (Shusta et al., 2000). 2C expresses the Vα3.1 region, which contains a tryptophan at Vα82 with a bulky side chain that is normally buried at the Cα interface. In human TCRs, and mouse and human VH and VL, position 82 is seldomly an aromatic residue. The absence of the C domain in the single-chain, exposing the tryptophan side chain, could limit surface display by increasing the scTCR’s susceptibility to aggregation during folding. In support of this argument, the crystal structure of 2C-T7 scTCR shows the arginine side chain is solvent-exposed (Figure 6B). The mutations HisVα81Arg and TrpVα85Arg in the mWT1 scTCR, as well as similar findings in Ab V domains (Nieba et al., 1997) further suggest that bulky residues in this region are disfavored in the single-chain format, and that substitution of these residues with an arginine could improve display.
Mutations at the Vα–Vβ interface
The identification of several mutations at the Vα-Vβ interface that enhance association suggests the linker is not sufficient to promote optimal VαVβ association and that additional forces can help compensate for the absence of the C domains in driving association.
Residue Vβ42: The most common amino acid, in mouse and human Vβ and VH, at Vβ42 is glycine. TCR structures show Vβ42Gly is in close proximity to the Vα, and is often engaged in main chain contact with Vα residues such as PheVα106, GlyVα107, and Vα108. Glutamic acid is the second most common amino acid at Vβ42 in human and mouse TCRs, and the GlyVβ42Glu mutation was isolated in two thermostable yeast display mutants of the 2C scTCR and a 3.L2 displayed mutant. In contrast to our expectation, the glutamic acid side chain at Vβ42 in the 2C scTCR-T7 structure did not make additional contacts with Vα but rather is angled away from the V domains toward solvent (Figure 6B). Therefore, it appears that GluVβ42 exerts its positive influence on surface display via a mechanism other than simply providing additional direct contacts with the Vα, perhaps by exposure to solvent.
Residue Vβ91: The side chain of this position on the f strand is angled toward the Vα, and in many TCR structures participates in the Vα–Vβ interaction, contacting GlnVα37, Vα40, Vα42, Vα43, or GlyVα109, depending on the TCR. In mouse TCRs and Ab VH and VL, this position is most often aromatic (phenylalanine or tyrosine). However, in human Vβs, such as CE10, leucine is most common at Vβ91, followed closely by phenylalanine. Mutation from leucine to phenylalanine at this position was found to be critical for CE10 scTCR surface expression. While crystal structures show that leucine at Vβ91 can contact Vα residues, phenylalanine has the potential for more Vα contacts, and tyrosine has the additional possibility of a hydrogen bond. Although mWT1 scTCR tolerated a leucine at Vβ91, the mWT1 scTCR Vα-Vβ interface was presumably strengthened by the introduced LeuVα43Pro mutation (see below), perhaps overcoming the need for additional contacts from Vβ91. Thus, mutation of leucine at Vβ91 to phenylalanine or tyrosine would likely improve Vα-Vβ association of other scTCRs.
Residue Vα43: The importance of this position, in particular with respect to the conserved LeuVH43:ProVL43 has been discussed previously (Kieke et al., 1999). Mutation of Vα43 from leucine to proline, allowing the Ab-like LeuVβ-ProVα interaction, significantly improved surface scTCR levels of the 2C scTCR. Although position 43 in TCRs is typically either a leucine or a proline, the position has been highly restricted to leucine in heavy chain V regions and proline in light chain V regions, thereby forcing the Leu:Pro interaction that is not strictly conserved in TCRs. Consequently, many TCR αβ pairs such as 2C, mWT1, and CE10 contain Leu:Leu or Pro:Pro. In this report, the importance of the LeuVα43Pro mutation was confirmed using the mWT1 scTCR.
Residue Vα108: TCR crystal structures show this residue, encoded by the Jα gene, often participates in inter-domain interactions with Vβ40, Vβ41, and Vβ42. Isolated mWT1 mutants contained either AspVα108Asn or AspVα108Lys, and a LysVα108Arg mutation was isolated in a 3.L2 scTCR mutant. This position in J genes, located at the center of the conserved Phe-Gly-X-Gly sequence, is variable and is perhaps involved in fine tuning the Vα–Vβ pairing achieved by the more conserved residues (e.g. GlnVα37/GlnVβ37, Vα35, Vα89, Vβ33, Vβ35, etc.). A recently published TCR structure highlighted the role of a J-encoded residue on Vα-Vβ pairing geometry. (McBeth et al., 2008). The more extensive diversity present among TCR Jα regions complicates prediction of Vα108 mutations that enhance display, but could provide a mechanism for generating diversity in the orientation of the Vα and Vβ.
