Abstract
Aim
To study the relationship between circulating androgens (total testosterone [TT], free testosterone [fT] and dihydrotestosterone [DHT]) and HDL-C in men with and without CVD.
Methods
Cross-sectional analyses included 1 661 baseline samples from the Massachusetts Male Aging Study (MMAS), a population-based cohort of men ages 40–70 years. Serum hormones were measured by radioimmunoassay and HDL-C was determined following precipitation of the lower density lipoproteins. CVD was determined by self-report. Analyses were performed using multiple linear regression.
Results
TT and HDL-C were positively correlated in the entire sample (r = 0.11, P = 0.0001). After adjusting for confounders, we found this relationship was mostly limited to the 209 men with CVD. Among men with CVD, TT (P = 0.0004), fT (P = 0.0172) and DHT (P = 0.0128) were all positively correlated with HDL-C, whereas in men without CVD only TT correlated with HDL-C (P = 0.0099).
Conclusion
Our results suggest that if androgens contribute to CVD in middle-aged men, the effect is not related to a suppressive effect of endogenous T on HDL-C.
Keywords: testosterone, high-density lipoprotein cholesterol, androgens, epidemiology
1 Introduction
The incidence of cardiovascular disease (CVD) is greater in men than age-matched women [1]. Major cardiovascular risk factors also differ by gender, including lipid abnormalities, which might help to explain some of the increased risk of CVD in men [2]. In particular, age-adjusted levels of high-density lipoprotein cholesterol (HDL-C) are lower in men than in women [2]. Interestingly, this difference is only manifest after puberty, supporting the concept that androgens contribute to a reduction in HDL-C in men [3]. It has been proposed that sex hormones contribute to the discrepancy in incident of CVD in men compared to pre-menopausal women [4]. In men, low levels of serum androgens are associated with increased risk of developing type 2 diabetes [5] and the metabolic syndrome [6], which are both strongly linked to the development of CVD. In addition, androgens could affect CVD through their impact of androgen on lipoprotein metabolism and HDL-C. Exogenous testosterone (T) lowers HDL-C when given to eugonadal middle-aged men both in physiologic doses associated with male hormonal contraceptive regimens and in supraphysiologic doses to athletes [7]. Likewise, androgen deprivation, either experimental or for the treatment of prostate disease, increases HDL-C [7]. These data support the hypothesis that androgens inhibit HDL-C production, or, perhaps, increase HDL-C catabolism.
In contrast to data from intervention trials, epidemiologic analyses have found a positive relationship between androgens and HDL-C [8, 9]. Moreover, substitution of T in hypogonadal men with age-associated hypogonadism results in either no change or only minor decreases in HDL-C [10], whereas correction of hypogonadism secondary to Klinefelters or Kallmanns syndrome can increase HDL-C [11]. These apparently conflicting results suggest that the relationship between HDL-C and androgens is complex and might be host dependent.
Using data from the Massachusetts Male Aging Study (MMAS), a population-based cohort of 1 709 middle-aged and older men, we investigated whether T and HDL-C are associated in men with and without CVD. We hypothesized that if T is involved in the causal pathway between HDL-C and CVD, higher androgen levels would be associated with lower levels of HDL-C in men with CVD.
2 Materials and methods
2.1 Design
The MMAS is a prospective, community-based, observational study of aging in middle-aged and older men. The current report uses only baseline data (T1: collected from 1987–1989). The design has been described previously [12]. At baseline, men aged 40–70 years from 11 cities and towns in the Boston, Massachusetts metropolitan area, USA were randomly selected from annual state census listings. To obtain a sample with approximately equal percentages in each age decade (40–49, 50– 59, 60–69 years), age-stratified cluster sampling was used. Of those eligible, 1 709 (52%) agreed to participate at T1. This response rate likely reflects, in part, the early-morning phlebotomy, extensive in-home interview, and absence of financial incentive involved in this study. Trained interviewer/phlebotomists visited the men in their homes, administered a standardized interview, and obtained physical measures and blood samples. The New England Research Institutes’ Institutional Review Board approved all protocols, including informed consent procedures.
