Abstract
H-2Kb-restricted tumor epitope peptides, including tyrosinase-related protein 2 residues 181–188 (TRP-2) and connexin 37 residues 52–59 (MUT1), were applied to permeability barrier-disrupted C57BL/6 (B6) mouse skin from which the stratum corneum of the epidermis had been removed by tape-stripping. This procedure primed tumor-specific cytotoxic T lymphocytes (CTLs) in the lymph nodes and spleen, protected mice against subsequent challenge with corresponding tumor cells, and suppressed the growth of established tumors. Preventive and therapeutic effectiveness was correlated with the frequency of tumor-specific CTL precursors. MHC class II Iab+ cells separated from tape-stripped skin, compared with those from intact skin, exhibited a strong antigen-presenting capacity for CTL, suggesting that CTL expansion after peptide application is primarily mediated by epidermal Langerhans cells. Thus, percutaneous peptide immunization via barrier-disrupted skin provides a simple and noninvasive means of inducing potent anti-tumor immunity which may be exploited for cancer immunotherapy.
One of the principal goals in tumor immunoprophylaxis and therapy is induction of anti-tumor responses by generating sufficient numbers of tumor antigen-specific cytotoxic T lymphocytes (CTLs). In tumor bearers, however, not only effective CTL priming but also recognition and effective lysis of tumor cells by CTLs are often blocked or attenuated by down-regulated expression of major histocompatibility complex (MHC) class I antigens and of costimulatory and adhesion molecules on tumor-cell surfaces (1), suggesting one evasion mechanism of tumor cells which affects immune surveillance. Therefore, establishment of efficient CTL priming strategies is an important issue for cancer immunotherapy.
Dendritic cells are potent antigen-presenting cells distributed widely at epithelial surfaces which interface with the external environment to facilitate efficient antigen trapping (2, 3). Because of high levels of expression of MHC class I antigen and costimulatory and adhesion molecules, tumor-specific CTLs are efficiently induced with tumor antigen- or peptide-loaded dendritic cells (4–7). Studies in animal models have shown the potential of dendritic cell-based tumor immunotherapy to elicit protective anti-tumor immune responses, and the validity of such an approach has also been confirmed in humans (8–10). While understanding of the mechanism of propagation of dendritic cells under cytokine influence has progressed at a rapid pace, the intricate manipulations required for in vitro preparation of large numbers of dendritic cells in a form appropriate for immunotherapy seem to be a major drawback in current immunotherapeutic strategies.
We have reported that disruption of the skin barrier results in not only enhanced permeability but also alterations in the immunoregulatory function of the skin in such a way that epidermal Langerhans cells (LCs) function as vigorous antigen presenters for T-helper cells (11). Various studies have shown that LCs target tumor antigens for induction of anti-tumor CTL responses in vitro and in vivo (12–15). In the present study we demonstrate that topical application of tumor-associated peptide onto the stratum corneum barrier-disrupted skin in mice induces protective anti-tumor responses in vivo and in vitro. LCs with up-regulated expression of MHC molecules and costimulatory molecules seem to be responsible for increasing the frequency of active CTL precursors. This percutaneous peptide immunization is a different and noninvasive method of tumor immunoprophylaxis and immunotherapy.
Materials and Methods
Mice and Tumors.
Male C57BL/6 (B6) mice, 7–9 weeks old, were obtained from Japan SLC (Hamamatsu, Japan). Both B16 melanoma (B16) and Lewis lung carcinoma (3LL), highly metastatic and poorly immunogenic tumor cell lines (16, 17), were maintained in vitro in Dulbecco's modified Eagle's medium (Nissui Pharmaceuticals, Tokyo) supplemented with 10% fetal calf serum (FCS; Filtron, Brooklyn, Australia). Tumor cells were inoculated subcutaneously (s.c.) (1 × 105 cells per mouse) at the dorsal surface.
Synthetic Peptides.
The following H-2Kb-restricted peptides were synthesized with an automated solid-phase peptide synthesizer, and purified by reverse-phase HPLC at >97% purity: TRP-2, tyrosinase-related protein 2 residues 181–188, VYDFFVWL; MUT1, the mutated form of connexin 37 protein residues 52–59, FEQNTAQP; and OVA, ovalbumin residues 257–264, SIINFEKL (18). TRP-2 and MUT1 peptides are tumor-associated antigens on B16 (16) and 3LL (17) cells, respectively. Because of low solubility in water, TRP-2 and OVA peptides were dissolved in PBS, acetone/olive oil (4:1), or 70% ethanol at 2 mg/ml for skin application and at 5 mg/ml for intradermal injection. For in vitro pulsing, stock solutions of peptides in PBS (5 mg/ml) were added to cells (106 per ml) in RPMI medium 1640 (no FCS), giving a final concentration of 50 μg/ml.
