Abstract
The SIBLING protein family is a group of non-collagenous proteins (NCPs) that includes dentin sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP1), bone sialoprotein (BSP), and osteopontin (OPN). In the present study, we compared these four proteins in different phases of rat dentin and bone. First, we extracted NCPs in the unmineralized matrices and cellular compartments using guanidium-HCl (G1). Second, we extracted NCPs closely associated with hydroxyapatite using an EDTA solution (E). Last, we extracted the remaining NCPs again with guanidium-HCl (G2). Each fraction of Q-Sepharose ion-exchange chromatography was analyzed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), Stains-All stain, and with western immunoblotting. In dentin, the NH2-terminal fragment of DSPP and its proteoglycan form were primarily present in the G1 extract, whereas the COOH-terminal fragment of DSPP was present exclusively in the E extract. The processed NH2-terminal fragment of DMP1 was present in G1 and E extracts, whereas the COOH-terminal fragment of DMP1 existed mainly in the E extract. Bone sialoprotein was present in all three extracts of dentin and bone, whereas OPN was present only in the G1 and E extracts of bone. The difference in the distribution of the SIBLING proteins between organic and inorganic phases supports the belief that these molecular species play different roles in dentinogenesis and osteogenesis.
Keywords: bone sialoprotein, dentin matrix protein 1, dentin sialophosphoprotein, mineralized tissues, osteopontin
Dentin and bone are mineralized tissues that closely resemble each other in composition and mechanism of formation. Both are composed of collagen-rich organic matrices and of a mineral phase consisting of plate-like apatite crystals. During the formation of dentin and bone, odontoblasts and osteoblasts secrete an unmineralized collagen-rich matrix between a mineralization front and the cells, termed predentin and osteoid, respectively. Predentin and osteoid are mineralized when apatite crystals are deposited. In spite of many similarities, the two tissues have different developmental origins; odontoblasts originate from neural crest ectomesenchyme and experience epithelial–mesenchymal interactions during development, whereas osteoblasts of limb bone derive from somatic mesenchyme. Bone undergoes active remodeling, whereas dentin matrix is not remodeled.
In dentinogenesis and osteogenesis, type I collagen secreted by odontoblasts and osteoblasts forms the undergirding that is mineralized in a highly controlled manner. The importance of the correct collagen structure is clearly seen in patients with osteogenesis imperfecta resulting from mutations in the type I collagen gene. However, collagen alone does not initiate or control apatite crystal formation. The extracellular matrix (ECM) of dentin and bone contains a number of non-collagenous proteins (NCPs) that are believed to be responsible for initiating and modulating the mineralization of collagen fibers when predentin and osteoid are converted to dentin and bone, respectively. This belief is strongly supported by studies showing that mutations in, or knockout of, genes coding for certain NCPs are associated with phenotypic abnormalities in the mineralization of dentin and/or bone (1–10).
One category of NCPs is the SIBLING (Small Integrin-Binding LIgand, N-linked Glycoprotein) family, which includes dentin sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP1), bone sialoprotein (BSP), osteopontin (OPN), and matrix extracellular phosphoglycoprotein (MEPE) (11). The SIBLING family members, principally found in mineralized tissues, share some common features such as the presence of phosphorylation, glycosylation, and the RGD cell-binding sequence, as well as similarities in genomic organization and localization. In particular, DSPP and DMP1 share unique similarities in proteolytic processing and tissue localization (12, 13).
Dentin sialophosphoprotein is proteolytically processed into dentin sialoprotein (DSP) and dentin phosphoprotein (DPP), which originate from the NH2-terminal and COOH-terminal regions of the DSPP amino acid sequence, respectively (14). Dentin sialoprotein and DPP are abundant in the ECM of dentin, whereas the intact, full-length form, representing the whole sequence of DSPP, has never been identified. Genetic studies have linked mutations affecting DSP and DPP portions of DSPP with different forms of dentinogenesis imperfecta, suggesting distinct functions for each, which are related to the mineralization process (1–3). Dentin sialophosphoprotein null mice demonstrate phenotypes similar to the manifestations of human dentinogenesis imperfecta type III (4). These data demonstrate that DSPP and/or its processed fragments (DSP and DPP) are critical for the mineralization of dentin. Recently, a proteoglycan form of DSP (designated as DSP-PG in this investigation) has been isolated and characterized from dentin ECM (15–17). Thus, the ECM of dentin contains three variants derived from the DSPP amino acid sequence: (i) DSP, (ii) DPP, and (iii) DSP-PG, which vary greatly in biochemical structure. It is likely that these variants are distributed differently among individual compartments of the tooth and play different roles during dentinogenesis, although the exact mechanisms by which they function are largely unknown.
Dentin matrix protein 1 is an acidic phosphoprotein predominantly expressed in dentin and bone (18, 19); it is more prominent in the latter than in the former (20). The importance of DMP1 for dentin and bone mineralization has been demonstrated by knockout experiments in mice and by mutation studies in humans: DMP1 null mice show profound defects in the mineralization of bone and dentin (5, 6), and mutations in the Dmp1 gene result in autosomal-recessive hypophosphatemic rickets in humans (7). Like DSPP, DMP1 is present in the ECM of bone and dentin as (i) an NH2-terminal (37 kDa) fragment, (ii) a COOH-terminal (57 kDa) fragment (20), and (iii) a proteoglycan form (known as DMP1-PG) of the NH2-terminal fragment (21). These three forms, differing dramatically in structure, may be distributed differently among individual compartments of tooth and bone, and may have different functions in dentinogenesis and osteogenesis.
Bone sialoprotein is mainly expressed in bone, mineralizing cartilage, cementum, and dentin (22–26). The biological functions of BSP in mineralized tissues are largely unknown. Some data suggest that BSP acts as a nucleator for the formation of initial apatite crystals (27); then, as this mineral grows on the collagen matrix, it acts as an inhibitor in directing the growth of the crystals (28).
Although OPN is present in mineralized tissues in relatively large quantities, it is also expressed at a relatively high level in a variety of non-mineralized tissues and cells (29–31). In mineralized tissues, OPN is expressed in bone, cementum, predentin, and tertiary dentin. Some in vitro studies have shown that OPN is an effective inhibitor of apatite formation and growth (32–34); data from OPN null mice strengthen the conclusion that this protein may be a major inhibitory factor of mineralization (9).
