Abstract
The characterization and localization of peptides and proteins in tissues provides information that aids in understanding function and in characterizing disease states. Over the past decades, the use of mass spectrometry for the profiling and imaging of biological compounds from tissues has evolved into a powerful modality to accomplish these studies. One recently described sampling approach, the stretched sample method (Anal. Chem. 2006, 78, 6826), places a tissue section onto an array of glass beads embedded on a Parafilm M membrane. When the membrane is stretched, it separates the tissue section into thousands of cell-sized pieces for tissue profiling by MALDI MS. The physical separation between beads eliminates analyte redistribution during matrix application and allows long analyte extraction periods without loss of spatial resolution. Here we enhance this sampling approach by introducing algorithms that enable the reconstruction of ion images from these stretched samples. As the first step, a sample-tailored data acquisition method is devised to obtain mass spectra exclusively from the beads, thereby reducing the time, instrument resources, and data handling required for such MS imaging experiments. Next, an image reconstruction algorithm matches data acquired from the stretched sample to the initial bead locations. The efficacy of this method is demonstrated using peptide-coated beads with known peptide distributions and appears well-suited to the MS imaging of heterogeneous tissue samples.
Keywords: Automated data acquisition, Image reconstruction, Imaging mass spectrometry, Mass spectrometry imaging, Matrix-assisted laser desorption-ionization
1 Introduction
Mass spectrometry imaging (MSI) is a technique that collects hundreds to thousands of mass spectra to not only identify biochemicals in a tissue sample, but also the spatial distribution of these compounds across the tissue. MALDI MSI has been utilized in studies of the interactions of signaling molecules in neural tissue [1–3], the effects and distribution of pharmacological agents within tissue sections [4–7], and disease and cancer-related biomarkers [8–10]. MALDI MS is tailored for the study of biological compounds ranging from small molecules and peptides to proteins directly from tissues [11]. However, the extraction of analytes with the MALDI matrix contributes to analyte redistribution and often limits the spatial resolution of resulting ion images. Several recent studies have improved the spatial resolution of MALDI MSI by reducing the effective laser beam diameter [12, 13] and enhancing focusing optics [14, 15]. Other studies have focused on reducing analyte migration by applying the MALDI matrix via a nebulized spray coating [16], automated acoustic deposition [17], electrospray deposition [18], or matrix seeding [19]. Often, a balance must be struck between sensitivity and spatial resolution—increased analyte extraction periods result in improved signals but lead to analyte redistribution and a reduction of spatial resolution.
To address this issue, our group developed the stretched sample method [20], successfully decoupling extraction/matrix incorporation from the spatial resolution of an imaging experiment. Briefly, a tissue section is adhered to an array of glass beads that have been embedded onto a Parafilm M membrane. Upon manual stretching, the tissue is rapidly separated into thousands of cell-sized fragments for study with MALDI MS. For both invertebrate and mammalian nervous system tissue sections, the connections between the tissue are overcome by adhesion of the tissue to the glass beads so that sufficiently thin (<10 μm) tissue sections are pulled apart into bead-sized fragments. The physical separation between the beads, combined with the hydrophobic nature of the Parafilm M surface, eliminates analyte spreading during matrix application, even following extended periods of matrix extraction. Longer extraction periods also reduce the presence of cationic salt adducts [21]. Although this method is well-suited to the mass spectral profiling of tissue sections, sample stretching is inherently a nonuniform process and complicates the production of ion images. Here we describe computational methods to obtain mass spectra solely from the individual bead substrates, rather than from a predefined raster pattern, and reconstruct ion images from samples following stretching.
In traditional MALDI MSI experiments, mass spectra are obtained in a regular raster pattern of evenly spaced locations across the tissue via the creation of a virtual target plate [22]. However, with the ~100-μm, yet uneven, separation between samples in the stretched sample method, a raster pattern generates extraneous data and instrument usage by also sampling the empty Parafilm M substrate. In this new approach, we take optical images of the membrane both before and after stretching and automatically determine the location of each bead. Using the data obtained, we create geometry files for input into the mass spectrometer as a sample-specific data acquisition strategy for imaging the stretched samples.