Barrier Disruption.
The skin barrier was disrupted by stripping both sides of the earlobes and depilated abdomen with cellophane tape (Nichiban, Tokyo), 8 and 15 times, respectively (11). Transepidermal water loss measured with an electrolytic water analyzer (Meeco, Warrington, PA) was maximal (8.7 ± 0.1 and 5.2 ± 0.2 mg/cm−2 per hr at earlobe and abdominal skin, respectively) immediately after tape stripping, and returned to about 40% of the maximal level at 24 hr.
In Vivo Induction of Peptide-Specific CTLs.
At 12, 24, and 48 hr after tape stripping, peptide was painted on both sides of the earlobes at doses of 24, 48, and 96 μg per mouse. For the second immunization, peptide at 24, 48, and 96 μg per mouse was applied to abdominal skin 2 weeks after the first treatment, which was depilated and tape-stripped 24 hr before peptide application. Cervical lymph nodes and spleens were removed 10 days after the first peptide application or 5 days after the second sensitization. After hemolysis, spleen cells were enriched for T cells by passage through nylon wool. Flow cytometric analysis revealed that more than 90% of the cells were CD3+. Lymph node cells or T-cell-enriched splenocytes (effector cells) were cultured in RPMI 1640 complete medium (CM) supplemented with 2.5 × 10−3 M Hepes, 5 × 10−5 M 2-mercaptoethanol, 2 × 10−5 M l-glutamine, 10−5 M sodium pyruvate, 1% nonessential amino acids, 100 mg/ml gentamycin, 10% FCS, and 5 units/ml human recombinant IL-2 for 3 days to expand sensitized cells, and were then subjected to CTL assay. In some experiments, peptide was injected intradermally at the base of the earlobes (96 μg per mouse). Control mice received peptide treatment on intact skin.
CTL Assay.
51Cr-release CTL assays used as target cells both nontransfected Ltk- cells and Ltk- cells transfected with the H-2Kb gene, both kindly provided by K. Egawa (Institute Tumor Laboratory, Tokyo). Target cells were first labeled with 51Cr for 1 hr at 37°C, washed thoroughly, and then pulsed for 1 hr in RPMI medium 1640 (no FCS) with TRP-2, MUT1, or OVA peptide at a concentration of 50 μg/ml and a density of 106 cells per ml. 51Cr-labeled target cells (104 cells per well) were then cocultured with effector cells at several effector-to-target ratios, and 6 hr later, supernatants were harvested and counted in a γ counter. Percentage specific lysis was calculated as described previously (19).
Peptide Immunization and Tumor Growth.
For prophylaxis of tumor development, mice were immunized twice by applying TRP-2 or MUT1 peptide to earlobes (96 μg per mouse) and, 2 weeks later, to abdominal skin (96 μg per mouse) (total 192 μg of peptide per mouse). Both sites were tape-stripped 24 hr before peptide application. Ten days after boosting, mice were inoculated s.c. with B16 or 3LL tumor cells (1 × 105 cells per mouse), and subsequent tumor growth was assessed every 3 days by measuring an average of perpendicular tumor diameters with a caliper. For treatment of established tumors, mice were implanted s.c. with B16 or 3LL tumor cells. One week after tumor inoculation (approximately 5–7 mm average tumor diameter), TRP-2 or MUT1 peptide was applied first to earlobes (96 μg per mouse) and 48 hr later to depilated abdominal skin (96 μg per mouse) (total peptide applied, 192 μg per mouse). All sites were tape-stripped 24 hr before peptide application. Tumor growth and survival rate were followed by measuring tumor diameter and counting surviving mice, respectively. In both experimental protocols, control mice were treated by application of peptide to intact skin or irrelevant peptide to tape-stripped skin, or mice were not treated with peptide.
Measurement of Precursor CTL (pCTL) Frequencies.
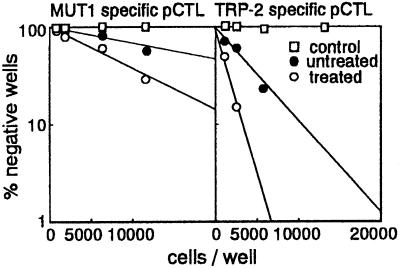
Frequencies of pCTLs in spleens of naive or immunized mice were determined by limiting dilution analysis (20, 21). Briefly, graded numbers of spleen cells (1,000–12,000 cells per well) in RPMI medium 1640 (10% FCS) supplemented with 50 units per ml IL-2 were cocultured for 10 days with LKb cells (104 per well) pulsed immediately before with TRP-2 or MUT1 (see CTL Assay above for pulsing conditions). Wells were assayed for CTL activity by adding 51Cr-labeled, peptide-pulsed LKb cells (104 per well) for 6 hr at 37°C. Wells with specific lysis of >10% were scored as positive (21). The spontaneous release in all assays was 1.18%. Regression analysis was used in a semilogarithmic plot of the data obtained in this way against the log of the percentage of negative wells. The intersection of 37% negative wells with the regression line was used to estimate the frequency.
