Abstract
Post-translational modification of proteins, such as glycosylation, can impact cell signaling and function. ST6Gal I, a glycosyltransferase expressed by B cells, catalyzes the addition of alpha-2, 6 sialic acid to galactose (Siaα2-6Gal), a modification found on N-linked glycoproteins such as CD22, a negative regulator of B cell activation. We show that SNA lectin, which binds Siaα2-6Gal, shows high binding on plasma blasts and germinal center B cells following viral infection, suggesting ST6Gal I expression remains high on activated B cells in vivo. To understand the relevance of this modification on the antiviral B cell immune response, we infected ST6Gal I−/− mice with influenza A/HKx31. We demonstrate that the loss of ST6Gal I expression results in similar influenza infectivity in the lung, but significantly reduced early influenza-specific IgM and IgG levels in the serum, as well as significantly reduced numbers of early viral specific antibody-secreting cells (ASCs). At later memory time points, ST6Gal I−/− mice show comparable numbers of IgG influenza-specific memory B cells and long-lived plasma cells, with similarly high antiviral IgG titers, with the exception of IgG2c. Finally, we adoptively transfer purified B cells from WT or ST6Gal I−/− mice into B cell deficient (μMT−/−) mice. Recipient mice that received ST6Gal I−/− B cells demonstrated reduced influenza-specific IgM levels, but similar levels of influenza-specific IgG, compared to mice that received WT B cells. These data suggest that a B cell intrinsic defect partially contributes to the impaired antiviral humoral response.
Introduction
Humoral immunity is characterized by prolonged antibody production, even after resolution of infection, in stark contrast to the T cell response, where effector T cell function is relatively short-lived. Antibodies, by neutralizing or opsonizing free extracellular pathogens, serve as a critical first-line defense against infection. Humoral immunity is comprised of pre-existing antibody produced by antibody secreting cells (ASCs) termed long-lived plasma cells (LLPCs), as well as a population of memory B cells (MBCs) that can differentiate following antigenic stimulation into plasma cells (1–4).
The enzyme ST6Gal I is a glycosyltransferase highly expressed by B and T cells that synthesizes the glycan sequence lpha 2,6 sialic acid linked to galactose (Siaα2-6Gal) (5). This structure is typically found on N-linked glycans of glycoproteins, such as the mammalian lectin CD22, a transmembrane glycoprotein expressed by B cells that has high specificity for (Siaα2-6Gal). CD22 has been shown to be a negative regulator of B cell signaling by recruiting SHP-1 to the BCR, thus attenuating signals via the BCR (5–7). The plant lectin Sambucas nigra agglutinin (SNA) binds the product of ST6Gal I, Siaα2-6Gal, so SNA lectin binding can be used as an indicator of ST6Gal I expression and/or activity (5).
ST6Gal I null mice were initially described several years ago (5). These mice demonstrate normal T cell activation to anti-CD3 cross-linking in vitro, but defective BCR signaling in vitro, and reduced antibody production following immunization with T-independent and T-dependent antigens (DNP-Ficoll or DNP-KLH) in vivo (5). However, despite indications that B cell immune function may be compromised in these mice, as well as evidence that addition of cytokines could abrogate in vitro B cell proliferation differences, no studies have documented whether or not protective antibody and memory B cell responses are compromised in these mice when exposed to pathogenic microorganisms such as live viral infection.
In this study, we have compared the outcome of influenza viral infection of wild type or ST6Gal I deficient mice. The haemagglutinin (HA) glycoprotein plays a key role in influenza pathogenicity and is involved in host-cell recognition (8). First, we show that effective replication of influenza A/HKx31 (Orthomyxoviridae, Influenza A) does occur in the lungs of ST6Gal I−/− mice, however these mice demonstrate defective early influenza-specific B cell responses. Next, we determine that expansion of CD4 and CD8 influenza-specific T cell numbers in the lung and lymph nodes is normal in ST6Gal I−/− mice. Further, we demonstrate similar overall numbers of influenza specific IgG MBCs and LLPCs, though we note both a reduction in serum levels of influenza specific IgG2c and IgG2c producing LLPCs in the bone marrow. Finally, we demonstrate an impaired influenza specific IgM response in B cell deficient mice that receive ST6Gal I−/− B cells, suggesting that a B cell intrinsic defect partially contributes to the impaired humoral response. Overall, our findings suggest that lack of ST6Gal I expression appears to prominently impair the generation of a viral specific humoral response, but plays a lesser role in influenza-specific memory.
Materials and Methods
Mice and immunizations
ST6Gal I deficient mice were generated by J. Marth and obtained from the Consortium for Functional Glycomics and bred in-house (5). C57BL/6 female mice and B6.129S2-Igh-6tm1Cgn/J (μMT−/− mice) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained under specific pathogen-free conditions at the University of Tennessee in accordance with university IACUC guidelines and used at 6–12 weeks of age. Anesthetized mice were intra-nasally infected with 106.8 EID50 influenza A/HKx31 in 30 μl PBS as previously described (9, 10). μMT−/− mice were primed with 107.4 EID50 influenza A/HKx31 i.p.