The components of dentin and bone can be divided into two major phases: the inorganic phase and the organic phase; the former is composed of apatite crystals while the latter includes unmineralized collagen matrices along with NCPs (predentin and osteoid) and cellular compartments (odontoblasts, odontoblast processes, osteoblasts, osteocytes, and osteocyte processes). Based on the belief that these SIBLING members and/or their processed fragments may have different distribution patterns between the inorganic and organic phases of dentin and bone, we systematically analyzed DSPP, DMP1, BSP, and OPN in the two phases, employing a three-step extraction approach (35–37). After separation by ion-exchange chromatography, the processed fragments of DSPP and DMP1, BSP, and OPN were analyzed in each of the three extracts. From these studies, we found clear differences in the distribution of these SIBLING proteins, which provide newer information and clues about the potentially different roles of these molecules in dentinogenesis and/or osteogenesis.
Material and methods
Tissue preparation
To obtain NCPs from dentin, 400 incisors from rats (≈ 10 wk of age) were used. Approximately one-quarter of the apical portion of the incisor was cut off and discarded. Then, the dental pulp was first removed using a dental barbed broach and then by using vacuum aspiration. The periodontal tissues were removed by scraping on the root surface with a scalpel. After these pretreatments, the incisors were split into halves and placed in 4 M guanidium-HCl (Gdm-HCl) containing protease inhibitors (0.78 mg ml−1 of benzamidine-HCl, 0.18 mg ml−1 of sodium iodoacetate, 1.8 μg ml−1 of soybean trypsin inhibitor, 0.17 mg ml−1 of phenylmethylsufonyl fluoride, and 5 μg ml−1 of pepstatin) at 4°C for ≈ 15 h. Then, the Gdm-HCl solution was discarded. From 400 incisors, we obtained 28.15 g of dentin that was devoid of pulpal and periodontal tissues. The 28.15 g of dentin was then manually ground into powders that were ≈ 2–3 mm in diameter. These dentin powders were the starting materials, which were subject to a three-step extraction protocol as described below.
To obtain NCPs from bone, 100 hind legs from rats (≈ 10 wk of age) were used. The epiphyseal regions were cut off and discarded. After the soft tissues on the bone surface (including periosteum) had been removed by scraping with a scalpel, the bone shafts were split into halves. Bone marrow was removed by scraping and vacuum aspiration. Then, the bone shafts were placed in 4 M Gdm-HCl, containing protease inhibitors, at 4°C for ≈ 15 h, and the Gdm-HCl solution was discarded. From 100 legs, 56.25 g of bone shafts were obtained that were devoid of soft tissues. The 56.25 g of bone shafts were manually ground into powders that were ≈ 2–3 mm in diameter. These bone powders were the starting materials for extraction.
Extraction of NCPs from rat dentin and bone
The dentin and bone powders were subject to a three-step extraction protocol, as reported previously (35–37). Briefly, the dentin or bone powders were first extracted by 4 M Gdm-HCl containing protease inhibitors, but without EDTA, for 96 h (48 h, twice). This initial step extracted NCPs present in the unmineralized matrices (predentin and osteoid) as well as those existing in the cellular compartments (odontoblast processes, osteocytes, and osteocyte processes). Non-collagenous proteins derived from the first-step extraction were designated as G1 extract. Subsequently, NCPs were extracted with 0.5 M EDTA containing protease inhibitors (named E extract), but without Gdm-HCl, for 96 h (48 h, twice); this second step extracted proteins that were embedded in the mineralized phase (i.e. in the mineralized ECM of dentin and bone) and were tightly bound to apatite crystals. Lastly, the remainder of the demineralized dentin or bone matrices (after EDTA extraction) was extracted again with the solution of 4 M Gdm-HCl containing protease inhibitors, but without EDTA, for 96 h (48 h, twice), as in step 1; this last step extracted NCPs that were bound tightly to the non-extractable matrix and that were exposed after demineralization. The extracts from the last step of extraction were referred to as G2.
Separation of NCPs from rat dentin and bone
The three types of extracts – G1, E, and G2 – were first subjected to gel chromatography on a Sephacryl S-200 (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK) column (bed volume, 500 ml) at room temperature. For Sephacryl S-200 chromatography, all of the extract from each step was loaded onto the column; the elution buffer was 4 M Gdm-HCl in phosphate buffer (pH 7.4) and the flow rate was 0.5 ml min−1. The Sephacryl S-200 column separated NCPs into four major fractions: an earlier fraction known as ES1 (38, 39) contained a group of proteins of higher molecular weight, which included the processed fragments of DSPP and DMP1, BSP, and OPN. The later three fractions contained molecules of lower molecular weight, such as osteocalcin (38, 39), proteinase inhibitors, and EDTA; western immunoblotting analyses confirmed that these later fractions did not contain the above SIBLING proteins or their processed fragments (data not shown).
Next, all the ES1 fraction from the extract of each step was loaded onto a Q-Sepharose (Amersham Biosciences, Piscataway, NJ, USA) ion-exchange column (bed volume, 210 ml) connected to a fast protein liquid chromatography system. Western immunoblotting analyses performed on the flow-through samples (containing molecules unbound to the column) showed that a negligible amount of the four SIBLING proteins was present in the flow-through, indicating that nearly all of the DSPP, DMP1, BSP, and OPN proteins were bound to the column. These were eluted within a gradient ranging from 0.1 to 0.8 M NaCl in 6 M urea (pH 7.4) and the flow rate was set to 0.5 ml min−1 at room temperature. It should be noted that because of the extreme heterogeneity in size and electric charge among different molecular species of DSPP and DMP1, it was essential to first separate the NCPs by ion-exchange chromatography before these DSPP and DMP1 variants could be clearly detected. In ion-exchange chromatography, the proteoglycan forms of these molecules eluted in later fractions than the core protein forms (15, 21). Additionally, the ion-exchange chromatography greatly enriches components eluted at a given NaCl concentration.
Our objectives in this investigation were to compare qualitatively the presence of the four SIBLING proteins –DSPP, DMP1, BSP, and OPN – between the organic and inorganic phases of dentin and bone, and to assess their relative quantities in each phase. Q-Sepharose chromatography separated each of the six extracts (G1, E, and G2 for dentin and G1, E, and G2 for bone) into 92 fractions, of 8 ml each, in 6 M urea solution. Each chromatographic fraction was further separated with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed using Stains-All staining along with western immunoblotting. For all of the experiments, 40 μl of urea containing sample from a single chromatographic run of each extract was loaded for Stains-All staining and 10 μl of sample was used for western immunoblotting. To prevent potential artifactual degradation of NCPs, the samples were kept in either 4 M Gdm-HCl or 6 M urea during the entire extraction or separation procedure, and samples in 6 M urea taken directly from the chromatographic fractions were loaded onto SDS-PAGE.