The effort to evenly stretch the membrane spreads the beads out in the x and y dimensions roughly uniformly. However, because Parafilm M stretching is not exact and distortions occur, a one-to-one mapping of the initial and final bead positions is made difficult. Yet, in order to adapt the stretched sample methodology to imaging, the final bead positions must be related to their corresponding initial positions. This is accomplished using a simple free transform mechanism [23, 24] that closely mimics the actual stretching process, wherein the height, width, rotation, and position of an image are altered. Computational methods are also needed to calculate how the free transform process affects the positions of individual bead samples and to produce selected ion images. Using these algorithms, the image reconstruction methodology relates the MS spectra to their corresponding initial bead locations. We demonstrate this technique by using a sample of peptide-coated beads of known chemical distribution to mimic cells in a tissue sample.
2 Materials and methods
2.1 Preparing peptide-coated bead substrates
Separate 115-μL solutions of 300 μM of angiotensin I or angiotensin II (both from Sigma-Aldrich, St. Louis, MO) were prepared in deionized water. The angiotensin I solution was added to approximately 100 mg of ~40-μm diameter clear glass beads (Mo-Sci Corporation, Rolla, MO) and the angiotensin II solution was added to a similar amount of ~40-μm diameter colored beads (Mo-Sci Corporation). The samples were shaken for 30 min and allowed to incubate overnight before removing the liquid by vacuum drying to ensure the adsorption of the peptides to the beads.
The beads were affixed to the Parafilm M (Pechiney, Neenah, WI) substrate similar to the means previously reported [20]. To isolate a small sample area, however, a 5-mm diameter mask was used during bead application, with this smaller area used to simplify the validation process. The mask was then removed and the beads affixed to the Parafilm M via the application of vertical pressure to glass slides placed over the substrate [20].
2.2 Locating bead positions
On an initial transmission mode optical microscopic image of the sample, the coordinates of the bead positions were identified by using a color thresholding plugin [25] (http://www.dentistry.bham.ac.uk/landinig/software/software.html) for ImageJ, version 1.38 (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/ij/). This threshold allows for elimination of non-specific highlights of the background Parafilm M substrate, while selecting only bead regions. The “analyze particles” command was then used to report the x–y coordinates of the beads, with a circularity parameter of 0.6 to further exclude non-bead artifacts.
Next, the Parafilm M substrate was stretched, the stretched sample affixed to a glass slide, and a second transmission optical image taken. Often, the size of the stretched sample exceeded the available field of the microscope; in such cases, several overlapping images were stitched together using the Photomerge function of Photoshop CS, version 8.0 (Adobe Systems Inc., San Jose, CA). Thresholding and bead identification for the final stretched sample image was performed in the same manner as for the initial image.
2.3 Creating custom geometry files
In order to selectively acquire mass spectra from individual beads in an automated manner, sample-tailored geometry files (for the mass spectrometer software controlling MS image acquisition) were created from the final bead locations, collected as described above. Optical images were calibrated by imaging a 1-mm calibration bar at the same magnification as the stretched sample image to calculate the pixel spacing of the sample image. This spacing was used to calculate the fractional distance (fraction of the distance from the origin at the center of the glass slide to the edge of the slide) [22] used by the Bruker (Bruker Daltonics, Billerica, MA) geometry file format (1 fractional distance ~ 50.8 mm).
A custom computer code was written in the Java programming language, version JDK 1.6.0 (http://java.sun.com), to perform the conversion of pixel x–y bead coordinates into the fractional distance coordinate system and to create new. xeo Bruker geometry files. This code accounts for differences in rotation angle between the stretched sample image and the orientation of the glass slide in the slide adapter, and is available at http://neuroproteomics.scs.uiuc.edu/imaging.html.
The code uses the manual linking of known points between the sample as it is observed within the instrument control software and the pixel coordinates of the optical image. These points of reference serve to connect the two coordinate systems. The use of several (2–4) calibration points greatly improves the fidelity of the created target file. This matching procedure was performed prior to MALDI matrix application and special care was taken to ensure that the slide was in a constant position during the geometry file creation process. Following matrix application, the slide must be placed in the same position within the slide adapter.
2.4 MALDI MS preparation and analysis
Subsequent to creation of the geometry file, the sample was coated with a matrix solution of 30 mg/mL of 2,5-dihydroxybenzoic acid (Sigma-Aldrich, St. Louis, MO) in 75/25 acetone:water by use of a commercial airbrush (Badger, Franklin Park, IL) at a distance of ~15 cm. Following matrix application, recondensation procedures were applied as previously described to further increase analyte incorporation into the MALDI matrix [20].