LC-Enriched and LC-Depleted Epidermal Cells.
Epidermal cell suspensions were prepared from earlobes (11). Interface cells isolated from epidermal cell suspensions by centrifugation on Histopaque 1083 (Sigma) were preincubated and incubated with anti-mouse CD16/CD32 Fab mAb (PharMingen; 5 μg per 106 cells, 15 min, 4°C) and anti-mouse Iab mAb (PharMingen; 5 μg per 106 cells, 30 min, 4°C), respectively, and subsequently with anti-mouse IgG-conjugated magnetic beads (Dynal, Oslo, Norway) at a ratio of three beads per cell for 1 hr at 4°C to obtain Iab+ cells. Unseparated epidermal cells stained with Iab mAb were bound to anti-mouse IgG-conjugated magnetic beads at a ratio of 20 beads per cell to deplete Iab+ cells. LC-enriched and LC-depleted epidermal cells obtained by this method (manufacturer's suggestion) contained >72% Iab+ cells and <0.01% Thy-1+ cells, and <0.1% Iab+ cells, respectively.
In Vitro CTL Priming Capacity of Epidermal Cells.
LC-enriched and LC-depleted epidermal cells from intact and tape-stripped skin were pulsed with TRP-2, MUT1, or OVA peptide at 37°C for 90 min in RPMI 1640 (without FCS) at a peptide concentration of 50 μg/ml and a cell density of 106 cells per ml. Cervical lymph node cells from naive mice were cultured with the pulsed cells at a ratio of 50:1 in CM supplemented with 5 units/ml recombinant IL-2 and 10% FCS for 7 days before assaying for CTL activity.
Depletion of CD4+ or CD8+ Cells in Vivo.
B6 mice were treated once i.v. with 500 μg of anti-CD4 mAb (YTS 177.9; Serotec, Oxford, England), anti-CD8 mAb (YTS 105.18; Serotec) or control mouse IgG Ab in 200 μl of PBS per mouse. In protocols for prophylaxis and therapy, these mAbs were injected 5 days before tape-stripping and tumor inoculation, respectively. Disappearance of CD4+ or CD8+ cells in the mAb-treated mice was confirmed by flow cytometric analysis of lymph node cells in comparison with those from control mouse IgG-treated mice. Dysfunction of CD4+ cells after treatment with anti-CD4 mAb was assessed by measuring the delayed type hypersensitivity (DTH) response to sheep red blood cells, in which footpad challenge was performed 7 days after sensitization (22).
Results
Specific CTL Priming by Application of Peptide to Barrier-Disrupted Skin.
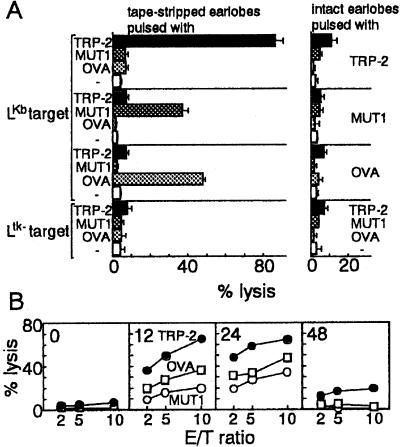
Stripping of the skin with adhesive tape is a method commonly used to remove the stratum corneum and induce subsequent immunologic alterations (11, 23, 24). We therefore explored specific CTL priming by application of H-2Kb-restricted CTL epitope peptides, including TRP-2, MUT1, and OVA, on tape-stripped earlobes of B6 mice. When the same peptide was used for immunization and pulsing of LKb cells, cervical lymph node cells cultured short term exerted marked killing activity against peptide-pulsed LKb cells (Fig. 1A). Moreover, optimal CTL priming was observed 12 and 24 hr after tape-stripping (Fig. 1B) at peptide doses of 48 and 96 μg per mouse (Fig. 2A). Peptides at 24 μg per mouse were less effective, compared with these doses. On the other hand, CTL priming was virtually absent when peptide was applied to intact earlobes.
Figure 1.
