Abstract
OBJECTIVE
To estimate the incidence of medically treated thyroid disease in children with Down syndrome enrolled in Tennessee Medicaid (TennCare) during 1995–2005 and determine whether rates increased following re-release of American Academy of Pediatrics guidelines in 2001.
PATIENTS AND METHODS
We conducted a population-based retrospective cohort study in which we identified children with Down syndrome using TennCare files and birth certificates. We included 1–18 year olds who were continuously enrolled in TennCare and did not fill a prescription for thyroid medication during a 90-day pre-study period. The rate of medically treated thyroid disease (a prescription filled for thyroid medication) was the main outcome. We determined rates of medically treated thyroid disease by study year, age, sex, race, region of residence, and payer type. We used Poisson regression to estimate predictors.
RESULTS
During the eleven year study period, 1257 children with Down syndrome (28% black, 72% white) met inclusion criteria. Overall, 10.8% filled a new prescription for thyroid medication. Rates of medically treated thyroid disease per 1000/child years were 13.25 (1995–1997), 13.34 (1998–1999), 13.62 (2000–2001), 22.34 (2002–2003) and 22.51 (2004–2005). In multivariable analyses, there was an increased rate of medically treated thyroid disease in 2002–03 (adjusted IR 1.73, 95% CI 1.02–2.93) and 2004–05 (adjusted IR 1.72, 95% CI 1.01–2.91) compared to 1995–1997. In a comparison cohort of children without Down syndrome, there was a smaller increase in the rate of medically treated thyroid disease when comparing 2002–03 (adjusted IR 1.26, 95% CI 1.03–1.53) and 2004–05 (adjusted IR 1.22, 95% CI 0.99–1.49) to 1995–1997.
CONCLUSIONS
The incidence of medically treated thyroid disease in children with Down syndrome in the TennCare cohort from 1995 to 2005 was 10.8%. A 73% increase in rate occurred following re-release of American Academy of Pediatrics guidelines which may have influenced screening for thyroid disease.
Keywords: Down syndrome, thyroid diseases, guidelines
INTRODUCTION
Down Syndrome (DS) is the most common known genetic cause of moderate to severe intellectual disability, and approximately 10,000 children with Down syndrome are born in the United States each year.1 A number of medical conditions are associated with DS, including thyroid dysfunction. Although reports vary, prevalence rates of any thyroid dysfunction in children with Down Syndrome have been estimated up to approximately 15%.2–4 Hypothyroidism has a subtle presentation and can be particularly challenging to detect in patients with intellectual disabilities and communication and language impairments. Furthermore, symptoms of hypothyroidism overlap with features of DS including impaired intellectual development in young children, decreased linear growth, dry skin, dentition abnormalities, and decreased physical activity.5–8 Although routine screening for thyroid disease is recommended for children with Down syndrome, it is not known how recommendations may influence physician practice.
The American Academy of Pediatrics (AAP) recommends annual screening for thyroid disease for individuals one year and older.2 Several cross-sectional and longitudinal studies have contributed to our understanding of the association of Down syndrome and thyroid disease.3;4;9–12 However, there are no large population-based estimates of the incidence of medically treated thyroid disease in children with DS. It is also not known whether re-release of AAP guidelines, recommending routine thyroid screening in children with DS, would be associated with increased identification of new cases of disease. The objectives of the study were to investigate whether an increase in medically treated thyroid disease occurred following re-release of AAP guidelines in 2001 and estimate the population based incidence of medically treated thyroid disease in children with Down syndrome enrolled in a state public insurance plan from 1995–2005.
PATIENTS AND METHODS
We conducted a population-based retrospective cohort study from 1995–2005 including children with Down syndrome enrolled in TennCare, Tennessee’s managed care program for persons with disabilities (Social Security Insurance), financial need (Temporary Aid to Needy Families), and children without health insurance (uninsured). The study population was limited to children who were continuously enrolled in TennCare 90 days prior to study entry and with no prescription for thyroid medication during the 90 days. Continuous enrollment was defined as no gap of more than 30 consecutive days during the 90 day pre-study entry period. The study was approved by the Institutional Review Boards of Vanderbilt University and the Tennessee Department of Health.