Mass spectra were collected in positive ion mode on an Ultraflex II ToF-ToF MS (Bruker Daltonics) with a solid-state UV laser and an accelerating voltage of +25 kV. Using the AutoXecute function in FlexControl 3.0 (Bruker Daltonics), the sample-tailored geometry file was read and mass spectra accumulated from each bead location from 100 laser shots at 50 Hz. It takes approximately 8 min to acquire data from 100 beads.
Collected mass spectra were converted into text files for reading by the in-house image reconstruction code using the batch conversion command in CompassXport (Bruker Daltonics) to produce mzXML files that were further converted into text files using MATLAB, version 7.2 (R2006a) (The MathWorks, Natick, MA) with the mzxmlread. m function from the Bioinformatics Toolbox 3.0, along with an in-house-written batch conversion wrapper code. The MATLAB wrapper code is freely available at http://neuroproteomics.scs.uiuc.edu/imaging.html.
2.5 Image reconstruction
To perform image reconstruction, the Free Transform command in Photoshop CS is used to adjust the translation, height, width, and rotation of the image of the initial bead locations. Both the initial and stretched sample images are loaded as separate layers into a blank Photoshop CS file. By aligning the initial position image on top of the stretched sample image, the initial position image layer is manually transformed to match the dimensions of the stretched sample image layer. The Info palette reports the changes in the free transform parameters of the initial image, which are used as inputs to calculate the effect of the free transform on the position of each bead. By overlaying the two images in the same plane, the bead positions between the stretched sample image and transformed initial image can be matched based on closest spatial proximity with the Cartesian distance formula using the custom written code. To reduce matching errors during image reconstruction, if more than one stretched position is matched to a single initial position, the algorithm reassigns one of the positions to the next-nearest initial position in an iterative fashion.
The image reconstruction is validated by determining the number of bead position matches that are correct between the initial and final configurations. First, an array of true matches is manually created for each bead with the aid of a custom macro in ImageJ. Then the classification rate is generated by comparing this array of “true” matches to the array of matches created by the image reconstruction algorithm.
3 Results and discussion
3.1 Sample-tailored data acquisition
After preparing the bead substrate, the subsequent workflow for the stretched sample imaging method is outlined in Figure 1. Optical images of the sample before and after stretching are taken in a transmission mode, wherein the beads appear brighter than the Parafilm M substrate. The color thresholding plugin is used with ImageJ, which allows for greater control of thresholding parameters than the original ImageJ thresholding algorithm, to exclusively find the bead positions (see the initial sample image in Figure 2).
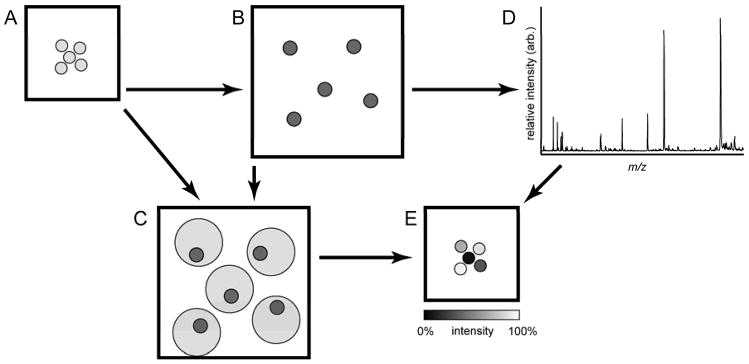
Figure 1.
Schematic of the image reconstruction process. (A) Substrates are prepared and an optical image is taken prior to (B) stretching, after which another image is collected. Bead positions are identified from both images and a sample-specific geometry file is created from the stretched sample image. (C) Bead positions from each image are linked though a free transformation and computational algorithm. (D) Mass spectra are collected from each bead position from the stretched sample and combined with the linked bead position information to (E) create ion images by plotting selected signals from the mass spectra in false color at the initial bead locations.
