Abstract
The capacity to anticipate and prepare for future events is thought to be critical for cognitive control. Dominant accounts of cognitive control treat the developing system as merely a weaker version of the adult system, progressively strengthening over time. Using the AX Continuous Performance Task (AX-CPT) in combination with high-resolution pupillometry, we find that whereas 8-year-old children resemble adults in their proactive use of cognitive control, 3.5-year-old children exhibit a qualitatively different, reactive form of cognitive control, responding to events only as they unfold and retrieving information from memory as needed in the moment. These results demonstrate the need to reconsider the origins of cognitive control and the basis for children's behaviors across domains.
Keywords: cognitive development, context processing, pupillometry, reactive control, proactive control
Although sometimes derided as “creatures of habit,” humans develop an unparalleled ability to adaptively control thought and behavior in accordance with current goals and plans. Dominant theories of cognitive control suggest that this flexibility is enabled by the proactive regulation of behavior through sustained inhibition of inappropriate thoughts and actions (1, 2), the active biasing of task-relevant thoughts (3–6), or construction of rule-like representations (7–8).
Theories of the developmental origins of cognitive control converge in positing that children engage these same proactive processes, but in a weaker form, with less strength or stability (9, 10), less resistance toward habitual responses (1, 2), or degraded complexity (8, 11). For example, according to one influential theory (8), developmental change in cognitive control is driven by “age-related improvements in the complexity and scope of children's intentional, top-down processes.” Similarly, dynamical systems accounts posit that deficits in cognitive control—as revealed by inappropriate reaching in putative object permanence tasks—arise “from the same multiple processes that produce goal-directed reaching at any age” (emphasis in original; ref. 10). Finally, neural network models have simulated developmental improvements in cognitive control via parametric increases in the strength of recurrent connections for maintaining task-relevant representations (9).
However, children can be notoriously constrained to the present (e.g., refs. 12 and 13), raising the possibility that the temporal dynamics of immature cognitive control are fundamentally different from that of adults. Specifically, we hypothesized that young children may show “reactive” as opposed to “proactive” context processing (14–17), characterized by a failure to proactively prepare for even the predictable future (18) and a tendency to react to events only as they occur, retrieving information from memory as needed in the moment. For lack of age-appropriate methods, the possibility of this qualitative developmental shift has not been directly tested.
Here, we distinguish proactive and reactive mechanisms by adapting a paradigm previously used at the other end of the lifespan: the dual-response expectancy AX-CPT (Fig. 1A). Modifications to the AX-CPT enhanced task understanding and sustained attention (see Materials and Methods), allowing us to test unprecedentedly young children (in previous work, the youngest to complete the expectancy AX-CPT was a 5.11-year-old) (19) and to measure mental effort through pupillometry (20, 21).
Fig. 1.
Overview of the child-adapted AX-CPT and children's behavioral performance. (A) In the AX-CPT, 4 stimuli are presented sequentially; AX trials occur with 70% frequency, and other trials with 10% probability each. Stimuli were replaced with cartoon characters; an adaptive allowable response time was set per subject. (B) Only 8-year-olds (white bars) show the significantly exaggerated reaction times on AY trials expected of proactive control. (C) Simple accuracy measures do not significantly distinguish reactive vs. proactive control, but only 8-year-olds show numerically fewer BX than AY errors, as expected of proactive control. Conditionalized accuracy measures reveal distinct reactive and proactive profiles (see SI Text).
In the AX-CPT, subjects provide a target response to a particular probe (“X”) if it follows a specific contextual cue (“A”). Nontarget responses are provided to other cue–probe sequences (“A” then “Y,” “B” then “X,” or “B” then “Y”), each occurring with lower probability than the target pair.
This asymmetry in trial type frequency is critical for revealing distinct behavioral profiles for proactive versus reactive control (22). Proactive control supports good BX trial performance at the expense of AY trials. Maintenance of the “B” cue supports a nontarget response to the subsequent “X” probe; however, maintenance of the “A” cue leads to anticipation of an X and thus a target response (due to the expectancy effect cultivated by the asymmetry in trial type frequencies), which can lead to false alarms in AY trials (23–25). Reactive control leads to the opposite pattern. The preceding cue is retrieved when needed, that is, in response to “X” probes but not to “Y” probes. Such retrieval renders BX trials vulnerable to retrieval-based interference; the lack of such retrieval on AY trials means that false alarms are less likely in this case. Similarly, proactive control should lead to increased delay-period effort, whereas reactive control should lead to increased effort to probes.