Using previously described methods we identified eligible children and obtained all study data from linked Tennessee Medicaid administrative data files and Tennessee State vital records.13;14 We identified children with Down syndrome between age 1 and 18 years from International Classification of Diseases (ICD) diagnoses (72.67%), birth certificate data (7.50%), or both (19.84%). Inpatient and outpatient claims of visits up to age 12 years with ICD-9 codes (758.0) or ICD-8 (759.3 used 1977–1978) were used. Children with a single inpatient ICD-9 or ICD-8 diagnosis of DS or 2 outpatient ICD-9 or ICD-8 diagnoses met our study definition of DS.
Our main outcome variable was the rate of medically treated thyroid disease, defined as a new prescription filled for thyroid medication. To be eligible for the study, individuals could not have filled a prescription for thyroid medication during the 90 days prior to study entry. Therefore, a new prescription was considered to represent the onset of medically treated thyroid disease. Thyroid replacement medications included liotrix, levothyroxine sodium, thyroglobulin, thyroid, and liothyronine sodium. Prescriptions for two anti-thyroid medications (propylthiouracil and methimazole) were also captured and included in our definition. We followed children until they filled a prescription for thyroid medication, had more than 30 days of consecutive non-enrollment, had out of state enrollment, died, or the study ended.
We assessed the rate of medically treated thyroid disease pre and post the February 2001 release of the AAP guidelines for health maintenance for individuals with Down syndrome. We combined adjacent years to give 5 categories: 1995–97, 1998–99, 2000–01, 2002–03, and 2004–05. We were also particularly interested in whether there were differences in medically treated thyroid disease by participant race. As participant race was a variable of interest, children with unknown race (7.8%) were excluded. We conducted sensitivity analyses before exclusion of children with unknown race. Results of bivariate analyses of race and new thyroid medication prescriptions were similar whether the unknown group was separate or combined with either the white or black group. In addition, children of other racial or ethnic groups were excluded as they were too few to study (4.7% combined for Latino, Asian, and other). Other predictor variables examined included participant age, participant sex, region of residence in Tennessee (urban, suburban, rural), and TennCare enrollment category. TennCare enrollment categories included, Temporary Aid to Needy Families (TANF), Social Security Insurance (SSI), or otherwise uninsured. For overlapping enrollment segments with different enrollment categories, if there were any TANF, the category was classified as TANF. If there were overlapping SSI and uninsured categories, enrollment was classified as SSI. For overlapping enrollment segments with different regions, if there were any rural regions the enrollment was classified as rural. If there were suburban and urban regions, region was classified as suburban. Using TennCare claims (1 inpatient and/or 2 outpatient physician claims) we captured the date of the first ICD-9 diagnosis of specific chronic diagnoses including congenital cardiac disease (745,746, 747.0–747.4), gastrointestinal conditions (751.0–751.5), or leukemia (204–208). ICD-8 codes were used 1977–1978.
As a parallel comparison group, we assembled a cohort of children without Down syndrome enrolled in TennCare 1995–2005. Children in the non-Down syndrome cohort did not have Down syndrome indicated on the birth certificate or an ICD-9 diagnosis of Down syndrome. As with the primary Down syndrome cohort, children in the non-Down syndrome comparison group were continuously enrolled during the 90 days prior to study entry and did not fill a prescription for thyroid medication during this study period.
