Summary
Embryologically, cardiac chambers differ in their morphological and contractile properties from the beginning. We hypothesized that a non-invasive ultrasonic backscatter investigation might illustrate the fundamental differences in myocardial morphologic properties of the two ventricles during heart development.
Objectives
The goals of this investigation were: 1) to explore the feasibility of measuring the magnitude of cyclic variation of ultrasonic backscatter from the left and right ventricular free walls of fetal hearts; 2) to compare measurements of the magnitude of cyclic variation from the left and right sides of the heart; and 3) to determine if the observed results are consistent with predictions relating the overall backscatter level and the magnitude of cyclic variation.
Methods
Cyclic variation data from the left and right ventricular free walls were generated from analyses of the backscatter from echocardiographic images of 16 structurally normal fetal hearts at mid-gestation.
Results
The magnitude of cyclic variation was found to be greater for the left ventricular free wall than for the right ventricular free wall (4.5 ± 1.1 dB vs. 2.3 ± 0.9 dB, respectively; mean ± SD; p < 0.0001, paired t-test).
Conclusion
Measurements of the cyclic variation of backscatter can be obtained from both the left and right sides of fetal hearts demonstrating a significant difference between the measured magnitude of cyclic variation in the left and right ventricular myocardium. This observation is consistent with predictions relating the overall backscatter level and the magnitude of cyclic variation. Results of this study suggest cyclic variation measurements may offer a useful approach for characterizing intrinsic differences in myocardial properties of the two ventricles in assessing fetal heart development.
Introduction
Formation of the heart involves an orchestrated series of morphogenetic events. Although individual cardiac chambers do not become morphologically distinguishable until after cardiac looping, each cardiac chamber differs in its morphological and contractile properties from the beginning.1 How chamber identities are established is unknown, but identification of morphologic properties may provide a window to appraise perturbations in the developmental process that can have consequences in the form of myopathic or congenital heart disease. Both in developng and developed hearts, global and local structural anisotropy of myocardial fibers and other ventricular wall constituents (e.g., the types and concentrations of proteins) produce mechanical and electrical properties that may be anisotropic, time varying, and spatially inhomogeneous and may differ between left and right ventricles.2–7 Understanding and establishing the normal patterns of these intrinsic properties in normally developing hearts will be a necessary prerequisite to their use in discerning the evolution of myopathic or congenital heart disease.
We hypothesized that a non-invasive ultrasonic backscatter investigation might illustrate the fundamental differences in myocardial morphologic properties of the two ventricles during heart development. The rationale that underlies this prospective study is that the intrinsic myoarchitectural properties of the developing heart are reflected in the ultrasonic properties and, hence, an assessment of myoarchitecture can be achieved through echocardiography-based analyses. Several investigations have demonstrated the relationship between measured ultrasonic parameters (attenuation, backscatter, speed of sound) and the inherent properties of myocardial tissue. The nature of the intrinsic myocardial properties (e.g., the types and concentrations of proteins present resulting in specific intra- and extracellular viscoelastic properties) as well as the geometrical properties (structural morphology) of the myocardium combine to produce the observed ultrasonic parameters.8–13 For example, several studies have been published illustrating the relationship between the measured ultrasonic backscatter properties and collagen content in myocardial tissue.8, 14–21 Results demonstrate an increase in both the measured ultrasonic attenuation and backscatter correlate well with increased collagen concentration determined biochemically or histologically.
Measurement of the systematic variation of backscattered ultrasonic energy from the myocardium over the heart cycle (i.e., the cyclic variation of backscatter) is a validated approach for investigating intrinsic myocardial characteristics in vivo13, 22 and has been successfully applied to characterize a number of cardiac pathologies23. However, relatively few studies have explored the feasibility of measurements of cyclic variation to characterize myoarchitecture in developing fetal heart, which may be relevant to postnatal ventricular structure and function.24, 25
Measurements of the cyclic variation of backscatter represent a clinically useful approach for characterizing the nature of backscatter properties from a myocardial region because each heart serves as its own reference. In this approach only the relative change in myocardial backscatter over the heart cycle is measured, not the absolute level of backscatter. Estimates of the absolute level of ultrasonic backscatter require explicit compensations for imaging system dependent contributions (e.g., system gain, beam volume, etc.) and ultrasonic field propagation effects (e.g., effects of overlying attenuation, etc.). By measuring the cyclic variation of myocardial backscatter, the effects of imaging system gain, body habitus, and other effects that can influence the overall backscattered signal are largely mitigated as long as the acquired images are not saturated.
The objectives of this initial investigation were three-fold: 1) to explore the feasibility of measuring the magnitude of cyclic variation of backscatter from the left and right ventricular free walls from the hearts of human fetuses at mid-trimester; 2) to compare measurements of the magnitude of cyclic variation from the left and right sides of the heart as an approach to understand and establish the normal patterns of the morphologic properties in normally developing hearts; and 3) to determine if the observed results are consistent with predictions based on a previously described model26 demonstrating a relationship between overall backscatter level and the magnitude of cyclic variation using recently published backscatter measurements27 in a fetal animal model.
Methods
Subjects
Data from sixteen human fetuses ranging in age from 17 to 29 weeks gestation were included in this study. Eligible fetuses were those whose mothers had been referred for a comprehensive fetal echocardiogram at St. Louis Children’s Hospital, Washington University School of Medicine because of a family history of congenital heart defects or suspected maternal exposure to teratogens. A full fetal echocardiographic examination was performed and only fetuses exhibiting structurally normal hearts were included in the study. Signed informed consent for participation was obtained from one of the parents under a human studies protocol approved by the Washington University investigation review board (IRB).