Tissue harvest and flow cytometry
At specified time points (day 7, 10, > 40 post-infection), animals were sacrificed and tissues harvested for viral titer (lungs only), immunofluorescence, or isolation of lymphocytes. For isolation of lymphocytes from lungs, lungs were perfused with 5 ml cold PBS before removal and processed as previously described (11). Single cell suspensions were stained with mAbs purchased from BD Pharmingen (CD4, CD8, CD44, B220, CD138, FAS, GL7, IFNγ and TNFα), Vector Labs (SNA and PNA lectins), or Southern Biotech (IgD). Intracellular cytokine staining was assayed on cells from spleen, lung, and MedLN. All samples were run on a FACSCalibur (BD Biosciences). All data were analyzed with FlowJo software (Tree Star). Unpaired student t tests were performed to determine statistical significance with *denoting p<.05 ** denoting P<0.01, and *** denoting P<0.001.
Immunofluorescence
MedLNs were removed from mice on day 7 p.i. and frozen in OCT at −80°C. The frozen tissues were cut at 8-μm thickness, thaw-mounted onto slides, air dried and fixed in cold acetone for 10 minutes. The sections were then blocked with 3% BSA and stained for germinal centers with PNA-FITC (Vector Laboratories), anti-B220-PE, and/or GL7-FITC (BD Pharmingen) as indicated. After staining, sections were washed with PBS, mounted using Vectashield mounting medium (Vector Laboratories), and analyzed using a laser scanning confocal microscope (Leica SP2).
Influenza viral titer determination
Lungs were collected on indicated days post-infection and homogenized in Hanks media with 0.1% BSA (GIBCO). Eight replicates of the homogenized lung were serially diluted tenfold across 96-well U-bottom plates in 1xMEM with 0.3% BSA, 1μg/ml Trypsin TPCK-treated (Worthington), penicillin (100IU/ml), streptomycin (100 μg/ml), and 5% FBS. The titrated lung samples were added to serum-free MEM media on a MDCK cell monolayer prepared in 96-well tissue culture plates and incubated for 48h at 37°C and 5% CO2. Wells that were positive for viral growth were identified by testing supernatants for hemagglutinating activity using 0.5% chicken red blood cells.
ELISPOT assay to detect influenza specific ASCs
A preparation of concentrated viral particles was disrupted for 10 min at room temperature in a 1:10 dilution of disruption buffer (0.5% Triton X-100, 0.6 M KCl, and 0.05 M Tris-HCl, pH 7.5) in PBS, further diluted in PBS, and plated at 1 μg/well in nitrocellulose-bottomed 96-well Multiscreen HA filtration plates (Millipore, Bedford, MA). After overnight incubation at 4°C, plates were washed with PBS, and blocked with BCM containing 10% FBS. Plates were emptied by flicking, and cell suspensions (including resuspended cells from 96-well MBC assay plates) were added in volumes of approximately 100 μl/well. After incubation for 3–4 h at 37°C in a humidified atmosphere containing 5% CO2, plates were thoroughly washed with PBS alone and PBS containing 0.1% Tween 20. Alkaline phosphatase-conjugated goat anti-mouse IgG, IgM, or IgA (Southern Biotechnology, Birmingham, AL) diluted to 2 μg/ml in PBS containing 5% bovine serum albumin was added (100 μl/well), and the plates were incubated overnight at 4°C. The plates were then washed extensively with PBS alone and PBS containing 0.1% Tween 20, including washing the underside of the nitrocellulose filters. Spots were developed at room temperature by the addition of 1 mg/ml of 5-bromo-4-chloro-3-indolyl phosphate (Sigma, St. Louis, MO) in diethanolamine buffer (10% diethanolamine, 0.1 M NaCl, 5 mM MgCl2, and 0.1 M Tris-HCl, pH 9.5), 100 μl/well. After spot development, plates were washed with PBS and dried, and spots representing individual ASCs were counted using an Olympus SZX9 stereozoom microscope.
Memory B cell assay
Influenza-specific MBC frequencies were determined by a previously described LDA based on in vitro stimulation of MBC to differentiate into ASCs (10, 12). Briefly, twofold dilutions of cells were incubated in 96-well tissue culture plates (routinely 12 wells per dilution), together with 106 irradiated (3000 rad) syngeneic naïve spleen cell feeders plus β-propiolactone-inactivated HKx31 (Charles River, Wilmington, MA). After incubation, cells in each well were transferred to ELISPOT plates for the enumeration of influenza-specific IgG ASCs. Pre-existing virus-specific ASC numbers at the time of sampling were determined by direct ex vivo ELISPOT assay. After in vitro MBC activation and ELISPOT analysis, individual wells were scored positive for virus-specific MBC if progeny ASC numbers were greater than twice the mean pre-existing ASC. The virus-specific MBC frequency was calculated from the number of negative wells per cell dilution by extrapolation to the dilution that gave 37% negative wells (12). Linearity between the proportion of negative cultures and the input cell dose indicated direct measurement of MBC. No influenza-specific IgG ASCs were detected after in vitro stimulation of lymphocytes from naïve mice or from mice infected i.n 8 wk previously with an unrelated virus.