2.2 Hormones and lipids
Two non-fasting blood samples were collected within 4 h of the subject’s awakening to control for diurnal variations in hormone levels. Samples were drawn 30 min apart, pooled to help smooth episodic secretion, transported in ice-cooled containers, and centrifuged within 6 h. The samples were stored at −20°C until shipment on dry ice to the central laboratory and then stored frozen at −70°C until being assayed.
All assays were performed at The Endocrine Laboratory, University of Massachusetts Medical School (Worcester, MA, USA). Total T (TT) was determined by radioimmunoassay (RIA) kit (Diagnostic Products Corporation, Los Angeles, CA, USA). Hormones and lipids were assayed in 1994 on sera stored since collection in 1987–1989. A structural equation model, equivalent to a Deming regression, showed negligible change as a result of assay drift or storage. The assay cross-reactivity with dihydrotestosterone (DHT) was 2.8%. The intra-assay and inter-assay coefficients of variation (CV) were 5.4% and 8.0%, respectively. As noted previously, the distribution of MMAS serum TT levels is similar to that reported by other large epidemiologic studies that have used RIA techniques [12].
Sex hormone-binding globulin (SHBG) was measured by RIA using kits (Farmos Diagnostica, Farmos Group, Oulunsalo, Finland). The intra-assay and inter-assay CVs were 8% and 10.9%, respectively. DHT was measured by RIA column chromatography [13]. The intra-assay and inter-assay CV were 10.9% and 12.2%, respectively. The Södergard equation was used to calculate free T (fT), assuming a fixed albumin-bound concentration [14]. The Södergard equation produces estimates for fT, which closely approximate those obtained from equilibrium dialysis [14]. Serum lipids were measured at the Lipid Research Laboratory at Miriam Hospital, Brown University (Providence, RI, USA). This lab participates in the national survey for clinical laboratories sponsored by the College of American Pathologists. HDL-C was determined on non-fasting serum samples following precipitation of the lower density lipoproteins using Heparin Manganese reagent [15].
2.3 Confounders and cardiovascular disease ascertainment
Well-validated instruments were used: alcohol [16], physical activity (Stanford Five-City Physical Activity Questionnaire [17]). Height and weight, waist and hip circumference were measured using standard techniques [18]. Smoking and chronic disease (diabetes, hypertension and CVD) were ascertained by self-report. Self-report of heart disease was assessed at every timepoint by asking “Have you ever been told by a health professional that you have heart disease?”. As reported previously, using longitudinal data from MMAS, we have found the concordance of self-report data compared to medical report and National Death Index data combined was approximately 80%. This is comparable to the concordance rate between self-report and medical records data reported in the published literature for ischemic heart disease and cardiovascular conditions in general [19].
To determine prescription and non-prescription medication use, the interviewer copied the medication name from the label and queried the reason for use. Medications were coded using a system based on the American Hospital Formulary Service, as described previously [20].
2.4 Statistical analysis
Of the 1 709 in the original cohort, men who were missing HDL-C data (n = 44) or all hormone data (n = 4) were excluded from the analyses, resulting in a sample size of 1 661.
Because of their skewed distributions, HDL-C, SHBG, DHT, body mass index (BMI), waist circumference (WC), and waist to hip ratio (WHR) were log transformed prior to modeling (Table 4 and Figure 1). However, results are presented on the original scale. To test whether HDL-C differed by categories, a two sample unpaired t-test was used. Tests of whether a variable differed by CVD status were done with a χ2-test or Fisher exact test for categorical variables and a two sample unpaired t-test for continuous variables. Correlations between HDL-C and continuous predictors were assessed by the Pearson correlation coefficient.
Table 4.
Association between log high density lipoprotein cholesterol (HDL-C) and continuous predictors (n = 1 661), Massachusetts Male Aging Study, 1987–1989.
| Characteristic | Ra | P valueb |
|---|---|---|
| Hormones (nmol/L) | ||
| TT | 0.11 | 0.0001 |
| fT | 0.01 | 0.5510 |
| Log SHBG | 0.16 | 0.0001 |
| Log DHT | 0.06 | 0.0133 |
| Age | −0.04 | 0.1435 |
| Adiposity | ||
| Log body mass index (kg/m2) | −0.30 | 0.0001 |
| Log waist circumference (cm) | −0.31 | 0.0001 |
| Log waist to hip ratio | −0.25 | 0.0001 |
Pearson correlation between characteristic and log HDL-C.