Image Acquisition
Fetal echocardiographic images to be used for this study were obtained with a Siemens/Acuson Sequoia C256 imaging system (Mountain View, CA) utilizing a 6C2-S curvilinear array using an approach similar to that previously described by our laboratory in which the imaging system is configured to provide grayscale images linearly proportional to the measured level of backscatter.28–30 Images were acquired in the harmonic imaging mode at 5.0 MHz with the imaging system configured to provide an approximately linear relationship between a change in backscattered ultrasound intensity (expressed in decibels, dB) and a change in the displayed grayscale value (i.e., a linear compression curve) over the useful dynamic range of the imaging system. For this study, the Siemens/Acuson Sequoia C256 imaging system was configured with settings: “space/time” = T1; “edge” = 0; “persistence” = 0; “post-process” = 1; “delta” = 1; “dynamic range” = 40 dB.
Transverse, long-axis cross-sectional images of each fetal heart were acquired with the imaging system in the “Zoom” mode and the overall (2D) receive gain and time-gain compensation (TGC) controls adjusted to provide relatively strong backscatter (unsaturated, mid-level grayscale values) from the mid-myocardial regions. Approximately 6 heart cycles worth of images were acquired and stored as 8-bit digital cineloops (AVI format) for subsequent analyses. The transverse, long-axis cross-sectional echocardiographic view provides images with the insonifying ultrasonic field perpendicular to the predominant myocardial fiber orientation and, hence, reduces the confounding effects of tissue anisotropy on measurements.29, 31–35
Generation of Cyclic Variation Data
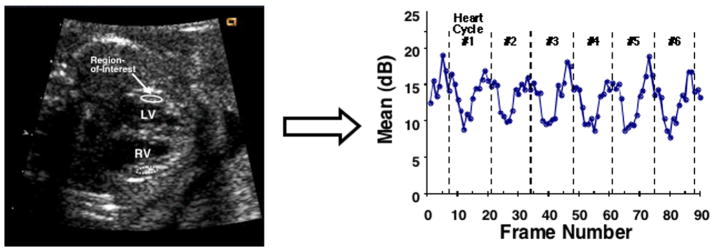
Cyclic variation of backscatter data were generated by analyzing the acquired echocardiographic images offline using the NIH ImageJ™ (National Institutes of Health, Bethesda, MD) software package. The acquired cineloops were opened as Quicktime™ (Apple Inc., Cupertino, CA) movies and separate regions-of-interest were placed in the myocardial free walls of the left and right ventricles. The position of each region-of-interest was manually adjusted in every acquired image frame such that approximately the same two-dimensional area of myocardium was measured over the heart cycles. The mean grayscale value within each region-of-interest was measured for each image frame thus producing a trace of mean backscatter values as a function of frame number (i.e., time). These data represent the measured cyclic variation of backscatter waveforms. Changes in measured mean grayscale values representing the cyclic variation of backscatter were converted to changes in ultrasonic backscatter values expressed in decibels (dB) using a previously described system calibration procedure.28–30, 36 Figure 1 illustrates an example showing the typical placements of regions-of-interest in a fetal echocardiogram and a representative cyclic variation of backscatter curve generated from the region-of-interest in the left-ventricular free wall.
Figure 1.
Example fetal echocardiographic image showing the typical placements of regions-of-interest and representative cyclic variation of backscatter data for 6 heart cycles generated from the region-of-interest in the left-ventricular free wall.
Measurement of the Magnitude of Cyclic Variation of Backscatter
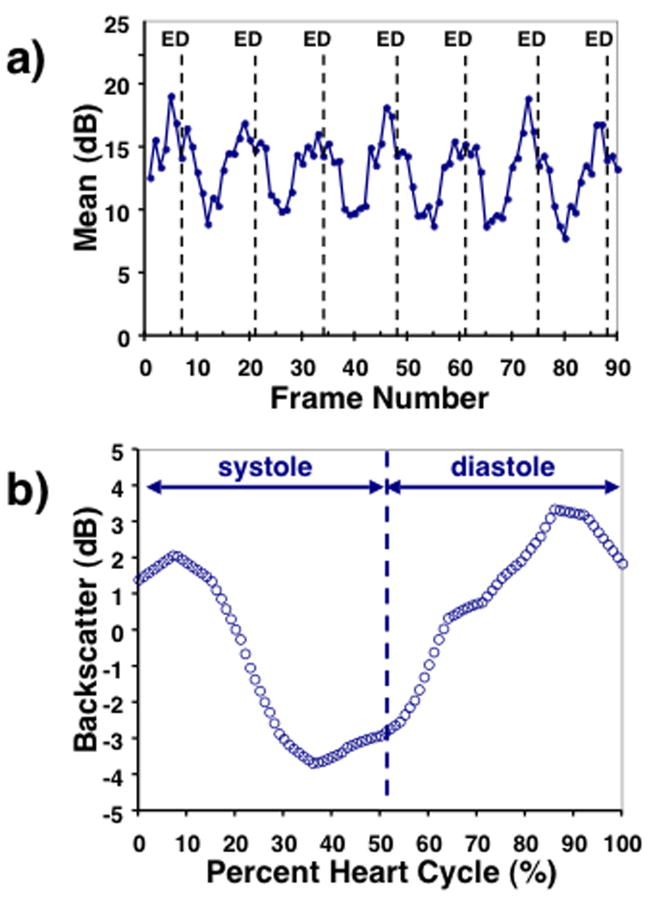
We define the magnitude of cyclic variation as the difference between the average peak and average nadir values of backscatter.31, 37–39 Individual heart cycles were delineated by determining the image frames closest to end-diastole from analysis of the wall motion in the acquired echocardiogram. Similarly, the end-systolic frames for each heart cycle were identified. For each set of cyclic variation data, a composite cyclic variation waveform was constructed by averaging the individual cyclic variation data for each heart cycle. This was accomplished by plotting the measured backscatter as a function of the percent of the individual heart cycle, linearly interpolating values between measured frames, and averaging the individual cyclic variation data. The composite cyclic variation data was expressed as a zero-mean waveform and low-pass binomial filtering was applied to reduce noise. The magnitude of cyclic variation was determined using a previously described automated algorithm based on Fourier decomposition of the cyclic variation data and the average systolic and diastolic intervals.37, 38 Figure 2 illustrates an example of the measured cyclic variation data and the corresponding composite cyclic variation curve from the left-ventricular free wall of one of the fetuses.