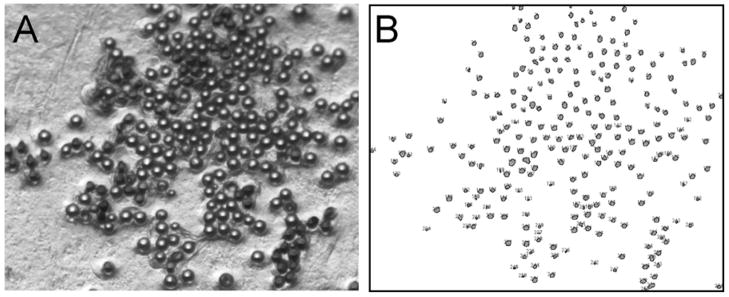
Figure 2.
Automated bead identification. (A) A microphotograph of the prepared substrate is used to (B) automatically determine the bead locations. The automated process outputs a list of positions as well as an image that numbers and marks each bead position.
Following thresholding and bead location identification, the coordinates from the sample images are used to create sample-tailored geometry files. By manually directing the sample stage to a position on the slide that is located within the area of the sample, that position and its fractional distance coordinates can be recorded and matched with the pixel coordinates of the same position in the stretched sample image. To create a sample-tailored geometry file, the translation between these common points and the scaling between the two coordinate systems are used to calculate the coordinates of the individual beads. After the geometry file is created, selected sample positions are checked in FlexControl to ensure that the probe has located the position of each bead at a point that is within its 40-μm diameter region. In practice, the geometry files show a high level of accuracy in locating the beads. Automatically acquired mass spectra produced appreciable peptide signals from each bead without any apparent collection of mass spectra from the Parafilm M substrate.
Acquiring data in a sample-tailored fashion from the stretched samples saves time and instrument resources, and eases data handling. In the traditional method of obtaining imaging data via a regular raster, the total analysis time depends on the number of locations interrogated, the laser repetition rate, and the number of laser shots acquired at each spot. For example, a 1-mm2 area of intact tissue requires ~600 beads and after stretching ~4-fold in each direction, results in a 16-mm2 area. To collect mass spectra from the stretched sample, and assuming a 4-sec collection period (100 shots @ 50 Hz and 2 sec for stage motion, etc.) at each spot, a 50-μm raster to ensure that each bead location is interrogated would require 6400 spots, or >7 hours to collect. In contrast, using the sample-tailored acquisition strategy to interrogate the same stretched sample, requires only 600 spots and is completed in ~1 hour. The time savings does not scale linearly with the reduction in the number of spots due, in large part, to the increased distance the sample stage must move between consecutive collections. Regardless, not only are 580,000 laser shots saved over the course of the data acquisition process, but data handling is greatly simplified by eliminating the collection of extraneous information produced by collecting mass spectra from the Parafilm M substrate alone or from only a portion of a bead that might fall on the edge of the laser beam profile when sampled in a raster pattern.
3.2 Image reconstruction
As described in the materials and methods section, during the reconstruction process an image of the initial bead positions is transformed and overlaid on top of the stretched sample image. Each bead makes a circular indentation in the membrane that is enlarged during the stretching process; thus, compared to the initial microphotograph, the free transformation procedure increases the apparent size of the beads in the initial sample image, complicating the overlay process (see part C of Figure 1). Following the bead position identification process, an image containing the small marks at the initial bead positions is created by our in-house written Java code. The bead indentations in the stretched sample image serve as guides and margins of error during the alignment process; the small bead markers align randomly within the indentation regions as shown in Figure 3. The position, height, width, and rotation angle of the initial positions image is recorded before and after alignment.
Figure 3.
Overlay of free-transformed initial positions (white squares) with an optical image of the stretched sample. The indentations due to the partial embedding of the beads into the Parafilm M surface are visible and greatly aid in the alignment process. As individual beads may end up anywhere within the indented regions, the free transform aims to place positions in the middle of the deformed regions.