Test of the null hypothesis that correlation between log HDL-C and characteristic equals 0.
T, testosterone; TT, Total T; fT, free T; SHBG; sex hormone-binding globulin; DHT, dihydrotestosterone.
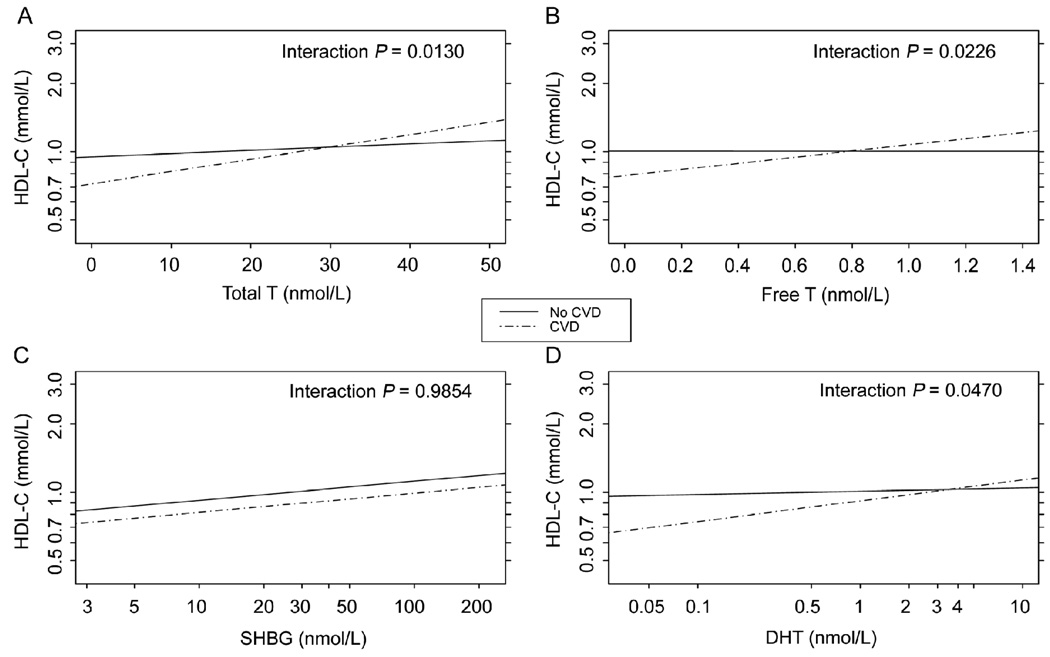
Figure 1.
Cross-sectional association (at T1) between hormones and high density lipoprotein cholesterol (HDL-C) by cardiovascular disease (CVD) status (n = 1 661). Lines represent adjusted regression of log HDL-C on hormone. (A): total testosterone (TT); (B): free T (fT); (C): log sex hormone-binding globulin (SHBG); or (D): log dihydrotestosterone (DHT). All models contain the following T1 variables: hormone, CVD, interaction between CVD and hormone, log waist to hip ratio (WHR), smoking, alcohol intake, age and hormone medications. For the purposes of display, the covariates were set to the following values: log waist (log median = log 96.5 cm), smoking (non-smoker), alcohol intake (< 1 drink/day), age (mean = 55 years), and hormone medications (none). P-values for the interaction between CVD and hormone are shown in the upper right corner of each panel. Massachusetts Male Aging Study, 1987–1989.
Multiple linear regression analysis was used to model HDL-C level as a function of hormone level and potential confounders. The impact of TT, fT, SHBG and DHT was examined. Separate models were fit for each variable.
The confounding effects of the following variables were examined: age, chronic disease (diabetes, hypertension and CVD), medication (lipid-lowering, prescription medications known to impact hormone levels), smoking, alcohol intake and adiposity (BMI, WC, WHR). Because of their correlation, the three adiposity measures were modeled separately.
For all models, we tested for possible two-way interactions between each hormone and each confounder. An interaction is present if the impact of the hormone on HDL-C varies by the level of a third variable. For many of the models, we found a statistically significant interaction between CVD and the hormone. This indicates that the relationship between the hormone and HDL-C differed depending on whether or not the man had CVD (e.g. positively correlated in one group, negatively in other; or association strong in one group and weak or nonexistent in another). When an interaction was present, we performed tests among the men with and without CVD to determine if the hormone–HDL-C association existed within each group.
