Summary
We produced six versions of recombinant human hoxb4 proteins and evaluated their effectiveness in expanding human hematopoietic stem and progenitor cells in vitro and in vivo. An N-terminal-tat and C-terminal histidine-tagged version of hoxb4 (T-hoxb4-H) had the highest activity in expanding colony forming cells (CFCs) and long-term culture-initiating cells (LTC-ICs) when used at 50nM concentration in cell culture. Human cord blood CD34+ cells cultured with 50nM T-hoxb4-H had a significant increase in severe-combined immunodeficient mouse-repopulating cells (SRCs). In a mouse model of immune-mediated BM failure, T-hoxb4-H showed an additive effect with cyclosporine in alleviating pancytopenia. In addition, T-hoxb4-H expanded CFC and LTC-IC on BM samples from patients with refractory severe aplastic anemia and myelodysplastic syndromes: after culturing with 50nM T-hoxb4-H for four days, BM cells from 10 of the 11 patients showed increases in CFC and LTC-IC, and the increase in LTC-IC was statistically significant in samples from 4 patients. Recombinant human hoxb4 could be a promising therapeutic agent for BM failure.
Keywords: Recombinant homobox b4, hematopoietic stem and progenitor cells, CD34, BM failure, mouse models
Introduction
Deficiency in long-term hematopoietic stem cells (HSCs) and short-term progenitor cells is characteristic of human bone marrow (BM) failure syndromes such as aplastic anemia (AA) and hypocellular myelodysplastic syndromes (MDS), and leads to the clinical manifestation of severe BM aplasia and fatal pancytopenia (Young et al, 2006; Nakao, 2008). CD34+ cell number, and hematopoietic progenitor cell colony formation as reflected in quantitation of hematopoietic progenitor cells using the long-term culture initiating cell (LTC-IC) assay, are markedly decreased in AA and MDS (Maciejewski et al, 1996; Maciejewski et al, 1994; Sato et al, 1998). Immunosuppressive therapy (IST) is effective in improving blood counts in 60−70% AA patients. However, low blood counts often persist and relapse is frequent, requiring repeated treatment (Scheinberg et al, 2006). While growth factors have been used in addition to IST to support these patients, responses are usually limited to a single cell lineage, and in many cases, patients are not responsive to the treatment (Young & Maciejewski, 1997). Stem cell replacement therapy provides a definitive cure, but difficulties in obtaining well-matched donors and transplantation-associated complications limit this option to only a minority of AA patients (Young et al, 2006).
An agent that could expand patient residual HSCs would be useful in the treatment of AA and other BM failure syndromes. Many attempts have been made to expand HSCs in vitro using different combinations of cytokines, with disappointing outcomes despite maintenance of long-term repopulating stem cells can be achieved in the best circumstances (Tisdale et al, 1998; Conneally et al, 1997; Ueda et al, 2000; Gammaitoni et al, 2003). Thus, attention has shifted to transcription factors that govern stem and progenitor cell fate decisions. One well studied factor is the homo-box gene B4 (HOXB4), a member of the homo-box family of transcription factors. Retroviral expression of HOXB4 in mice significantly improved HSC regeneration in vivo, with three log increases of HSCs in both primary and secondary recipients (Antonchuk et al, 2001; Sauvageau et al, 1995; Thorsteinsdottir et al, 1999), without affecting normal differentiation or inducing cell transformation (Sauvageau et al, 1995). Similarly, retroviral expressed hoxb4 expanded mouse HSCs by more than 1000 fold in vitro with the expanded HSCs remained multipotent (B, T, and myeloid) and competitive in repopulating primary and secondary recipients (Antonchuk et al, 2002). In one report, retroviral-expressed human hoxb4 expanded human HSCs in vitro, but the effect was much less than that observed in the mouse (Buske et al, 2002). Recombinant hoxb4 has been produced in order to avoid the potential toxicities of retroviral vectors. A recombinant hoxb4, tat-hoxb4, expanded mouse HSCs (Krosl et al, 2003). However, the effect of recombinant human hoxb4 on HSCs from normal human donors and BM failure patients is unknown.
In this study, we produced six versions of recombinant human hoxb4 with the purification tag, six histidines, and the cell permealizaition tag, tat, at different locations relative to hoxb4, and tested their effects on human HSCs using colony-forming-cell (CFC) and LTC-IC assays. We selected one version of hoxb4 with the tat tag at the N-terminal and the histidine tag at the C-terminal (T-hoxb4-H) that had the highest HSC-expansion activity, determined its optimum working concentration, and tested its effectiveness in expanding severe-combined immunodeficient (SCID) mouse-repopulating cells (SRC) in human cord blood CD34+ cells. T-hoxb4-H was also tested in vivo in a mouse model of BM failure in conjunction with the immunosuppressive agent, cyclosporine. Further, the effectiveness of T-hoxb4-H was examined in ex vivo expansion of CFC and LTC-IC from normal volunteers, as well as from AA and MDS patients. Our results show that recombinant hoxb4 can expand HSCs and could be a potential therapeutic agent for BM failure syndromes.
Materials and methods
Cloning, expression, and purification of recombinant human hoxb4
A commercial pET-21(+) vector (Novagen, WI, USA) was used to clone three expression vectors, Pet I, II and III, that expressed target proteins with tat and histidine tags at the C terminal (I), tat at the N-terminal and histidines at the C terminal (II), and both histidine and tat tags located at the N-terminal (III). The pET-21(+) vector DNA was digested by either XhoI or BamH1, and was dephosphorylated with calf intestinal phosphatase. Three primer sets (listed in the references) were melted at 95°C and annealed at gradually decreasing temperature to room temperature. The annealed primers were phosphorylated and ligated to either the XhoI or BamH1 site of linerized pET-21(+) vector in order to generate the pET-I, II and III vectors.
All versions of recombinant hoxb4 constructs were generated by DNA polymerase chain reaction (PCR) with primers (shown in the references) using a full-length HOXB4 cDNA clone (MHS1010−9205037, accession number BC049204), purchased from Open Biosystems, Florida, USA. Because of the high GC content in HOXB4 cDNA, Clontech advantage GC2 PCR kit (Clontech, CA, USA) was used for the PCR. Next, PCR fragments were purified and cloned into PCR2.1 vector (Invitrogen, MD, USA). The desired fragments were subcloned further into pET-I, II and III vectors. All final clones were verified by sequencing and transformed into BL21 (DE3) cells (Novagen, WI, USA).