Likewise, the requirement to respond on nontarget trials is also critical for dissociating proactive and reactive control. For example, because difficulty with AY trials reflects proactive control, correct responses should be more likely on slow than fast trials, where faster responses indicate response preparation in advance. In contrast, correct responses on BX trials may reflect proactive use of the B cue and therefore should be more likely on fast than slow trials. These effects are not predicted of reactive control, in which advance preparation of responses does not occur. Similarly, proactive control should lead to correlations among an individual's reaction times on nontarget trials that share the same cue (BX and BY trials) but not those that share the same probe (AY and BY trials), whereas reactive control should lead to correlations between those that that share the same probe but not those that share the same cue. Therefore, reactive and proactive control yield these dissociations only in the dual-response expectancy AX-CPT.
If young children only react to the future as it unfolds, then this task should reveal exclusively reactive mechanisms in 3.5-year-olds, contrasting with 8-year-olds who begin to recruit proactive, adult-like parietofrontal mechanisms in tasks requiring cognitive control (26). The results confirmed our predictions: Although both groups of children demonstrated an ability to understand and follow the task rules, 3.5-year-olds performed in a reactive way, whereas 8-year-olds performed in a proactive way. These differences were evident in reaction times, pupillometric indices of mental effort, and conditionalized accuracy (Table 1).
Table 1.
Dissociations between proactive and reactive control as observed in the AX-CPT
| Proactive preparation to cue (observed in 8-year-olds) | Reactive retrieval to probe (observed in 3.5-year-olds) | |
|---|---|---|
| Reaction times | AY trials slower than B trials | No differential slowing* |
| Effort | During delay period (after cue) | During probe period |
| Individual differences | Worse performance with more probe-period effort; nontarget reaction times predictable based on cues | Better performance with more probe-period effort; nontarget reaction times predictable based on probes |
| Sequence-conditionalized accuracy† | Worse AY performance with more preceding AX trials; better BX performance with more preceding AX trials | Better AY performance with more preceding AX trials; worse BX performance with more preceding AX trials |
| Speed–accuracy tradeoffs† | More AY errors on fast trials; fewer BX errors on fast trials | No speed–accuracy tradeoffs |
*Although reactive control might be predicted to slow BX relative to Y trials, the rare Y probes (occurring on 20% of trials) may themselves capture the attention of reactive subjects. This attentional capture during the probe period and its concomitant response slowing would obscure any differential reaction times among that group.
Results
As fully described in Materials and Methods, children understood the task rules and were capable of following them. First, the instructions were repeated for each trial type until children responded correctly. Second, all children correctly responded on all trial types during a subsequent verification phase. Third, performance was above chance overall and on the AX and BY trials that reflect task understanding independent of context processing. Finally, we minimized the chances that perseverative responding was driving the observed effects by excluding all children with <60% accuracy on BY trials, yielding an average accuracy of 83% across all trial types and 87% on BY trials. Similar patterns were observed in the larger group, and the main findings were unaffected by this performance-based filtering.
Pupillometry: Group Differences.
Moment-by-moment changes in the temporal dynamics of control were assessed with the task-evoked pupillary response, a measure known to correlate with mental effort across ages and tasks (27) due to cortical modulation of the reticular formation (21). Notably, 8-year-olds showed increased delay-period effort, reflecting proactive maintenance of context in preparation for the subsequent response (Fig. 2A). In direct contrast, 3.5-year-olds showed relatively greater probe-period effort, reflecting the retrieval of contextual information. Indeed, a pronounced reactive peak was observed particularly in BX trials (Fig. 2B) precisely when reactive control is required to override a prepotent target response. This pattern of results closely resembles an age dissociation at the other end of the lifespan, whereby young adults show greater proactive prefrontal cortical activity to cues whereas older adults show greater reactive activity to probes (28).
Fig. 2.