Mutations near the HV4 loop of Vβ
It has been known since the discovery of TCR genes that the sequence variability of residues Vβ68 to Vβ77 is unique to TCRs, as Ab V domain sequences are more conserved in this region. Accordingly, this region was defined as a hypervariable 4 region (HV4) in Vβ genes. Furthermore, the two beta strands that support this loop appear to have more beta sheet character in antibodies than in TCRs, perhaps indicating a greater flexibility of this region in the TCR. The TCR HV4 loop is directed toward the pepMHC, but has not been shown to directly contact ligand (although an HV4Vα residue has been observed to mediate CDR1α and CDR2α rearrangement upon ligand binding (Kjer-Nielsen et al., 2003)). This sequence diversity is intriguing given the apparent lack of direct involvement in antigen contact and the conserved nature of the equivalent loop in antibodies. However, there is virtually nothing known about the function of this loop.
Residues that flank the HV4 Vβ loop (e.g. Vβ68 and Vβ78) emerged as a hot spot for mutations that improved scTCR yeast surface display. While both of these residues are exposed in the known TCR structures, there was not a consistent hydrophobic to hydrophilic substitution that seems to account for the mechanism of these mutations. For example, in C18 and 3.L2 the threonine at Vβ68 was mutated to an isoleucine or alanine, respectively. In contrast, 3.L2 and 2C scTCRs benefited from substitutions of a leucine at Vβ78 to a less hydrophobic valine or a threonine, respectively (the IleVβ78Thr mutation in 2C was introduced to improve solubility of the scTCR expressed from E. coli, as has been done for two other Vβ8.2-positive TCRs (Garcia et al., 2001)).
Although residue Vβ47 is not within the HV4, it appears to be involved in stabilizing the HV4 loop and a HisVβ47Tyr mutation was isolated in a yeast display mutant of the 2C scTCR (Shusta et al., 2000). Vβ47 exhibits modest variability among TCR Vβ genes but the position is dominated by histidine or tyrosine. This residue reaches from the c’ strand just before the CDR2, through the core of the Vβ, to contact main chain atoms on residues such as 65 and 66 on or near the d strand. In the wild-type 2C structure, HisVβ47 hydrogen bonds to the main chain oxygen of TyrVβ65. In 2C-T7, the general direction of the Vβ47 side chain is maintained, however the tyrosine side chain hydroxyl is contacting a water molecule, and the side chain is further away (3.39 Å) from the TyrVβ65 main chain oxygen, suggesting that this mutation enhances stability via the additional solvent contact (Figure 6B).
Mutation of another residue near the HV4, Vβ81, has been identified in yeast display selections of the 2C scTCR (a LeuVβ81Ser mutation). Vβ81 is solvent exposed and in most TCRs, as well as VH and VL domains, it is a serine. However, some Vβ genes encode a leucine at this position, exposing the aliphatic side chain to solvent. Thus, this residue can be mutated in the scTCR format, adhering to the relatively straightforward principle that replacing exposed hydrophobic residues with hydrophilic ones can improve protein folding/stability (Novotny et al., 1991).
Concluding remarks
The long-standing observation that many TCRs are difficult to express and crystallize has led to the suggestion that Vα and Vβ regions must confer unique biochemical properties on these proteins (Rudolph et al., 2006). We have shown that inherent properties of wild type Vα and Vβ regions also make expression of a single-chain format of the TCR difficult to express, unlike the analogous single-chain formats (scFv) of antibodies. This difficulty with the scTCR format extends to those TCRs (e.g. 2C) where the Vα and Vβ have proven successful in crystallographic studies of the full-length, C-region containing form.