The Java code performs image reconstruction using the free transform parameters as inputs to link the bead locations, and handles the mass spectra for the creation of ion images. During image reconstruction, the positions are translated between the difference in the starting and final position of the initial positions image. Next, the predicted positions in the free transformed image are calculated using the trigonometric rotation functions. The individual positions are rotated by angle of rotation of the transformed image using Eq. 1.
| (1) |
As the changes in height and width must be applied along the axis of rotation, the coordinate system is rotated to match this rotation using Eq. 2.
| (2) |
The rotation of the coordinate system allows the dimensions to be changed with a simple multiplication operation. Hence, the x-coordinate of each position is multiplied by the fractional change in width of the image. Likewise, the y-coordinate is multiplied by the fractional change in height. The final bead coordinates must then be rotated to match the axes of the coordinate system defined on the MALDI target by rotating the rotated coordinate system to match the x and y axes of the target plate at zero degrees using Eq. 2. This gives the calculated free transformed coordinates which, if plotted for verification purposes, should overlap with the transformed initial positions image. Each stretched sample position and its associated mass spectrum are matched to the transformed position that is closest in distance. Matching error occurs when two stretched sample positions are matched to the same transformed initial position. To reduce error, of the two stretched positions, the one that is nearer to the closest previously unassigned transformed initial position is reassigned to that unassigned position. Finally, the transformed initial positions are associated with mass spectra taken from the nearest stretched positions and are replaced with the corresponding initial positions.
To create ion images, the intensity of a selected signal, defined as the local maximum within a selected m/z range of the selected value, is plotted for each individual bead location in a false color scale corresponding to the signal intensity. In addition, when prompted, the classification rate of the automated reconstruction is calculated when a manual validation array of image reconstruction matches is present. This image reconstruction code is freely available at http://neuroproteomics.scs.uiuc.edu/imaging.html.
Although robust in terms of locating bead locations for a range of stretched samples, it is useful to perform the image reconstruction with the optical sample images prior to data acquisition in order to ensure that successful image reconstruction is possible. Generally, the free transform process is successful and rapid. Complications signify a problem in the stretching or substrate preparation such as large deviations from linear stretching, beads missing in a region, or a torn membrane. These samples are discarded prior to matrix application and mass spectra acquisition. The alignment process may be aided by the addition of a small percentage of colored beads to the substrate such that the resulting visual patterns can help to identify the rotational orientation of the sample before and after stretching.
The reproducibility and efficacy of the reconstruction methodology was investigated using the validation strategy detailed below. Six samples, containing between 129–139 beads each, were correctly classified at a 84.1 ± 6.4% rate. Most of the misclassifications resulted from nearest-neighbor or near-neighbor misassignments. The presence of multiple beads within a region of Parafilm M that was originally presumed to be an individual bead and localized “clumping” of 2–3 beads at the junction between several circular indentations results in the majority of the misclassification events. Because these are not common and only cause nearest-neighbor misassignments, these do not adversely affect the analyses as a whole.
3.3 Imaging a stretched sample of peptide standards
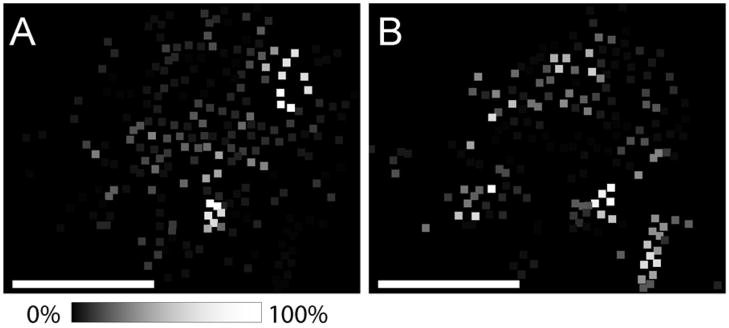
Reconstructed ion images of the angiotensin-coated bead sample containing 230 total beads are shown in Figure 4. Clear glass beads were coated with angiotensin I (Figure 4A), and the colored beads with angiotensin II (Figure 4B). The ion images closely correspond to the observed initial arrangement of the beads, as shown in Figure 2A. As opposed to biological samples that have variable and undefined distributions of analytes, peptide-coated beads allow validation of the image reconstruction process through the visual alignment of peptide ion distributions with the patterns of clear and colored beads. Owing to the desire to restrict the sample size for this trial illustration, the sample substrate is not entirely comprised of closest-packed beads, although such packing is otherwise readily achievable for larger substrates as would be used for tissue experiments. The large physical separation between beads in the stretched sample allows for single bead resolution and the production of ion images with a high degree of uniformity. In relation to tissue section analyses, this bead separation allows for the detection of rare signals that may arise from a single or small number of cells to be more accurately located when localized to a single or small number of individual beads. The spatial resolution in these images approximates 40 μm, the diameter of the individual beads. Although this value is appropriate for most tissue imaging applications, higher spatial resolution can be obtained by using smaller diameter beads.