Antibodies
A monoclonal antibody against BSP was recently generated in our laboratory using BSP isolated from rat bone as the antigen, employing approaches described previously (13, 15, 21). Briefly, BSP isolated from rat long bone was injected into the foot pad of BALB/c mice. Lymphocytes obtained from local lymphonodi were fused with P3 myeloma cells. Fusion cells showing positive reactions to BSP were further subcloned and expanded. We obtained a number of positive clones, among which clone 10D9.2 demonstrated a strong and highly specific immunoreaction to rat and human BSP on western immunoblotting. Clone 10D9.2, with an isotype of IgG1, was expanded in nude mice and purified by affinity chromatography through a protein G column (Harlan Bioproducts, Indianapolis, IN, USA).
The polyclonal antibody against the COOH-terminal region of mouse DMP1 (designated as anti-DMP1-C) was generated by Sigma Genosys (Woodlands, TX, USA), using an oligopeptide with the sequence AYHNKPIGDQDDND, which matches amino acid residues 485–498 of mouse DMP1. Note that this sequence is extremely highly conserved across species and is identical among mouse, rat, bovine, and human DMP1.
The other antibodies used in this study included monoclonal anti-DSP IgG2b 2G7.3 that is reactive to DSP and DSP-PG (15), monoclonal anti-DMP1 IgG2b 9B6.3 that specifically recognizes the NH2-terminal fragment of DMP1 including DMP1-PG (21), and polyclonal rabbit anti-OPN serum (39).
SDS-PAGE and western immunoblotting
For Stains-All staining and western immunoblotting, 5–15% SDS-PAGE gradient gels were utilized for all the experiments. Western immunoblotting was performed using a chemiluminescence protocol. Briefly, SDS-PAGE gels were transferred to a cationic membrane, Zeta Probe (Bio-Rad Laboratories, Hercules, CA, USA), at 100 V in transfer buffer (0.025 M Tris-HCl, 0.2 M glycine, 20% methanol) for 60 min. Blots were blocked in phosphate-buffered saline (PBS) containing 5% non-fat bovine milk and 0.1% Tween-20 at room temperature overnight. Blots were then incubated in PBS containing 5% non-fat bovine milk, 0.1% Tween-20, and a primary antibody at room temperature for 60 min. The dilution for the antibodies against DSP (2G7.3), the NH2-terminal fragment of DMP1 (9B6.3), BSP (10D9.2), and OPN was 1:2,000, while that for the antibody against the COOH-terminal fragment of DMP1 (anti-DMP1-C) was 1:1,000. Blots were then washed three times (for 15 min each wash) in PBS containing 0.2% Tween-20. Next, the blots were incubated in PBS containing 5% non-fat bovine milk and 0.1% Tween-20, together with a 1:5,000 dilution of alkaline phosphatase-conjugated anti-mouse IgG or anti-rabbit IgG. The blots were then washed again (three times, for 15 min each wash) in PBS containing 0.2% Tween-20. The blots were then incubated in the chemiluminescent substrate, CDP-Star (Ambion, Austin, TX, USA), for 5 min and exposed in a Kodak imaging system for 20 min.
Results
We analyzed every chromatographic fraction from each of the extracts by using SDS-PAGE, Stains-All, and western immunoblotting. To illustrate our results, Stains-All staining for all the fractions that might potentially contain any of the four SIBLING components or their processed fragments is shown, while for western immunoblotting analyses, representative fractions are shown.
SIBLING proteins in G1, E, and G2 extracts of dentin
The Q-Sepharose chromatography separated rat dentin NCPs into 92 fractions. The ion-exchange chromatography separation profile of dentin E extract (data not shown) resembled that reported previously by Linde et al. (35) and our group (39). Using relative chromatographic elution positions, Stains-All staining characteristics, and western immunoblotting analysis, the identities of the SIBLING proteins were determined.
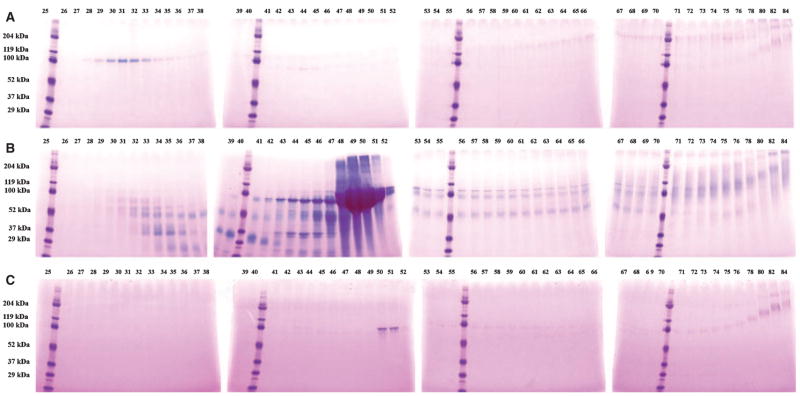
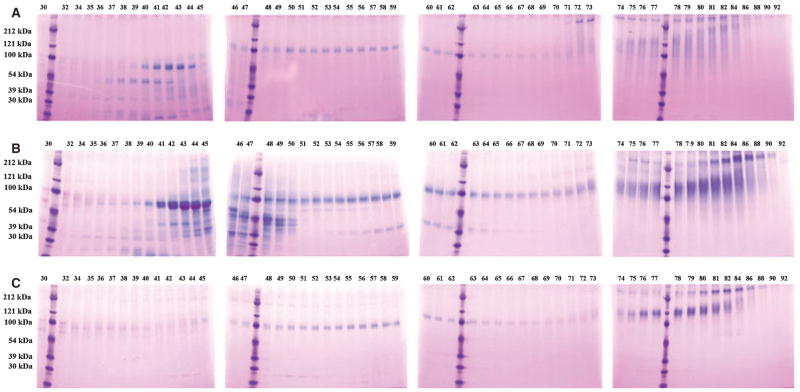
Figure 1 shows Stains-All staining of NCPs from fractions 25 to 84 of the three dentin extracts: DSP/DPP, DMP1 fragments, and BSP, and the proteoglycan forms of DSP and DMP1 (i.e. DSP-PG and DMP1-PG, respectively) were observed in these fractions. The broad protein bands migrating at 90–100 kDa in fractions 48–51 of E extract represent DPP, which is extremely abundant in the EDTA extract (Fig. 1B), but undetectable in the G1 (Fig. 1A) or G2 (Fig. 1C) extracts by Stains-All staining. The blue bands in fractions 29 to 33 of G1 extract, migrating around 100 kDa, represent DSP (also see Fig. 2A). The weak purple bands that migrate at ≈ 110 kDa in fraction 42 and progress to > 200 kDa in fraction 76 of G1 extract (Fig. 1A) contain the proteoglycan form of DSP (DSP-PG), which was confirmed by western immunoblotting (Fig. 2A).