Our findings have shown that there are multiple regions of the Vα/Vβ protein that require mutations in order for a scTCR to be optimally expressed on the surface of yeast. These include residues that are exposed at the Vα and Vβ surface in the C-region deleted forms, and residues that appear to improve the interaction of the Vα and the Vβ. Unexpectedly, residues on the face of the Vβ near the HV4 that are exposed in the native TCR format are also important. This finding, and the distinct differences between scFv and scTCRs, raises the question of whether in the native form the TCR complex may actually have regions that are “protected” by other surface molecules including CD3 subunits or by glycosylation. A recent study by Kuhns and Davis showed that regions of extracellular association of the αβ heterodimer with CD3 subunits are critical for assembly, but it has been predicted that these associations involve the C-regions not the V regions of the TCR (Kuhns and Davis, 2007). Nevertheless, the CD3γε dimer, which has been hypothesized to interact with the Cβ FG loop, could also influence the Vβ region near the base of the HV4 loop. It is also possible that glycosylation effects, including the binding of galectin-3 (Demotte et al., 2008), could provide further stabilization of the V regions within the αβ/CD3 complex. While these possibilities remain yet to be tested, it is worth noting that each of the TCRs examined here as scTCRs, and those examined as full-length heterodimeric TCRs (2C and CE10) in the yeast display system, have been successfully expressed as heterodimers in their native membrane bound form when introduced into CD3-positive T cells (Donermeyer et al., 2006; Hanson et al., 2000; Holler and Kranz, 2003; Kuball et al., 2007). The relatively low levels of heterodimers displayed on yeast in the presence or absence of the C-region stabilizing disulfides, supports the notion that the entire TCR/CD3 assembly serves to stabilize the heterodimer. The difficulties encountered in the expression and crystallization of many TCR heterodimers may in part be due to the absence of this stabilization.
Finally, it is worth considering whether it may now be possible to rationally design scTCR mutants that can be expressed in display or secretion systems. Mutation of key exposed residues as outlined here, from hydrophobic to hydrophilic side chains, has thus far been done on an ad hoc basis. We have shown that changing the interface residue at Vα43 provided further improvements in the mWT1 scTCR. However, other changes which involve intramolecular stabilizations are more difficult to predict and will likely need to be generated through a directed evolution approach, at least until we have additional information about a larger collection of Vα and Vβ regions.
Supplementary Material
Mutations isolated de novo are shown in bold. Deviations from the template sequence that were carried through from a previous generation are shown in grey. Mutations that were introduced by site-directed mutagenesis are indicated by an asterisk. Conserved immunoglobulin fold residues are highlighted in orange. 2C-T7, 2C-LWHI, hVβ2.1, and 3.L2 mutations have been published previously.
Acknowledgments
We thank Scott Weber, Rebecca Buonpane, Jennifer Stone, Raven Huang, Leremy Colf, and Chris Garcia for discussions and Barbara Pilas and Ben Montez at the University of Illinois Flow Cytometry Facility for technical assistance. The work was supported by NIH grants R01 GM55767 and R21 CA111877 (to DMK) and CA33084, CA18029, and Leukemia & Lymphoma Society LLS 7040-03 (to PDG). SAR was supported in part by an NIH NRSA 1 F31 ES013571.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison JP, McIntyre BW, Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J Immunol. 1982;129:2293–2300. [PubMed] [Google Scholar]
- Belmont HJ, Price-Schiavi S, Liu B, Card KF, Lee HI, Han KP, Wen J, Tang S, Zhu X, Merrill J, Chavillaz PA, Wong JL, Rhode PR, Wong HC. Potent antitumor activity of a tumor-specific soluble TCR/IL-2 fusion protein. Clin Immunol. 2006;121:29–39. doi: 10.1016/j.clim.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Bentley GA, Boulot G, Karjalainen K, Mariuzza RA. Crystal structure of the β chain of a T cell antigen receptor. Science. 1995;267:1984–1987. doi: 10.1126/science.7701320. [DOI] [PubMed] [Google Scholar]
- Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–12. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Boder ET, Wittrup KD. Yeast surface display for directed evolution of protein expression, affinity, and stability. Methods Enzymol. 2000;328:430–44. doi: 10.1016/s0076-6879(00)28410-3. [DOI] [PubMed] [Google Scholar]
- Boulter JM, Glick M, Todorov PT, Baston E, Sami M, Rizkallah P, Jakobsen BK. Stable, soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng. 2003;16:707–11. doi: 10.1093/protein/gzg087. [DOI] [PubMed] [Google Scholar]
- Buonpane RA, Moza B, Sundberg EJ, Kranz DM. Characterization of T cell receptors engineered for high affinity against toxic shock syndrome toxin-1. J Mol Biol. 2005;353:308–21. doi: 10.1016/j.jmb.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Call ME, Schnell JR, Xu C, Lutz RA, Chou JJ, Wucherpfennig KW. The structure of the zetazeta transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell. 2006;127:355–68. doi: 10.1016/j.cell.2006.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Bao Z, Yao Y, Tse AGD, Goyarts EC, Madsen M, Kawasaki E, Brauer PP, Sacchettini JC, Nathenson SG, Reinherz EL. A general method for facilitating heterodimeric pairing between two proteins: Application to expression of α and β T-cell receptor extracellular segments. Proc Natl Acad Sci. 1994;91:11408–11412. doi: 10.1073/pnas.91.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC. How a single T cell receptor recognizes both self and foreign MHC. Cell. 2007;129:135–46. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Daugherty PS, Chen G, Iverson BL, Georgiou G. Quantitative analysis of the effect of the mutation frequency on the affinity maturation of single chain Fv antibodies. Proc Natl Acad Sci U S A. 2000;97:2029–34. doi: 10.1073/pnas.030527597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Demotte N, Stroobant V, Courtoy PJ, Van Der Smissen P, Colau D, Luescher IF, Hivroz C, Nicaise J, Squifflet JL, Mourad M, Godelaine D, Boon T, van der Bruggen P. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28:414–24. doi: 10.1016/j.immuni.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Donermeyer DL, Weber KS, Kranz DM, Allen PM. The study of high-affinity TCRs reveals duality in T cell recognition of antigen: specificity and degeneracy. J Immunol. 2006;177:6911–9. doi: 10.4049/jimmunol.177.10.6911. [DOI] [PubMed] [Google Scholar]
- Dunn SM, Rizkallah PJ, Baston E, Mahon T, Cameron B, Moysey R, Gao F, Sami M, Boulter J, Li Y, Jakobsen BK. Directed evolution of human T cell receptor CDR2 residues by phage display dramatically enhances affinity for cognate peptide-MHC without increasing apparent cross-reactivity. Protein Sci. 2006;15:710–21. doi: 10.1110/ps.051936406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen HN. Specificity and degeneracy in antigen recognition: yin and yang in the immune system. Annu Rev Immunol. 2001;19:1–21. doi: 10.1146/annurev.immunol.19.1.1. [DOI] [PubMed] [Google Scholar]
- Ewert S, Honegger A, Pluckthun A. Structure-based improvement of the biophysical properties of immunoglobulin VH domains with a generalizable approach. Biochemistry. 2003;42:1517–28. doi: 10.1021/bi026448p. [DOI] [PubMed] [Google Scholar]
- Feldhaus MJ, Siegel RW. Yeast display of antibody fragments: a discovery and characterization platform. J Immunol Methods. 2004;290:69–80. doi: 10.1016/j.jim.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Fischmann TO, Bentley GA, Bhat TN, Boulot G, Mariuzza RA, Phillips SE, Tello D, Poljak RJ. Crystallographic refinement of the three-dimensional structure of the FabD1.3-lysozyme complex at 2.5-A resolution. J Biol Chem. 1991;266:12915–20. [PubMed] [Google Scholar]