B cell transfer and ELISA
MACS B220 beads (Miltenyi Biotech) were used for purification of B cells according to the manufacturer’s instructions. Purity of B220+ B cells was 95% determined by FACS. 2×107 B220+ spleen cells were adoptively transferred into individual primed μMT deficient mice i.v. followed by infection with influenza i.n. 24 hrs later. For serum antibody determination by ELISA, plates were coated with purified, detergent disrupted influenza A/HKx31 virus (0.5μg/well) and incubated at 4°C overnight. Plates were washed with PBS-Tween20 (0.05%), blocked with PBS/FBS (3%), and washed again. Serial three-fold serum dilutions were prepared in PBS-Tween20 (0.05%)-BSA (0.5%). The plates were incubated at RT 4 h or overnight at 4°C, then washed thoroughly. Plate-bound secreted Abs were detected using alkaline phosphatase-conjugated goat anti-mouse Abs with specificity for IgM, IgG, IgG1, IgG2b, IgG2c or IgG3 (Southern Biotechnology, Birmingham, AL) diluted in PBS-BSA (1%). After 4 h incubation at RT, plates were washed extensively, and color development with p-nitrophenyl phosphate (Sigma) in diethanolamine buffer was read at 405 nm using a Synergy2 Multi-Detection Microplate Reader (BioTek Instruments, Inc.). The virus-specific serum Ab titer is expressed as the reciprocal of the highest dilution giving an absorbance value more than twice that for simultaneously titrated samples from naïve mice.
Results
SNA binding is high on naïve and in vivo activated B cells
The plant lectin SNA preferentially binds α2-6-linked sialic acids and previous reports have demonstrated high SNA binding on naïve lymphocytes that is abrogated in lymphocytes lacking ST6Gal I expression (5). Gene expression studies comparing naïve B cells to in vitro activated B cells revealed that ST6Gal I expression remains high on activated B cells (13).
We examined SNA lectin binding by flow cytometry on B cells following in vivo B cell activation (Figure 1). We observed that on day 8 following viral infection, B220+ CD138+ plasma blasts remain SNA high similar to B220+ IgD+ naïve B cells in uninfected mice. Additionally, on day 15 post-infection, B220+, Fas+, IgDlo germinal center B cells also show high SNA lectin binding (Figure 1).
Figure 1. Binding of SNA on B cell populations.
Splenocytes from uninfected C57BL/6 mice, or mice infected with 2×105 pfu LCMV Armstrong i.p. either 8 or 15 days earlier were used for the analysis. The left panel in B–D shows the gating, and the right panel SNA binding as histograms of the gated population. A, Cartoon depicting the glycosyltransferase ST6Gal I which catalyzes addition of terminal α2,6 sialic acids to galactose. SNA lectin binds Sia α 2-6Galβ1-4GlcNac. B, Naive B cells from uninfected mice gated on B220+/IgD+ cells C, CD138+/B220lo plasma blasts on day 8 and D, B220+FAS+/IgD− germinal center cells all show high SNA binding.
Impaired generation of influenza specific humoral responses in ST6Gal I deficient mice
To examine the functional relevance of this modification for antiviral humoral responses, we obtained ST6Gal I null mice. ST6Gal I−/− mice develop normally and show a normal lymphoid compartment and architecture (5, 14). However, B cells from ST6Gal I−/− mice show impaired proliferation in vitro to anti-IgM, anti-CD40, and LPS stimulation that can be rescued by the addition of IL-4, or gene ablation of CD22 (5, 6, 15). These mice also show defective T-dependent and T-independent humoral responses to hapten-protein immunization. Previous studies examining antibody responses following viral infection have sometimes contrasted with results using hapten-protein immunizations (16, 17). We infected ST6Gal I−/− mice with influenza A/HKx31 and serially bled them to determine influenza-specific IgM and IgG by serum ELISA (Figure 2a). We observed ~10-fold reductions and ~5-fold reductions in the levels of influenza-specific IgM and IgG, respectively on day 7 p.i. (Figure 2a). Analysis of IgG isotypes on day 7 p.i. revealed that all isotypes were significantly reduced compared to wild type mice, with IgG3 and IgG2c showing the most impairment of the IgG isotypes (3- and 7-fold, respectively) (Figure 2b). By day 14 p.i., influenza-specific IgG was similar comparing wild-type and ST6Gal I−/− mice, while influenza-specific IgM still remained impaired.
Figure 2. Influenza-specific B cell responses in ST6Gal I−/− mice during acute infection.