Specificity and time course of in vivo CTL priming after application of peptide to tape-stripped skin. (A) Cervical lymph node cells (effectors) obtained from mice (n = 5) immunized 10 days earlier with TRP-2, MUT1, or OVA (96 μg per mouse) either through intact earlobes or earlobes tape-stripped 12 hr earlier were subjected to CTL assay using LKb or Ltk- target cells pulsed with TRP-2, MUT1, or OVA. CTL assays were performed at effector-to-target ratio of 10. (B) TRP-2, MUT1, or OVA (96 μg per mouse) was painted on intact earlobes (0 hr) and those tape-stripped 12, 24, and 48 hr before. Cervical lymph node cells (effectors) obtained 10 days after peptide application were tested in a CTL assay using LKb target cells pulsed with the peptide used for immunization. Data are expressed as the average of the mean of two independent experiments. E/T, effector-to-target ratio.
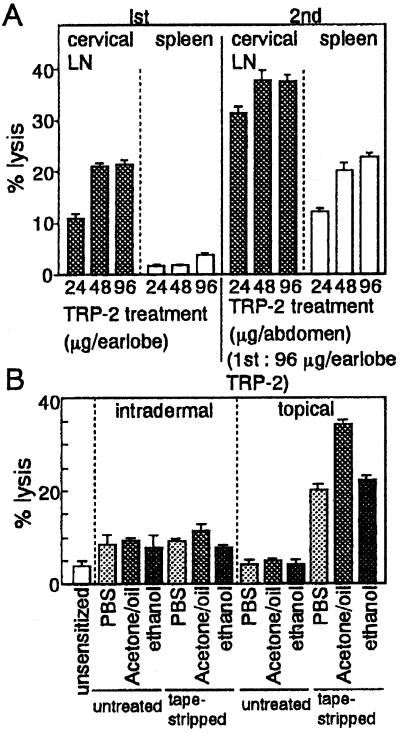
Figure 2.
Augmentation of specific CTL induction by peptide application topically on tape-stripped skin. (A) Indicated doses of TRP-2 peptide dissolved in acetone/oil were painted on tape-stripped earlobes (1st) and abdominal skin (2nd) 2 weeks later. Cervical lymph node cells and splenocytes were assayed for lysis against TRP-2-pulsed LKb target cells at an effector-to-target ratio of 10. (B) Mice were immunized with TRP-2 (96 μg per mouse) dissolved in PBS, acetone/oil, or 70% ethanol by painting on earlobes or with intradermal injection into the base of earlobes, which were either untreated or tape-stripped. Cervical lymph node cells were assayed with TRP-2-pulsed LKb target cells at an effector-to-target ratio of 20. All data are expressed as mean ± SE of two independent experiments.
Fig. 2A also showed that TRP-2 peptide application on earlobes generated CTLs preferentially in cervical lymph nodes but rarely in spleen. However, reapplication of TRP-2 peptide to tape-stripped abdominal skin in preimmunized mice augmented specific CTL activity in both lymph nodes and spleen. Therefore, CTL sensitization by this strategy seemed to operate in the draining lymphoid organs. Peptide painting on tape-stripped skin is more effective in generating CTLs than is intradermal injection because priming efficacy was low even when TRP-2 dissolved in PBS, acetone/oil, or 70% ethanol was injected intradermally at tape-stripped earlobes (Fig. 2B).
Peptide Immunization via Barrier-Disrupted Skin for Tumor Immunotherapy.
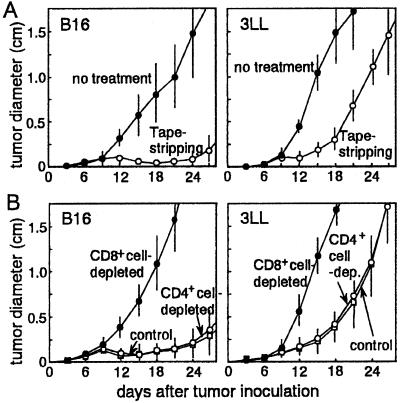
Mice were immunized twice with TRP-2 or MUT1 at barrier-disrupted or intact earlobes and abdominal skin, and were challenged s.c. on the back lateral to the midline with B16 or 3LL tumor cells (1 × 105 cells per mouse) at the dorsum. B16 tumor cells were virtually completely rejected after TRP-2 immunization via barrier-disrupted skin (Fig. 3A); these mice all survived for more than 40 days. In contrast, no protection was observed in mice immunized via intact skin. MUT1-immunized mice showed significant attenuation of 3LL tumor proliferation compared with control mice until day 20. However, 90% of the mice died of continuous tumor progression by day 30. In both tumor types, mice immunized with irrelevant peptide and untreated mice showed the same tumor growth rate as mice immunized via intact skin (data not shown). CD8+ cell-depleted mice prepared by i.v. injection of specific mAb demonstrated no resistance to either B16 or 3LL tumor challenge after treatment with specific peptide at tape-stripped skin sites (Fig. 3B). On the other hand, CD4+ cell-depleted mice still had resistance against B16 or 3LL cell challenge by treatment with specific peptides at tape-stripped skin, suggesting T-helper cell-independent generation of CTLs. As assessed by flow cytometry, depletion of CD4+ cells was >98% 5 days after anti-CD4 mAb treatment. However, by 14 days, CD4+ cells had recovered to 48% of control levels. Foot pad swelling delayed type hypersensitivity responses to sheep red blood cells (SRBCs) were suppressed by 73% in mice injected with the same dose of anti-CD4 mAb 10 days before SRBC sensitization, but were suppressed by only 37% in mice injected with mAb 14 days before sensitization. These results do not definitively eliminate the possibility that CD8-dependent resistance to tumor growth may be helped to some degree by residual and/or recovering CD4+ cells in the mAb-injected hosts.