SAS software (SAS System for Windows 9.1; Cary, NC, USA) was used to perform statistical analysis. The level of significance was set at P < 0.05.
3 Results
3.1 Baseline characteristics
Baseline demographic characteristics of the study cohort (n = 1 709) have been presented previously [12] and demographics of the analysis sample (n = 1 661) were similar. The sample was predominantly white (95.5%), employed (78%), with at least a high school education (88%). By design (e.g. random sampling), these demographics closely match those of the population of Massachusetts in 1990 according to census data. Descriptive statistics for baseline confounding variables, hormone levels, and HDL-C are shown in Table 1. Subjects ranged from 40–70 years old with a mean of 55.2 ± 8.7. The prevalence of CVD was 13% (n = 209), similar to other community-based studies of older men [2, 21]. Lipid-lowering medication usage was rare (1%) while 9% of subjects reported a prescription medication that could lower hormone levels. Mean (standard deviation) TT was 17.9 (6.1) nmol/L, SHBG was 32.3 (16.3) nmol/L, and average HDL-C was 1.10 (0.36) mmol/L (equivalent to 42.5 mg/dL). Characteristics of the subjects with and without CVD are shown in Table 2. As expected, men with CVD were older, more likely to take lipid-lowering medication, have hypertension and/or diabetes mellitus, and had a greater degree of central adiposity, as assessed by waist circumference and WHR, than men without CVD. Men with CVD also had higher total cholesterol, lower HDL-C, and lower total and free testosterone than those without known CVD.
Table 1.
Descriptive statistics of the analysis sample for confounding variables, hormones and high density lipoprotein cholesterol, (n = 1 661), Massachusetts Male Aging Study, 1987–1989.
| Characteristic | Analysis Sample (n = 1 661) |
|---|---|
| Age (year), mean ± SDa | 55.2 ± 8.7 |
| Chronic diseasea, n (%) | |
| CVD | 209 (13) |
| Diabetes | 128 (8) |
| Hypertension | 506 (30) |
| Medication, n (%) | |
| Lipid-lowering | 23 (1) |
| Affecting hormones | 153 (9) |
| Lifestyle | |
| Current cigarette smoking, n (%) | 405 (24) |
| Drinks/dayb, n (%) | |
| < 1 | 888 (54) |
| 1–3 | 450 (27) |
| > 3 | 309 (19) |
| Physical activity (kcal/day), mean ± SD | 3 072 ± 622 |
| Adiposity, mean ± SD | |
| Body mass index (kg/m2) | 27.3 ± 4.4 |
| Waist circumference (cm) | 97.4 ± 11.3 |
| Waist to hip ratio | 0.95 ± 0.06 |
| Hormones, mean ± SD | |
| Total T (nmol/Lc) | 17.9 ± 6.1 |
| Free T (nmol/L) | 0.45 ± 0.18 |
| SHBG (nmol/L) | 32.3 ± 16.3 |
| DHT (nmol/L) | 0.92 ± 0.59 |
| HDL-C (mmol/Ld), mean ± SD | 1.10 ± 0.36 |
Self report
One drink is equivalent to 15 mL ethanol (10 oz beer, 4 oz wine or 1.5 oz spirits)
nmol/L may be converted to ng/dL by dividing by 0.0347
mmol/L may be converted to mg/dL by dividing by 0.0259.
CVD, cardiovascular disease; T, testosterone; SHBG, sex hormone-binding globulin; DHT, dihydrotestosterone, HDL-C, high density lipoprotein cholesterol.
Table 2.