We determined the rate of medically treated thyroid disease in eligible children with Down syndrome enrolled in the Tennessee Medicaid Program and the comparison group of children without Down syndrome 1995–2005. The children’s baseline characteristics and the presence of associated chronic conditions present before diagnosis of thyroid disease were determined and presented using proportions. In person-time analyses, we determined rates of medically treated thyroid disease pre and post re-release of the 2001 AAP guidelines. Child-days were converted to child years and results were determined per 1000 child years. We used Poisson regression to estimate predictors of medically treated thyroid disease.15 We included in the final model factors that could potentially confound the relationship between year of study and rate of medically treated thyroid disease. We estimated identifying at least 1200 eligible children with Down syndrome.1
RESULTS
A total of 1257 black and white children with Down syndrome were included in the study cohort. Overall, 28% of the children were black/African-American and 55% were males (Table 1). At study entry, 75% of the children were in the SSI insurance payer category, 22% TANF, and 3% were otherwise uninsured. Thirty-four percent of the children lived in urban regions of the state, 27% suburban, and 39% rural. As expected, a substantial portion of the children with Down syndrome experienced co-morbidities including, congenital heart disease (40%) and gastrointestinal disorders (5.65 %).
Table 1.
Demographics and Baseline Characteristics of Children With Down Syndrome enrolled in TennCare, 1995–2005.
| Characteristics | N−1257 | % | |
|---|---|---|---|
| Age at study entry (years) | ≥ 1 to <3 | 567 | 50.8 |
| 3 to 5 | 137 | 12.3 | |
| 6 to <12 | 241 | 21.6 | |
| 12 to <18 | 171 | 15.3 | |
| Race | White | 808 | 72.4 |
| Black | 308 | 27.6 | |
| Sex | Male | 604 | 54.1 |
| Female | 512 | 45.9 | |
| Payer Category | Uninsured | 29 | 2.6 |
| Disability | 858 | 76.9 | |
| Temporary Aid to Need Families (TANF) | 229 | 20.5 | |
| Region of residence | Urban | 382 | 34.2 |
| Suburban | 295 | 26.4 | |
| Rural | 439 | 39.3 | |
| Co-morbid conditions | |||
| Cardiac | 443 | 39.7 | |
| Gastrointestinal | 56 | 5.0 | |
| Leukemia | 4 | 0.4 | |
Over the 11 year study period, 10.8% of children in the cohort filled a new prescription for a thyroid medication. The majority of medications filled were for thyroid hormone replacement (95.6%). By study years, rates of medically treated thyroid disease per 1000/ child years were 13.25 (1995–1997), 13.34 (1998–1999), 13.62 (2000–2001), 22.34 (2002–2003) and 22.51 (2004–2005), Table 2. Blacks had a lower rate of medically treated thyroid disease than whites. Rates of medically treated thyroid disease differed by patient age. The highest incidence of medically treated thyroid disease occurred in children aged 1 to less than 3 years, and the lowest incidence occurred in the late childhood age group of 6 to <12 year olds. Children with a pre-existing medical condition were more likely to have medically treated thyroid disease than children without a medical condition,