B6 or ST6Gal I−/− mice were infected with influenza A/HKx31 i.n. and bled on indicated days. A, Serum virus-specific IgM (left), IgG (right) and B, IgG isotypes on day 7 were measured by ELISA. Data is expressed as the reciprocal endpoint antibody titer on serial titration of influenza antibody. C, Cell suspensions of CLN, MedLN and spleen were prepared from individual mice on day 7 p.i., and the ELISPOT assay was used to measure the number of cells producing virus-specific IgM, IgG1, IgG2b, IgG2c, IgG3 or IgA as indicated, with N =6–8 mice per group. Results are expressed as the number of ASC/105 nucleated cells. (*, indicate p < 0.05, ** indicate p < 0.01, *** indicate p < 0.001)
One possible explanation for the differences observed could be aberrantly glycosylated antibodies are preferentially scavenged in vivo by lectin bearing macrophages (18). We determined whether ST6Gal I−/− mice are defective in the generation of viral specific plasma cells (Figure 2c). Examination of influenza-specific ASCs by ELISPOT in the CLN, MedLN, and spleen show significantly reduced numbers at day 7. Influenza-specific IgM producing ASCs showed the most impairment, especially in the spleen. Influenza-specific IgA producing ASCs were reduced, but the reduction was not statistically significant (Figure 2c). These results show that the reduced serum levels of antiviral antibodies are likely not the effect of preferential scavenging by macrophages, but rather a defect in the generation of the humoral immune response.
Germinal centers of ST6Gal I−/− mice do not bind GL7
To determine if any differences in germinal center formation are present in ST6Gal I−/− mice, we performed immuno-staining in the lymph nodes. Our analysis of the MedLN by immuno-fluorescence on day 7 p.i. revealed that germinal center B cells in ST6Gal I−/− mice show no expression of the germinal center marker GL7, but show high binding by the PNA lectin (Figure 3a). These data are consistent with results demonstrating that the monoclonal antibody GL7 shows specificity for a sialylated glycan epitope expressed on germinal center B cells (19). FACS analysis of lymphocytes from the CLN of these mice gating on IgDloB220+ cells showed similar percentages and numbers of GC cells as measured by expression of germinal center markers Fas and PNA, and confirmed loss of GL7 binding (Figure 3b,d). We also noted that the MFI of PNA binding was significantly higher in ST6Gal I−/− GC cells compared to WT (MFI = 1178 vs 549). The frequencies of total plasma cells (CD138+B220+) were reduced in ST6Gal I−/− mice, consistent with the reduction in viral specific ASCs and as expected, showed no binding of the SNA lectin (Figure 2c and 3c).
Figure 3. Germinal center B cells of ST6Gal I−/− mice do not bind GL7.
Mice were infected with influenza x31 i.n. and on day 7 p.i. lymph nodes were isolated and either frozen for immunofluorescence, or stained for FACS analysis using germinal center or plasma cell markers. Numbers show percentages of gated population. A, Immunofluorescence staining of MedLN using B220 and GL7 or B220 and PNA as indicated. B220 in red and GL7 or PNA staining in green. B, FACS analysis of germinal center B cells in CLN of same mice, gating on IgDloB220+ cells. Histogram shows GL7 binding on gated IgDloB220+ cells. Dot plot shows PNAhiFas+ cell population of gated IgDloB220+ cells. C, FACS analysis of plasma cells in CLN gating on CD138+B220+ cells. Histogram overlay of SNA binding of gated population. Filled histogram shows ST6Gal I−/−, open histogram shows B6. Bar graph shows frequencies of total plasma cells in indicated mice. D, Total number of germinal center B cells in CLN gating on IgDloB220+ PNAhiFas+ or IgDloB220+ GL7+ cells. Bar graphs show data from n=5 mice. (*, indicates p < 0.05 and NS= not significant).
Productive infection and replication of influenza in ST6Gal I−/− mice
Entry of influenza virus, as well as the productive infection and replication in lung epithelial cells is dependent on sialic acid modification of glycoproteins. Productive influenza infection in humans depends specifically on sialyloligosaccharides containing terminal N-acetyl sialic acid linked to galactose by an α2,6-linkage, while in mice the data suggests α2,3-linked sialic acids are important (20, 21). Influenza viral titers were determined in the lungs of mice infected with A/HKx31 (Figure 4). We confirm that similar to wild-type mice, lungs from ST6Gal I−/− mice become productively infected with influenza A/HKx31 and show comparable viral titers at day 3 and 5 p.i. By day 7 p.i., we see a trend towards higher viral titers in the lungs of ST6Gal I−/− mice, which coincides with the early impairment of viral specific antibody responses observed in these mice. However, this difference is not statistically significant (p=.07) and by day 10 virus was cleared by both wild-type and ST6Gal I−/− mice. These results extend the findings in a recently published report showing several human influenza A viruses are able to productively infect the respiratory tract of mice lacking ST6Gal I expression (22).
Figure 4. Influenza viral infection and clearance in lungs.
B6 and ST6Gal I−/− mice were infected i.n. and on indicated days post infection, lungs were collected and assayed for influenza viral titer. Black/filled symbols are B6 mice and white/open symbols are ST6Gal I−/− mice. Each symbol represents one mouse tested. The limit of detection is shown by the dotted line.