Descriptive statistics for men with and without cardiovascular disease (CVD) at baseline, Massachusetts Male Aging Study, 1987–1989.
| Characteristic | Non-CVD | CVD | P value |
|---|---|---|---|
| Sample size, n | 1 452 | 209 | |
| Age (years), mean ± SD | 54.5 ± 8.6 | 59.9 ± 7.3 | < 0.0001a |
| Medication | |||
| Lipid-lowering (%) | 1.0 | 4.3 | 0.0011b |
| Affecting hormones (%) | 9.2 | 9.6 | 0.5829c |
| Current cigarette smoking, n (%) | 25.0 | 20.6 | 0.1671c |
| Physical activity (kcal/day), mean ± SD | 3 085 ± 633 | 2 994 ± 533 | 0.0543a |
| Hypertension (%) | 28.2 | 46.4 | < 0.0001c |
| Diabetes (%) | 6.1 | 18.7 | < 0.0001c |
| Adiposity, mean ± SD | |||
| Body mass index (kg/m2) | 27.2 ± 4.3 | 28.2 ± 4.5 | 0.0015a |
| Waist circumference (cm) | 97.0 ± 11.3 | 100.8 ± 10.7 | < 0.0001a |
| Waist to hip ratio | 0.94 ± 0.06 | 0.96 ± 0.06 | 0.0002a |
| Hormones, mean ± SD | |||
| Total T (nmol/L) | 18.1 ± 6.1 | 16.7 ± 5.8 | 0.0030a |
| Free T (nmol/L) | 0.46 ± 0.18 | 0.41 ± 0.15 | < 0.0001a |
| SHBG (nmol/L) | 32.1 ± 16.2 | 33.6 ± 16.9 | 0.2336a |
| DHT (nmol/L) | 0.93 ± 0.61 | 0.85 ± 0.46 | 0.0876a |
| Total cholesterol (mmol/L), mean ± SD | 5.4 ± 1.3 | 5.6 ± 1.5 | 0.0497a |
| HDL-C (mmol/L), mean ± SD | 1.12 ± 0.36 | 0.97 ± 0.32 | < 0.0001a |
P-value from two sample t-test
P-value from Fisher’s exact test
P-value from χ2-test.
T, testosterone; SHBG, sex hormonebinding globulin; DHT, dihydrotestosterone, HDL-C, high density lipoprotein cholesterol.
3.2 Relationship between HDL-C and CVD and established cardiac disease risk factors
The association between mean HDL-C and established coronary risk factors in the study cohort is shown in Table 3. As expected, mean HDL-C was significantly lower in men with CVD, diabetes and hypertension. Men with higher alcohol intake had higher HDL-C. Lipid-lowering medication had little impact on HDL-C, possibly because of the low prevalence of use. Mean HDL-C did not differ by hormone medication or smoking.
Table 3.
Association between high density lipoprotein cholesterol (HDL-C) and categorical predictors other than cardiovascular disease (CVD) (n = 1 661), Massachusetts Male Aging Study, 1987–1989.
| Characteristic | HDL-C (mmol/L), mean ± SD | P valuec |
|---|---|---|
| Chronic diseasea | 0.0031 | |
| Diabetes | ||
| No | 1.11 ± 0.36 | |
| Yes | 1.02 ± 0.34 | |
| Hypertension | 0.0002 | |
| No | 1.13 ± 0.35 | |
| Yes | 1.06 ± 0.36 | |
| Medication | ||
| Affecting hormones | 0.2360 | |
| No | 1.10 ± 0.35 | |
| Yes | 1.14 ± 0.38 | |
| Lipid-lowering | 0.1300 | |
| No | 1.11 ± 0.36 | |
| Yes | 0.99 ± 0.28 | |
| Lifestyle | ||
| Current smoking | 0.4945 | |
| No | 1.11 ± 0.34 | |
| Yes | 1.09 ± 0.39 | |
| Drinks/dayb | 0.0001 | |
| < 1 | 1.03 ± 0.31 | |
| 1–3 | 1.17 ± 0.38 | |
| > 3 | 1.23 ± 0.41 |
Self-report
One drink is equivalent to 15 mL ethanol (10 oz beer, 4 oz wine or 1.5 oz spirits)
Test of null hypothesis that mean of HDL-C does not differ by levels of the characteristic, two sample unpaired t-test. Means are presented on original scale.
3.3 Androgens and HDL-C
Overall, in unadjusted analyses, TT was positively correlated with HDL-C (r = 0.11, P = 0.0001; Table 4) although the magnitude of the correlation coefficient was small. SHBG and DHT were also positively correlated with HDL-C (Table 4; r = 0.16 and r = 0.06, respectively), whereas fT was not (r = 0.01).