Figure 2.
a) Example of the measured cyclic variation of backscatter expressed in dB for 6 heart cycles from the left-ventricular free wall of one of the fetuses with the end-diastolic frames indicated by “ED”. b) Corresponding composite cyclic variation curve. The average systolic and diastolic intervals are indicated.
Results
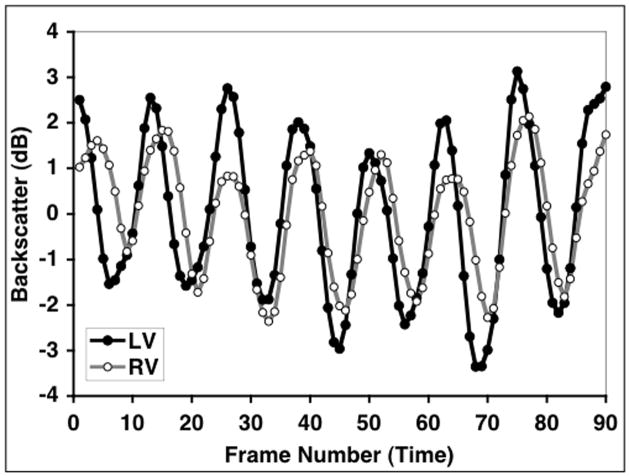
Echocardiographic images from a total of 21 fetuses were analyzed for inclusion in this study of which the cyclic variation data from both the left- and right-ventricular free walls from 16 of them was of sufficient quality to make meaningful magnitude estimates. Fetal position, motion, or acoustic shadowing from ribs, spine, and/or limbs prohibited measurements to be made simultaneously from both sides of the heart in the remaining 5 subjects. Figure 3 illustrates an example of the cyclic variation data from the left- and right-ventricular free walls acquired from one of the fetal subjects. (In order to illustrate differences in the magnitudes of the cyclic variation from the two sides of the heart more clearly, these data are plotted as low-pass binomial filtered, zero-mean curves.)
Figure 3.
Cyclic variation data from the left- and right-ventricular free walls acquired from one of the fetal subjects. These data have been low-pass (binomial) filtered and plotted as zero-mean curves in order to illustrate differences in the magnitudes of the cyclic variation from the two sides of the heart.
Figure 4 depicts the measured magnitudes of the cyclic variation from the left and the right ventricular free walls of each fetus along with the corresponding mean values. Results demonstrate that the magnitude of cyclic variation is greater for the left ventricular free wall than for the right ventricular free wall, with measured mean magnitudes of cyclic variation of 4.5 ± 1.1 dB and 2.3 ± 0.9 dB (mean ± SD; p < 0.0001, paired t-test; p < 0.0001, unpaired t-test) for the left and right ventricular free walls, respectively. To examine the intra- and inter-observer variability of the cyclic variation measurements, a randomly chosen subset of 8 fetal echocardiograms were reanalyzed. A Bland-Altman type of analysis was utilized to quantify the intra- and inter-observer variability in comparing the original measurements with the reanalyzed data.40 The intra-observer variability of the measured magnitude of cyclic variation was found to be 0.0 ± 1.7 dB and 0.2 ± 1.8 dB (mean difference ± 2 standard deviations) for the left and right ventricular free walls, respectively. The corresponding inter-observer variability was found to be 0.1 ± 1.8 dB and 0.2 ± 2.5 dB (mean difference ± 2 standard deviations) for the left and right ventricular free walls, respectively.
Figure 4.
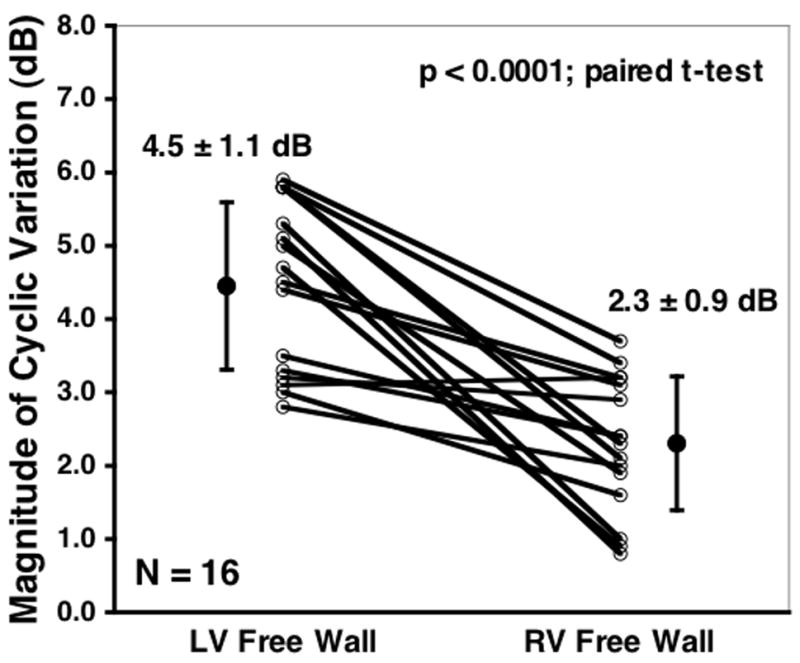
Measured magnitudes of the cyclic variation of backscatter from the left and the right ventricular free walls of each fetus along with the corresponding mean values.
Measurements of the thicknesses of the left and right ventricular walls for the 16 subjects analyzed ranged from 0.12 cm to 0.30 cm for the left ventricular free wall and from 0.12 cm to 0.30 cm for the right ventricular free wall. Fetal wall thickness was primarily determined by the gestational age of the fetus.