Transformed bacteria were grown in LB culture media (with ampicillin) to optical density of 0.8 followed by induction with 1mM IPTG (Isopropyl β-D-1-thiogalactopyranoside) for 2 to 3 hours. Bacteria were harvested, centrifuged (5000 g, 10 minutes at 4°C) and sonicated in binding buffer (6 M GnHCl, 100 mM NaH2PO4, 10 mM Tris, PH 8.0). Cell lysates were centrifuged (20,000 g, 30 min at 4°C), resuspended in the binding buffer with 10mM imidazole, loaded on HisTrap (ni-affinity) chelating columns, and eluted with a 10 to 500mM imidazole gradient in binding buffer using AKTA FPLC (Amersham Biosciences). Hoxb4-containing fractions were desalted on a PD-10 column and eluted with ion-exchange buffer (10 mM Tris, pH 8.0, 10 mM NaCl). Next, the samples were loaded on HiTrap SP HP (ion-exchange) column and eluted with a 10 to 500mM NaCl gradient in ion-exchange buffer on AKTA FPLC. High protein-containing fractions were again desalted and eluted in PBS (Quality Biologicals, Gaithersberg, MD, USA). Endotoxin was removed by mixing samples with 1% Triton X-114 at 4°C for 10 minutes, transferred to a 37°C water bath for 10 minutes followed by centrifugation at 15,000 g for 10 minutes at room temperature, and the upper liquid phase was collected and subject to the above process two more times. Preparations were tested endotoxin-free by the Limuslus Amebocyte Lysate QCL-1000 (Cabrex Bioscience, Walkersville, MD, USA) endotoxin detection kit. The concentration of each protein was determined by first measuring total protein concentration using a BCA protein assay kit (Pierce, Rockford, IL USA), then multiplied by the proportion of the protein in the major band from all bands in the silver-stained polyacrylamide gel through image quantification using the ImageQuant TL software (Amersham Biosciences). The final products were supplemented with 5% glycerol, aliquoted and frozen at −80°C. All chromatography and desalting columns were purchased from Amersham Biosciences. All chemicals were purchased from Sigma-Aldrich, MO, USA.
Sample collection
Human cord blood cells were kindly provided by the New York Blood Center. CD34+ cells were isolated using the indirect CD34 MicroBead Kit (Miltenyi Biotec). Heparinized human BM samples were obtained from the posterior iliac crest of patients and normal donors in 5 separate 0.5-ml aspirations. Cells were separated according to density differences by centrifugation on a Ficoll-hypaque gradient by the method of Boyum (Boyum, 1968). Cells present at the plasma/Ficoll-hypaque interface were collected and the red blood cells lysed with ACK lysis buffer (Quality Biologicals, Gaithersburg, MD). The cells were then washed in Hanks solution, frozen down in DMEM with 5% DMSO and stored in −70°C using cryo-preservation boxes overnight before transferring to the liquid nitrogen tank the next day.
BM cells from nine patients with refractory AA and two patients with MDS were used in the experiments; one patient had cytogenetics inv 9 and t (7, 18), the other had marked morphologic dysplasia in both erythroid and myeloid cells with normal cytogenetics. Informed consent was acquired from each patient according to a protocol approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute to collect patients’ samples for research studies. Samples were obtained in accordance with the Declaration of Helsinki for all patients.
Cell culture, CFC and LTC-IC assays
One to five × 105 BM cells were first cultured for 2 days in StemSpan medium (Stem Cell Technologies, Vancouver, Canada) with 20ng/ml recombinant human IL3, 20ng/ml IL6, 100ng/ml Flt-3, 100ng/ml SCF, 50ng/ml TPO, 100u/ml penicillin and 100μg/ml gentamycin in 12 well plates. Then recombinant hoxb4 protein and the control (BSA) were added every 6 hours at desired concentrations. Cells were resuspended in fresh medium containing fresh cytokines and antibiotics every 24 hours, and were harvested after culturing for four more days.
Human clonogenic progenitors were assayed by plating cells in 1 ml MethoCult with cytokines (Stem Cell Technologies) in 35mm culture dishes for 15 days at 37°C with 5% CO2 and 95% humidity. CFCs were then scored by observation under light microscope. LTC-IC were measured by culturing human hematopoietic cells (1,000 cells/cm2) on 80-Gy pre-irradiated M2−10B4 murine fibroblasts (ATCC, Manassas, VA, USA) as feeder-layer cells (3 × 104 cells/cm2) in bulk (100mm dish) or under limiting-dilution conditions (96 well plate) in MyeloCult 5100 containing 10−4 M hydrocortisone (Stem Cell Technologies). Cell cultures were maintained for five weeks, with half of the medium replaced every week. Cells were harvested and assayed for CFC as LTC-IC output. Total LTC-IC number was obtained by multiplying the frequency of LTC-ICs, as determined in the secondary CFC assay, by the total number of cells present after the 5-week primary long-term co-culture.
SRC assay
Human cord blood CD34+ cells were cultured at 105 cells per well for four days with or without 50nM of the recombinant T-hoxb4-H protein. Cells were harvested and injected into sub-lethally irradiated (2.5Gy) NOD-SCID mice (the Jackson Laboratory, Bar Harbor, Maine) with each recipient received cells equivalent to 104 or 5×103 starting CD34+ cells together with 105 CD34-accessory cells. Recipients were euthanized eight weeks after the transplantation and BM nucleated cells were analyzed for the presence of human CD45+ cells by flow cytometry. Mice were considered positive for human HSC engraftment when at least 0.1% CD45+ human cells were detected in the mouse BM cells. SRC frequency was calculated based on the Poission Distribution using equation Pi=e(-N)×(Ni/i!) at P0 (Chen et al, 1999). BM cells from positive mice were further analyzed by flow cytometry using antibodies for human CD14, CD15, CD19 and CD34 antigens (BD Biosciences, CA, USA).