Figure 4.
Reconstructed ion images from labeled bead substrates. Ion images for (A) angiotensin I and (B) angiotensin II correspond well with the expected results as clear beads were labeled with angiotensin I while angiotensin II was adsorbed to colored beads. Scale bars = 500 μm.
4 Concluding remarks
Through the development of sample-tailored data acquisition and image reconstruction algorithms, the stretched sample method has been adapted from profiling to allow imaging as well. Using sample-tailored data acquisition significantly reduces analysis time by reducing the number of mass spectra that must be obtained, thus greatly easing data handling. The advantages of the stretched imaging method are particularly well-suited to the study of small and heterogeneous structures present throughout the nervous system. In addition, the parallel detection of isolated bead-sized samples may enable the detection of rare-cellular events or processes, where the origin of rare signals is isolated to the location of individual beads. Future work will both validate these approaches using thin tissue sections and compare the stretched sample approach to more traditional direct tissue mass spectrometric imaging.
Acknowledgments
This material is based upon work supported by the National Institutes of Health under Award No. DE018866, and the National Institute on Drug Abuse under Award No. DA017940 and No. DA018310 to the UIUC Neuroproteomics Center on Cell-Cell Signaling. Also acknowledged is the fellowship support to E. M. from the ACS Division of Analytical Chemistry sponsored by Proctor & Gamble. The authors would like to acknowledge Dr. Michael L. Heien and Kevin R. Tucker for valuable discussion and assistance.
References
- 1.Rubakhin SS, Hatcher NG, Monroe EB, Heien ML, Sweedler JV. Mass spectrometric imaging of the nervous system. Curr Pharma Design. 2007;13:3325–3334. doi: 10.2174/138161207782360708. [DOI] [PubMed] [Google Scholar]
- 2.DeKeyser SS, Kutz-Naber KK, Schmidt JJ, Barrett-Wilt GA, Li L. Imaging mass spectrometry of neuropeptides in decapod crustacean neuronal tissues. J Proteome Res. 2007;6:1782–1791. doi: 10.1021/pr060603v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoeckli M, Staab D, Staufenbiel M, Wiederhold KH, Signor L. Molecular imaging of amyloid beta peptides in mouse brain sections using mass spectrometry. Anal Biochem. 2002;311:33–39. doi: 10.1016/s0003-2697(02)00386-x. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh Y, Casale R, Fukuda E, Chen J, et al. Matrix-assisted laser desorption/ionization imaging mass spectrometry for direct measurement of clozapine in rate brain tissue. Rapid Commun Mass Spectrom. 2006;20:965–972. doi: 10.1002/rcm.2397. [DOI] [PubMed] [Google Scholar]
- 5.Meistermann H, Norris JL, Aerni HR, Cornett DS, et al. Biomarker discovery by imaging mass spectrometry: transthyretin is a biomarker for gentamicin-induced nephrotoxicity in rat. Mol Cell. 2006;5:1876–1886. doi: 10.1074/mcp.M500399-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Khatib-Shahidi S, Andersson M, Herman JL, Gillespie TA, Caprioli RM. Direct molecular analysis of whole-body animal tissue sections by imaging MALDI mass spectrometry. Anal Chem. 2006;78:6448–6456. doi: 10.1021/ac060788p. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh Y, Chen J, Korfmacher WA. Mapping pharmaceuticals in tissues using MALDI imaging mass spectrometry. Pharmacol Toxicol Methods. 2007;55:193–200. doi: 10.1016/j.vascn.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Lemaire R, Menguellet SA, Stauber J, Marchaudon V, et al. Specific MALDI imaging and profiling for biomarker hunting and validation: fragment of the 11S proteasome activator complex, reg alpha fragment, is a new potential ovary cancer biomarker. J Proteome Res. 2007;6:4127–4134. doi: 10.1021/pr0702722. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MD, Floyd JL, Caprioli RM. Proteomics in diagnostic neuropathology. J Neuropathol Exp Neurol. 2006;65:837–845. doi: 10.1097/01.jnen.0000235116.67558.24. [DOI] [PubMed] [Google Scholar]