Discussion
Our study shows the feasibility of an in vivo process for the assessment of myocardial properties that can be achieved through echocardiography-based analyses in developing hearts of human fetuses. The study identifies intrinsic ultrasonic myocardial properties of developing right and left ventricles in normal human fetuses at mid-trimester, which distinguish them from each other and substantiates the concept that they differ from early stages in their morphological and mechanical properties despite the exposure to similar prenatal myocardial maturation process and loading conditions. These intrinsic myocardial properties can be inferred from measurements of magnitude of cyclic variation.
Measurements show that the magnitude of cyclic variation is significantly greater for the left ventricular free wall than for the right ventricular free wall in human fetuses at mid-gestation. It may be instructive to determine whether this observation is consistent with what might be anticipated based on previous measurements of the apparent ultrasonic backscatter properties of developing hearts of fetal pigs27 and the predicted relationship between the overall scattering level of myocardium and the magnitude of cyclic variation based on a previously-described model of the backscatter properties of myocardium26.
Recently our laboratory reported measurements of the backscatter properties of fetal pig hearts at mid-gestation.27 In this study, integrated backscatter measurements from excised, formalin-fixed fetal pig heart specimens were acquired using a high-frequency measurement system. Results demonstrated that the backscatter level from the right ventricular myocardium was larger than that from the left ventricular myocardium (−35.9 ± 2.0 dB vs. −40.1 ± 1.9 dB for the right- and left-ventricular myocardium, respectively; mean ± standard deviation; N = 16; p < 0.001). This study suggests that the intrinsic ultrasonic backscatter properties of the left and right ventricular myocardium are distinct in fetal pig hearts at mid-gestation. These fetal pig results are consistent with previous measurements comparing the backscatter properties of the left and right ventricular myocardium in mature adult human and canine hearts that appear to correlate well with collagen content.15
Model Relating the Level of Backscatter and the Predicted Magnitude of Cyclic Variation
A relationship between the measured magnitude of cyclic variation and the overall level of myocardial backscatter has been previously described in a paper from our laboratory.26 In this approach, contributions to the observed cyclic variation of backscattered energy results from a systematic variation in the relative differences between intracellular and extracellular characteristic acoustic impedances (ΔZ) over the heart cycle.13, 26 During diastole the difference between intracellular and extracellular characteristic acoustic impedances is relatively large, resulting in a relatively large level of backscatter. However, during systole, the intracellular acoustic impedance becomes closer in value to the larger extracellular impedance resulting in a reduction in acoustic impedance mismatch and, hence, the level of backscatter. It is thought that this may represent one of the mechanisms contributing to the observed cyclic variation of backscatter over the heart cycle.
Establishing a relationship between the observed magnitude of cyclic variation and the level of myocardial backscatter can be achieved by keeping the respective systolic and diastolic intracellular acoustic impedances the same while systematically increasing the extracellular acoustic impedance (keeping the systolic and diastolic values of the extracellular acoustic impedance equal). This approach is schematically illustrated in Figure 5a. Systematically increasing the difference between the intracellular and extracellular impedances results in a larger overall level of backscatter. As this impedance mismatch increases, the fractional change in impedance difference between the intra- and extracellular components over the heart cycle is altered, resulting in predicted changes in the magnitude of cyclic variation of backscatter. Figure 5b depicts the relationship between the magnitude of cyclic variation as a function of the overall myocardial backscatter level predicted from this model. This figure shows that the predicted magnitude of cyclic variation decreases as the backscatter level (e.g., end-diastolic backscatter level) increases.
Figure 5.
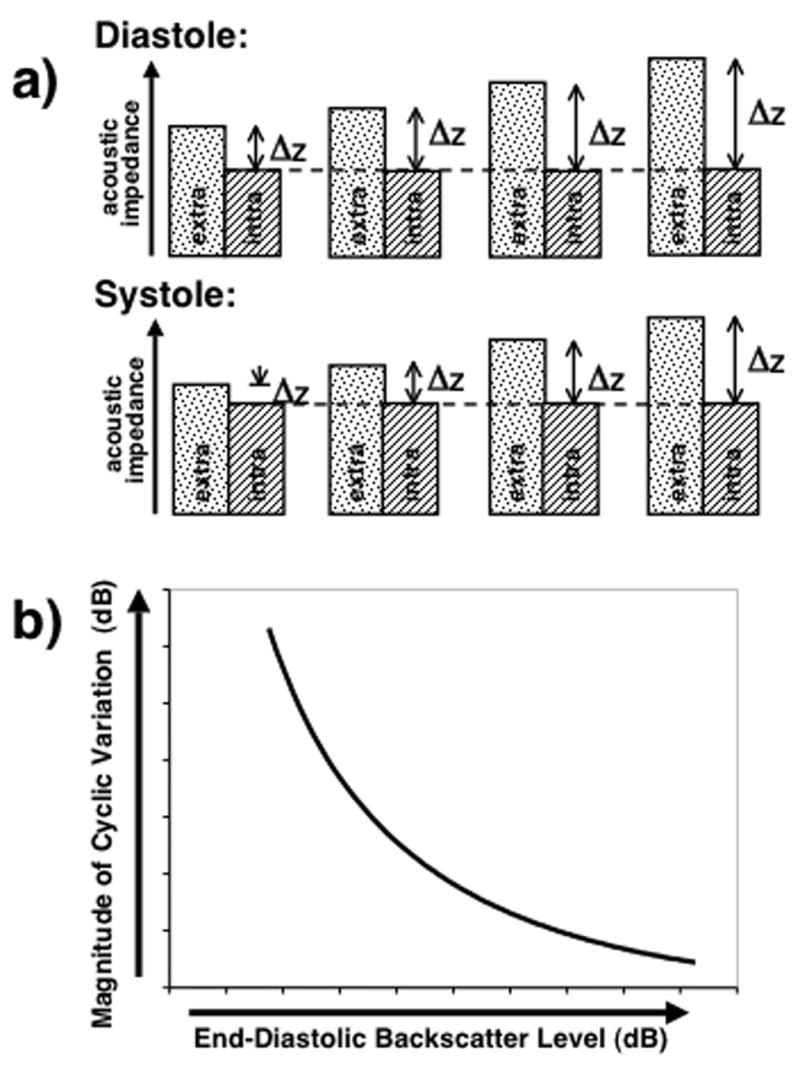
a) Schematic illustration showing the method of establishing a relationship between the magnitude of cyclic variation and the overall myocardial backscatter level by keeping the respective systolic and diastolic intracellular acoustic impedances the same while systematically increasing the extracellular acoustic impedance (keeping the systolic and diastolic values of the extracellular acoustic impedance equal). b) Predicted relationship between the magnitude of cyclic variation and the overall myocardial backscatter level.