Recombinant hoxb4 treatment in a mouse model of immune-mediated BM failure
Twelve C.B10-H2b/LilMcdJ (C.B10) mice (The Jackson Laboratory, Bar Harbor, Maine, USA) each received 5Gy sublethal irradiation followed by the infusion of 5 × 106 B6 lymph node (LN) cells. The mice were then divided into four groups of three animals each: 1) no treatment control; 2) cyclosporine 50 μg/g/day i.p. once daily from days 0 − 6; 3) cyclosporine 50 μg/g/day i.p. once daily from days 0 − 6 plus T-hoxb4-H 2 μg/g/day i.p. once daily from days 7 − 13; 4) T-hoxb4-H 2 μg/g/day i.p once daily from days 7−13. Animals were bled on weeks two, three, five, six, and fourteen for complete blood counts. Animal survival was assessed at weeks five, six, and fourteen. All animal study protocols were approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute.
Statistical analysis
Data were analyzed by the JMP statistical software on the fit-model platform (SAS Institute Inc., NC, USA). Results are presented as means with standard errors. Statistic significances were declared at P<0.05 and P<0.01 respectively.
Results
Expression and purification of recombinant human hoxb4
We expressed six versions of recombinant human hoxb4 with the tat and histidine tags at different locations (Figure 1A). Version V is the same as described by Krosl et al (Krosl et al, 2003). Proteins were purified by nickel-affinity chromatography followed by ion-exchange chromatography. Version III could not be purified by nickel-affinity chromatography, possibly because the histidine residues were not accessible to the nickel-affinity beads. After purification, endotoxin was removed using Triton X-114 extraction. Purified hoxb4 proteins were visualized by silver staining after PAGE (Figure 1B).
Figure 1.
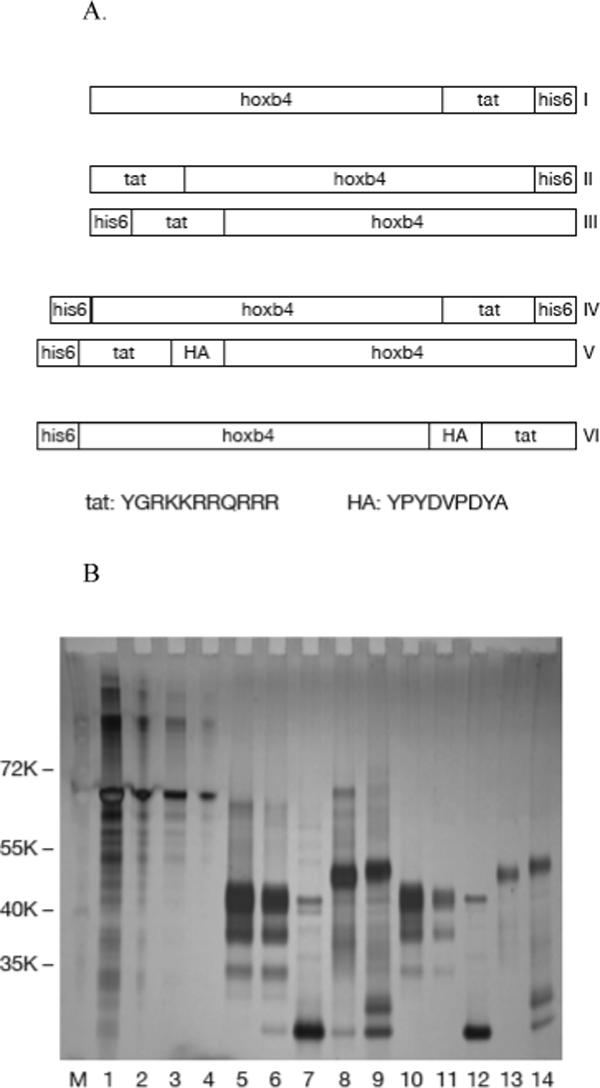
Expression of recombinant human hoxb4. Diagram of six versions of recombinant hoxb4 with different locations of the histidine and tat tags (A). E. coli expressed, ni-affinity chromatography and HiTrap SP HP ion-exchange chromatography purified hoxb4 proteins were analyzed by SDS-PAGE and stained with Silver Quest staining kit. Lanes 1, 2, 3 and 4: BSA 1 μg, 0.2 μg, 0.1 μg and 0.02 μg; lanes 5, 6, 7, 8 and 9: version I, II, IV, V and VI hoxb4 each 0.6 μg (3ul) before endotoxin removal; lanes 10, 11, 12, 13 and 14: version I, II, IV, V and VI hoxb4 each 3ul after endotoxin removal (B).
Expansion of CFC and LTC-IC by recombinant human hoxb4
We first tested the effects of the five versions of purified hoxb4 protein on fresh human BM cells. Normal BM mononuclear cells were cultured in StemSpan medium supplemented with recombinant human IL3, IL6, Flt-3, SCF and TPO for 2 days at 5 × 105 cells/well. Different versions of hoxb4 or control (BSA) were added at 10nM or 50nM respectively every 6 hours for four days, similar to the published condition (Krosl et al, 2003). Cells were then collected and assayed for CFC and LTC-IC. Version II hoxb4 (T-boxb4-H) at 10nM and versions I, II, IV and V at 50nM all induced significant CFC expansion (P<0.05, Figure 2A), while versions I, II, V and VI at 50nM showed significant expansion of LTC-IC (P<0.05, Figure 2B) compared with the control (BSA). T-hoxb4-H demonstrated the largest expansion in CFC (9.6 ± 0.7 fold, Figure 2A) and LTCIC (14.2 ± 1.0 fold, Figure 2B). We further determined an optimum concentration for T-hoxb4-H by culturing 5 × 105/well frozen normal BM cells with 0, 10, 50, 100, 150, 200 and 300nM T-hoxb4-H, with the protein added every 6 hours. After four days in culture, cells were harvested and assayed for CFC. The expansion of CFC reached a peak at 50nM (Figure 2C). Therefore, 50nM of T-hoxb4-H was chosen for further experiments.
Figure 2.
