Abstract
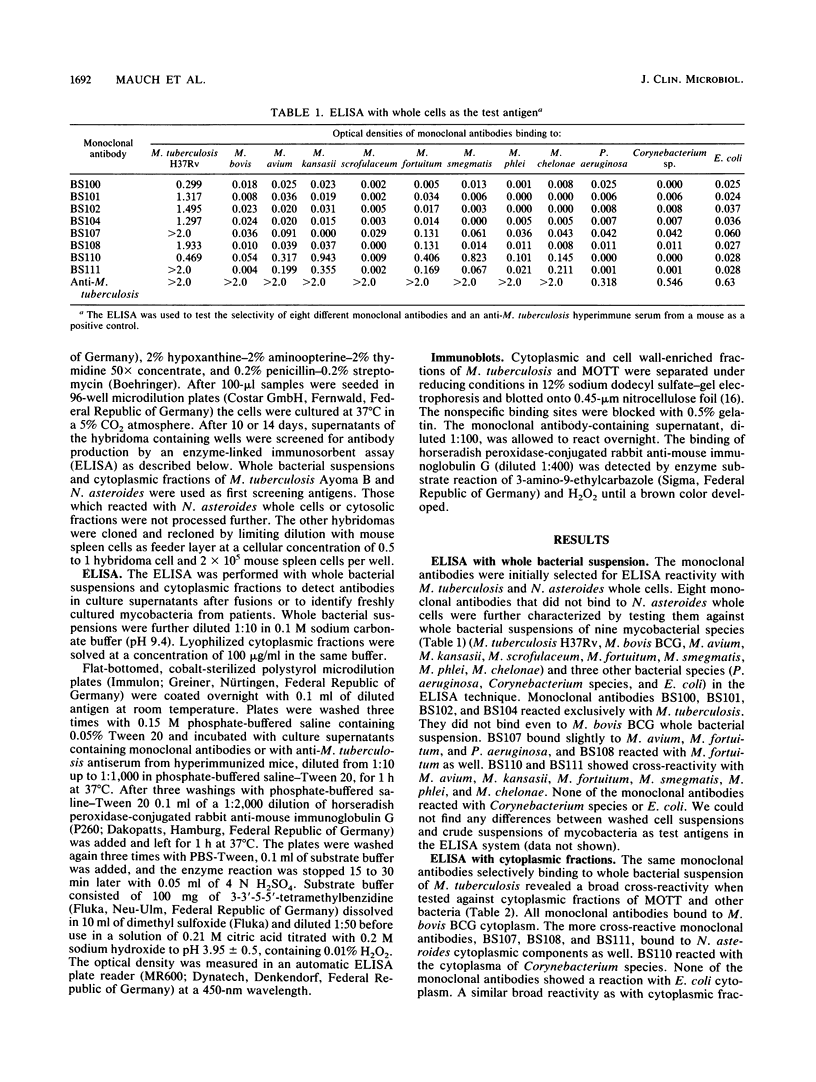
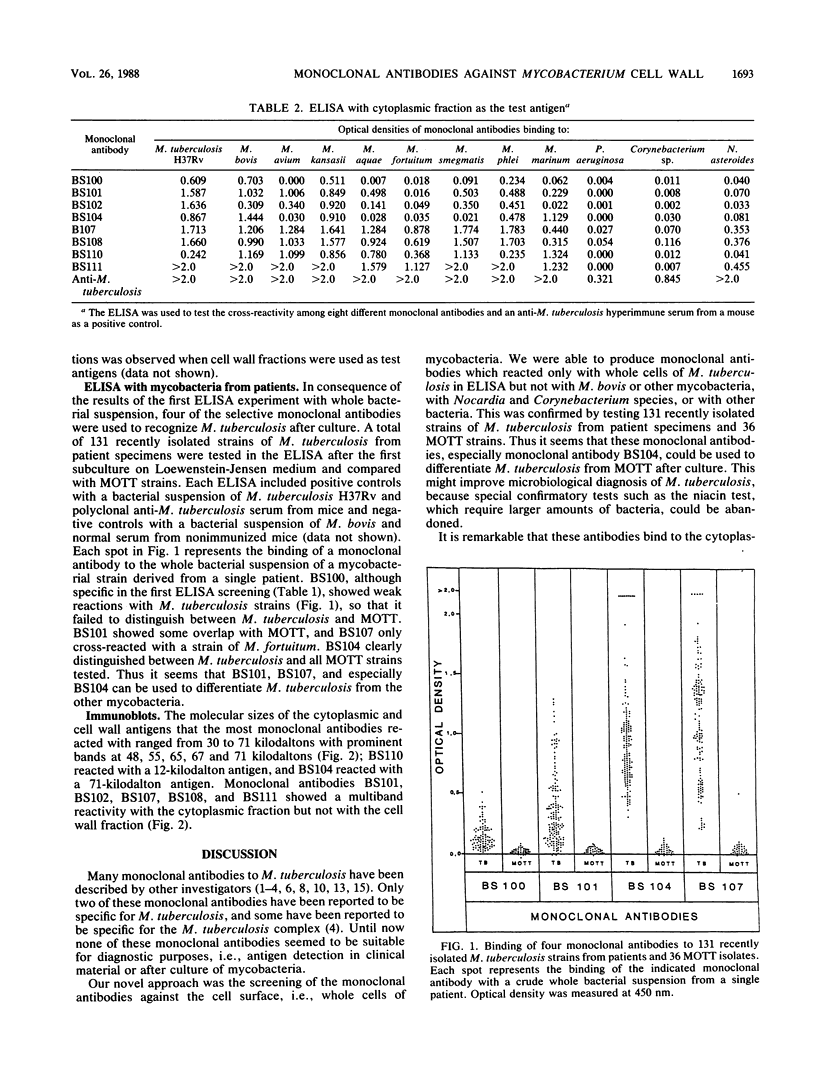
In the last few years several monoclonal antibodies against Mycobacterium tuberculosis have been described, but their use as diagnostic tools has been limited. In this study we describe eight monoclonal antibodies against M. tuberculosis for diagnostic purposes. The monoclonal antibodies were selected after enzyme-linked immunosorbent assay screening with whole bacterial suspensions of mycobacteria and other bacterial species. Four monoclonal antibodies (BS100, BS101, BS102, and BS104) reacted with a whole bacterial suspension of M. tuberculosis but not with the other mycobacteria. When tested with a cytoplasmic fraction of mycobacteria the same monoclonal antibodies showed a broad cross-reactivity. Therefore the monoclonal antibodies showed not specific but selective binding to M. tuberculosis. The molecular size of the recognized antigens ranged from 12 to 71 kilodaltons as determined by the immunoblotting technique. The ability to differentiate M. tuberculosis from mycobacteria other than tuberculosis was investigated by enzyme-linked immunosorbent assay with 131 freshly cultured strains of M. tuberculosis from patients and 36 strains of mycobacteria other than tuberculosis. The monoclonal antibody BS104 could clearly distinguish between M. tuberculosis and other mycobacterial species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Yuan Z. L., Hasløv K., Vergmann B., Bennedsen J. Interspecies reactivity of five monoclonal antibodies to Mycobacterium tuberculosis as examined by immunoblotting and enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Mar;23(3):446–451. doi: 10.1128/jcm.23.3.446-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Olds G. R. Demonstration of a shared epitope among mycobacterial antigens using a monoclonal antibody. Clin Exp Immunol. 1985 May;60(2):249–258. [PMC free article] [PubMed] [Google Scholar]
- Kadival G. V., Chaparas S. D. Production, characterization, and species specificity of five monoclonal antibodies to Mycobacterium tuberculosis. J Clin Microbiol. 1987 Jan;25(1):76–80. doi: 10.1128/jcm.25.1.76-80.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk A. H., Ho M. L., Klatser P. R., Eggelte T. A., Kuijper S., de Jonge S., van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984 Dec;58(3):511–521. [PMC free article] [PubMed] [Google Scholar]