Table 2.
Rates and Univariate Analyses of Predictors of Medically Treated Thyroid Disease in Children with Down Syndrome Enrolled in TennCare, 1995–2003
| Characteristics | # Thyroid prescriptions | Child-years | Rate/1000 child yrs | Rate Ratio | |
|---|---|---|---|---|---|
| Age at study entry (years) | ≥ 1 to <3 | 39 | 1043.17 | 33.93 | 1 |
| 3 to 5 | 16 | 1430.31 | 11.19 | 0.30 (0.17–0.54) | |
| 6 to <12 | 28 | 2620.62 | 10.68 | 0.29 (0.18–0.46) | |
| 12 to <18 | 41 | 2300.50 | 17.82 | 0.48 (0.31–0.74) | |
| Race | White | 102 | 5262.28 | 19.38 | 1 |
| Black | 22 | 2132.32 | 10.32 | 0.53 (0.34–0.85) | |
| Sex | Male | 64 | 4116.11 | 15.55 | 1 |
| Female | 60 | 3278.48 | 18.30 | 0.85 (0.60–1.21) | |
| Payer Category | Uninsured | 3 | 197.72 | 15.17 | 1 |
| SSI | 109 | 6371.28 | 17.11 | 1.26 (0.79–2.02) | |
| TANF* | 12 | 825.59 | 14.54 | 1.31 (0.86–2.00) | |
| Region of residence | Urban | 36 | 2559.21 | 14.06 | 1 |
| Suburban | 34 | 1912.74 | 17.78 | 1.26 (0.79–2.02) | |
| Rural | 54 | 2922.64 | 18.48 | 1.31 (0.86–2.00) | |
| Co-morbid conditions | Y | 65 | 3168.91 | 20.51 | 1.47 (1.03–2.09) |
| N | 59 | 4225.68 | 13.96 | 1 | |
| Study Year | 1995–1997 | 25 | 1887.33 | 13.25 | 1 |
| 1998–1999 | 18 | 1348.98 | 13.34 | 1.01 (0.55–1.85) | |
| 2000–2001 | 19 | 1394.97 | 13.62 | 1.03 (0.57–1.87) | |
| 2002–2003 | 31 | 1385.98 | 22.37 | 1.69 (1.00–2.86) | |
| 2004–2005 | 31 | 1377.34 | 22.51 | 1.70 (1.00–2.88) | |
Temporary Aid to Needy Families
In the multivariable model including patient age and race, there was an increased rate of medically treated thyroid disease in 2002–03 and 2004–05 compared to 1995–1997. Older children were less likely to receive a new prescription for thyroid medication compared to toddlers, including children aged 3 to 5 years, 6 to<12 years, and 12 to 18 years. There was a lower rate of medically treated thyroid disease in blacks compared to whites in the multivariable model.
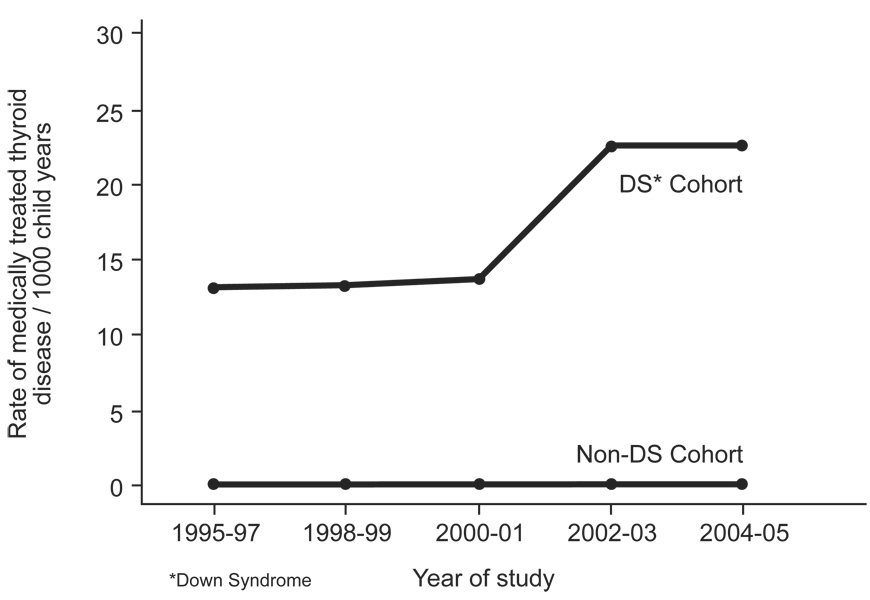
Over the 11 year study period, 0.21% of children in the cohort without Down syndrome filled a new prescription for a thyroid medication. The majority of medications filled were for thyroid hormone replacement (93.2%). By years, rates of medically treated thyroid disease per 1000/ child years were 0.19 (1995–1997), 0.19 (1998–1999), 0.19 (2000–2001), 0.24 (2002–2003), and 0.23 (2004–2005). Figure 1 demonstrates the rate of medically treated thyroid disease over the study period in children with and without DS. In multivariate analyses, the adjusted rates of medically treated thyroid disease were increased in 2002–03 [adjusted incidence ratio (IR) 1.26, 95% CI 1.03–1.53] and 2004–05 (adjusted IR 1.22, 95% CI 0.99–1.49) compared to 1995–97. The rate of medically treated thyroid disease was lower in blacks (0.16/1000 child years) compared to whites (0.24/1000 child years) and this decreased rate was also seen in the multivariable model (adjusted IR 0.68, 95% CI 0.59–0.79).