Using this approach, we predicted that the magnitude of cyclic variation should be less for regions of myocardium exhibiting a greater level of backscatter. Hence, based on the previously reported measurements from our laboratory of mid-gestation fetal pig hearts27 where it was found that the apparent backscatter from the right ventricular myocardium is greater than that of the left, we anticipated that the magnitude of the cyclic variation from the left ventricular free wall to be greater than that of the right ventricular free wall as depicted in Figure 6. This prediction was consistent with the results of our measurements that demonstrate that the magnitude of the cyclic variation from the left ventricular free wall is greater than that of the right ventricular free wall in fetal human hearts.
Figure 6.
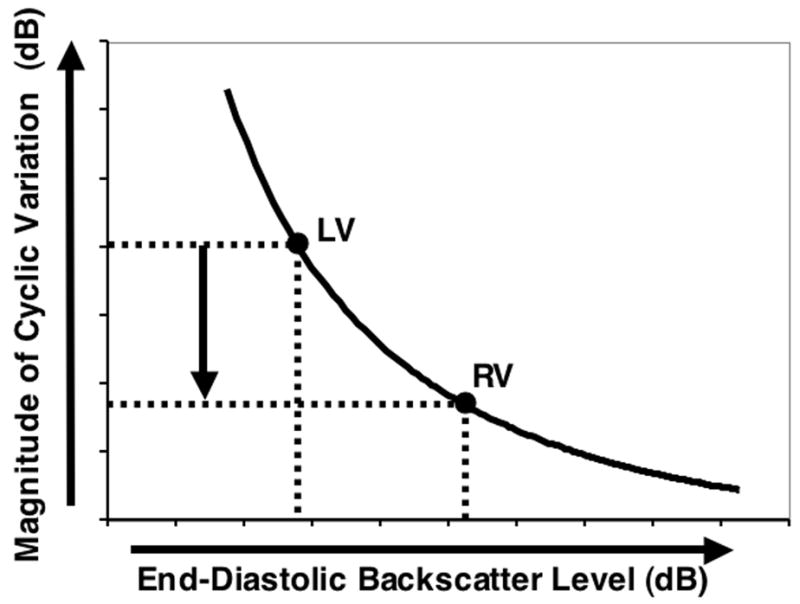
Predicted relative magnitude of the cyclic variation of backscatter from the left ventricular free wall compared with that from the right ventricular free wall. The predicted magnitude from the left ventricular free wall is greater than that from the right ventricular free wall consistent with the measured values.
Clinical Implication
These observed differences in the acoustic properties of the left and right sides of fetal hearts appear consistent with previously published studies demonstrating differences in the constituent properties of each side. Embryologically, the heart develops from a linear cardiac tube, which is segmentally patterned along the anterior–posterior axis into precursors of different cardiac chambers. Although individual cardiac chambers do not become morphologically distinguishable until after cardiac looping, their cell fates seem to be genetically programmed much earlier. Each cardiac chamber differs in its morphological and contractile properties1 and studies suggest that the left- and right-ventricular myocardium develop differently.41–43
In a study of sheep hearts, Smolich et al.42 describe distinct differences between the left and right ventricular myocardium during fetal and postnatal development. They report measurements of larger myocyte cross-sectional areas, capillary luminal areas, and intercapillary distances in the right ventricular myocardium than those found in the left. In addition, they found smaller myocyte densities, capillary densities, and myocyte matrix volume densities in the right ventricular myocardium than those associated with the left. In a study by Salih et al.43 examining neonatal human heart specimens, they report a higher percentage of collagen in the right-ventricular myocardium than that found in the left-ventricular myocardium. However, how these chamber morphological identities are established is unknown, and how they can be prenatally characterized non-invasively is not well described. Formation of the heart involves a precisely orchestrated series of molecular and morphogenetic events and even subtle perturbation of this process can affect the ultimate phenotype manifesting in the form of structural congenital and myopathic heart disease.1 A prenatal recognition of early phenotypic changes may provide a clue to the development of such congenital and myopathic heart disease and, perhaps, subsequent manifestation of adult onset cardiovascular diseases44. To this end, early recognition of normal chamber morphological identities is a first step.
As a specific example of how cyclic variation measurements may provide clinically applicable indices for characterizing the fetal heart, we speculate that these measurements may help elucidate the intrinsic compositional and myoarchitectural properties of the myocardium of fetuses with Hypoplastic Left Heart Syndrome (HLHS) and Tetralogy of Fallot (TOF). Studies suggest that abnormal myoarchitecture and extracellular collagenous matrix exist from the fetal stage in the right ventricle of HLHS and TOF 43, 45, which, in the presence of patient- and procedure-specific “risk” factors may not lend the right ventricle to favorably remodel to the hemodynamic load both prior to and after surgery, resulting in detrimental ventricular diastolic and systolic pump function. Knowledge of the intrinsic myocardial properties during the fetal stage may help in appraisal of the suitability of the right ventricle as the systemic ventricle for maintaining systemic circulation after postnatal reconstructive cardiac surgery.