Figure 3.
Protection against tumor cell challenge after peptide immunization via barrier-disrupted skin. (A) Mice (n = 10) were treated with TRP-2 or MUT1 on untreated or tape-stripped skin. B16 or 3LL tumor cells (1 × 105 cells per mouse) were inoculated s.c. into TRP-2- or MUT1-treated mice, respectively, and tumor sizes were measured every 3 days. (B) CD4+ or CD8+ cell-depleted, or control rat IgG-treated mice (n = 10) were painted with TRP-2 or MUT1 on tape-stripped skin. B16 or 3LL tumor cells (1 × 105 cells per mouse) were inoculated s.c. into TRP-2- or MUT1-treated mice, respectively, and tumor sizes were measured every 3 days.
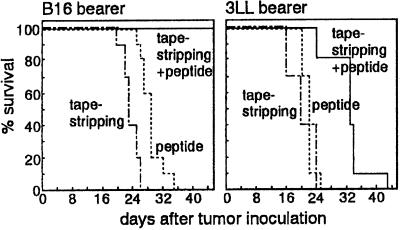
When B16 or 3LL tumor cells that were inoculated to mice attained approximately 5 mm in diameter, tumor-bearing animals were treated with TRP-2 or MUT1 on tape-stripped skin as described above. B16 tumors regressed with TRP-2 application, and 100% of the mice survived for 1 month and 90% for over 50 days (Fig. 4). Mice treated with tape-stripping only, or peptide application to intact skin, died by 26 and 34 days, respectively. Mice treated with irrelevant peptide also died by 25 days (data not shown). Immunization of 3LL-bearing mice with MUT1 on tape-stripped skin markedly attenuated 3LL tumor growth compared with control mice, although 90% of the mice died within 33 days. When CD8+ cell-depleted mice were inoculated with B16 and 3LL cells and then treated with the corresponding tumor peptide, 40% of B16-bearing mice and 95% of 3LL-bearing mice died by day 20. Furthermore, no mice in either group survived 40 days. On the other hand, almost all of CD4+ cell-depleted mice bearing B16 and 3LL tumor still survived at day 40 and 20, respectively (Table 1). Control Ab treatment did not affect the survival of tumor-bearing mice.
Figure 4.
Tumor immunotherapy by peptide application to barrier-disrupted skin. Mice inoculated with B16 (n = 20) or 3LL (n = 20) were treated with TRP-2 or MUT1, respectively, on tape-stripped earlobes and then abdomen 2 days later. Percentage survival of the mice treated with peptide at tape-stripped skin (solid line), treated with tape-stripping only (dash–dot line), or treated with peptide at intact skin (dotted line) was monitored.
Table 1.
CD8+ cells are essential for survival of tumor bearers
| Mice | % survival after tumor inoculation
|
||
|---|---|---|---|
| 20 days | 40 days | 60 days | |
| TRP-2-treated, B16-bearer control | 100 | 100 | 95 |
| Rat IgG-treated | 100 | 100 | 85 |
| CD4+ cell-depleted | 100 | 95 | 70 |
| CD8+ cell-depleted | 60 | 0 | 0 |
| MUT-1-treated, 3LL-bearer control | 100 | 35 | 0 |
| Rat IgG-treated | 100 | 40 | 0 |
| CD4+ cell-depleted | 95 | 20 | 0 |
| CD8+ cell-depleted | 5 | 0 | 0 |
Ear skin and adbominal skin were tape-stripped 24 hr before application of peptide; the first topical application to ear skin was performed 7 days after tumor inoculation, and the second application was 2 days later to abdominal skin.
These results suggest that growth inhibition of established tumors is mediated primarily by CD8+ effector cells; CD4+ cells, however, may also contribute to the development of anti-tumor effector cells.