Figure 1.
Rates of Medically Treated Thyroid Disease in Children with and without Down Syndrome Enrolled in TennCare, 1995–2005
DISCUSSION
Although racial disparities in the life expectancy of individuals with DS exist, overall the median life expectancy has increased dramatically in recent decades.16;17 Despite improvements in quality of life, people with DS remain a vulnerable population. Screening for thyroid disease has been recommended for a number of reasons including: the high prevalence in the population, the adverse medical effects associated with the disorder, and the availability of safe testing and an effective treatment. Over the 11 year study pear, the population-based incidence of medically treated thyroid disease in this cohort of over 1000 children with Down syndrome was 10.8%. Although several studies have investigated the prevalence and incidence of thyroid dysfunction in children and adults with Down syndrome, to our knowledge, this is the first population-based estimate of the incidence of medically treated thyroid disease in children with DS.4;9–11;18–24
The primary objective of this investigation was to determine whether an increase in medically treated thyroid disease occurred following re-release in February 2001 of AAP guidelines recommending annual screening for thyroid disease starting at age one year. Compared to 1995–1997, rates of medically treated thyroid disease increased 73% in 2002–2003 and 72% in 2004–2005.
We determined rates of medically treated thyroid disease in a comparison cohort of children without DS, in whom an increase in identification of new cases of medically treated thyroid disease would not be expected following re-release of DS specific guidelines. In a Scottish cohort which included over 100,000 children aged 1 to 22 years, the prevalence of receiving 2 or more prescriptions for thyroid medication was 0.135% over the 3 year study period.25 In the TennCare cohort of children without DS, the incidence of medically treated thyroid disease over the 11 year study period was 0.21%. In contrast to the 73% increase in the cohort of children with Down syndrome there was only a 26% increase in the rate of medically treated thyroid disease in 2002–03 compared to 1995–1997.
There were also differences in medically treated thyroid disease by race and age. African-American children with Down syndrome were less likely to have medically treated thyroid disease than white children. It is important to note that the decreased rate in African-Americans compared to whites was also seen in the parallel cohort of children without Down syndrome. Therefore we do not know whether racial differences in rates of medically treated thyroid disease in children with Down syndrome are due to differences in disease incidence or in screening. Lastly, there were differences in risk of medically treated thyroid disease by age group. In children with Down syndrome, all of the older age groups were less likely to have medically treated thyroid disease than the 1 to < 3 year age group. This could be the result of a number of factors, including the highest incidence occurring in this age group or the higher likelihood of undergoing screening due to multiple contacts with the healthcare system. In addition, physicians may be more likely to prescribe medication for young children with abnormal thyroid function studies as children less than age three are particularly vulnerable to the adverse effects of untreated hypothyroidism on intellectual development.
There are several limitations to consider. The outcome of medically treated thyroid disease is dependent on screening for thyroid disease, which is likely subject to variation in physician practice. We were not able to capture screening for thyroid disease or the results of laboratory tests in this study. In addition, practice patterns may vary among physicians in treatment of abnormal thyroid function. For example, the treatment of compensated thyroid disease, a condition that may be transient, is a topic of debate.3;10 However this study was designed to determine the rate of medically treated thyroid disease in this population, not the appropriateness of treatment.
Although we assessed the level of medically treated thyroid disease after the re-release of the policy guideline statement, we were not able to directly evaluate the impact of a particular guideline on screening for thyroid disease. However, this study does provide a population based estimation of the incidence of medically treated thyroid disease and investigates the temporal relationship of the change in incidence and the re-release of guidelines. It is important to note that across the eleven year study period there were no major changes in the structure of TennCare or in how the primary outcome, prescription filled for thyroid medication, was captured. Furthermore, changes in the sensitivity of thyroid function testing were not temporally related to re-release of the guidelines.