All three measures of adiposity, BMI, WC and WHR, were inversely correlated with HDL-C (Table 4).
3.4 Androgens and HDL-C in men with and without CVD
We went on to examine the relationship between androgens and HDL-C in men with and without CVD after controlling for confounders, including age and central adiposity (Figure 1). The regression lines were adjusted for WHR (as a measure of central adiposity), smoking, alcohol consumption, age and the use of medications that might affect hormone measures. The association between TT and HDL-C differed depending on whether the man had CVD (P-value for interaction term 0.0130). Among men with CVD, TT was significantly and positively associated with HDL-C (P = 0.0004 for association between T and HDL-C among CVD cases). Figure 1A illustrates the magnitude of this relationship: if two men with CVD differed by 5 nmol/L in TT and all other characteristics in the model were equal, the man with the higher T would be expected to have a 6% higher HDL-C. The association between TT and HDL-C among men without CVD (P = 0.0099) was much weaker; a 5 nmol/L difference in TT would result in a 2% higher HDL-C. Moreover, when WC or BMI were controlled for instead of WHR, the hormone–HDL-C association also varied by CVD status (interaction P = 0.0144 and 0.0289, respectively). Among the men with CVD, a high TT was still associated with high HDL-C (P = 0.0028 WC model; P = 0.0053 BMI model). However, there was no longer any association between TT and HDL-C among the men without CVD when we adjusted for WC or BMI instead of WHR (P = 0.2875 and P = 0.1925, respectively).
The results for fT and DHT were similar to those for TT (Figure 1B and 1D). When WHR was controlled for, the association between fT and HDL-C varied by CVD status and was present only among men with CVD (interaction P = 0.0226). High fT was associated with high HDL-C in this group (P = 0.0172). Similar results were found when controlling for WC (interaction P = 0.0389), although the interaction term did not reach significance when adjusting for BMI (P = 0.0610). Analogous to the results for TT, a man with 0.2 nmol/L higher fT would be expected to have 6% higher HDL-C than a man with similar WHR, cardiac risk factors and lower serum fT. Moreover, in men with CVD there was a positive association between DHT and HDL-C when WHR was controlled for (interaction P = 0.0470; P = 0.0128 for the correlation between DHT and HDL-C in this model); this relationship, however, did not reach statistical significance if WC or BMI replaced WC in the model (interaction P = 0.1010 and P = 0.1560, respectively).
Unlike TT, fT and DHT, the association between SHBG and HDL-C in the adjusted model did not differ by CVD status (Figure 1C, P = 0.9854 for the interaction term). Controlling for WC or BMI did not alter these results (data not shown). However, SHBG was very highly positively associated with HDL-C regardless of CVD status or which adiposity measure was controlled for (P = 0.0001 for SHBG in all three adiposity adjusted models without interaction terms; data not shown).
4 Discussion
Similar to other cross-sectional analyses of middle aged men [8, 9], in the present study of 1 661 men enrolled in the MMAS, we found a positive relationship between HDL-C and TT. HDL-C was inversely related to chronic disease, including CVD, diabetes and hypertension. After adjustment for confounders (age, WHR, smoking, alcohol consumption and medications), we found that the relationship between androgens and HDL-C was mostly limited to the 209 men with CVD within the cohort. Importantly, the positive relationship between HDL-C and androgens was consistent, whether the assessment of androgen level used TT, fT or DHT measurement. Moreover, although SHBG, which binds 60% of circulating T, was strongly and positively correlated with HDL-C, the strength of this relationship did not vary between men with and without CVD. These results suggest that the dichotomy between androgens and HDL-C in men with and without CVD that we observed is androgen specific, and not a function of the carrier protein.