Fig. 1.
Stains-All staining of fractions 25–84 of dentin G1 (A), E (B), and G2 (C) extracts. The digits at the top of a figure represent the fraction number after Q-Sepharose chromatography. Note the presence of a blue band at ≈ 100 kDa (representing dentin sialoprotein) in fractions 29–33 of the G1 extract (A) and the absence of this protein band in the E extract (B) and the G2 extract (C).
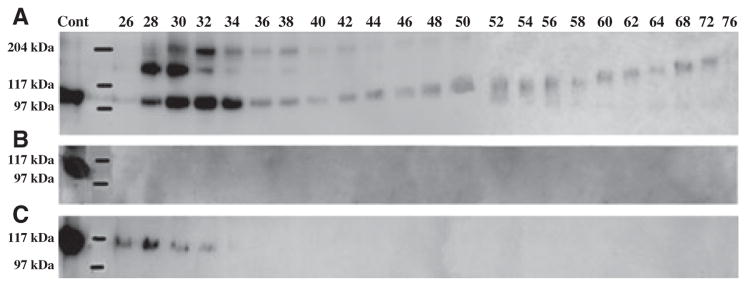
Fig. 2.
Western immunoblotting of dentin extracts using monoclonal anti-DSP IgG2b 2G7.3 as a probe. Dentin sialoprotein (DSP) (≈ 100 kDa in fractions 26–34) and the proteoglycan form of DSP (DSP-PG) (≈ 110 to ≈ 200 kDa in fractions 42–76) were detected in G1 extract (A), but not in E extract (B). Note the small amount of DSP in fractions 26–32 in G2 extract (C). Cont, 2 μg of DSP purified from rat dentin was used as a positive control.
Figure 2 shows the results of western immunoblotting using monoclonal anti-DSP IgG2b 2G7.3. Dentin sialoprotein was detected at the highest relative concentration in G1 (Fig. 2A), was absent in E (Fig. 2B), and was present in minor amounts in G2 (Fig. 2C) extracts. The proteoglycan form of dentin sialoprotein, migrating at > 110 kDa in earlier fractions and progressing up to > 200 kDa in later fractions, was only observed in G1. Previously, we have established the elution position of DSP-PG; the migration rates of DSP-PG observed in this study were very similar to those described in our previous publication (15). Recently, we performed disaccharide analyses on DSP-PG isolated from these fractions by elution through a monoclonal antibody affinity column and found that the glycosaminoglycan side-chain of DSP-PG was made of chondroitin-4-sulphates.
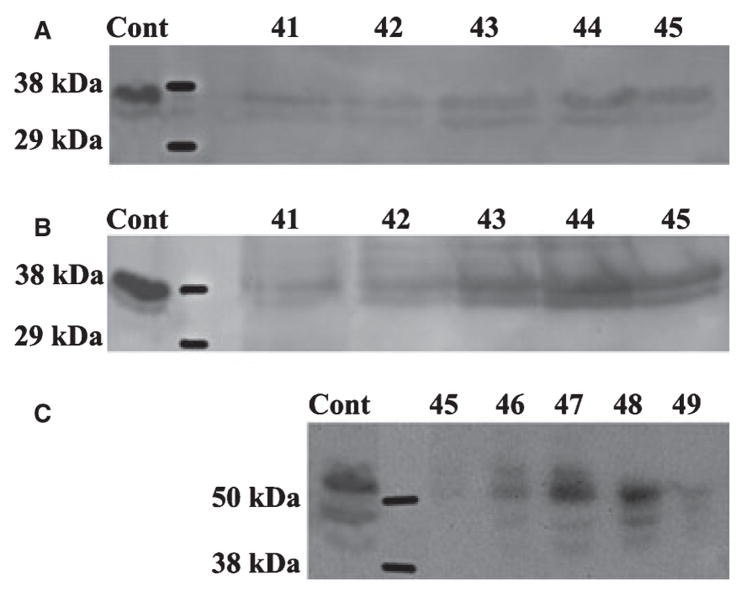
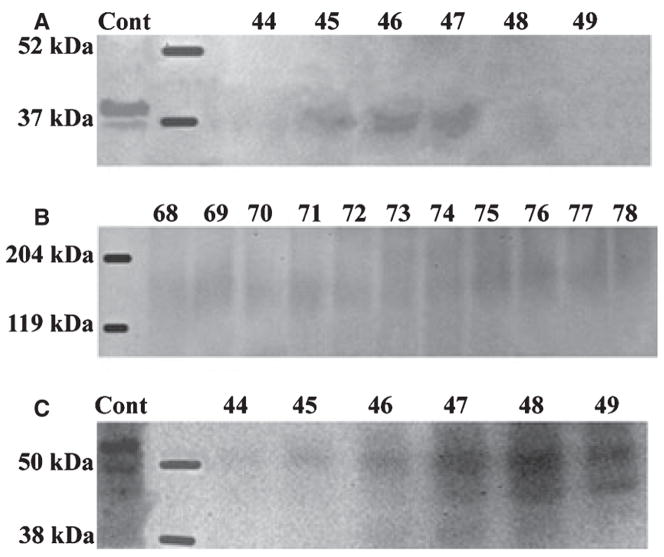
The NH2-terminal (≈ 37 kDa) fragment of DMP1 was detected in G1 and E (Fig. 3A,B) extracts, was absent in G2 extract, while the COOH-terminal (≈ 57 kDa) fragment of this protein was primarily found in E extract (Fig. 3C). The 37 kDa fragment eluted in earlier fractions (i.e. at a lower concentration of NaCl) than the 57 kDa fragment; at the end of the elution of the 37 kDa fragment (fraction 45), these two fragments co-eluted. This elution profile was in agreement with the fact that the 57 kDa fragment is more acidic than the 37 kDa fragment (20). A proteoglycan form of dentin matrix protein 1 was observed in the G1 extract.