Study Limitations
The results of this study demonstrating a significant difference in the measured magnitude of cyclic variation from the left and right ventricular free walls from human fetuses, consistent with model predictions, are promising. However, there are limitations that may need to be considered. The acquisition of useful cyclic variation data from fetuses can be difficult. As described in the results section, suboptimal fetal positioning and the presence of artifacts (e.g., spine shadowing) can make the acquisition of suitable images technically challenging. In order to reduce the effects of myocardial anisotropy on cyclic variation measurements31, only long-axis transverse views of the fetal heart were acquired and analyzed. In this view, the insonifying beam remains primarily perpendicular to the predominate myofiber orientation, reducing the effects of anisotropy.29, 32–35
The relationship between the level of backscatter from the left and right ventricular free walls was based on high-frequency measurements from excised, formalin-fixed fetal pig hearts and used as a basis for predicting the corresponding relationship of the magnitudes of cyclic variation in fetal human hearts. In spite of this limitation, the reported similarity between human and canine backscatter measurements comparing the left and right ventricular free walls in mature hearts15 suggests that observed differences are preserved across species. Furthermore, because high-frequency measurements from excised, formalin-fixed fetal pig hearts were used, only the relative magnitude of cyclic variation was predicted.
There are several mechanisms that may contribute to the observed cyclic variation of backscatter from myocardium over the heart cycle.8–10, 12, 13, 26, 46, 47 For example, changes in blood volume, fiber orientation, scatterer size, shape and concentration as well as relative intra-and extracellular acoustic impedances over the heart cycle may all play a role. In the model utilized in this study, only the relative changes in intra- and extracellular acoustic impedances over the heart cycle were incorporated. By obtaining long-axis transverse views of the heart, the insonifying beam remains primarily perpendicular to the predominant myofiber orientation over the heart cycle, thus reducing the effects of changes in fiber orientation. Despite these limitations, the model utilized in this study appears to work well and seems to predict the observed results in this study as well as other cyclic variation studies.26
Conclusions
Results of this study demonstrate that it is feasible to measure the cyclic variation of backscatter from the hearts of fetuses at mid-gestation and that there is a significant difference in the measured magnitude of cyclic variation from the left and right ventricular free walls. This observation is consistent with that predicted using a model of the relationship between overall backscatter level and the magnitude of cyclic variation incorporating previously reported differences in the level of backscatter between the left and right ventricular myocardium of fetal pig hearts. The results of this study suggest fetal cyclic variation measurements may offer a useful approach for investigating fundamental differences in myocardial properties of the two ventricles during heart development. This method may provide a noninvasive approach for assessing relationships among myocardial stiffness properties and contractile performance in developing hearts and may contribute to the development of new and improved methods for assessing cardiac development that complement conventional fetal echocardiographic imaging techniques.
Acknowledgments
We would like to thank James G. Miller, PhD of Washington University in St. Louis for his useful suggestions regarding this study and manuscript preparation. This study was supported, in part, by NIH R01 HL040302.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Srivastava D, Olson EN. A Genetic Blueprint for Cardiac Development. Nature. 2000;407:221–227. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 2.Buckberg GD, Weisfeldt ML, Ballester M, Beyar R, Burkhoff D, Coghlan HC, et al. Left ventricular form and function: scientific priorities and strategic planning for development of new views of disease. Circulation. 2004;110:e333–6. doi: 10.1161/01.CIR.0000143625.56882.5C. [DOI] [PubMed] [Google Scholar]
- 3.Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH. Left Ventricular Fibre Architecture in Man. Br Heart Journ. 1981;45:248–263. doi: 10.1136/hrt.45.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jouk PS, Usson Y, Michalowicz G, Grossi L. Three-dimensional cartography of the pattern of the myofibres in the second trimester fetal human heart. Anat Embryol (Berl) 2000;202:103–18. doi: 10.1007/s004290000103. [DOI] [PubMed] [Google Scholar]
- 5.LeGrice IJ, Smaill BH, Chai LZ, Edgar SG, Gavin JB, Hunter PJ. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol. 1995;269:H571–82. doi: 10.1152/ajpheart.1995.269.2.H571. [DOI] [PubMed] [Google Scholar]
- 6.Scollan DF, Holmes A, Winslow R, Forder J. Histological validation of myocardial microstructure obtained from diffusion tensor magnetic resonance imaging. Am J Physiol. 1998;275:H2308–18. doi: 10.1152/ajpheart.1998.275.6.H2308. [DOI] [PubMed] [Google Scholar]