Viral specific CD4 and CD8 T cells in the lungs and MedLN
Since T cells also express ST6Gal I, we enumerated influenza-specific T cells isolated from the lungs or draining nodes (MedLN) of wild-type or ST6Gal I−/− mice on day 10 using CD4 specific (NP311–325) or CD8 specific (NP366–374) peptides performing intracellular cytokine staining to detect IFNγ (23). ST6Gal I−/− mice generate similar numbers of viral specific CD4 and CD8 T cells in the lung and MedLN, show similar co-production of TNFα and IL-2, and show similar numbers of CD44high CD4 and CD8 T cells in these tissues (Figure 5 and data not shown). However, we did note reduced numbers of viral specific CD8 T cells in the spleen on day 10 consistent with our observations of reduced viral specific T cells following LCMV infection in these mice (Onami et al manuscript in prep).
Figure 5. Influenza-specific T cell responses.
At day 10 p.i., lymphocytes from MedLN and lung were stimulated with indicated peptides for 5 hrs, and stained for intracellular cytokine production of IFNγ. A, Representative staining for each tissue gating on CD4 or CD8 T cells. Numbers indicate the percentage of CD4 or CD8 T cells specific for each peptide. B, Total NP311–325 specific CD4 or NP366–374 CD8 T cells were enumerated in MedLN and lung at day 10, n = 4 mice in each group.
Viral specific memory B cell responses
ST6Gal I−/− mice control influenza infection by day 10, despite impaired influenza specific humoral responses (Figure 4). The strong antiviral T cell responses in the lungs of these mice likely contribute to this effective viral clearance. Immune ST6Gal I−/− mice show high levels of class switched IgG antibody; however, a significant reduction in serum levels of IgG2c was observed, supported by a significant reduction in frequencies of influenza-specific IgG2c producing LLPCs in the bone marrow (Figure 6a,b). Examination of MBCs in the spleen and MedLN reveal comparable frequencies of total influenza-specific IgG MBCs (Figure 6c). These results clearly indicate that with the exception of the reduced IgG2c levels, immune ST6Gal I−/− mice generated similar high titers of antiviral specific antibody.
Figure 6. Immune influenza-specific IgG responses.
A, Influenza specific IgG isotypes from day 180 sera samples of B6 or ST6Gal I−/− mice. B, ELISPOT assay was used to determine the virus-specific IgG1, IgG2b, IgG2c and IgG3 antibody secreting cells in bone marrow of mice (LLPCs). C, Memory B cell numbers from spleen and MedLN; cells are pooled from 4 mice for MedLN, n = 4–8 mice per group.
B cell expression of ST6Gal I is required to generate optimal levels of viral specific IgM
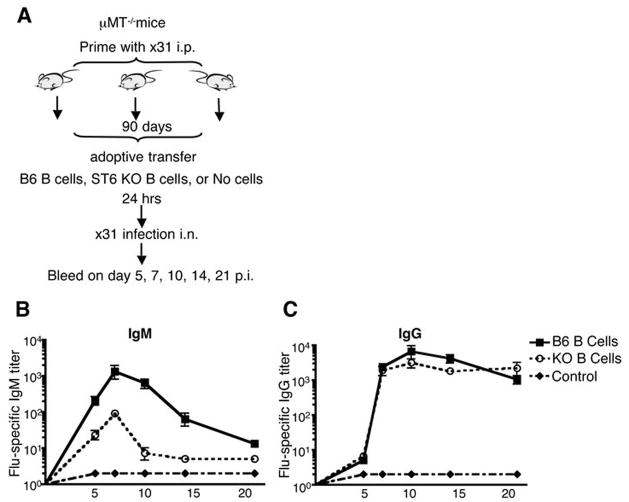
Our data indicate that ST6Gal I−/− mice are able to generate strong antiviral humoral memory despite early impairments in the generation of the viral specific humoral response (Figures 2 and 6). To determine whether it is the expression of ST6Gal I by B cells alone that results in impairment of the early antiviral IgM and IgG response, we transferred purified naïve B cells from either wild-type or ST6Gal I−/− mice into primed B cell deficient μMT−/− mice. Recipient mice were intra-nasally infected with influenza, and serially bled to determine influenza specific antibody levels. Mice that received ST6Gal I−/− B cells show impaired influenza specific IgM levels, but similar viral specific IgG (Figure 7). These data argue that the observed impairment in production of viral specific IgM in ST6Gal I−/− mice is likely B cell intrinsic, however the impairment in early production of viral specific IgG is not B cell intrinsic. Thus, expression of ST6Gal I by B cells, at least in part, is required for normal antiviral humoral immunity.
Figure 7. Loss of expression of ST6Gal I by B cells results in defective influenza specific IgM.