The findings may also have limited generalizability. The TennCare population in Tennessee represents individuals with disabilities, who are low-income, or unable to otherwise obtain medical insurance. Therefore, we did not capture participants with only private insurance, the uninsured, or individuals who did not seek medical care. The practice patterns of physicians that serve patients in TennCare may not reflect those of providers that primarily work with other patient populations. Therefore, the rate of medically treated thyroid disease in the TennCare cohort may not reflect the rate of children who were privately insured or that did not have any type of insurance. However, because TennCare provides insurance for children with disabilities, the study likely includes the majority of children with Down syndrome in the state.
CONCLUSION
A 73% increase in the rate of medically treated thyroid disease in children with Down syndrome enrolled in TennCare was seen following re-release of AAP guidelines. The population-based incidence of medically treated thyroid disease in the cohort was 10.8%, over the 11 year period 1995 to 2005. The temporal relationship suggests that the re-release of the AAP guidelines may have increased physician awareness, resulting in increased rates of screening for and identification of thyroid dysfunction.
Table 3.
Predictors of Medically Treated Thyroid Disease in Children with Down Syndrome Enrolled in TennCare, 1995–2005.
| Characteristics | Adjusted Rate Ratios | |
|---|---|---|
| Age at study entry (years) | ≥ 1 to <3 | 1 |
| 3 to 5 | 0.30 (0.17–0.54) | |
| 6 to <12 | 0.29 (0.18–0.47) | |
| 12 to <18 | 0.46 (0.30–0.72) | |
| Race | white | 1 |
| black | 0.53 (0.33–0.84) | |
| Study Year | 1995–1997 | 1 |
| 1998–1999 | 1.07 (0.58–1.96) | |
| 2000–2001 | 1.03 (0.57–1.88) | |
| 2002–2003 | 1.73 (1.02–2.93) | |
| 2004–2005 | 1.72 (1.01–2.91) | |
ACKNOWLEDGMENT
The authors are indebted to the Tennessee Bureau of TennCare of the Department of Finance and Administration, and the Tennessee Department of Health, Office of Policy, Planning & Assessment, for providing the data.
This study was supported by grants from the following sources: Ambulatory Pediatric Association/Agency for Healthcare Research and Quality Young Investigators Grant Program; National Institutes of Health (K12 RR17697)
Abbreviations
- DS
Down syndrome
- AAP
American Academy of Pediatrics
- ICD
International Classification of Diseases
- TANF
Temporary Aid to Needy Families
- SSI
Social Security Insurance
- IR
Incidence ratio
References
- 1.Roizen NJ, Patterson D. Down's syndrome. Lancet. 2003 Apr 12;361(9365):1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Genetics. American Academy of Pediatrics: Health supervision for children with Down syndrome. Pediatrics. 2001 Feb;107(2):442–449. doi: 10.1542/peds.107.2.442. [DOI] [PubMed] [Google Scholar]
- 3.Gibson PA, Newton RW, Selby K, et al. Longitudinal study of thyroid function in Down's syndrome in the first two decades. Arch Dis Child. 2005 Jun;90(6):574–578. doi: 10.1136/adc.2004.049536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pueschel SM, Jackson IM, Giesswein P, et al. Thyroid function in Down syndrome. Res Dev Disabil. 1991;12(3):287–296. doi: 10.1016/0891-4222(91)90013-i. [DOI] [PubMed] [Google Scholar]
- 5.Hardy O, Worley G, Lee MM, et al. Hypothyroidism in Down syndrome: screening guidelines and testing methodology. Am J Med Genet A. 2004 Feb 1;124(4):436–437. doi: 10.1002/ajmg.a.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morreale de EG, Obregon MJ, Escobar del RF. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004 Nov;151 Suppl 3:U25–U37. doi: 10.1530/eje.0.151u025. U25–U37. [DOI] [PubMed] [Google Scholar]