Pupillometry. (A) Averaged across trials, 8-year-olds show larger pupils than 3.5-year-olds during the delay period, reflecting increased mental effort for proactive context maintenance (marked by *). In contrast, 3.5-year-olds show larger pupils than 8-year-olds during the probe period, reflecting increased mental effort for context retrieval (marked by **). (B) The 3.5-year-olds show a large reactive peak during probes on BX trials, precisely when context reactivation is required (marked by ***). (C) Across all trials, 8-year-olds show larger changes in pupil diameter during the delay period than 3.5-year-olds, indicating effortful context maintenance. The 3.5-year-olds show a more reactive profile, with large changes in pupil diameter during cues and probes.
Reactive control is characterized by transient rather than sustained effort, and individual subjects may differ in the onset, offset, and duration of this transient effort. Consequently, traditional time-series pupillometry analysis at the group level may underestimate reactive control efforts by averaging across asynchronous peaks of transient mental effort and thus washing out the effect in aggregate time-series analysis. To address this problem, we calculated the coefficient of variation in pupil diameter (CVPD) during each trial phase (Fig. 2C) for each subject, thereby measuring instability in pupil diameter irrespective of its synchrony between subjects. Reactive control should yield increased effort expenditure on encoding and retrieval; accordingly, 3.5-year-olds showed less CVPD during the delay period both relative to 8-year-olds [t(50) = 2.02, P < 0.05] and relative to the probe [F(1,20) = 24.15, P < 0.01] or cue [F(1,20) = 12.16, P < 0.01] periods. In contrast, 8-year-olds showed greater CVPD during the delay than cue period [F(1,30) = 52.97, P < 0.01], as expected for individuals maintaining a representation that is newly lacking sensory input and yielding a significant interaction with age [F(2,50) = 19.31, P < 0.01]. Thus, the CVPD transformation of the pupillometry time series reveals effortful delay-period context maintenance among 8-year-olds and increased cue- and probe-related activity among 3.5-year-olds.
Pupillometry: Individual Differences.
This CVPD transformation also reveals opposite patterns at the level of individual differences due to the use of proactive or reactive control in the relation between probe-period effort and behavioral context sensitivity (in terms of d′context calculated on the basis of AX hits and BX false alarms; refs. 23–25). As expected, greater context sensitivity was associated with relatively less need for cognitive effort during the probe period among 8-year-olds (Pearson's r = −0.361, P < 0.05), whereas 3.5-year-olds showed the opposite but nonsignificant trend (Pearson's r = 0.273, P = 0.23). The contrasting relationships between context sensitivity and probe-period effort across age groups was confirmed by a significant difference in these correlations (Fisher's z = 2.18, P < 0.03). In addition, context sensitivity increased with age [t(1,55) = 9.01, P < 0.01], perhaps reflecting the benefits conferred by proactive control.
Reaction Time Analyses: Group Differences.
In terms of reaction times (RTs), proactive control should incur a cost on AY trials (due to advance preparation of an incorrect target response that must then be overridden) but lead to a benefit on B trials (due to advance preparation of a nontarget response).* Indeed, 8-year-olds were more slowed on AY than on BX and BY trials [F(1,33) = 45.62, P < 0.01 and F(1,33) = 58.75, P < 0.01, respectively], whereas 3.5-year-olds showed no differential slowing on these trials (P's > 0.15). A significant interaction with age indicates that 8-year-olds showed significantly more proactive control than 3.5-year-olds [F(1,50) = 7.55, P < 0.01].
Reaction Time Analyses: Individual Differences.
Individual differences in RT on the nontarget BY trials were differentially predictive of individual differences in AY and BX RT depending on age group. Because reactive control is associated with probe-driven processing, individual differences in RT on trials with Y probes should correlate under a reactive regime: Both trial types involve controlled processing of the Y probe (and perhaps some attentional capture, due to the Y probe's rarity). Consistent with this prediction, only 3.5-year-olds showed a significant correlation between Y probe RTs, after controlling for general reaction time to AX trials (3.5-year-olds, Pearson's r = .587, P < 0.02; 8-year-olds, Pearson's r = −0.12, P > 0.5). A Fisher z test indicated that these correlations were significantly different (z = 2.23, P = 0.01).