TRP-2- and MUT1-specific precursor CTL frequencies in naive splenocytes were 1/6055 and 1/28550, respectively (Fig. 5). The frequency of TRP-2-specific pCTLs (1/1216) was more markedly elevated than that of MUT1-specific pCTLs (1/11625) after two applications of peptide to tape-stripped skin. These findings suggest that the effect of peptide immunization on tumor growth depends in part on specific pCTL frequency.
Figure 5.
MUT1 and TRP-2 peptide-specific pCTL frequencies. Splenocytes obtained from untreated mice or mice treated twice with MUT1 or TRP-2 at tape-stripped skin were subjected to limiting dilution analysis. The number of wells containing peptide-specific CTLs determined by CTL assay was counted. OVA-pulsed LKb target cells were used as control.
Epidermal LCs as the Main and Effective Inducer of CTLs in Barrier-Disrupted Skin.
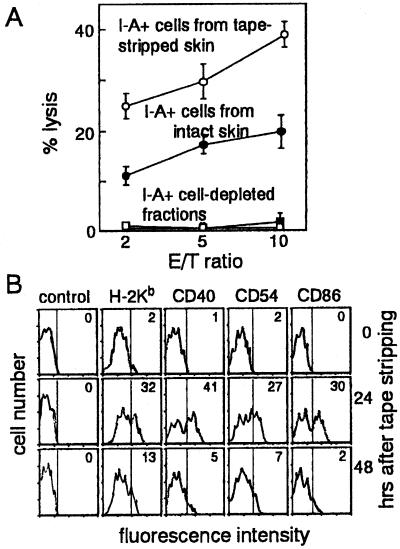
Epidermal cell populations responsible for initiation of CTL priming were identified in barrier-disrupted skin. LC-enriched and LC-depleted epidermal cells were prepared from tape-stripped and intact earlobes and pulsed with peptide. Cervical lymph node cells from intact mice, upon culture with TRP-2-pulsed LC populations, killed TRP-2-pulsed LKb cells, whereas no cytotoxicity was induced with LC-depleted epidermal cells (Fig. 6A). Notably, LCs from barrier-disrupted skin more effectively presented TRP-2 to specific CTLs than did those from intact skin. Flow cytometric analysis showed that substantial numbers of LCs expressed H-2Kb, CD40, CD54, and CD86 molecules vigorously at 24 hr after tape-stripping (Fig. 6B). Expression levels of these molecules returned to untreated levels 48 hr after tape stripping. Thus, LCs with elevated expression of H-2Kb and other accessory molecules were responsible for effective CTL-priming in percutaneous peptide immunization.
Figure 6.
Augmentation of CTL priming capacity of epidermal LCs by barrier disruption. (A) Ia+ cell-enriched and -depleted epidermal cells were prepared from tape-stripped (○) or intact (●) earlobes by separation with anti-Iab mA-conjugated magnetic beads. Cervical lymph node cells (1 × 105) from intact mice were primed in vitro with TRP-2-pulsed epidermal cell populations (1 × 104) at a ratio of 20 lymph node cells to 1 epidermal cell. Cytotoxicity was assayed by using TRP-2-pulsed LKb target cells at indicated effector-to-target (E/T) ratios. Data are expressed as mean ± SE of the results of two independent experiments. (B) Epidermal cells prepared from intact earlobes (0 hr) or those tape-stripped 12, 24, or 48 hr before were incubated with FITC-conjugated H-2Kb (AF6–88.5), CD40 (HM40–3), CD54 (3E2), CD86 (GL-1), or control mouse IgG mAb and phycoerythrin-conjugated anti-Iab mA (AF6–120.3). By flow cytometry, strongly Iab+ cells, representing LCs, were gated, and H-2Kb, CD40, CD54, or CD86 expression was visualized as a histogram. Numbers indicate percentage of LC populations that express H-2Kb, CD40, CD54, and CD86.
Discussion
In the present study, we have demonstrated that immunization with tumor-associated epitopes through barrier-disrupted skin can result in preventive and therapeutic effects on in vivo tumor growth in mice. In the B16 model, TRP-2 peptide vaccination completely protected against subsequent tumor challenge. Furthermore, even when peptide immunization through tape-stripped skin was initiated after tumor growth had already been established, small tumors were partly rejected. On the other hand, the effects of MUT1 immunization were less striking: 3LL cell growth was only retarded in both naive and tumor-bearing mice. When pCTL frequencies were examined, anti-tumor effects were found to be related partly to the degree of generation and expansion of tumor-specific CTLs in lymphoid organs. Two findings indicated that epidermal LCs, but not dermal components, were primarily responsible for CTL priming. First, regional lymph node cells showed cytolytic activity only when peptide was applied to barrier-disrupted skin, not when peptide was introduced in the dermis. This observation further indicated that efficient CTL priming did not rely solely on enhanced peptide delivery. Second, LC-enriched, but not LC-depleted, epidermal cell populations generated anti-tumor effects in vitro. Notably, LCs with up-regulated expression of MHC class I antigen and costimulatory molecules in barrier-disrupted skin were superior in CTL induction to LCs in intact skin.