- 7.Spotnitz HM. Macro design, structure, and mechanics of the left ventricle. J Thorac Cardiovasc Surg. 2000;119:1053–77. doi: 10.1016/S0022-5223(00)70106-1. [DOI] [PubMed] [Google Scholar]
- 8.Hall CS, Scott MJ, Lanza GM, Miller JG, Wickline SA. The extracellular matrix is an important source of ultrasound backscatter from myocardium. J Acoust Soc Am. 2000;107:612–9. doi: 10.1121/1.428327. [DOI] [PubMed] [Google Scholar]
- 9.Kumar KN, Mottley JG. Quantitative Modeling of the Anisotropy of Ultrasonic Backscatter from Canine Myocardium. IEEE Trans Ultrasonics Ferroelect Freq Contr. 1994;41:441–450. [Google Scholar]
- 10.O’Brien PD, O’Brien WD, Jr, Rhyne TL, Warltier DC, Sagar KB. Relation of ultrasonic backscatter and acoustic propagation properties to myofibrillar length and myocardial thickness. Circulation. 1995;91:171–5. doi: 10.1161/01.cir.91.1.171. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien WD, Jr, Sagar KB, Warltier DC, Rhyne TL. Acoustic Propagation Properties of Normal, Stunned, and Infarcted Myocardium: Morphological and Biochemical Determinants. Circulation. 1995;91:154–160. doi: 10.1161/01.cir.91.1.154. [DOI] [PubMed] [Google Scholar]
- 12.Rose JH, Kaufmann MR, Wickline SA, Hall CS, Miller JG. A Proposed Microscopic Elastic Wave Theory for Ultrasonic Backscatter from Myocardial Tissue. J Acoust Soc Am. 1995;97:656–668. doi: 10.1121/1.412288. [DOI] [PubMed] [Google Scholar]
- 13.Wickline SA, Thomas LJ, 3rd, Miller JG, Sobel BE, Perez JE. A relationship between ultrasonic integrated backscatter and myocardial contractile function. J Clin Invest. 1985;76:2151–60. doi: 10.1172/JCI112221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyt RH, Collins SM, Skorton DJ, Ericksen EE, Conyers D. Assessment of fibrosis in infarcted human hearts by analysis of ultrasonic backscatter. Circulation. 1985;71:740–4. doi: 10.1161/01.cir.71.4.740. [DOI] [PubMed] [Google Scholar]
- 15.Hoyt RM, Skorton DJ, Collins SM, Melton HE., Jr Ultrasonic backscatter and collagen in normal ventricular myocardium. Circulation. 1984;69:775–82. doi: 10.1161/01.cir.69.4.775. [DOI] [PubMed] [Google Scholar]
- 16.Mimbs JW, O’Donnell M, Bauwens D, Miller JG, Sobel BE. The Dependence of Ultrasonic Attenuation and Backscatter on Collagen Content in Dog and Rabbit Hearts. Circulation Research. 1980;47:49–58. doi: 10.1161/01.res.47.1.49. [DOI] [PubMed] [Google Scholar]
- 17.Mimbs JW, O’Donnell M, Miller JG, Sobel BE. Detection of cardiomyopathic changes induced by doxorubicin based on quantitative analysis of ultrasonic backscatter. Am J Cardiol. 1981;47:1056–60. doi: 10.1016/0002-9149(81)90212-5. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen CT, Hall CS, Scott MJ, Zhu Q, Marsh J, Wickline SA. Age-related alterations of cardiac tissue microstructure and material properties in Fischer 344 rats. Ultrasound Med Biol. 2001;27:611–9. doi: 10.1016/s0301-5629(01)00343-x. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell M, Mimbs JW, Miller JG. Relationship between collagen and ultrasonic backscatter in myocardial tissue. J Acoust Soc Am. 1981;69:580–8. doi: 10.1121/1.385433. [DOI] [PubMed] [Google Scholar]
- 20.Pohlhammer J, O’Brien WD., Jr Dependence of the ultrasonic scatter coefficient on collagen concentration in mammalian tissues. J Acoust Soc Am. 1981;69:283–5. doi: 10.1121/1.385349. [DOI] [PubMed] [Google Scholar]
- 21.Wong AK, Osborn TG, Miller JG, Wickline SA. Quantification of ventricular remodeling in the tight-skin mouse cardiomyopathy with acoustic microscopy. Ultrasound Med Biol. 1993;19:365–74. doi: 10.1016/0301-5629(93)90055-s. [DOI] [PubMed] [Google Scholar]
- 22.Madaras EI, Barzilai B, Perez JE, Sobel BE, Miller JG. Changes in Myocardial Backscatter Throughout the Cardiac Cycle. Ultrasonic Imaging. 1983;5:229–239. doi: 10.1177/016173468300500303. [DOI] [PubMed] [Google Scholar]
- 23.Perez JE, Holland MR, Barzailai B, Handley SM, Vandenberg BF, Miller JG, et al. Ultrasonic Characterization of Cardiovascular Tissue. In: Skorton DJ, Seelbert HR, Wolf GL, Brundage BH, editors. Cardiac Imaging - A Companion to Braunwald’s Heart Disease. 2. Vol. 1. W. B. Saunders Co.; 1996. pp. 606–622. [Google Scholar]
- 24.Goens MB, Karr SS, Martin GR. Cyclic variation of integrated ultrasound backscatter: normal and abnormal myocardial patterns in children. J Am Soc Echocardiogr. 1996;9:616–21. doi: 10.1016/s0894-7317(96)90056-5. [DOI] [PubMed] [Google Scholar]
- 25.Mori K, Manabe T, Nii M, Hayabuchi Y, Kuroda Y, Kitahata H. Cyclic variation of integrated ultrasound backscatter in the left ventricle during the early neonatal period. American Heart Journal. 2000;140:463–468. doi: 10.1067/mhj.2000.108515. [DOI] [PubMed] [Google Scholar]
- 26.Holland MR, Wallace KD, Miller JG. Potential relationships among myocardial stiffness, the measured level of myocardial backscatter (“image brightness”), and the magnitude of the systematic variation of backscatter (cyclic variation) over the heart cycle. J Am Soc Echocardiogr. 2004;17:1131–7. doi: 10.1016/j.echo.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Gibson AA, Singh GK, Kulikowska A, Wallace KD, Hoffman JJ, Ludomirsky A, et al. Regional variation in the measured apparent ultrasonic backscatter of mid-gestational fetal pig hearts. Ultrasound Med Biol. 2007;33:1955–1962. doi: 10.1016/j.ultrasmedbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Holland MR, Gibson AA, Peterson LR, Areces M, Schaffer JE, Perez JE, et al. Measurements of the Cyclic Variation of Myocardial Backscatter From Two-Dimensional Echocardiographic Images as an Approach for Characterizing Diabetic Cardiomyopathy. J CardioMetabolic Syndrome. 2006;1:149–152. doi: 10.1111/j.1559-4564.2006.05493.x. [DOI] [PubMed] [Google Scholar]