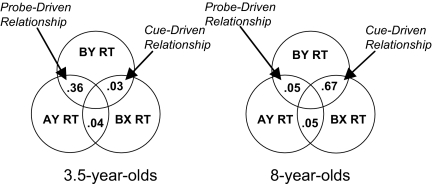
Conversely, proactive control is associated with cue-driven processing; in that case, individual differences in RT on trials with B cues should correlate, because both trial types involve controlled processing of the B cue. Accordingly, only 8-year-olds showed a significant correlation between RT on trials with B cues after controlling for general reaction time to AX trials (3.5-year-olds: Pearson's r = .129, P > 0.6; 8-year-olds: Pearson's r = 0.8, P < 0.01). Again, a Fisher z test indicated that these correlations were significantly different (z = 2.70, P < 0.01). We constructed ballantines to illustrate the overlapping variance among AY, BX, and BY trial reaction times (Fig. 3) using squared semipartial correlations. Negative commonality among all 3 predictors was set to zero (as in other commonality analyses; refs. 29 and 30) and is therefore not illustrated.
Fig. 3.
Squared semipartial correlations on nontarget trials, controlling for AX RTs. AY and BY trials share the Y probe and should thus correlate under a probe-driven and reactive regime. Accordingly, only 3.5-year-olds show individual differences in BY trial reaction times that have large overlapping variance with AY trials. Conversely, BY and BX trials share the B cue and should correlate under a cue-driven and proactive regime; only 8-year-olds show this pattern of individual differences in BY trial reaction times that have large overlapping variance with BX trials.
Additional evidence for a qualitative shift from reactive to proactive control is provided from conditionalized accuracy and other analyses (see SI Text).
Discussion
By dissociating proactive and reactive control mechanisms in children, our findings call into question a previously untested assumption of developmental theories of cognitive control, that is, relative to young adults, weaker but qualitatively similar control processes guide the task performance of children. Of course, children and even infants may be capable of sustaining context representations over shorter delays than the 1.2 s used here, but such limited proactive mechanisms would seem unlikely to strongly influence most behaviors.
Further research is needed to determine the processes that drive the developmental transition from reactive to proactive control. This qualitative shift could reflect genuinely qualitative changes, for example, in metacognitive strategies that allow children to engage proactive control (31). Alternatively (or additionally), the underlying mechanisms for this qualitative shift could be continuous. For example, the gradual strengthening of task-relevant representations (9) could allow proactive control to become effective, thus supporting a shift in the temporal dynamics of control. In any case, the developmental progression to be addressed is a shift from reactive to proactive control rather than merely positing incremental improvements with development.
These results reflect dual methodological advances, establishing the utility of both pupillometry for assessing the temporal dynamics of cognitive control and the AX-CPT for use in preschoolers. These findings may also shed light on the profound disruption of prefrontal development resulting from early hippocampal damage relative to that occurring later (32): reactive control may engage prefrontal mechanisms in conjunction with hippocampal recall mechanisms (ref. 14, cf. ref. 33), such that young children's dependence on reactive control contributes to the dependence of prefrontal development on hippocampal integrity early in life.
More generally, this evidence indicates the need to reconsider the mechanisms guiding a wide variety of children's behaviors and the implications for improving performance. Failures of reactive control, in the absence of proactive mechanisms, might explain children's difficulties in switching from one task to another (34), reasoning about others' thoughts (35), and thinking outside of the moment (12, 13). In each case, children may fail to engage the reactive control that is needed to respond appropriately, due to insufficient conflict at the moment that retrieval of relevant information is required. Further, even if reactive control is engaged, children may mistakenly retrieve incorrect information due to retrieval-based interference. For example, in the widely used appearance–reality and false-belief tasks meant to assess reasoning about others' thoughts, the beliefs of others are based on a preceding situation that is visually similar to the child's current context, thereby increasing interference on the retrieval of the preceding context on which others' thoughts are based. From this perspective, children's performance across domains is less likely to be improved via manipulations that rely on proactive control, such as repeating instructions that must be maintained (34), than by manipulations aimed at supporting reactive control, such as highlighting conflict to increase the chances of engaging reactive control (36) and using distinctive contexts to reduce retrieval-based interference (37, 38).
In conclusion, a dependence on reactive control may explain why even highly capable children show an apparent inability to heed warnings or instructions if they pertain to a context that is cognitively distal, previously unassociated with conflict, or highly similar to other contexts.
Materials and Methods
Participants.