Dendritic cell-based strategies for cancer treatment have been shown to be promising in animal models and humans. Of note is the recent development in methods in which dendritic cell progenitors present in blood and bone marrow in low numbers are expanded and matured in vitro with the use of different cytokines and growth factors (25–28). Administration of autologous dendritic cells thus manipulated and further pulsed with tumor antigen in vitro has been shown to induce protective anti-tumor immune responses in vivo (29, 30). However, despite enormous progress in the yield of dendritic cells, the intricate manipulations required for large-scale in vitro preparations of dendritic cells remain an obstacle in clinical application to cancer patients. On the other hand, the method described here utilized the host's own LCs in the skin as therapeutic vectors. LCs form a reservoir of immature dendritic cells that are distributed throughout the epidermis (2, 3). Whereas LCs are poor T-cell activators in intact skin, they mature to potent antigen-presenting cells by increasing surface expression levels of MHC antigens and costimulatory molecules in response to tissue perturbations caused by pathogenic invasion (10, 31). Tumor necrosis factor-α and IL-1α are secreted by keratinocytes soon after barrier disruption, resulting in the maturation and activation of LCs (11).
A number of recent studies have indicated that CD4+ cells can play important roles in tumor immunoprophylaxis and therapy (32). However, the results in this study indicate an essential role for CD8+ cells and minimal roles (if any) for CD4+ cells. In the induction of CD8+ cell activity, participation of CD4+ cells should be considered because of gradual recovery of functional CD4+ cells throughout the depletion experiments. Nevertheless, CD4-independent generation of CTLs can be achieved by high antigen concentrations and increased expression of costimulatory molecules (33, 34); the present results have shown effective peptide immunization at higher peptide doses (Fig. 2A) as well as the strong expression of CD40, CD54, and CD86 on epidermal LCs after barrier disruption (Fig. 6).
One concern is the induction of autoimmune diseases in association with promotion of a CTL response by immunization with peptide-loaded dendritic cells. TRP-2 is a normal tissue differentiation antigen produced by melanocytes (16). It is assumed that the relatively high frequencies of TRP-2-reactive CTLs in naive mice, compared with MUT1 pCTL frequencies, are due to an expansion of CTL progenitors by continuous stimulation with melanocyte-derived antigens. However, during the 6-month follow-up period of animals surviving B16 inoculation, we noted neither the significant development of white hair nor a decline in physical condition that would suggest underlying autoimmune manifestations.
Several critical issues should be considered to improve strategic efficacy of the present method. Because peptide-specific CTL frequency seems to be correlated with therapeutic effectiveness, identification of tumor peptides recognized by large-scale CTL populations is of prime importance. It has been reported that antigen-presenting cells pulsed with heat shock protein-conjugated peptide (35, 36) or RNA encoding antigen protein (37) are more powerful in the in vivo and in vitro induction of CTLs than those pulsed merely with peptide. By applying these modified molecules or even naked DNA to perturbed skin (38), peptide may be expressed on LCs with high efficiency. Furthermore, peptide substitution is affected by the strength of peptide-binding affinity with MHC class I molecules. It is possible that the relative inefficacy in the treatment of 3LL tumors resulted from the low substitution rate of MUT1 for peptides already bound to MHC class I molecules on LCs. Application of unfractionated tumor-derived peptides eluted from class I molecules may be more effective than simple peptide painting in maximizing peptide loading (30).
In summary, these data demonstrate that percutaneous peptide immunization via skin with impaired barrier function is a simple and noninvasive strategy to develop effective immune responses against tumors. It has recently been shown that the application of DNA encoding the antigenic protein to skin with increased permeability raises the antigen-specific antibody responses (39, 40). Because the skin represents an easily accessible site for immunization and vaccination, the method reported here is an alternative to injection of CTL-activating molecules and can be readily exploited for cancer treatment in humans. Furthermore, the effective induction of CTLs suggests that this method could potentially be applied to treatment of virus and helminth infections with the use of appropriate antigenic peptides.
Acknowledgments
We thank Dr. Robert E. Tigelaar (Department of Dermatology, Yale University School of Medicine) for critical reading of the manuscript. This work was supported by grants from the Lydia O'Leary Memorial Foundation Fund, the Shiseido Fund, and the Ministry of Health of Japan.