- Mauch H., Brehmer W. Mycobacterial antibodies after tuberculin testing, BCG- vaccination, BCG-immunotherapy and against cross-reacting antigens in a solid-phase radioimmunoassay. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Mar;251(3):380–388. [PubMed] [Google Scholar]
- Miller R. A., Buchanan T. M. Production and characterization of a murine monoclonal antibody recognizing a shared mycobacterial polysaccharide. Int J Lepr Other Mycobact Dis. 1984 Dec;52(4):461–467. [PubMed] [Google Scholar]
- Minden P., Houghten R. A., Spear J. R., Shinnick T. M. A chemically synthesized peptide which elicits humoral and cellular immune responses to mycobacterial antigens. Infect Immun. 1986 Sep;53(3):560–564. doi: 10.1128/iai.53.3.560-564.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden P., Kelleher P. J., Freed J. H., Nielsen L. D., Brennan P. J., McPheron L., McClatchy J. K. Immunological evaluation of a component isolated from Mycobacterium bovis BCG with a monoclonal antibody to M. bovis BCG. Infect Immun. 1984 Nov;46(2):519–525. doi: 10.1128/iai.46.2.519-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
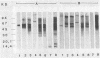
- Olds G. R., Sanson A. J., Daniel T. M. Characterization of Mycobacterium tuberculosis antigen 5 epitopes by using a panel of 19 monoclonal antibodies. J Clin Microbiol. 1987 Mar;25(3):471–475. doi: 10.1128/jcm.25.3.471-475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajki K., Brehmer W., Hammer H. J., Fischer W., Daus H., Mauch H. Analysis of the soluble cytoplasmic components of Mycobacteria and Nocardia by crossed immunoelectrofocusing. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Mar;251(3):389–398. [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986 Feb;51(2):718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou C., Yuan Z. L., Andersen A. B., Bennedsen J. Production and partial characterization of monoclonal hybridoma antibodies to Mycobacterium tuberculosis. Acta Pathol Microbiol Immunol Scand C. 1985 Dec;93(6):265–272. doi: 10.1111/j.1699-0463.1985.tb02955.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]