Fig. 3.
Western immunoblotting of dentin extracts using anti-DMP1 IgG2b. (A) The 37 kDa fragment of dentin matrix protein 1 (DMP1) was detected in fractions 41–45 of the dentin G1 extract by monoclonal anti-DMP1 IgG2b 9B6.3 that specifically recognizes the NH2-terminal region of DMP1. (B) The 37 kDa fragment of DMP1 was also detected in similar fractions of dentin E extract by antibody 9B6.3. (C) The 57 kDa fragment of DMP1 was detected in fractions 45–49 of dentin E extract by polyclonal anti-DMP1-C that specifically recognizes the COOH-terminal region of DMP1. Note that more than one immunoreactive band for anti-DMP1 was present. We believe that the double or triple bands of DMP1 represent the processed products of DMP1 resulting from cleavage at several sites (20). The positive control (Cont) in panels A and B was 4 μg of 37 kDa fragment purified from rat bone. The positive control (Cont) in panel C was 4 μg of 57 kDa fragment purified from rat bone.
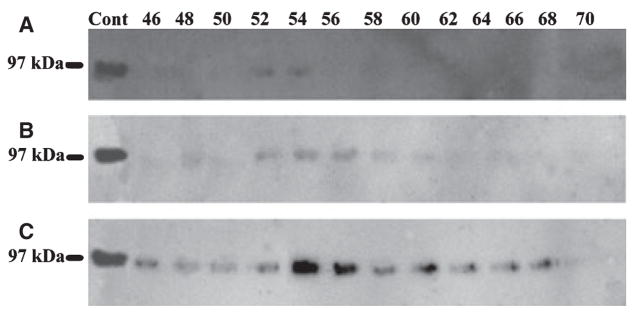
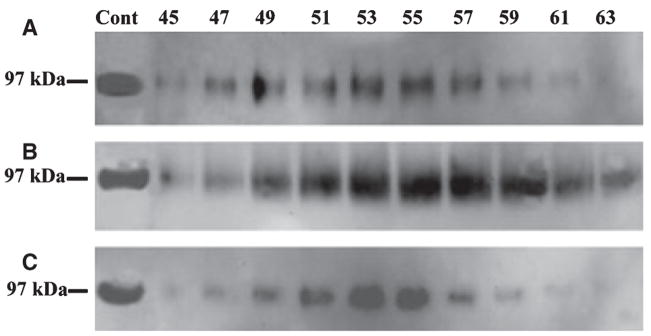
Bone sialoprotein was found in all three extracts; it was least abundant in G1 and present in the greatest amounts in G2 (Fig. 4). Osteopontin was undetectable in any of the extracts, which was probably a result of the fact that relatively smaller amounts of sample were loaded onto SDS-PAGE during this investigation; previous studies in our laboratory showed that dentin ECM contains only small amounts of OPN (39).
Fig. 4.
Western immunoblotting of dentin extracts using monoclonal anti-BSP IgG1 10D9.2 as a probe. Bone sialoprotein (BSP) was detected in G1 (A), E (B), and G2 (C) extracts of dentin. BSP was least abundant in G1 extract and most abundant in G2 extract. The positive control (Cont) was 2 μg of BSP purified from rat bone.
SIBLING proteins in G1, E, and G2 extracts of bone
Bone ECM proteins from each of the three extracts were also separated into 92 fractions, each containing 8 ml of 6 M urea. The Q-Sepharose chromatography-separation profile of bone E extract (data not shown) was similar to that reported previously (39, 40). Non-collagenous proteins in fractions 30 to 92 of each extract were initially visualized with Stains-All staining (Fig. 5); these fractions contained DMP1 fragments, DMP1-PG, BSP, and OPN. Dentin sialoprotein and DSP-PG were not detected in any of the extracts from bone, which was probably a result of the fact that this protein is present in trace amounts in bone (41) and that the loading volume in this investigation was relatively small.
Fig. 5.
Stains-All staining of fractions 30–92 of bone G1 (A), E (B), and G2 (C) extracts. Bone sialoprotein (BSP), a blue band just below the 100 kDa molecular weight marker, was present in all three extracts; this blue band was recognized by anti-BSP IgG1 10D9.2 (see Fig. 7). Note the presence of osteopontin (OPN) migrating between the 54- and 100-kDa molecular weight markers in fractions 40–45 of G1 extract and E extract, but not in G2 extract; these bands were immunoreactive to anti-OPN serum (see Fig 8).
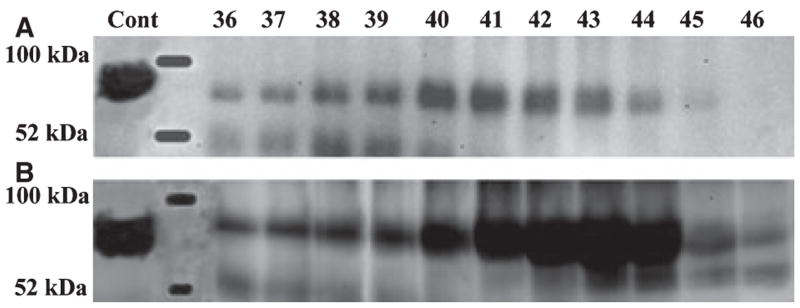
The NH2-terminal (37 kDa) fragment (Fig. 6A) and DMP1-PG (Fig. 6B) were observed in G1 and E extracts, but undetectable in the G2 extract. The COOH-terminal (57 kDa) fragment of DMP1 was detected in the E extract (Fig. 6C) but not in G1 or G2 extracts.
Fig. 6.
Western immunoblotting for E extract of bone using anti-DMP1 IgG2b. (A) The 37 kDa fragment of dentin matrix protein 1 (DMP1) was mainly detected in fractions 45–47 of bone E extract by monoclonal anti-DMP1 IgG2b 9B6.3. (B) The proteoglycan form of DMP1 (DMP1-PG) was detected in fractions 68–78 of bone E extract by antibody 9B6.3. (C) The 57 kDa fragment of DMP1 was primarily detected in fractions 46–49 of bone E extract by polyclonal anti-DMP1-C serum. The positive control (Cont) in panel A was 4 μg of 37 kDa fragment purified from rat bone. The positive control (Cont) in panel C was 4 μg of 57 kDa fragment purified from rat bone.