Thirty-four 3.5-year-old (M = 3.58 years, SD = 7 days) and thirty-four 8-year-old (M = 8.38 years, SD = 3.6 months) children were recruited using a participant database and completed the expectancy AX-CPT. Fourteen additional 3.5-year-old children were excluded for completing <20 trials in the AX-CPT (n = 10) or for failure to cooperate with the experimenter (n = 4). Two additional 8-year-old children were excluded for mental illness reported by the parent (n = 1) and experimenter error (n = 1). Parents received $5 compensation for travel expenses, and children were given a small toy.
Procedure.
A child-adapted AX-CPT was modified from the standard dual-response expectancy task to improve engagement, task understanding, and sustained attention. First, the AX-CPT's abstract letter stimuli were replaced with popular cartoon characters and the instructions took the form of character preferences. For example, subjects were told, “Spongebob likes watermelon, so press the happy face when you see Spongebob and then the watermelon,” and, “Blue doesn't like the slinky, so press the sad face when you see Blue and then the slinky.” The pairings of characters, objects, and preferences were all counterbalanced. The target and nontarget buttons appeared only with the presentation of each probe; therefore, subjects responded only to probes, as in other versions of the expectancy AX-CPT (39, 40), preventing premature responses.
To ensure that subjects understood the instructions and were capable of following rules, a “verification” phase followed, during which each of the cue–probe pairs was presented. Subjects then responded to each pair of stimuli before moving on to the next pair. If subjects responded incorrectly, then the relevant rule was repeated (“Remember, when you see [A,B] and then you see [X,Y], tap this button [appropriate button blinks] as quickly as you can!”) and subjects were allowed to try again.
Sustained attention was encouraged with 3 additional modifications. First, a “score bar” appeared at the top of the screen and incremented after the completion of each trial. Second, children were given limited time to respond, after which the trial was terminated and the child was asked to respond more quickly. On the basis of pilot work showing that most 3.5-year-old children could respond within 5 s when attentive, this allowable response time was initially set to 6 s and was thereafter adjusted using an adaptive tracking procedure such that children had to respond within 150% of their mean RT on the previous 8 trials. If subjects did not respond within the allotted time, then the image of a sleeping alarm clock appeared, and subjects were told, “Oops! Too slow. Try to tap the button faster next time.” Third, verbal feedback was provided after each timely response, either “Good job!” (for correct responses) or “Remember the rules!” (for incorrect responses).
Stimuli were displayed via computer using E-Prime (version 1.1), and all responses were collected through a MagicTouch touchscreen interface. Participants were instructed to use a “magic pointing stick” to respond on the touchscreen. A Tobii x50 eyetracker recorded pupil diameter under constant illumination every 20 ms between the onset of cues and the provision of a response. Cues were presented for 500 ms, followed by a blank delay interval of 1,200 ms, followed by the presentation of a probe stimulus and the 2 response buttons for the allowable response time. Each block consisted of 30 trials, after which children were provided with a congratulatory image on the computer, a small prize, and then encouragement to complete another block of trials. The task concluded when children expressed and confirmed a desire to stop; this possibility was checked at the conclusion of every block.
Data Trimming and Transforming.
Responses made <200 ms after the presentation of the probe were removed from the analysis, resulting in the exclusion of <1% of all trials. As in previous work (28), average RTs from correct trials on each trial type were z-transformed with respect to each subject's grand mean RT across all correct trials to increase statistical power and correct for individual differences in overall processing speed.
Pupillometry was limited to the first 4.5 s of data from correct trials where both eyes were successfully tracked, and measurements were averaged into consecutive 60-ms bins. To control for any effects of age-related differences in baseline pupil diameter on pupil variability, all pupil measurements were transformed with respect to each subject's mean pupil diameter in one of two ways: For time-series analyses, the data were transformed into percentage change from the subject's grand mean across all recorded samples. For repeated-measures analyses, the data were transformed into the standard deviation/mean ratio of each subject's pupil diameter (i.e., the CVPD) during the cue, delay, and probe periods.
Preliminary Analyses.
The 3.5-year-old children completed 44 trials on average, and the 8-year-old children completed 103 trials on average. Although younger children completed fewer trials than older children, similar patterns were observed when analyzing only the first 44 trials completed by 8-year-olds, demonstrating that the observed reactive-to-proactive shift is not driven by the difference in the number of trials completed by each age group.