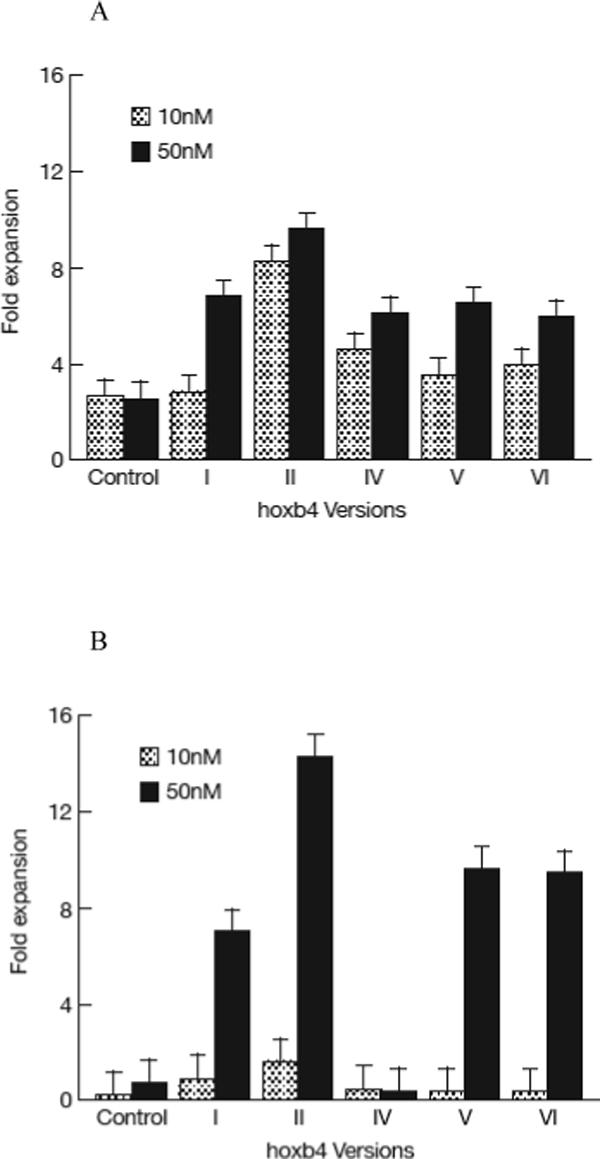
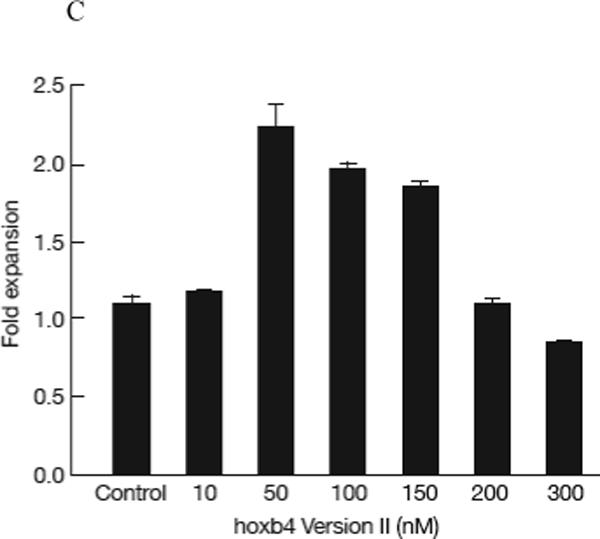
Expansion of HSCs by recombinant human hoxb4. 1−5 × 105 normal human BM cells were cultured in 1ml cytokine-supplemented StemSpan medium for 2 days, then recombinant hoxb4 proteins were added at desired concentrations once every 6 hours for 4 more days. Cells were harvested, 10% of the cells were used for CFC assay and 90% of the cells were used for LTC-IC assay. Overall, hoxb4 had a significant effect (P<0.01) in CFC expansion, of which version II at 10nM concentration and versions I, II, IV and V at 50nM concentrations significantly expanded CFC (P<0.05) compared with cultures without hoxb4 (A). Hoxb4 also had a significant effect (P<0.01) in expanding LTC-IC, of which all hoxb4 versions at 50nM concentration significantly expanded LTC-IC compared the controls (B). Among all five versions of hoxb4, version II had the highest activity in expanding fresh human BM cells. Dose response of version II hoxb4 (T-hoxb4-H) was tested in frozen human BM cells. T-hoxb4-H used at 50, 100, and 150nM concentrations showed significantly higher (P<0.05) CFC expansion than controls (C).
Expansion of human cord blood SRC by recombinant hoxb4
Next, we cultured human cord blood CD34+ cells with or without 50nM T-hoxb4-H for four days. The cells were harvested and injected into sublethally irradiated NOD/SCID mice, with each recipient receiving cells equivalent to 104 or 5×103 starting CD34+ cells. Eight weeks post cell transplantation, BM cells were harvested from the recipients and subjected to flow cytometric analysis with an anti-human CD45 antibody. Based on the number of recipients that did not have at least 0.1% of human CD45+ cells in the peripheral blood, we calculated SRC concentration using the Poisson probability at P0 . In three separate experiments, cord blood CD34+ cells cultured with T-hoxb4-H had significantly higher (P<0.01) SRC frequencies than did cells cultured without T-hoxb4-H (Table 1). The engrafted cells showed similar myeloid and lymphoid differentiation in hoxb4 treated and control groups as analyzed by flow cytometry with myeloid and lymphoid markers (Figure 3).
Table I.
Expansion of SRC by recombinant hoxb4
| Experiment | Treatment | Positive/Total | % Positive | SRC frequency |
|---|---|---|---|---|
| I | Control | 9/15 | 60 | 1 in10989 |
| I | T-hoxb4-H | 13/17 | 76 | 1 in 6910 |
| II | Control | 4/15 | 26 | 1 in 16118 |
| II | T-hoxb4-H | 7/14 | 50 | 1 in 7213 |
| III | Control | 4/15 | 26 | 1 in 16118 |
| III | T-hoxb4-H | 8/14 | 57 | 1 in 5901 |
Human cord blood CD34+ cells were cultured for four days in the StemSpam media with 50nM BSA control or T-hoxb4-H. Cells were harvested, washed and injected into sub-lethally-irradiated (2.5Gy) NOD/SCID mice, each mouse received cells equivalent to 104 (experiment I) or 5 × 103 (experiment II and III) starting CD34+ cells. Animals were euthanized eight weeks later; the presence of human CD45+ cells in the recipient BM cells was analyzed by flow cytometry. Mice with 0.1% or high human CD45+ cell in the BM were recorded as positive of human HSC engraftment. SRC frequency was calculated by using the Poission equation Pi=e(-N) × (Ni/i!) to calculate P0. Data shown were from three independent experiments.
Figure 3.