Bone sialoprotein was detected in all three extracts; it was least abundant in G1, moderately abundant in G2, and most abundant in E (Fig. 7). One interesting finding about BSP was that this protein was distributed in a very broad range of ion-exchange chromatographic fractions, suggesting that it must be very heterogeneous in electric charges. By contrast, OPN was eluted in a relatively narrow range of chromatographic fractions of G1 and E (Fig. 8), and was undetectable in G2.
Fig. 7.
Western immunoblotting for bone extracts using monoclonal anti-BSP IgG1 10D9.2. Bone sialoprotein (BSP) was detected in G1 (A), E (B), and G2 (C) extracts of bone. The positive control (Cont) was 2 μg of BSP purified from rat bone.
Fig. 8.
Western immunoblotting for bone extracts using polyclonal anti-OPN. Osteopontin (OPN) was detected in G1 (A) and E (B) extracts of bone. OPN eluted mainly in fractions 40–45. The positive control (Cont) was 2 μg of OPN purified from rat bone.
The distribution of the SIBLING proteins, including their proteoglycan forms, is summarized in Table 1. It is worth noting that the failure to detect a SIBLING component in an extract of either dentin or bone does not indicate that this individual protein is definitely not present in this extract, because detection of a molecule requires a sufficient quantity of a component to be loaded for analyses. In other words, certain components that were not detected in the current assay might have been observed if a large volume of a sample had been loaded. As the objectives of this investigation were to compare the relative amounts of the four SIBLING proteins among the three extracts that were from the same starting materials, we could draw conclusions about which component is relatively more abundant in a specific extract and thus provide information about each protein’s relative location in organic vs. inorganic phases in vivo.
Table 1.
SIBLING components in different extracts of dentin and bone
| DSP | DSP-PG | DPP | DMP1–37 kDa | DMP1-PG | DMP1–57 kDa | BSP | OPN | |
|---|---|---|---|---|---|---|---|---|
| Dentin-G1 | + | + | − | + | + | − | + | − |
| Dentin-E | − | − | + | + | − | + | + | − |
| Dentin-G2 | + | − | − | − | − | − | + | − |
| Bone-GI | − | − | − | + | + | − | + | + |
| Bone-E | − | − | − | + | + | + | + | + |
| Bone-G2 | − | − | − | − | − | − | + | − |
BSP, bone sialoprotein; Dentin-G1, G1 extract of dentin; Dentin-E, E extract of dentin; Dentin-G2, G2 extract of dentin; Bone-G1, G1 extract of bone; Bone-E, E extract of bone; Bone-G2, G2 extract of bone; DSP, dentin sialoprotein; DPP, dentin phosphoprotein; DSP-PG, proteoglycan form of DSP; DMP1-PG, proteoglycan form of the NH2-terminal fragment of DMP1; DMP1–37 kDa, the NH2-terminal (37 kDa) fragment of DMP1; DMP1–57 kDa, the COOH-terminal (57 kDa) fragment of DMP1; OPN, osteopontin. +, a component is detected; −, a component is not detected.
Discussion
Dentinogenesis or osteogenesis occurs by a two-stage process: (i) formation of predentin or osteoid (unmineralized precursors); and (ii) the subsequent mineralization of these precursors at the mineralization front. Predentin and osteoid lie between the mineralization front and the cells; they are transformed to mineralized tissues when hydroxyapatite crystals are deposited. This process involves mechanisms that precisely control the site and the rate of apatite formation. For example, under normal conditions of growth, a rather uniform layer of predentin and osteoid is maintained, indicating that the rate of formation of the unmineralized precursor layer must equal the rate of mineralization. The width of predentin ranges from 15 to 40 μm depending on the species; in the human incisor, its thickness is approximately 15 μm and the osteoid seam is much thinner than predentin. The dynamic process of biomineralization involves interplays among a number of molecules, including type I collagen, NCPs, and proteoglycans.
In the present investigation, we employed a three-step approach to extract NCPs from dentin and bone. This approach essentially followed the methodology used in earlier studies (35–37). In the last decade, new proteins, such as some of those in the SIBLING family, have been introduced, and newer tools (e.g. new antibodies) have been made available for studying the NCPs of dentin and bone. These established extraction protocols, along with new tools, can be used to study mineralized tissues to provide newer information concerning NCPs in these tissues. The SIBLING proteins are believed to play important roles in the biomineralization of dentin and bone (11, 31). Recent studies have indicated that some members of the SIBLING family also have signaling functions; they are involved in activating certain pathways and play roles in tissue remodeling during organ development (42, 43). In this study, we systematically compared four SIBLING family members (DSPP, DMP1, BSP, and OPN) between the organic and inorganic phases of dentin and bone. Clear differences in the distribution of these SIBLING proteins were observed in the two mineralized tissues.
Dentin sialophosphoprotein is proteolytically processed into NH2-terminal and COOH-terminal fragments, referred to as DSP and DPP, respectively. The proteoglycan form of dentin sialoprotein (15–17) represents a third variant of the DSPP entity. The results from this investigation showed that DSP and DSP-PG were present primarily in the G1 (organic) extract, not in the E (mineral) extract, indicating that these two molecules are mainly present in predentin and not associated with hydroxyapatite crystals. The presence of the highly glycosylated DSP-PG in predentin, but not in mineralized dentin, indicates that a major portion, or all, of the proteoglycan forms of DSP would be metabolized or removed prior to mineralization of collagen fibrils and the conversion of predentin to dentin. Previous studies showed that the DSP had little or no effects on in vitro mineralization (44). Clearly, additional studies are warranted to examine the effects of DSP-PG on the formation and growth of hydroxyapatite crystals. The highly phosphorylated DPP was detected exclusively in the E (mineral) extract, indicating a strong binding of this highly acidic protein with hydroxyapatite crystals. These observations are consistent with the purported role of DPP as an initiator and modulator of hydroxyapatite formation and growth (45–49).