Both groups provided sufficient data to confirm an ability to follow rules and reveal converging evidence for distinct dynamics in cognitive control. First, before the testing phase, all subjects successfully completed 12 practice trials, confirming they could understand and follow the task rules. Second, accuracy was significantly >50% overall [t(67) = 24.69, P < 0.01], and for each age group individually [3.5-year-olds, t(33) = 14.41, P < 0.01); 8-year-olds, t(33) = 99.8, P < 0.01]. Although 70% accuracy could be achieved simply by perseverating on the target response (due to the preponderance of AX trials), BY trial accuracy was also significantly >50% overall [t(67) = 8.94, P < 0.01] and for each age group individually [3.5-year-olds, t(33) = 2.59, P = 0.01; 8-year-olds, t(33) = 120.17, P < 0.01], indicating that children were not simply perseverating.
Finally, we minimized the chances that perseverative responding was driving the observed effects by excluding all children with <60% accuracy on BY trials, yielding a total of twenty-three 3.5-year-olds and thirty-four 8-year-olds for inclusion in the analyses. This performance-based filtering of 3.5-year-olds yielded an average accuracy of 83% across all trial types and 87% on BY trials (above chance according to a conservative binomial test; z = 4.34, P < 0.01). Similar patterns were observed in the larger group, and all of the figures and analyses reported in Results and the SI Text refer to this smaller group. Thus, the distinct patterns observed in proactive and reactive controls are not driven by a failure to understand the task rules or a more general difficulty in following rules.
Supplementary Material
Acknowledgments.
We thank T. Braver, C. A. Chatham, B. Depue, L. Gindin, A. Miyake, E. Nyhus, R. O'Reilly, and Cognitive Development Center members for their helpful comments and suggestions. This work was funded by National Institutes of Health Grants R01 HD37163 and P50-MH079485.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810002106/DCSupplemental.
RTs on correct AX trials are uninformative, because they can reflect either prepotent responding or proactive preparation of a target response.
References
- 1.Kirkham NZ, Cruess LM, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Dev Sci. 2003;6:449–467. [Google Scholar]
- 2.Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kane MJ, Engle. RW. Working memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol Gen. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JD, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 6.Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 7.Rougier NP, Noelle DC, Braver TS, Cohen JD, O'Reilly RC. Prefrontal cortex and flexible cognitive control: Rules without symbols. Proc Natl Acad Sci USA. 2005;102:7338–7343. doi: 10.1073/pnas.0502455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zelazo PD, et al. The development of executive function in early childhood. Monogr Soc Res Child Dev. 2003;68:vii–137. doi: 10.1111/j.0037-976x.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 9.Stedron JM, Sahni SD, Munakata Y. Common mechanisms for working memory and attention: The case of perseveration with visible solutions. J Cognit Neurosci. 2005;17:623–631. doi: 10.1162/0898929053467622. [DOI] [PubMed] [Google Scholar]
- 10.Thelen E, Schoner G, Scheier C, Smith LB. The dynamics of embodiment: A field theory of infant perseverative reaching. Behav Brain Sci. 2001;24:1–86. doi: 10.1017/s0140525x01003910. [DOI] [PubMed] [Google Scholar]
- 11.Bunge SA, Zelazo PD. A brain-based account of the development of rule use in childhood. Curr Dir Psychol Sci. 2006;15:118–121. [Google Scholar]
- 12.Atance CM, Meltzoff AN. Young children's ability to anticipate future states of the self. Cogn Dev. 2005;20:341–361. doi: 10.1016/j.cogdev.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atance CM, Meltzoff AN. Preschoolers' current desires warp their choices for the future. Psychol Sci. 2006;17:583–587. doi: 10.1111/j.1467-9280.2006.01748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braver TS, Gray JR, Burgess GC. In: Variation in Working Memory. Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse J, editors. New York: Oxford Univ. Press; 2007. pp. 76–106. [Google Scholar]