The hypothesis that T adversely impacts CVD risk is largely based upon interventional trials that indicate that exogenous androgens suppress HDL-C in men, even when given in physiologic doses [4]. T has been demonstrated to decrease HDL-C by increasing both hepatic lipase (HL) activity [22] and scavenger receptor B1 expression [23], resulting in increased HDL-C uptake by hepatocytes [23, 24]. However, it is difficult to extrapolate these data to increases in CVD risk [23, 24], because atherosclerosis is inhibited in transgenic animal models with enhanced HL activity despite decreased HDL-C [24]. Furthermore, epidemiologic data have failed to demonstrate an association between T levels and CVD [24, 25] and, in fact, lower androgen levels are associated with the development of type 2 diabetes, which would increase the risk of CVD [25]. Moreover, high circulating T levels are associated with high, not low, HDL-C levels in cross-sectional studies [9]. If high levels of circulating androgens contribute to CVD by lowering HDL-C, one might expect that in men with CVD, androgens would be associated with lower HDL-C levels; in fact, our results showed just the opposite. Our data support the hypothesis that if T increases CVD risk, this effect is unlikely to be mediated through a negative impact of T on HDL-C.
Because there is a very strong, positive association between SHBG and HDL-C [26], analyses of free hormone is critical to discerning the effects of androgens. In addition, although serum concentrations of DHT (the product of 5α-reduction of T) are significantly lower than T in men, DHT is a significantly more potent androgen than T, at least in vitro. A strength of our data is the consistency in the relationships between T, fT and DHT and HDL-C in men with CVD. Although most studies find a positive relationship between TT and HDL-C, some have suggested that this likely reflects the contribution of SHBG (bound to T) [26], and that increased fT results in a more atherogenic lipid profile, including lower HDL [27]. Our data argues against this, because both higher fT and DHT levels were associated with higher HDL-C in men with CVD. In addition, T levels might be impacted by the presence of acute or chronic illness, body composition, age and smoking [24]. Others have argued that the association between T and HDL-C is mostly, if not completely, mediated by body composition, and central adiposity in particular, because obesity is associated with low T and low HDL-C [24]. However, when we controlled for BMI, WHR or WC in our analyses, androgens still were clearly related to HDL-C in men with CVD. Such factors, or a selection bias, might have influenced previous, small case control studies in men undergoing coronary angiography, which failed to find a relationship, or found a negative relationship, between androgens and HDL-C in men with or without CVD [8, 28].
In conclusion, using cross-sectional analyses of a large cohort of community dwelling, middle-aged men, we demonstrate a strong, positive relationship between androgens and HDL-C in men with CVD. This is in contrast to men without CVD, where only TT, and not fT or DHT, weakly correlated with increased HDL-C. Our data suggest that any androgenic effect on CVD risk is not mediated by an inhibitory effect of endogenous T on HDL-C levels.
Acknowledgment
This work was supported by grants from the Endocrine Society (S. T. P.), VA Special Fellowship Program in Advanced Geriatrics (S. T. P.), National Institute on Aging (AG04763), and the National Institute of Diabetes and Digestive and Kidney Diseases (DK51345 and DK44995).
References
- 1.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 2.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99:1165–1172. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 3.Kirkland RT, Keenan BS, Probstfield JL, Patsch W, Lin TL, Clayton GW, et al. Decrease in plasma high-density lipoprotein cholesterol levels at puberty in boys with delayed adolescence. Correlation with plasma testosterone levels. JAMA. 1987;257:502–507. [PubMed] [Google Scholar]
- 4.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 5.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 6.Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91:843–850. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 7.Hurley BF, Seals DR, Hagberg JM, Goldberg AC, Ostrove SM, Holloszy JO, et al. High-density-lipoprotein cholesterol in bodybuilders v powerlifters. Negative effects of androgen use. JAMA. 1984;252:507–513. [PubMed] [Google Scholar]