- Foote J, Eisen HN. Breaking the affinity ceiling for antibodies and T cell receptors. Proc Natl Acad Sci U S A. 2000;97:10679–81. doi: 10.1073/pnas.97.20.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Degano M, Pease LR, Huang M, Peterson P, Teyton L, Wilson IA. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Degano M, Stanfield RL, Brunmark A, Jackson MR, Peterson PA, Teyton L, Wilson IA. An αβ T cell receptor structure at 2.5 angstrom and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. doi: 10.1126/science.274.5285.209. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Radu CG, Ho J, Ober RJ, Ward ES. Kinetics and thermodynamics of T cell receptor- autoantigen interactions in murine experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2001;98:6818–23. doi: 10.1073/pnas.111161198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–76. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983;157:1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods. 2006;310:40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Holler PD, Chlewicki LK, Kranz DM. TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol. 2003;4:55–62. doi: 10.1038/ni863. [DOI] [PubMed] [Google Scholar]
- Holler PD, Holman PO, Shusta EV, O’Herrin S, Wittrup KD, Kranz DM. In vitro evolution of a T cell receptor with high affinity for peptide/MHC. Proc Natl Acad Sci U S A. 2000;97:5387–92. doi: 10.1073/pnas.080078297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105–16. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- Hoyer W, Ramm K, Pluckthun A. A kinetic trap is an intrinsic feature in the folding pathway of single-chain Fv fragments. Biophys Chem. 2002;96:273–84. doi: 10.1016/s0301-4622(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Ohta N, Furukawa K, Miyazaki H, Wang L, Kuribayashi K, Old LJ, Shiku H. Mutated mitogen-activated protein kinase: a tumor rejection antigen of mouse sarcoma. Proc Natl Acad Sci U S A. 1997;94:6375–9. doi: 10.1073/pnas.94.12.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Brophy SE, Bankovich AJ, Colf LA, Hanick NA, Garcia KC, Kranz DM. Engineering and characterization of a stabilized alpha1/alpha2 module of the class I major histocompatibility complex product Ld. J Biol Chem. 2006;281:25734–44. doi: 10.1074/jbc.M604343200. [DOI] [PubMed] [Google Scholar]
- Kieke MC, Shusta EV, Boder ET, Teyton L, Wittrup KD, Kranz DM. Selection of functional T cell receptor mutants from a yeast surface- display library. Proc Natl Acad Sci U S A. 1999;96:5651–6. doi: 10.1073/pnas.96.10.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieke MC, Sundberg E, Shusta EV, Mariuzza RA, Wittrup KD, Kranz DM. High affinity T cell receptors from yeast display libraries block T cell activation by superantigens. J Mol Biol. 2001;307:1305–15. doi: 10.1006/jmbi.2001.4560. [DOI] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Clements CS, Purcell AW, Brooks AG, Whisstock JC, Burrows SR, McCluskey J, Rossjohn J. A structural basis for the selection of dominant alphabeta T cell receptors in antiviral immunity. Immunity. 2003;18:53–64. doi: 10.1016/s1074-7613(02)00513-7. [DOI] [PubMed] [Google Scholar]
- Kranz DM, Sherman DH, Sitkovsky MV, Pasternack MS, Eisen HN. Immunoprecipitation of cell surface structure of cloned cytotoxic T lymphocytes by clone-specific antisera. Proc Natl Acad Sci USA. 1984;81:573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, Greenberg PD. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–8. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhns MS, Davis MM. Disruption of extracellular interactions impairs T cell receptor-CD3 complex stability and signaling. Immunity. 2007;26:357–69. doi: 10.1016/j.immuni.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Lee B, Richards FM. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971;55:379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, Liddy N, Jacob J, Jakobsen BK, Boulter JM. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23:349–54. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- Maynard J, Adams EJ, Krogsgaard M, Petersson K, Liu CW, Garcia KC. High-level bacterial secretion of single-chain alphabeta T-cell receptors. J Immunol Methods. 2005;306:51–67. doi: 10.1016/j.jim.2005.07.022. [DOI] [PubMed] [Google Scholar]
- McBeth C, Seamons A, Pizarro JC, Fleishman SJ, Baker D, Kortemme T, Goverman JM, Strong RK. A new twist in TCR diversity revealed by a forbidden alphabeta TCR. J Mol Biol. 2008;375:1306–19. doi: 10.1016/j.jmb.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer SC, Acuto O, Hussey RE, Hodgdon JC, Fitzgerald KA, Schlossman SF, Reinherz EL. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983;303:808–10. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieba L, Honegger A, Krebber C, Pluckthun A. Disrupting the hydrophobic patches at the antibody variable/constant domain interface: improved in vivo folding and physical characterization of an engineered scFv fragment. Protein Eng. 1997;10:435–44. doi: 10.1093/protein/10.4.435. [DOI] [PubMed] [Google Scholar]