- 15.Unsworth N, Engle RW. The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychol Rev. 2007;114:104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- 16.Einstein GO, et al. Multiple processes in prospective memory retrieval: Factors determining monitoring versus spontaneous retrieval. J Exp Psychol Gen. 2005;134:327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- 17.Dosenbach UF, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shallice T, Vallesi A. Developmental dissociations of preparation over time: Deconstructing the variable foreperiod phenomena. J Exp Psychol Hum Percept Perform. 2007;33:1377–1388. doi: 10.1037/0096-1523.33.6.1377. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths P, Campbell R, Robinson P. Executive function in treated phenylketonuria as measured by the one-back and two-back versions of the continuous performance test. J Inherit Metab Dis. 1998;21:125–135. doi: 10.1023/a:1005339524847. [DOI] [PubMed] [Google Scholar]
- 20.Beatty J, Lucero-Wagoner B. In: Psychophysiology: Systems, Processes, and Applications. Coles MGH, Donchin E, Porges SW, editors. New York: Guilford Press; 2000. pp. 43–50. [Google Scholar]
- 21.Just MA, Carpenter PA, Miyake A. Neuroindices of cognitive workload: Neuroimaging, pupillometric, and event-related potential studies of brain work. Theor Issues Ergon Sci. 2003;4:56–88. [Google Scholar]
- 22.Braver TS, et al. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. J Exp Psychol Gen. 2001;130:746–763. [PubMed] [Google Scholar]
- 23.Barch DM, et al. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 24.Barch DM, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 25.Frank MJ, Santamaria A, O'Reilly R, Willcutt E. Testing computational models of dopamine and noradrenaline dysfunction in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2007;32:1583–1599. doi: 10.1038/sj.npp.1301278. [DOI] [PubMed] [Google Scholar]
- 26.Bunge SA, et al. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karatekin C, Marcus DJ, Couperus JW. Regulation of cognitive resources during sustained attention and working memory in 10-year-olds and adults. Psychophysiology. 2007;44:128–144. doi: 10.1111/j.1469-8986.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 28.Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frederick BN. In: Advances in Social Sciences Methodology. Thompson B, editor. Stamford, CT: JAI Press; 1999. pp. 305–318. [Google Scholar]
- 30.Capraro RM, Capraro MM. Bigger is not better: Seeking parsimony in canonical correlation analysis via variable deletion strategies. Multiple Linear Regression Viewpoints. 2001;27:16–23. [Google Scholar]
- 31.Gathercole SE. The development of memory. J Child Psychol Psychiatry. 1998;39:3–27. [PubMed] [Google Scholar]
- 32.Blair C. How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. Behav Brain Sci. 2006;29:109–125. doi: 10.1017/S0140525X06009034. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds JR, Braver TS, Brown JW, van der Stigchel S. Computational and neural mechanisms of task switching. Neurocomputing. 2006;69:1332–1336. [Google Scholar]
- 34.Brace JJ, Morton JB, Munakata Y. When actions speak louder than words: Improving children's flexibility in a card-sorting task. Psychol Sci. 2006;17:665–669. doi: 10.1111/j.1467-9280.2006.01763.x. [DOI] [PubMed] [Google Scholar]
- 35.Flavell JH. Children's knowledge about the mind. Annu Rev Psychol. 1999;50:21–45. doi: 10.1146/annurev.psych.50.1.21. [DOI] [PubMed] [Google Scholar]
- 36.Mack W. Improving postswitch performance in the dimensional change card-sorting task: The importance of the switch and of pretraining by redescribing the test cards. J Exp Child Psychol. 2007;98:243–251. doi: 10.1016/j.jecp.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Sahakyan L, Kelley CM. A contextual change account of the directed forgetting effect. J Exp Psychol Learn Mem Cogn. 2002;28:1064–1072. doi: 10.1037//0278-7393.28.6.1064. [DOI] [PubMed] [Google Scholar]
- 38.Delaney PF, Sahakyan L. Unexpected costs of high working memory capacity following directed forgetting and contextual change manipulations. Mem Cognit. 2007;35:1074–1082. doi: 10.3758/bf03193479. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald AW, et al. A convergent-divergent approach to context processing, general intellectual functioning, and the genetic liability to schizophrenia. Neuropsychology. 2005;19:814–821. doi: 10.1037/0894-4105.19.6.814. [DOI] [PubMed] [Google Scholar]
- 40.Haarmann HJ, Ashling GE, Davelaar EJ, Usher M. Age-related declines in context maintenance and semantic short-term memory. Q J Exp Psychol A. 2005;58:34–53. doi: 10.1080/02724980443000214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.