A, Cartoon depicting experimental protocol where μMT−/− recipient mice were primed with 107.4 EID50 i.p; 90 days later, 2×107 purified naïve B220+ B cells from B6 or ST6Gal I−/− mice were adoptively transferred i.v. into mice. Control mice received no transferred cells. Mice were infected with 106.8 EID50 influenza A/HKx31 i.n. one day later and bled on indicated days. B, Serum virus-specific antibodies for IgM or C, IgG levels were detected by ELISA, n = 3 mice in each group.
Discussion
In this report, we have evaluated the influence of post-translational modification of glycoproteins on the immune response to influenza virus infection. Changes in the post-translational modification of glycoproteins during immune responses have been reported for decades, but their functional importance remains obscure (24–32). We demonstrate that in contrast to observations with PNA lectin binding, which changes during activation/differentiation of B cells, naïve and activated B cells in vivo show similar high binding of SNA (Figure 1). This supports recently published data showing that ST6Gal I gene expression does not change following B cell activation in vitro (13). Using ST6Gal I−/− mice, which lack the ability to catalyze the addition of α2,6 sialic acids to N-linked glycoproteins, we investigated the role of this modification on the generation of an influenza-specific humoral response. Serum levels of influenza-specific antibodies in these mice are reduced early in the immune response, with influenza-specific IgM responses showing the greatest impairment (Figure 2a). These data are supported by reductions in the frequencies of viral specific plasma cells, suggesting that the impaired viral specific antibody levels are likely a result of altered generation of short-lived viral specific plasma cells, and not a result of preferential scavenging of antibodies lacking α2,6 sialic acids. (Figure 2c and 3c). Additionally, ST6Gal I−/− mice show no differences in influenza viral replication in the lungs, so the reduced antibody levels are not due to reduced viral load or antigen availability in these mice (Figure 4). We did observe comparable influenza-specific CD4 and CD8 T cell responses in the lung and draining lymph nodes of these mice, and this most likely explains why ST6Gal I−/− mice are able to control and clear influenza viral infection in the lungs by day 10, similar to wild-type mice (Figure 5). Despite significant impairments in the generation of an early antiviral humoral response, ST6Gal I−/− mice are able to generate potent antiviral humoral immunity, with high levels of class switched IgG antibodies by day 14 and memory time-points (Figure 2a, and Figure 6). This is the first reported analysis of antiviral immune responses in ST6Gal I−/− mice.
To determine whether the observed impairment of antiviral humoral responses were B cell intrinsic, we adoptively transferred ST6Gal I+/+ or −/− B cells into primed B cell deficient μMT−/− mice (Figure 7). Primed mice were used to ensure adequate CD4 T help in these mice. The antiviral IgM response to infection was substantially impaired in mice that received ST6Gal I−/− B cells. However, the antiviral IgG response was similar compared to mice that received wild-type B cells. These results suggest that the defective antiviral IgM response observed in ST6Gal I−/− mice is likely B cell intrinsic. While we must be cautious with our interpretations of antiviral responses in B cell deficient mice due to known alterations in lymphoid architecture, the results raise the possibility that subtle defects in an additional cell compartment in ST6Gal I−/− mice may contribute to the delayed antiviral IgG response. Future studies will investigate how ST6Gal I deficiency affects other lymphoid cells, and how this loss contributes to the defective anti-viral response.
Recent reports have demonstrated that loss of expression of ST6Gal I by B cells results in preferential co-localization of the BCR with CD22, a negative regulator of BCR signaling, in clathrin-rich membrane microdomains (6, 15). Aged CD22 deficient mice have been reported to develop IgM autoantibodies (33). Ablation of CD22 expression in ST6Gal I−/− mice rescues BCR signaling defects (6). In naïve B cells, modification of CD22 by ST6Gal I normally results in segregation of CD22 molecules, due to homotypic interactions with the α2, 6 sialic acid cis-ligands of other CD22 molecules (6). Following antigen receptor engagement, CD22 is tyrosine phosphorylated, and phosphorylated CD22 recruits SHP-1 (Src homology domain2-containing tyrosine phosphatase), mediating signal inhibition via the BCR (33, 34). Due to the absence of these cis-CD22 ligands in ST6Gal I−/− mice, there is increased association of sIgM with the inhibitory receptor CD22 on naïve B cells; following antigen receptor engagement in vivo, this could result in immediate attenuated BCR signaling. We speculate that a possible in vivo outcome of this could be significantly impaired differentiation of naïve B cells into extra-follicular short-lived plasma cells following viral infection of ST6Gal I−/− mice (33, 35–38). Viral-specific B cells in the ST6Gal I−/− mice, with “weak or moderate BCR” signaling could enter germinal centers and undergo affinity maturation and differentiation into plasma cells. This could explain why we observe that after day 14 p.i, influenza-specific IgG responses are quite comparable to wild-type mice. Thus in a normal immune response, ST6Gal I modification of glycoproteins on naïve B cells may influence the signaling threshold required following antigen receptor signaling—and the biological outcome in vivo would favor differentiation of a greater proportion of Ag-specific B cells into extra-follicular short-lived plasma cells, important in the early control of pathogens. Alternatively, the repertoire of B cells available in ST6Gal I−/− mice could be different from wild-type mice due to differences in signaling strength during development (39, 40).