Dentin matrix protein 1 is also processed into the NH2-terminal (37 kDa) and COOH-terminal (57 kDa) fragments. In addition to the 37 kDa form, the NH2-terminal fragment also occurs as a proteoglycan known as DMP1-PG (21). Previous protein chemistry work demonstrated that the 57 kDa fragment of DMP1 has a much higher level of phosphorylation than the 37 kDa fragment (20), and in vitro mineralization studies showed that the highly phosphorylated COOH-terminal fragment promotes mineralization by acting as a nucleator for hydroxyapatite formation (50–52). Information regarding the 37 kDa fragment and DMP1-PG is lacking. In the present investigation, the 37 kDa fragment and DMP1-PG were detected in the G1 extract of dentin and bone, whereas the highly phosphorylated 57 kDa fragment was primarily present in the E extract. Our recent immunolocalization experiments, using an antibody reactive to the NH2-terminal region of DMP1, showed that the NH2-terminal fragment of DMP1 was primarily present in the predentin of rat molars (Qin C., unpublished observations). The observation that the highly phosphorylated COOH-terminal fragment of DMP1 is primarily distributed in the inorganic phase is in agreement with previous studies showing that the 57 kDa fragment of DMP1 acts as an initiator for hydroxyapatite crystals (50–52). The difference in tissue distribution between the NH2-terminal and COOH-terminal fragments of DMP1 suggests that the biological role of DMP1-PG and/or the 37 kDa form must be different from that of the 57 kDa fragment.
Bone sialoprotein has been shown to initiate hydroxyapatite formation (27), and a number of studies have shown consistently that OPN inhibits mineralization (32–34). The presence of BSP in all of the three extracts of dentin and bone indicates that this protein has a broad interaction with different ECM components in the two tissues: BSP molecules extracted in G1 may only bind to type I collagen (in predentin or osteoid); others (in E extract) may only bind to hydroxyapatite crystals; and the remainder (only extractable in G2) may bind tightly to both type I collagen and hydroxyapatite crystals.
In summary, we assessed the presence and relative quantities of four SIBLING proteins in the organic and inorganic phases of rat dentin and bone by employing a three-step, sequential extraction approach. The findings from this study have provided information about the difference in the distribution of these proteins in the non-mineralized predentin and osteoid vs. their mineralized counterparts, mineralized dentin and bone, respectively. The observations that the processed NH2-terminal fragment and proteoglycan forms of DSPP and DMP1 are primarily present in the non-mineralized phase, whereas their highly phosphorylated COOH-terminal fragments exist mainly in the mineralized matrix, suggest that the biological functions of the NH2-terminal fragments of DSPP and DMP1 must differ from the fragments originating from the COOH-terminal region of the two proteins. Additionally, we observed that sufficient amounts of BSP remained in the matrix (G2) after the first two steps of extraction, whereas no significant amounts of other SIBLING components were left in G2. The clear SIBLING proteins in rat dentin and bone differences in the distribution pattern among these four SIBLING members indicate that these macromolecules interact with different components in the ECM while working collectively to control the bimineralization process of dentin and bone.
Acknowledgments
This work was supported by the National Institutes of Health Grant DE 005092 (to CQ).
References
- 1.Xiao S, Yu C, Chou X, Yuan W, Wang Y, Bu L, Fu G, Qian M, Yang J, Shi Y, Hu L, Han B, Wang Z, Huang W, Liu J, Chen Z, Zhao G, Kong X. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 2001;27:201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Zhao J, Li C, Gao S, Qiu C, Liu P, Wu G, Qiang B, Lo WH, Shen Y. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001;27:151–152. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- 3.Dong J, Gu T, Jeffords L, Macdougall M. Dentin phosphoprotein compound mutation in dentin sialophosphoprotein causes dentinogenesis imperfecta type III. Am J Med Genet A. 2005;132:305–309. doi: 10.1002/ajmg.a.30460. [DOI] [PubMed] [Google Scholar]
- 4.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’souza R, Hong S, Wright JT, Macdougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 5.Ye L, Macdougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 6.Ye L, Mishina Y, Chen D, Huang H, Dallas SL, Dallas MR, Sivakumar P, Kunieda T, Tsutsui TW, Boskey A, Bonewald LF, Feng JQ. Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J Biol Chem. 2005;280:6197–6203. doi: 10.1074/jbc.M412911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1230–1235. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rittling SR, Matsumoto HN, Mckee MD, Nanci A, An XR, Novick KE, Kowalski AJ, Noda M, Denhardt DT. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res. 1998;13:1101–1111. doi: 10.1359/jbmr.1998.13.7.1101. [DOI] [PubMed] [Google Scholar]
- 9.Boskey AL, Spevak L, Paschalis E, Doty SB, Mckee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- 10.Alford AI, Hankenson KD. Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- 12.Qin C, Baba O, Brunn JC, Mckee MD, Bonewald L, Butler WT. Dentin matrix protein 1 (DMP1) and dentin sialophosphoprotein (DSPP) share unique properties including tissue localization, proteolytic processing, and high molecular weight forms. In: Sodek J, Landis W, editors. Proceedings of the 8th IC-CBMT. Banff, Alberta, Canada: University of Toronto Press; 2005. pp. 174–177. [Google Scholar]
- 13.Baba O, Qin C, Brunn JC, Wygant JN, Mcintyre BW, Butler WT. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 2004;23:371–379. doi: 10.1016/j.matbio.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Macdougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 15.Qin C, Brunn JC, Baba O, Wygant JN, Mcintyre BW, Butler WT. Dentin sialoprotein isoforms: detection and characterization of a high molecular weight dentin sialoprotein. Eur J Oral Sci. 2003;111:235–242. doi: 10.1034/j.1600-0722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamakoshi Y, Hu JC, Fukae M, Iwata T, Kim JW, Zhang H, Simmer JP. Porcine dentin sialoprotein is a proteoglycan with glycosaminoglycan chains containing chondroitin 6-sulfate. J Biol Chem. 2005;280:1552–1560. doi: 10.1074/jbc.M409606200. [DOI] [PubMed] [Google Scholar]
- 17.Sugars RV, Olsson ML, Waddington R, Wendel M. Substitution of bovine dentine sialoprotein with chondroitin sulfate glycosaminoglycan chains. Eur J Oral Sci. 2006;114:89–92. doi: 10.1111/j.1600-0722.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- 18.George A, Sabsay B, Simonian PAL, Veis A. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J Biol Chem. 1993;268:12624–12630. [PubMed] [Google Scholar]