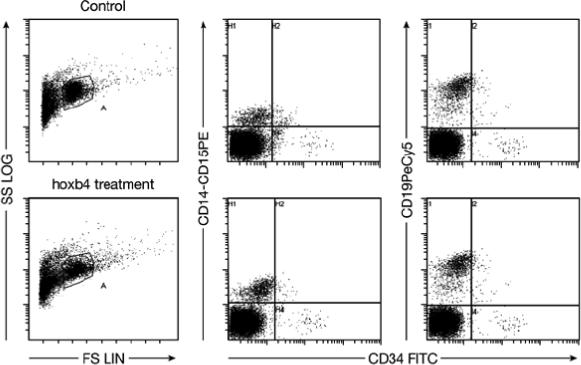
Multilineage engraftment of hoxb4-expanded human cord blood CD34+ cells. Representative flow cytometry profile of engrafted BM cells in mice received BSA or T-hoxb4-H treated human cord blood CD34+ cells. Human cord blood CD34+ cells were cultured for 4 days with 50nM BSA or T-hoxb4-H. Cells were then collected and injected into sublethally irradiated NOD/SCID mice, with each recipient received cells equivalent to 104 or 5×103 starting CD34+ cells in a SRC assay as detailed in Material and Methods. Recipient BM cells were harvested eight weeks later and stained for human myeloid (CD14/CD15) and lymphoid (CD19) markers.
Effect of human hoxb4 in a mouse model of immune-mediated BM failure
Human hoxb4 shares 96% protein sequence homology with mouse hoxb4, thus, we tested whether the recombinant T-hoxb4-H protein had protective role in a mouse model of immune-mediated BM failure. As we have reported, infusion of 5 × 106 C57BL/6 (B6) LN cells to minor-histocompatibility antigen mismatched C.B10 mice leads to rapid development of BM failure (Chen et al, 2007). We treated these mice with either cyclosporine 50μg/g/day, T-hoxb4-H 2μg /g/day (based on the optimum 50nM concentration for cell culture), or both and performed serial complete blood counts (CBC). Mice treated with both cyclosporine and T-hoxb4-H had higher blood cell counts, especially white blood cell and neutrophil counts, than did mice treated with cyclosporine alone, despite the differences were not large enough to be statistically significant (P>0.05, Figure 4A). Although T-hoxb4-H treatment alone did not protect the mice from BM failure, one mouse that received T-hoxb4-H survived and recovered, while all mice in the control group died within five weeks due to BM failure (Figure 4B). Animals survived to week 6 all survived to week 14 with normal blood counts. Recombinant human hoxb4 exerted a positive effect in alleviating pancytopenia in mice with immune-mediated BM failure.
Figure 4.
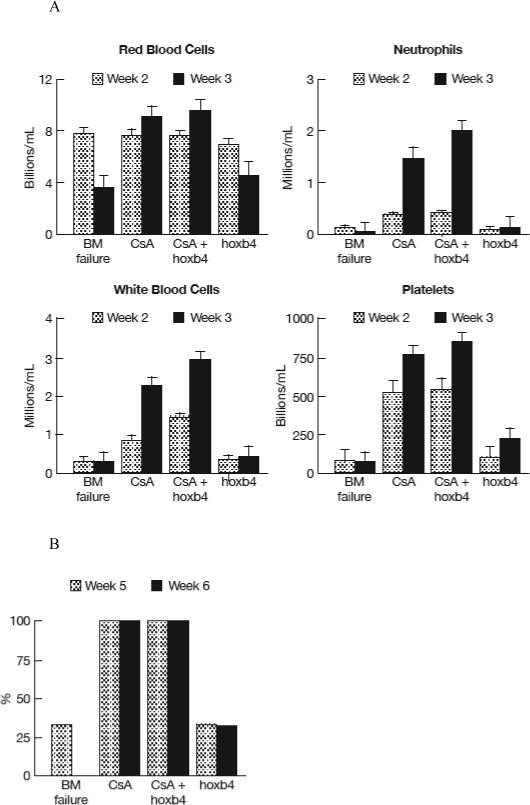
Hoxb4 enhances cyclosporine in rescuing animals in a mouse model of immune-mediated BM failure. Twelve C.B10 mice each received 5 Gy TBI and infused with 5 × 106 B6 LN cells were divided into four groups of three mice each: 1) No treatment control; 2) Cyclosporine 50 μg/g/day i.p. once per day for 7 days from day 0 to 6; 3) Cyclosporine 50 μg/g/day i.p. once per day for 7 days from day 0 to 6 plus hoxb4 at 2 μg/g/day i.p. once per day for 7 days from day 7 to 13; 4) hoxb4 at 2 μg/g/day i.p once per day for 7 days from day 7 to 13. Complete blood counts were analyzed weekly two weeks after LN cell infusions (A). Animal survival were recorded at week five and six (B). Animals survived to week six all survived to week fourteen when all animals were euthanized.
Effect of recombinant human hoxb4 on BM cells from patients with BM failure
To examine the effect of recombinant hoxb4 on patient BM cells, we cultured frozen BM cells from patients with refractory AA (n = 9) or MDS (n = 2) in comparison to a normal control (Table 2) with or without the addition of the 50nM T-hoxb4-H protein. After four days in culture, T-hoxb4-H-treated cells showed higher CFCs in 10 out of 11 patient samples as well as in the normal control sample, with the overall hoxb4 treatment effect being statistically significant (P<0.01, Figure 5A). Treatment with T-hoxbH resulted in a lower average CFC expansion in samples from AA patients (1.8 ± 0.2 fold) than in samples from MDS patients (2.6 ± 0.5 fold) and the normal control (2.4 ± 0.7 fold) (P<0.0918, Figure 5B). T-hoxb4-H treatment also increased LTC-IC in 10 out of 11 patient samples, with the overall effect being statistically significant (P<0.01, Figure 5C). Four of these 10 samples showed a significant (P<0.05) increase in LTC-IC by T-hoxb4-H treatment in comparison to the same four samples cultured with BSA. Again, T-hoxb4-H-induced LTC-IC expansion was lower in samples from AA patients (3.8 ± 0.5 fold) than in samples from MDS patients (12.2 ± 1.1 fold) and the normal control (17.4 ± 1.6 fold) (P<0.0001, Figure 5D).
Table II.