In conclusion, our data demonstrate that despite early defects in the generation of an antiviral humoral response, ST6Gal I−/− mice are able to clear virus and generate potent humoral memory. Further, our data suggests that loss of ST6Gal I expression by B cells may be responsible for the impaired antiviral IgM response. Finally, loss of expression of ST6Gal I in non-B cells likely contributes to the impaired IgG humoral response. Future experiments will elucidate how the expression of this enzyme by different lymphocyte populations, influences the generation of an antiviral immune response.
Acknowledgments
We thank Dr. Rafi Ahmed at the Emory Vaccine Center and Dr. Linda Baum at UCLA for helpful discussions and suggestions, Dr. Kendall Smith at Cornell University’s Weill Medical College for the NP311-325 CD4 peptide, Dr. Barry Rouse for critical review of the manuscript, and members of the Onami lab for technical assistance.
Footnotes
This work was supported by AI05771901 and University of Tennessee start-up funds to Thandi M. Onami and in part by NIGMS- The Consortium for Functional Glycomics GM62116
Abbreviations used in this paper: Ag, antigen; CLN, cervical lymph node; MedLN, mediastinal lymph node; ASC, antibody secreting cells; MBC, memory B cell; LLPC, long-lived plasma cell; SNA, Sambucus nigra agglutinin
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–264. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Slifka MK, Ahmed R. Limiting dilution analysis of virus-specific memory B cells by an ELISPOT assay. J Immunol Methods. 1996;199:37–46. doi: 10.1016/s0022-1759(96)00146-9. [DOI] [PubMed] [Google Scholar]
- 3.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10:252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 4.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 5.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci U S A. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins BE, Smith BA, Bengtson P, Paulson JC. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nat Immunol. 2006;7:199–206. doi: 10.1038/ni1283. [DOI] [PubMed] [Google Scholar]
- 7.Nitschke L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Curr Opin Immunol. 2005;17:290–297. doi: 10.1016/j.coi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- 9.Sangster MY, Riberdy JM, Gonzalez M, Topham DJ, Baumgarth N, Doherty PC. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J Exp Med. 2003;198:1011–1021. doi: 10.1084/jem.20021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Vanitha DJ, Joo HM, He Y, Rouse BT, Sangster MY. A strategy for selective, CD4+ T cell-independent activation of virus-specific memory B cells for limiting dilution analysis. J Immunol Methods. 2006;313:110–118. doi: 10.1016/j.jim.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 12.Joo HM, He Y, Sangster MY. Broad dispersion and lung localization of virus-specific memory B cells induced by influenza pneumonia. Proc Natl Acad Sci U S A. 2008;105:3485–3490. doi: 10.1073/pnas.0800003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comelli EM, Sutton-Smith M, Yan Q, Amado M, Panico M, Gilmartin T, Whisenant T, Lanigan CM, Head SR, Goldberg D, Morris HR, Dell A, Paulson JC. Activation of murine CD4+ and CD8+ T lymphocytes leads to dramatic remodeling of N-linked glycans. J Immunol. 2006;177:2431–2440. doi: 10.4049/jimmunol.177.4.2431. [DOI] [PubMed] [Google Scholar]
- 14.Martin LT, Marth JD, Varki A, Varki NM. Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J Biol Chem. 2002;277:32930–32938. doi: 10.1074/jbc.M203362200. [DOI] [PubMed] [Google Scholar]
- 15.Grewal PK, Boton M, Ramirez K, Collins BE, Saito A, Green RS, Ohtsubo K, Chui D, Marth JD. ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling. Mol Cell Biol. 2006;26:4970–4981. doi: 10.1128/MCB.00308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitmire JK, Flavell RA, Grewal IS, Larsen CP, Pearson TC, Ahmed R. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T cell responses but is dispensable for CD8 T cell responses. J Immunol. 1999;163:3194–3201. [PubMed] [Google Scholar]