- 19.Macdougall M, Gu T, Luan X, Simmons D, Chen J. Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res. 1998;13:422–431. doi: 10.1359/jbmr.1998.13.3.422. [DOI] [PubMed] [Google Scholar]
- 20.Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, Butler WT. Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem. 2003;278:34700–34708. doi: 10.1074/jbc.M305315200. [DOI] [PubMed] [Google Scholar]
- 21.Qin C, Huang B, Wygant JN, Mcintyre BW, Mcdonald CH, Cook RG, Butler WT. A chondroitin sulfate chain attached to the bone dentin matrix protein 1 nh2-terminal fragment. J Biol Chem. 2006;281:8034–8040. doi: 10.1074/jbc.M512964200. [DOI] [PubMed] [Google Scholar]
- 22.Oldberg A, Franzén A, Heinegård D. The primary structure of a cell-binding bone sialoprotein. J Biol Chem. 1988;263:19430–19432. [PubMed] [Google Scholar]
- 23.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression of bone sialoprotein (BSP) in developing human tissues. Calcif Tissue Int. 1991;49:421–426. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Mcculloch CA, Sodek J. Bone sialoprotein in developing porcine dental tissues: cellular expression and comparison of tissue localization with osteopontin and osteonectin. Arch Oral Biol. 1993;38:241–249. doi: 10.1016/0003-9969(93)90034-j. [DOI] [PubMed] [Google Scholar]
- 25.Somerman MJ, Sauk JJ, Foster RA, Norris K, Dickerson K, Argraves WS. Cell attachment activity of cementum: bone sialoprotein II identified in cementum. J Periodontal Res. 1991;26:10–16. doi: 10.1111/j.1600-0765.1991.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 26.Moses K, Butler WT, Qin C. Immunohistochemical study of SIBLING proteins in reactionary dentin of rat molars at different ages. Eur J Oral Sci. 2006;114:216–222. doi: 10.1111/j.1600-0722.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- 27.Hunter GK, Goldberg HA. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 1993;90:8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stubbs JT, III, Mintz KP, Eanes ED, Torchia DA, Fisher LW. Characterization of native and recombinant bone sialoprotein: delineation of the mineral-binding and cell adhesion domains and structural analysis of the RGD domain. J Bone Miner Res. 1997;12:1210–1222. doi: 10.1359/jbmr.1997.12.8.1210. [DOI] [PubMed] [Google Scholar]
- 29.Butler WT, Ridall AL, Mckee MD. Osteopontin. In: Bilezikian JP, Raisz LG, Rodan DA, editors. Principles of bone biology. San Diego: Academic Press; 1996. pp. 167–181. [Google Scholar]
- 30.Sodek J, Ganss B, Mckee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 31.Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 32.Boskey AL, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatingel. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- 33.Hunter GK, Kyle CL, Goldberg HA. Modulation of crystal formation by bone phosphoproteins: structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem J. 1994;300:723–728. doi: 10.1042/bj3000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wada T, Mckee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. 1999;84:166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 35.Linde A, Bhown M, Butler WT. Noncollagenous proteins of dentin. A re-examination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J Biol Chem. 1980;255:5931–5942. [PubMed] [Google Scholar]
- 36.Grynpas MD, Tupy JH, Sodek J. The distribution of soluble, mineral-bound, and matrix-bound proteins in osteoporotic and normal bones. Bone. 1994;15:505–513. doi: 10.1016/8756-3282(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 37.Sodek KL, Tupy JH, Sodek J, Grynpas MD. Relationships between bone protein and mineral in developing porcine long bone and calvaria. Bone. 2000;26:189–198. doi: 10.1016/s8756-3282(99)00251-3. [DOI] [PubMed] [Google Scholar]
- 38.Butler WT. Dentin-specific proteins. Methods Enzymol. 1987;145:290–303. doi: 10.1016/0076-6879(87)45017-9. [DOI] [PubMed] [Google Scholar]
- 39.Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT. A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci. 2001;109:133–141. doi: 10.1034/j.1600-0722.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 40.Linde A, Jontell M, Lundgren T, Nilson B, Svanberg U. Noncollagenous proteins of rat compact bone. J Biol Chem. 1983;258:1698–1705. [PubMed] [Google Scholar]
- 41.Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, Nagai N, Butler WT. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 2002;81:392–394. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- 42.Jadlowiec JA, Zhang X, Li J, Campbell PG, Sfeir C. Extracellular matrix-mediated signaling by dentin phosphophoryn involves activation of the Smad pathway independent of bone morphogenetic protein. J Biol Chem. 2006;281:5341–5347. doi: 10.1074/jbc.M506158200. [DOI] [PubMed] [Google Scholar]
- 43.Godovikova V, Ritchie HH. Dynamic processing of recombinant dentin sialoprotein-phosphophoryn protein. J Biol Chem. 2007;282:31341–31348. doi: 10.1074/jbc.M702605200. [DOI] [PubMed] [Google Scholar]
- 44.Boskey A, Spevak L, Tan M, Doty SB, Butler WT. Dentin sialoprotein (DSP) has limited effects on in vitro apatite formation and growth. Calcif Tissue Int. 2000;67:472–478. doi: 10.1007/s002230001169. [DOI] [PubMed] [Google Scholar]
- 45.Linde A, Lussi A, Crenshaw MA. Mineral induction by immobilized polyanionic proteins. Calcif Tissue Int. 1989;44:286–295. doi: 10.1007/BF02553763. [DOI] [PubMed] [Google Scholar]
- 46.Boskey AL, Maresca M, Doty S, Sabsay B, Veis A. Concentration-dependent effects of dentin phosphophoryn in the regulation of in vitro hydroxyapatite formation and growth. Bone Miner. 1990;11:55–65. doi: 10.1016/0169-6009(90)90015-8. [DOI] [PubMed] [Google Scholar]
- 47.Linde A, Goldberg M. Dentinogenesis. Crit Rev Oral Biol Med. 1993;4:679–728. doi: 10.1177/10454411930040050301. [DOI] [PubMed] [Google Scholar]
- 48.Saito T, Arsenault AL, Yamauchi M, Kuboki Y, Crenshaw MA. Mineral induction by immobilized phosphoproteins. Bone. 1997;21:305–311. doi: 10.1016/s8756-3282(97)00149-x. [DOI] [PubMed] [Google Scholar]
- 49.He G, Ramachandran A, Dahl T, George S, Schultz D, Cookson D, Veis A, George A. Phosphorylation of phosphophoryn is crucial for its function as a mediator of biomineralization. J Biol Chem. 2005;280:33109–33114. doi: 10.1074/jbc.M500159200. [DOI] [PubMed] [Google Scholar]
- 50.Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279:18115–18120. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- 51.He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, Hao J, George A. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005;44:16140–19148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–1204. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]