- 8.Kiel DP, Baron JA, Plymate SR, Chute CG. Sex hormones and lipoproteins in men. Am J Med. 1989;87:35–39. doi: 10.1016/s0002-9343(89)80480-2. [DOI] [PubMed] [Google Scholar]
- 9.Barrett-Connor EL. Testosterone and risk factors for cardiovascular disease in men. Diabete Metab. 1995;21:156–161. [PubMed] [Google Scholar]
- 10.Whitsel EA, Boyko EJ, Matsumoto AM, Anawalt BD, Siscovick DS. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med. 2001;111:261–269. doi: 10.1016/s0002-9343(01)00833-6. [DOI] [PubMed] [Google Scholar]
- 11.Ozata M, Yildirimkaya M, Bulur M, Yilmaz K, Bolu E, Corakci A, et al. Effects of gonadotropin and testosterone treatments on Lipoprotein(a), high density lipoprotein particles, and other lipoprotein levels in male hypogonadism. J Clin Endocrinol Metab. 1996;81:3372–3378. doi: 10.1210/jcem.81.9.8784099. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell AB, Araujo AB, McKinlay JB. The health of normally aging men: The Massachusetts Male Aging Study (1987–2004) Exp Gerontol. 2004;39:975–984. doi: 10.1016/j.exger.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Longcope C, Franz C, Morello C, Baker R, Johnston CC., Jr Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas. 1986;8:189–196. doi: 10.1016/0378-5122(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 15.National Heart and Lung Institute LRCPLMC. Manual of Laboratory Operations: Lipid and Lipoprotein Analysis. 2nd edn. Vol DHEW publication No. 75–628 Washington, DC: US Government Printing Office; 1974. [Google Scholar]
- 16.Khavari KA, Farber PD. A profile instrument for the quantification and assessment of alcohol consumption. The Khavari Alcohol Test. J Stud Alcohol. 1978;39:1525–1539. doi: 10.15288/jsa.1978.39.1525. [DOI] [PubMed] [Google Scholar]
- 17.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 18.McKinlay SM, Kipp DM, Johnson P, Downey K, Carelton RA. A field approach for obtaining physiological measures in surveys of general populations: Response rates, reliability and costs. Paper presented at: In: Proceedings of the Fourth Conference on Health Survey Research Methods; Washington DC. 1984. USDHHS-PHS Publication 84–3346. [Google Scholar]
- 19.St Sauver JL, Hagen PT, Cha SS, Bagniewski SM, Mandrekar JN, Curoe AM, et al. Agreement between patient reports of cardiovascular disease and patient medical records. Mayo Clin Proc. 2005;80:203–210. doi: 10.4065/80.2.203. [DOI] [PubMed] [Google Scholar]
- 20.Mohr BA, Guay AT, O’Donnell AB, McKinlay JB. Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 2005;62:64–73. doi: 10.1111/j.1365-2265.2004.02174.x. [DOI] [PubMed] [Google Scholar]
- 21.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78:539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 22.Herbst KL, Amory JK, Brunzell JD, Chansky HA, Bremner WJ. Testosterone administration to men increases hepatic lipase activity and decreases HDL and LDL size in 3 wk. Am J Physiol Endocrinol Metab. 2003;284:E1112–E1118. doi: 10.1152/ajpendo.00524.2002. [DOI] [PubMed] [Google Scholar]
- 23.Langer C, Gansz B, Goepfert C, Engel T, Uehara Y, von Dehn G, et al. Testosterone up-regulates scavenger receptor BI and stimulates cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2002;296:1051–1057. doi: 10.1016/s0006-291x(02)02038-7. [DOI] [PubMed] [Google Scholar]
- 24.Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24:183–217. doi: 10.1210/er.2001-0025. [DOI] [PubMed] [Google Scholar]
- 25.Yarnell JW, Beswick AD, Sweetnam PM, Riad-Fahmy D. Endogenous sex hormones and ischemic heart disease in men. The Caerphilly prospective study. Arterioscler Thromb. 1993;13:517–520. doi: 10.1161/01.atv.13.4.517. [DOI] [PubMed] [Google Scholar]
- 26.Bataille V, Perret B, Evans A, Amouyel P, Arveiler D, Ducimetière P, et al. Sex hormone-binding globulin is a major determinant of the lipid profile: the PRIME study. Atherosclerosis. 2005;179:369–373. doi: 10.1016/j.atherosclerosis.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Gyllenborg J, Rasmussen SL, Borch-Johnsen K, Heitmann BL, Skakkebaek NE, Juul A. Cardiovascular risk factors in men: The role of gonadal steroids and sex hormone-binding globulin. Metabolism. 2001;50:882–888. doi: 10.1053/meta.2001.24916. [DOI] [PubMed] [Google Scholar]
- 28.Kabakci G, Yildirir A, Can I, Unsal I, Erbas B. Relationship between endogenous sex hormone levels, lipoproteins and coronary atherosclerosis in men undergoing coronary angiography. Cardiology. 1999;92:221–225. doi: 10.1159/000006977. [DOI] [PubMed] [Google Scholar]