- 29.Holland MR, Kovacs A, Posdamer SH, Wallace KD, Miller JG. Anisotropy of Apparent Backscatter in the Short-Axis View of Mouse Hearts. Ultrasound Med Biol. 2005;31:1623–1629. doi: 10.1016/j.ultrasmedbio.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs A, Courtois MR, Weinheimer CJ, Posdamer SH, Wallace KD, Holland MR, et al. Ultrasonic Tissue Characterization of the Mouse Myocardium: Successful In-Vivo Cyclic Variation Measurements. J Am Soc Echocardiogr. 2004;17:883–892. doi: 10.1016/j.echo.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 31.Finch-Johnston AE, Gussak HM, Mobley J, Holland MR, Petrovic O, Pérez JE, et al. Cyclic Variation of Integrated Backscatter: Dependence of Time Delay on the Echocardiographic View Employed and the Myocardial Segment Analyzed. J Am Soc Echocardiogr. 2000;13:9–17. [PubMed] [Google Scholar]
- 32.Aygen M, Popp RL. Influence of the Orientation of Myocardial Fibers on Echocardiographic Images. Am J Cardiol. 1987;60:147–152. doi: 10.1016/0002-9149(87)91002-2. [DOI] [PubMed] [Google Scholar]
- 33.Holland MR, Wilkenshoff UM, Finch-Johnston AE, Handley SM, Perez JE, Miller JG. Effects of Myocardial Fiber Orientation in Echocardiography: Quantitative Measurements and Computer Simulation of the Regional Dependence of Backscattered Ultrasound in the Parasternal Short-Axis View. J Am Soc Echocardiogr. 1998;11:929–937. doi: 10.1016/s0894-7317(98)70134-8. [DOI] [PubMed] [Google Scholar]
- 34.Recchia D, Hall C, Shepard RK, Miller JG. Mechanisms of the View-Dependence of Ultrasonic Backscatter from Normal Myocardium. IEEE Trans Ultrason Ferroelec Freq Contr. 1995;42:91–98. [Google Scholar]
- 35.Recchia D, Miller JG, Wickline SA. Quantification of Ultrasonic Anisotropy in Normal Myocardium with Lateral Gain Compensation of Two-Dimensional Integrated Backscatter Images. Ultrasound Med Biol. 1993;19:497–505. doi: 10.1016/0301-5629(93)90125-8. [DOI] [PubMed] [Google Scholar]
- 36.Sosnovik DE, Baldwin SL, Lewis SH, Holland MR, Miller JG. Transmural Variation of Myocardial Attenuation Measured With a Clinical Imager. Ultrasound Med Biol. 2001;27:1643–1650. doi: 10.1016/s0301-5629(01)00485-9. [DOI] [PubMed] [Google Scholar]
- 37.Mobley J, Feinberg MS, Gussak HM, Banta CE, Perez JE, Miller JG. On-Line Implementation of Algorithm for Determination of Magnitude and Time Delay of Cyclic Variation of Integrated Backscatter in an Echocardiographic Imager. Ultrasonic Imaging. 1994;16:49. [Google Scholar]
- 38.Mohr GA, Vered Z, Barzilai B, Perez JE, Sobel BE, Miller JG. Automated Determination of the Magnitude and Time Delay (“Phase”) of the Cardiac Cycle Dependent Variation of Myocardial Ultrasonic Integrated Backscatter. Ultrasonic Imaging. 1989;11:245–259. doi: 10.1177/016173468901100403. [DOI] [PubMed] [Google Scholar]
- 39.Finch-Johnston AE, Gussak HM, Mobley J, Holland MR, Petrovic O, Perez JE, et al. Effect of Time Delay on the Apparent Magnitude of Cyclic Variation of Myocardial Ultrasonic Backscatter in Standard Echocardiographic Views. Ultrasonic Imaging. 1995;17:77. [Google Scholar]
- 40.Bland JM, Altman DG. Statistical Methods For Assessing Agreement Between Two Methods Of Clinical Measurement. Lancet. 1986:307–310. [PubMed] [Google Scholar]
- 41.Perles Z, Nir A, Gavri S, Rein AJ. Assessment of fetal myocardial performance using myocardial deformation analysis. Am J Cardiol. 2007;99:993–6. doi: 10.1016/j.amjcard.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 42.Smolich JJ, Walker AM, Campbell GR, Adamson TM. Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am J Physiol. 1989;257:H1–9. doi: 10.1152/ajpheart.1989.257.1.H1. [DOI] [PubMed] [Google Scholar]
- 43.Salih C, McCarthy KP, Ho SY. The fibrous matrix of ventricular myocardium in hypoplastic left heart syndrome: a quantitative and qualitative analysis. Ann Thorac Surg. 2004;77:36–40. doi: 10.1016/s0003-4975(03)01472-3. [DOI] [PubMed] [Google Scholar]
- 44.Barker DJ. The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proc Biol Sci. 1995;262:37–43. doi: 10.1098/rspb.1995.0173. [DOI] [PubMed] [Google Scholar]
- 45.Krymsky LD. Pathologic anatomy of congenital heart disease. Circulation. 1965;32:814–827. doi: 10.1161/01.cir.32.5.814. [DOI] [PubMed] [Google Scholar]
- 46.Gong L, Wang ZG, Ran HT, Ling ZY, Tang HL, Zheng YY, et al. Relationship between myocardial ultrasonic integrated backscatter and mitochondria of the myocardium in dogs. Clin Imaging. 2006;30:402–8. doi: 10.1016/j.clinimag.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 47.Micari A, Pascotto M, Jayaweera AR, Sklenar J, Goodman NC, Kaul S. Cyclic variation in ultrasonic myocardial integrated backscatter is due to phasic changes in the number of patent myocardial microvessels. J Ultrasound Med. 2006;25:1009–19. doi: 10.7863/jum.2006.25.8.1009. [DOI] [PubMed] [Google Scholar]