Patient information.
| Patient No | Date of Sample Collection | Cell Number | Diagnosis |
|---|---|---|---|
| Control | 08/2005 | 1.6 × 105 | Normal |
| 001 | 02/14/2006 | 3.0 × 105 | AA |
| 002 | 02/14/2006 | 2.6 × 105 | AA |
| 003 | 02/07/2006 | 1.4 × 105 | AA |
| 004 | 07/12/2005 | 2.6 × 105 | AA |
| 005 | 02/07/2006 | 2.4 × 105 | AA |
| 006 | 01/24/2006 | 1.0 × 105 | AA |
| 007 | 01/24/2006 | 5.5 × 105 | AA |
| 008 | 05/10/2005 | 0.4 × 105 | AA |
| 009 | 09/27/2005 | 2.0 × 105 | AA |
| 010 | 04/20/2004 | 0.2 × 105 | MDS |
| 011 | 06/01/2004 | 4.0 × 105 | MDS |
Information on 11 patients whose BM cells were used in the expansion experiment is summarized in the table 2. Both MDS patients had refractory cytopenia with multilineage dysplasia (RCMD).
Figure 5.
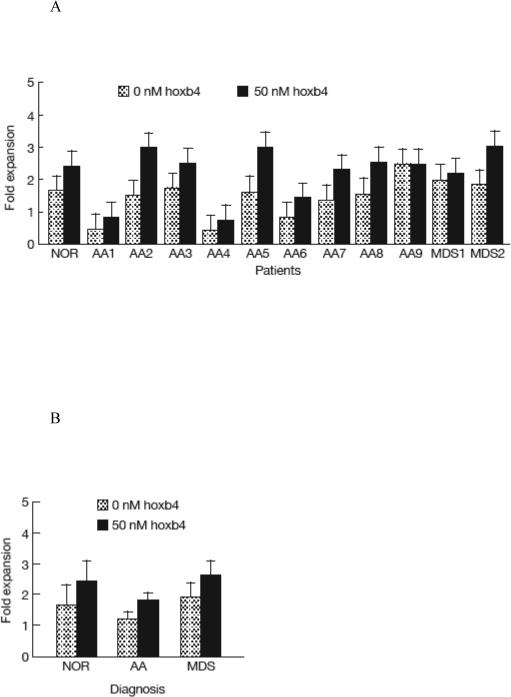

Expansion of HSCs from BM failure patients by recombinant hoxb4. Frozen BM cells (0.2−5.5 × 105) from a normal control and BM failure patients were cultured in 1ml cytokine-supplemented Stemspam medium for 2 days, then T-hoxb4-H protein was added at 50nM every 6 hours for 4 days. Harvested cells were tested for the expansion of CFC and LTC-IC. Treatment with hoxb4 significantly expanded CFC (P<0.01) in normal control and patient samples (A), samples from MDS patients had the higher CFC expansion than from AA patients although not statistically significant (P<0.0918, B). Similarly, hoxb4 treatment produced significant LTC-IC expansion (P<0.01) in samples from normal control and patients (C), and samples from the two MDS patients showed significant higher LTC-IC than samples from AA patients (P<0.0001, D).
Discussion
Primitive HSCs are the ultimate source of hematopoietic progenitor cells that sustain lifelong production of mature blood cells. In human BM failure diseases such as AA and hypocellular MDS, immune attack or intrinsic cellular defects cause a severe HSC deficiency, resulting in marrow hypoplasia and pancytopenia which is fatal if not treated (Maciejewski et al, 1996; Maciejewski et al, 1994; Yamaguchi et al, 2003). Counteracting autoimmunity and providing normal functional HSCs are used to treat patients with BM failure.
Our current study aimed to explore a novel treatment strategy for BM failure syndromes by expanding residual HSCs using recombinant human hoxb4, a transcription factor that has been extensively studied for its role in promoting HSC self-renewal (Sauvageau et al, 1995; Antonchuk et al, 2002; Krosl et al, 2003; Amsellem et al, 2003). The choice of hoxb4 was based on previous studies in mouse models, in which transduction of HSCs with a hoxb4-containing retroviral vector resulted in marked HSC expansion (Antonchuk et al, 2002; Sorrentino, 2004). In a mouse HSC transplantation model, lethally-irradiated recipients reconstituted with BM cells that had been transduced with a control vector had only 5−10% of normal number HSCs regenerated, while recipients reconstituted with BM cells transduced with a retroviral vector expressing hoxb4 had 100% of normal number of HSCs regenerated (Sauvageau et al, 1995). Expansion of HSCs did not continue after normal number of HSCs were regenerated, suggesting the effects of hoxb4 were subjected to normal homeostatic controls. When mouse HSCs were transduced with hoxb4 and cultured in vitro for 14 days, the number of HSCs expanded 40-fold from the initial numbers, or 2,000-fold relative to control cultures with cytokines only (Antonchuk et al, 2002).
The mechanism of hoxb4-mediated HSC expansion is not well understood. Gene expression profiling in combination with subsequent functional analysis with enriched adult murine HSCs has suggested that hoxb4 modulated the response of HSCs to multiple extrinsic signals in a concerted manner, thereby shifting the balance toward stem cell self-renewal (Schiedlmeier et al, 2007). Hoxb4 might protect adult HSCs from detrimental effects mediated by the pro-inflammatory cytokine TNF-α. This protection also likely contributes to the competitive repopulation advantage of hoxb4-expressing HSCs observed in vivo (Schiedlmeier et al, 2007).
The high level of hoxb4 expression achieved with viral vectors provided the possibility of using hoxb4 for HSC expansion ex vivo for clinical use. However, one major concern is that uncontrolled high levels of hoxb4 expression, achieved by using viral vectors with strong promoters, have been associated with leukemias in animal models, and abnormalities in myeloid differentiation in cell cultures (Zhang et al, 2008; Larochelle & Dunbar, 2008; Brun et al, 2004; Schiedlmeier et al, 2003). To avoid the use of viral vectors, recombinant hoxb4 fusion protein that contain a plasma-membrane permeabilization sequence “tat” to allow the entrance of hoxb4 protein into cell cytoplasm was shown to stimulate HSC-expansion (Krosl et al, 2003).