- Novotny J, Ganju RK, Smiley ST, Hussey RE, Luther MA, Recny MA, Siliciano RF, Reinherz EL. A soluble, single-chain T-cell receptor fragment endowed with antigen-combing properties. Proc Natl Acad Sci. 1991;88:8646–8650. doi: 10.1073/pnas.88.19.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr BA, Carr LM, Wittrup KD, Roy EJ, Kranz DM. Rapid Method for Measuring ScFv Thermal Stability by Yeast Surface Display. Biotechnol Prog. 2003;19:631–8. doi: 10.1021/bp0200797. [DOI] [PubMed] [Google Scholar]
- Park S, Xu Y, Stowell XF, Gai F, Saven JG, Boder ET. Limitations of yeast surface display in engineering proteins of high thermostability. Protein Eng Des Sel. 2006;19:211–7. doi: 10.1093/protein/gzl003. [DOI] [PubMed] [Google Scholar]
- Pecorari F, Tissot AC, Pluckthun A. Folding, heterodimeric association and specific peptide recognition of a murine alphabeta T-cell receptor expressed in Escherichia coli. J Mol Biol. 1999;285:1831–43. doi: 10.1006/jmbi.1998.2422. [DOI] [PubMed] [Google Scholar]
- Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–66. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- Segal DM, Padlan EA, Cohen GH, Rudikoff S, Potter M, Davies DR. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974;71:4298–302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusta EV, Holler PD, Kieke MC, Kranz DM, Wittrup KD. Directed evolution of a stable scaffold for T-cell receptor engineering. Nat Biotechnol. 2000;18:754–9. doi: 10.1038/77325. [DOI] [PubMed] [Google Scholar]
- Shusta EV, Kieke MC, Parke E, Kranz DM, Wittrup KD. Yeast polypeptide fusion surface display levels predict thermal stability and soluble secretion efficiency. J Mol Biol. 1999;292:949–56. doi: 10.1006/jmbi.1999.3130. [DOI] [PubMed] [Google Scholar]
- Soo Hoo WF, Lacy MJ, Denzin LK, Voss EWJ, Hardman KD, Kranz DM. Characterization of a single-chain T cell receptor expressed in E. Coli. Proc Natl Acad Sci. 1992;89:4759–4763. doi: 10.1073/pnas.89.10.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steipe B, Schiller B, Pluckthun A, Steinbacher S. Sequence statistics reliably predict stabilizing mutations in a protein domain. J Mol Biol. 1994;240:188–92. doi: 10.1006/jmbi.1994.1434. [DOI] [PubMed] [Google Scholar]
- Tynan FE, Reid HH, Kjer-Nielsen L, Miles JJ, Wilce MC, Kostenko L, Borg NA, Williamson NA, Beddoe T, Purcell AW, Burrows SR, McCluskey J, Rossjohn J. A T cell receptor flattens a bulged antigenic peptide presented by a major histocompatibility complex class I molecule. Nat Immunol. 2007;8:268–76. doi: 10.1038/ni1432. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao Y, Li Z, Guo Y, Jones LL, Kranz DM, Mourad W, Li H. Crystal structure of a complete ternary complex of TCR, superantigen and peptide-MHC. Nat Struct Mol Biol. 2007;14:169–71. doi: 10.1038/nsmb1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrens AN, Jones MD, Lechler RI. Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest. Gene. 1997;186:29–35. doi: 10.1016/s0378-1119(96)00674-9. [DOI] [PubMed] [Google Scholar]
- Weber KS, Donermeyer DL, Allen PM, Kranz DM. Class II-restricted T cell receptor engineered in vitro for higher affinity retains peptide specificity and function. Proc Natl Acad Sci U S A. 2005;102:19033–8. doi: 10.1073/pnas.0507554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidanz JA, Card KF, Edwards A, Perlstein E, Wong HC. Display of functional αβ single-chain T-cell receptor molecules on the surface of bacteriophage. J Immunol Meth. 1998;221:59–76. doi: 10.1016/s0022-1759(98)00153-7. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Garcia KC. T-cell receptor structure and TCR complexes. Curr Opin Struct Biol. 1997;7:839–48. doi: 10.1016/s0959-440x(97)80156-x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mutations isolated de novo are shown in bold. Deviations from the template sequence that were carried through from a previous generation are shown in grey. Mutations that were introduced by site-directed mutagenesis are indicated by an asterisk. Conserved immunoglobulin fold residues are highlighted in orange. 2C-T7, 2C-LWHI, hVβ2.1, and 3.L2 mutations have been published previously.