- 17.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 18.Onami TM, Lin MY, Page DM, Reynolds SA, Katayama CD, Marth JD, Irimura T, Varki A, Varki N, Hedrick SM. Generation of mice deficient for macrophage galactose- and N-acetylgalactosamine-specific lectin: limited role in lymphoid and erythroid homeostasis and evidence for multiple lectins. Mol Cell Biol. 2002;22:5173–5181. doi: 10.1128/MCB.22.14.5173-5181.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naito Y, Takematsu H, Koyama S, Miyake S, Yamamoto H, Fujinawa R, Sugai M, Okuno Y, Tsujimoto G, Yamaji T, Hashimoto Y, Itohara S, Kawasaki T, Suzuki A, Kozutsumi Y. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol Cell Biol. 2007;27:3008–3022. doi: 10.1128/MCB.02047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibricevic A, Pekosz A, Walter MJ, Newby C, Battaile JT, Brown EG, Holtzman MJ, Brody SL. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J Virol. 2006;80:7469–7480. doi: 10.1128/JVI.02677-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatakeyama S, Sakai-Tagawa Y, Kiso M, Goto H, Kawakami C, Mitamura K, Sugaya N, Suzuki Y, Kawaoka Y. Enhanced expression of an alpha2,6-linked sialic acid on MDCK cells improves isolation of human influenza viruses and evaluation of their sensitivity to a neuraminidase inhibitor. J Clin Microbiol. 2005;43:4139–4146. doi: 10.1128/JCM.43.8.4139-4146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaser L, Conenello G, Paulson J, Palese P. Effective replication of human influenza viruses in mice lacking a major alpha2,6 sialyltransferase. Virus Res. 2007;126:9–18. doi: 10.1016/j.virusres.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Crowe SR, Miller SC, Brown DM, Adams PS, Dutton RW, Harmsen AG, Lund FE, Randall TD, Swain SL, Woodland DL. Uneven distribution of MHC class II epitopes within the influenza virus. Vaccine. 2006;24:457–467. doi: 10.1016/j.vaccine.2005.07.096. [DOI] [PubMed] [Google Scholar]
- 24.London J, Berrih S, Bach JF. Peanut agglutinin. I. A new tool for studying T lymphocyte subpopulations. J Immunol. 1978;121:438–443. [PubMed] [Google Scholar]
- 25.London J, Horton MA. Peanut agglutinin. V. Thymocyte subpopulations in the mouse studied with peanut agglutinin and Ly-6.2 antiserum. J Immunol. 1980;124:1803–1807. [PubMed] [Google Scholar]
- 26.Galvan M, Murali-Krishna K, Ming LL, Baum L, Ahmed R. Alterations in cell surface carbohydrates on T cells from virally infected mice can distinguish effector/memory CD8+ T cells from naive cells. J Immunol. 1998;161:641–648. [PubMed] [Google Scholar]
- 27.Galvan M, Tsuboi S, Fukuda M, Baum LG. Expression of a specific glycosyltransferase enzyme regulates T cell death mediated by galectin-1. J Biol Chem. 2000;275:16730–16737. doi: 10.1074/jbc.M001117200. [DOI] [PubMed] [Google Scholar]
- 28.Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J Exp Med. 2000;191:1241–1246. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, Baum LG, Pearce BD, Ahmed R. Dynamic regulation of T cell immunity by CD43. J Immunol. 2002;168:6022–6031. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- 30.Schrader JW, Chen WF, Scollay R. The acquisition of receptors for peanut agglutinin by peanut agglutinin-negative thymocytes and peripheral T cells. J Immunol. 1982;129:545–549. [PubMed] [Google Scholar]
- 31.Baum LG, Derbin K, Perillo NL, Wu T, Pang M, Uittenbogaart C. Characterization of terminal sialic acid linkages on human thymocytes. Correlation between lectin-binding phenotype and sialyltransferase expression. J Biol Chem. 1996;271:10793–10799. doi: 10.1074/jbc.271.18.10793. [DOI] [PubMed] [Google Scholar]
- 32.Kosco MH, Szakal AK, Tew JG. In vivo obtained antigen presented by germinal center B cells to T cells in vitro. J Immunol. 1988;140:354–360. [PubMed] [Google Scholar]
- 33.Walker JA, Smith KG. CD22: an inhibitory enigma. Immunology. 2008;123:314–325. doi: 10.1111/j.1365-2567.2007.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horikawa K, Martin SW, Pogue SL, Silver K, Peng K, Takatsu K, Goodnow CC. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J Exp Med. 2007;204:759–769. doi: 10.1084/jem.20061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benson MJ, Erickson LD, Gleeson MW, Noelle RJ. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr Opin Immunol. 2007;19:275–280. doi: 10.1016/j.coi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujimoto M, Sato S. B cell signaling and autoimmune diseases: CD19/CD22 loop as a B cell signaling device to regulate the balance of autoimmunity. J Dermatol Sci. 2007;46:1–9. doi: 10.1016/j.jdermsci.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fink K, Manjarrez-Orduno N, Schildknecht A, Weber J, Senn BM, Zinkernagel RM, Hengartner H. B cell activation state-governed formation of germinal centers following viral infection. J Immunol. 2007;179:5877–5885. doi: 10.4049/jimmunol.179.9.5877. [DOI] [PubMed] [Google Scholar]
- 39.Santos L, Draves KE, Boton M, Grewal PK, Marth JD, Clark EA. Dendritic cell-dependent inhibition of B cell proliferation requires CD22. J Immunol. 2008;180:4561–4569. doi: 10.4049/jimmunol.180.7.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh S, Bandulet C, Nitschke L. Regulation of B cell development and B cell signalling by CD22 and its ligands alpha2,6-linked sialic acids. Int Immunol. 2006;18:603–611. doi: 10.1093/intimm/dxh402. [DOI] [PubMed] [Google Scholar]