- 10.Stauber J, Lemaire R, Franck J, Bonnel D, et al. MALDI imaging of formalin-fixed paraffin-embedded tissues: application to model animals of parkinson disease for biomarker hunting. J Proteome Res. 2008;7:969–978. doi: 10.1021/pr070464x. [DOI] [PubMed] [Google Scholar]
- 11.Rubakhin SS, Jurchen JC, Monroe EB, Sweedler JV. Imaging mass spectrometry: fundamentals and applications to drug discovery. Drug Discov Today. 2005;10:823–837. doi: 10.1016/S1359-6446(05)03458-6. [DOI] [PubMed] [Google Scholar]
- 12.Jurchen JC, Rubakhin SS, Sweedler JV. MALDI-MS imaging of features smaller than the size of the laser beam. J Am Soc Mass Spectrom. 2005;16:1654–1659. doi: 10.1016/j.jasms.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Spengler B, Hubert M. Scanning microprobe matrix-assisted laser desorption ionization (SMALDI) mass spectrometry: instrumentation for sub-micrometer resolved LDI and MALDI surface analysis. J Am Soc Mass Spectrom. 2002;13:735–748. doi: 10.1016/S1044-0305(02)00376-8. [DOI] [PubMed] [Google Scholar]
- 14.Luxembourg SL, Mize TH, McDonnell LA, Heeren RM. High-spatial resolution mass spectrometric imaging of peptide and protein distributions on a surface. Anal Chem. 2004;76:5339–5344. doi: 10.1021/ac049692q. [DOI] [PubMed] [Google Scholar]
- 15.Luxembourg SL, Vaezaddeh AR, Amstalden ER, Zimmermann-Ivol CG, et al. The molecular scanner in microscope mode. Rapid Commun Mass Spectrom. 2006;20:3435–3442. doi: 10.1002/rcm.2747. [DOI] [PubMed] [Google Scholar]
- 16.Chaurand P, Norris JL, Cornett DS, Mobley JA, Caprioli RM. New developments in profiling and imaging of proteins from tissue sections by MALDI mass spectrometry. J Proteome Res. 2006;5:2889–2900. doi: 10.1021/pr060346u. [DOI] [PubMed] [Google Scholar]
- 17.Aerni HR, Cornett DS, Caprioli RM. Automated acoustic matrix deposition for MALDI sample preparation. Anal Chem. 2006;78:827–834. doi: 10.1021/ac051534r. [DOI] [PubMed] [Google Scholar]
- 18.Kruse R, Sweedler JV. Spatial profiling invertebrate ganglia using MALDI MS. Am Soc Mass Spectrom. 2003;14:752–759. doi: 10.1016/S1044-0305(03)00288-5. [DOI] [PubMed] [Google Scholar]
- 19.Chaurand P, Schwartz SA, Reyzer ML, Caprioli RM. Imaging mass spectrometry: principles and potentials. Toxicol Pathol. 2005;33:92–101. doi: 10.1080/01926230590881862. [DOI] [PubMed] [Google Scholar]
- 20.Monroe EB, Jurchen JC, Koszczuk BA, Losh JL, et al. Massively parallel sample preparation for the MALDI MS analyses of tissues. Anal Chem. 2006;78:6826–6832. doi: 10.1021/ac060652r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monroe EB, Koszczuk BA, Losh JL, Jurchen JC, Sweedler JV. Measuring salty samples without adducts with MALDI MS. Int J Mass Spectrom. 2007;260:237–242. [Google Scholar]
- 22.Clerens S, Ceuppens R, Arckens L. Createtarget and analyze this!: new software assisting imaging mass spectrometry on Bruker Reflex IV and Ultraflex II instruments. Rapid Commun Mass Spectrom. 2006;20:3061–3066. doi: 10.1002/rcm.2698. [DOI] [PubMed] [Google Scholar]
- 23.Decker JD. Image editing. Am J Orthod Dentofacial Orthop. 2004;125:215–219. doi: 10.1016/j.ajodo.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Hackel RE, Saine PJ. Creating retinal fundus maps. J Opthalmic Photog. 2005;27:10–18. [Google Scholar]
- 25.Papadopulous F, Spinelli M, Valente S, Foroni L, et al. Common tasks in microscopic and ultrastructural image analysis in ImageJ. Ultrastruct Pathol. 2007;31:401–407. doi: 10.1080/01913120701719189. [DOI] [PubMed] [Google Scholar]