- 7.Bettendorf M. Thyroid disorders in children from birth to adolescence. Eur J Nucl Med Mol Imaging. 2002 Aug;29 Suppl 2:S439–S446. doi: 10.1007/s00259-002-0905-3. [DOI] [PubMed] [Google Scholar]
- 8.Demers LM. Thyroid disease: pathophysiology and diagnosis. Clin Lab Med. 2004 Mar;24(1):19–28. doi: 10.1016/j.cll.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Selikowitz M. A five-year longitudinal study of thyroid function in children with Down syndrome. Dev Med Child Neurol. 1993 May;35(5):396–401. doi: 10.1111/j.1469-8749.1993.tb11660.x. [DOI] [PubMed] [Google Scholar]
- 10.Tuysuz B, Beker DB. Thyroid dysfunction in children with Down's syndrome. Acta Paediatr. 2001 Dec;90(12):1389–1393. doi: 10.1080/08035250152708770. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson B, Gustafsson J, Hedov G, et al. Thyroid dysfunction in Down's syndrome: relation to age and thyroid autoimmunity. Arch Dis Child. 1998 Sep;79(3):242–245. doi: 10.1136/adc.79.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutler AT, ezra-Obeiter R, Brink SJ. Thyroid function in young children with Down syndrome. Am J Dis Child. 1986 May;140(5):479–483. doi: 10.1001/archpedi.1986.02140190089034. [DOI] [PubMed] [Google Scholar]
- 13.Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989 Apr;129(4):837–849. doi: 10.1093/oxfordjournals.aje.a115198. [DOI] [PubMed] [Google Scholar]
- 14.Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006 Jun 8;354(23):2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 15.McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. London: Chapman & Hall; 1989. [Google Scholar]
- 16.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002 Mar 23;359(9311):1019–1025. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Racial disparities in median age at death of persons with Down syndrome--United States, 1968–1997. MMWR Morb Mortal Wkly Rep. 2001 Jun 8;50(22):463–465. [PubMed] [Google Scholar]
- 18.Pueschel SM, Pezzullo JC. Thyroid dysfunction in Down syndrome. Am J Dis Child. 1985 Jun;139(6):636–639. doi: 10.1001/archpedi.1985.02140080106045. [DOI] [PubMed] [Google Scholar]
- 19.Fort P, Lifshitz F, Bellisario R, et al. Abnormalities of thyroid function in infants with Down syndrome. J Pediatr. 1984 Apr;104(4):545–549. doi: 10.1016/s0022-3476(84)80544-2. [DOI] [PubMed] [Google Scholar]
- 20.Rooney S, Walsh E. Prevalence of abnormal thyroid function tests in a Down's syndrome population. Ir J Med Sci. 1997 Apr;166(2):80–82. doi: 10.1007/BF02944192. [DOI] [PubMed] [Google Scholar]
- 21.Prasher V, Gomez G. Natural history of thyroid function in adults with Down syndrome--10-year follow-up study. J Intellect Disabil Res. 2007 Apr;51(Pt 4):312–317. doi: 10.1111/j.1365-2788.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- 22.Mani C. Hypothyroidism in Down's syndrome. Br J Psychiatry. 1988 Jul;153:102–104. doi: 10.1192/bjp.153.1.102. 102–4. [DOI] [PubMed] [Google Scholar]
- 23.Friedman DL, Kastner T, Pond WS, et al. Thyroid dysfunction in individuals with Down syndrome. Arch Intern Med. 1989 Sep;149(9):1990–1993. [PubMed] [Google Scholar]
- 24.Rubello D, Pozzan GB, Casara D, et al. Natural course of subclinical hypothyroidism in Down's syndrome: prospective study results and therapeutic considerations. J Endocrinol Invest. 1995 Jan;18(1):35–40. doi: 10.1007/BF03349694. [DOI] [PubMed] [Google Scholar]
- 25.Hunter I, Greene SA, MacDonald TM, et al. Prevalence and aetiology of hypothyroidism in the young. Arch Dis Child. 2000 Sep;83(3):207–210. doi: 10.1136/adc.83.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]