We reasoned that the position of the purification and permeabilization tags could affect hoxb4 activity. Therefore, we generated six versions of recombinant hoxb4, varying in the locations of the histidine and tat tags. Indeed, one version of recombinant hoxb4 could not be purified. Of the five versions of purified human hoxb4, including version V in which the histindine and tat tag at the N-terminal of the protein identical with the one already reported (Krosl et al, 2003), version II with an N-terminal tat and a c-terminal histidines showed the highest activity in expanding CFC (10 fold) and LTC-IC (15 fold) in fresh human BM cells in a four-day culture at 50nM concentration. In a study using lentiviral HOXB4-transduced feeder layers, passively diffused hoxb4 induced about 20-fold expansion of LTC-IC and 2.5-fold expansion of SRC in human cord blood cells when cells were cultured for five weeks (Amsellem et al, 2003); we achieved comparable levels of LTC-IC and SRC expansion on fresh human BM cells and human cord blood CD34+ cells by culturing with the version II T-hoxb4-H protein in four days. In our experiments, there were no significant difference in total cell numbers between T-hoxb4-H and BSA treated fresh human BM cells, cord blood CD34+ cells, or patient BM cells, yet T-hoxb4-H orchestrated significant CFC, LTC-IC and SRC expansion, with the expansion of LTC-IC being much greater than that of CFC. These data are consistent with the previous report that hoxb4 exert its activity on more primitive HSCs (Sauvageau et al, 1995).
Adding to the previously published data in murine models of marrow failure, in which IST with ATG, cyclosporine, or regulatory T cells ameliorated BM destruction (Bloom et al, 2004; Chen et al, 2007), recombinant hoxb4 demonstrated additional protection in conjunction with cyclosporine in preventing BM failure, possibly by expanding HSCs in vivo (It was not surprising the HSC expansion could not be achieved in this situation with hoxb4 alone without immunosuppression, as any gain in HSCs would be eliminated by active immune attack).
Using frozen BM cells, we found that recombinant hoxb4 expanded HSCs ex vivo from patients with AA and MDS. These patients are not candidates for hematopoietic stem cell transplant and had been refractory to immunosuppressive therapy. They could benefit from treatment to expand residual HSCs. Although the number of patient samples we tested was small, the in vitro results are promising since 90% of the samples responded positively to hoxb4 treatment as assayed by CFC and LTC-IC. Cells from both MDS patients responded favorably to hoxb4 stimulation, with significant LTC-IC expansion, and cells from three of nine AA patients also showed significant LTC-IC expansion. Together with in vivo data from the mouse model, we hypothesize that hoxb4 might be useful in treating BM failure patients when combined with immunosuppressive therapy, and patients with higher number of residual HSCs would be more likely to benefit.
Acknowledgments
We thank Cynthia Dunbar and Andre LaRochelle from Hematology Brach, NHLBI for helpful discussions; Jan Melenhorst from Hematology Branch, NHLBI for providing human cord blood cells; and William DeGraff from Radiation Biology Branch, NCI for providing assistance in feeder layer cell irradiation. This research was supported by the Intramural Research Program of the National Heart, Lung and Blood Institute.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
Primers:
Pet-I: primers
5’ TC GAG TAC GGA CGCAAA AAGAGAAGGCAACGGCGACGTG (forward)
5’ TCGACACGTCGCCGTTGCCTTCTCTTTTTGCGTCCGTAC (reverse)
Pet-II: Primers
5’GATCATGTACGGACGCAAAAAGAGAAGGCAACGGCGACGTG (forward)
5’ GATCCACGTCGCCGTTGCCTTCTCTTTTTGCGTCCGTACAT (reverse)
Pet-III: Primers
5’ GATCATGCACCATCACCATCACCATTACGGACGCAAAAAGAGAAGGCAACGGCGACGTGGTGGGGGCGGAG (forward)
5’GATCCTCCGCCCCCACCACGTCGCCGTTGCCTTCTCTTTTTGCGTCCGTAATGGTGATGGTGATGGTGCAT (reverse)
Version I: hox b4 tat his6
5’ CCACCTTGGATCCATG GCT ATG AGT TCT TTT TTGATC (forward)
5’GATCAGCTCGAGCGCGCGGGGGCCTCCATTG (reverse)
Version II: tat hoxb4 his6
5’ CCACCTTGGATCCATGGCTATGAGTTCTTTTTTGATC (forward)
5’ GATCAGCTCGAGCGCGCGGGGGCCTCCATTG (reverse)
Version III: his-tat-hoxb4
5’CCACCTTGGATCCATGGCTATGAGTTCTTTTTTGATC (forward)
5’GATCAGCTCGAGCTAGAGCGCGCGGGGGCCTCCATTG (reverse)
Version IV: his6-hoxb4-tat-his6
5’GATCATGCACCATCACCATCACCATTACGGACGCAAAAAGAGAAGGCAACGG CGA CGT GGT GGG GGC GGA G (forward)
5’GATCCTCCGCCCCCACCACGTCGCCGTTGCCTTCTCTTTTTGCGTCCGTAATGGTGATGGTGATGGTGCAT (reverse)
Version V: his6-tat-hoxb4
5’GATCATGCACCATCACCATCACCATGGTGGGGGCGGATACGGACGCAAAAAGAGAAGGCAACGGCGACGTGGTGGGGGCG (forward)
5’GATCCGCCCCCACCACGTCGCCGTTGCCTTCTCTTTTTGCGTCCGTATCCGCCCCCACCATGGTGATGGTGATGGTGCAT (reverse)
Version VI: his6-tat-HA-tat
5’GATCATGCACCATCACCATCACCATGGTGGGGGCGGATACGGACGCAAAAAGAGAAGGCAACGGCGACGTGGTGGGGGCGGTTACCCATACGATGTTCCAGATTAC GCTG (forward)
5’GATCCAGCGTAATCTGGAACATCGTATGGGTAACCGCCCCCACCACGTCGCCGTTGCCTTCTCTTTTTGCGTCCGTATCCGCCCCCACCATGGTGATGGTGATGGTGCAT (reverse)
- Amsellem S, Pflumio F, Bardinet D, Izac B, Charneau P, Romeo PH, Dubart-Kupperschmitt A, Fichelson S. Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat.Med. 2003;9:1423–1427. doi: 10.1038/nm953. [DOI] [PubMed] [Google Scholar]
- Antonchuk J, Sauvageau G, Humphries RK. HOXB4 overexpression mediates very rapid stem cell regeneration and competitive hematopoietic repopulation. Exp.Hematol. 2001;29:1125–1134. doi: 10.1016/s0301-472x(01)00681-6. [DOI] [PubMed] [Google Scholar]
- Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- Bloom ML, Wolk AG, Simon-Stoos KL, Bard JS, Chen J, Young NS. A mouse model of lymphocyte infusion-induced bone marrow failure. Exp.Hematol. 2004;32:1163–1172. doi: 10.1016/j.exphem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Boyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand.J.Clin.Lab Invest Suppl. 1968;97:7. [PubMed] [Google Scholar]
- Brun AC, Bjornsson JM, Magnusson M, Larsson N, Leveen P, Ehinger M, Nilsson E, Karlsson S. Hoxb4-deficient mice undergo normal hematopoietic development but exhibit a mild proliferation defect in hematopoietic stem cells. Blood. 2004;103:4126–4133. doi: 10.1182/blood-2003-10-3557. [DOI] [PubMed] [Google Scholar]
- Buske C, Feuring-Buske M, Abramovich C, Spiekermann K, Eaves CJ, Coulombel L, Sauvageau G, Hogge DE, Humphries RK. Deregulated expression of HOXB4 enhances the primitive growth activity of human hematopoietic cells. Blood. 2002;100:862–868. doi: 10.1182/blood-2002-01-0220. [DOI] [PubMed] [Google Scholar]
- Chen J, Astle CM, Harrison DE. Development and aging of primitive hematopoietic stem cells in BALB/cBy mice. Exp.Hematol. 1999;27:928–935. doi: 10.1016/s0301-472x(99)00018-1. [DOI] [PubMed] [Google Scholar]
- Chen J, Ellison FM, Eckhaus MA, Smith AL, Keyvanfar K, Calado RT, Young NS. Minor antigen h60-mediated aplastic anemia is ameliorated by immunosuppression and the infusion of regulatory T cells. J.Immunol. 2007;178:4159–4168. doi: 10.4049/jimmunol.178.7.4159. [DOI] [PubMed] [Google Scholar]
- Conneally E, Cashman J, Petzer A, Eaves C. Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lymphomyeloid repopulating activity in nonobese diabetic-scid/scid mice. Proc.Natl.Acad.Sci.U.S.A. 1997;94:9836–9841. doi: 10.1073/pnas.94.18.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammaitoni L, Bruno S, Sanavio F, Gunetti M, Kollet O, Cavalloni G, Falda M, Fagioli F, Lapidot T, Aglietta M, Piacibello W. Ex vivo expansion of human adult stem cells capable of primary and secondary hemopoietic reconstitution. Exp.Hematol. 2003;31:261–270. doi: 10.1016/s0301-472x(02)01077-9. [DOI] [PubMed] [Google Scholar]
- Krosl J, Austin P, Beslu N, Kroon E, Humphries RK, Sauvageau G. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat.Med. 2003;9:1428–1432. doi: 10.1038/nm951. [DOI] [PubMed] [Google Scholar]
- Larochelle A, Dunbar CE. HOXB4 and retroviral vectors: adding fuel to the fire. J.Clin.Invest. 2008 Apr;118(4):1350–3. doi: 10.1172/JCI35326. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski JP, Anderson S, Katevas P, Young NS. Phenotypic and functional analysis of bone marrow progenitor cell compartment in bone marrow failure. Br.J.Haematol. 1994;87:227–234. doi: 10.1111/j.1365-2141.1994.tb04903.x. [DOI] [PubMed] [Google Scholar]
- Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. A severe and consistent deficit in marrow and circulating primitive hematopoietic cells (long-term culture-initiating cells) in acquired aplastic anemia. Blood. 1996;88:1983–1991. [PubMed] [Google Scholar]
- Nakao S. [Immune pathophysiology of refractory anemias]. Nippon Rinsho. 2008;66:453–459. [PubMed] [Google Scholar]
- Sato T, Kim S, Selleri C, Young NS, Maciejewski JP. Measurement of secondary colony formation after 5 weeks in long-term cultures in patients with myelodysplastic syndrome. Leukemia. 1998;12:1187–1194. doi: 10.1038/sj.leu.2401084. [DOI] [PubMed] [Google Scholar]
- Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdorp PM, Humphries RK. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br.J.Haematol. 2006;133:622–627. doi: 10.1111/j.1365-2141.2006.06098.x. [DOI] [PubMed] [Google Scholar]
- Schiedlmeier B, Klump H, Will E, rman-Kalcek G, Li Z, Wang Z, Rimek A, Friel J, Baum C, Ostertag W. High-level ectopic HOXB4 expression confers a profound in vivo competitive growth advantage on human cord blood CD34+ cells, but impairs lymphomyeloid differentiation. Blood. 2003;101:1759–1768. doi: 10.1182/blood-2002-03-0767. [DOI] [PubMed] [Google Scholar]
- Schiedlmeier B, Santos AC, Ribeiro A, Moncaut N, Lesinski D, Auer H, Kornacker K, Ostertag W, Baum C, Mallo M, Klump H. HOXB4's road map to stem cell expansion. Proc.Natl.Acad.Sci.U.S.A. 2007;104:16952–16957. doi: 10.1073/pnas.0703082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino BP. Clinical strategies for expansion of haematopoietic stem cells. Nat.Rev.Immunol. 2004;4:878–888. doi: 10.1038/nri1487. [DOI] [PubMed] [Google Scholar]
- Thorsteinsdottir U, Sauvageau G, Humphries RK. Enhanced in vivo regenerative potential of HOXB4-transduced hematopoietic stem cells with regulation of their pool size. Blood. 1999;94:2605–2612. [PubMed] [Google Scholar]
- Tisdale JF, Hanazono Y, Sellers SE, Agricola BA, Metzger ME, Donahue RE, Dunbar CE. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- Ueda T, Tsuji K, Yoshino H, Ebihara Y, Yagasaki H, Hisakawa H, Mitsui T, Manabe A, Tanaka R, Kobayashi K, Ito M, Yasukawa K, Nakahata T. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J.Clin.Invest. 2000;105:1013–1021. doi: 10.1172/JCI8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NS, Maciejewski J. The pathophysiology of acquired aplastic anemia. N.Engl.J.Med. 1997;336:1365–1372. doi: 10.1056/NEJM199705083361906. [DOI] [PubMed] [Google Scholar]
- Zhang XB, Beard BC, Trobridge GD, Wood BL, Sale GE, Sud R, Humphries RK, Kiem HP. High incidence of leukemia in large animals after stem cell gene therapy with a HOXB4-expressing retroviral vector. J.Clin.Invest. 2008 Apr;118(4):1502–10. doi: 10.1172/JCI34371. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