Abbreviations
- CTL
cytotoxic T lymphocyte
- pCTL
precursor CTL
- LC
Langerhans cell
References
- 1.Chen L, Linsley S P, Hellstrom E K. Immunol Today. 1993;14:483–486. doi: 10.1016/0167-5699(93)90262-J. [DOI] [PubMed] [Google Scholar]
- 2.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 3.Watts C. Nature (London) 1997;388:724–725. doi: 10.1038/41900. [DOI] [PubMed] [Google Scholar]
- 4.Celluzzi C M, Mayordomo I J, Storkus J W, Lotze T M, Falo L D J. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zitvogel L, Mayordomo J I, Tjandrawan T, DeLeo A B, Clarke M R, Lotze M T, Storkus W J. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paglia P, Chiodoni C, Rodolfo M, Colombo P M. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young J W, Inaba K. J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu F J, Benike C, Fagnoni F, Liles T M, Czerwinski D, Taida B, Engleman E G. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg S A. Immunol Today. 1997;18:175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 10.Nestle F O, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 11.Nishijima T, Tokura Y, Imokawa G, Seo N, Furukawa F, Takigawa M. J Invest Dermatol. 1997;109:175–182. doi: 10.1111/1523-1747.ep12319282. [DOI] [PubMed] [Google Scholar]
- 12.Romani N, Schuler G. Springer Semin Immunopathol. 1992;13:265–279. doi: 10.1007/BF00200527. [DOI] [PubMed] [Google Scholar]
- 13.Cohen P J, Cohen P A, Rosenberg S A, Katz S I, Mule J J. Eur J Immunol. 1994;24:315–319. doi: 10.1002/eji.1830240206. [DOI] [PubMed] [Google Scholar]
- 14.Grabbe S, Beissert S, Schwarz T, Granstein R D. Immunol Today. 1995;16:117–121. doi: 10.1016/0167-5699(95)80125-1. [DOI] [PubMed] [Google Scholar]
- 15.Celluzzi C M, Falo L D J. J Invest Dermatol. 1997;108:716–720. doi: 10.1111/1523-1747.ep12292095. [DOI] [PubMed] [Google Scholar]
- 16.Bloom B M, Perry-Lalley D, Robbins P F, Li Y, El-Gamil M, Rosenberg S A, Yang J C. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandelboim O, Berke G, Frindkin M, Feldman M, Eisenstein M, Eisenbach L. Nature (London) 1994;369:67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- 18.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee H G. Nature (London) 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 19.Seo N, Egawa K. Cancer Immunol Immunother. 1995;40:358–366. doi: 10.1007/BF01525386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taswell C. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 21.Castro F R, Perlman S. Virology. 1996;222:247–251. doi: 10.1006/viro.1996.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terashita M, Kudo C, Yamashita T, Gresser I, Sendo F. J Immunol. 1996;156:4638–4643. [PubMed] [Google Scholar]
- 23.Wood L C, Jackson S M, Elias P M, Grunfeld C, Feingold K R. J Clin Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickoloff B J, Naidu Y. J Am Acad Dermatol. 1994;30:535–546. doi: 10.1016/s0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- 25.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R M. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. Nature (London) 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 27.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young J W, Szabolcs P, Moore M A. J Exp Med. 1995;182:1111–1119. doi: 10.1084/jem.182.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayordomo I J, Zorina T, Storkus W J, Zitvogel L, Celluzzi C, Falo L D, Melief C J, Ildstad S T, Kast M W, Deleo A B. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 30.Nair K S, Boczkowski D, Snyder D, Gilboa E. Eur J Immunol. 1997;27:589–597. doi: 10.1002/eji.1830270304. [DOI] [PubMed] [Google Scholar]
- 31.Aiba S, Katz S I. J Immunol. 1990;145:2791–2796. [PubMed] [Google Scholar]
- 32.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigal L J, Reiser H, Rock K L. J Immunol. 1998;161:2740–2745. [PubMed] [Google Scholar]
- 34.Rock K L, Clark K. J Immunol. 1996;156:3721–3726. [PubMed] [Google Scholar]
- 35.Suto R, Srivastava P K. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 36.Udono H, Srivastava K P. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boczkowski D, Nair K S, Snyder D, Gilboa E. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang D C, DeVit M, Johnston S A. Nature (London) 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 39.Tang D C, Shi Z, Curiel D T. Nature (London) 1997;388:729–730. doi: 10.1038/41917. [DOI] [PubMed] [Google Scholar]
- 40.Glenn G M, Rao M, Matyas G R, Alving C R. Nature (London) 1998;391:851. doi: 10.1038/36014. [DOI] [PubMed] [Google Scholar]