Abstract
Drug abuse is a worldwide health concern in which addiction involves activation of the dopaminergic signaling pathway in the brain. Here, we introduce a nanotechnology approach that utilizes gold nanorod-DARPP-32 siRNA complexes (nanoplexes) that target this dopaminergic signaling pathway in the brain. The shift in the localized longitudinal plasmon resonance peak of gold nanorods (GNRs) was used to show their interaction with siRNA. Plasmonic enhanced dark field imaging was used to visualize the uptake of these nanoplexes in dopaminergic neurons in vitro. Gene silencing of the nanoplexes in these cells was evidenced by the reduction in the expression of key proteins (DARPP-32, ERK, and PP-1) belonging to this pathway, with no observed cytotoxicity. Moreover, these nanoplexes were shown to transmigrate across an in vitro model of the blood–brain barrier (BBB). Therefore, these nanoplexes appear to be suited for brain-specific delivery of appropriate siRNA for therapy of drug addiction and other brain diseases.
Keywords: DARPP-32, dark field imaging, surface plasmon resonance, nanoplexes, blood–brain barrier
Nanotechnology is having an increasing impact in the healthcare industry, offering unprecedented capability of not only carrying multiple diagnostic/therapeutic payloads in the same “package,” but also facilitating the targeted delivery into specific sites and across complex biological barriers (1–4). The combination of diagnostic (imaging) and therapeutic capability enables the “real-time” monitoring of therapeutic progression, thus bringing “personalized medicine” closer to clinical reality (5).
Substance abuse is a major worldwide health concern. Opiate addiction involves activation of the dopaminergic signaling pathway in the brain, in which adenosine 3′,5′-monophosphate-regulated phosphoprotein (DARPP-32) plays a significant role (6–8). Neurotransmitters such as dopamine activate protein kinase A (PKA)-mediated phosphorylation of DARPP-32 (6, 7, 9–11). Recently, both dopamine and glutamate receptors have been implicated in inducing modulation of DARPP-32, resulting in the activation of the extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase cascades (12–14). ERK activity has been known to be important in neuronal plasticity and its pharmacologic blockade prevents the transcriptional and behavioral effects of various drugs of abuse. All of the above observations indicate that the DARPP-32 is the central molecular “trigger” that underlies the neurobiological alterations related to drug abuse. Therefore, we hypothesize that the suppression of DARPP-32 gene expression using delivery of its siRNA antagonist in dopaminergic neuronal (DAN) cells will lead to significant behavioral inhibition of the drug addiction process.
However, a major hurdle limiting the use of the gene silencing technology is the lack of methods to safely and efficiently deliver siRNA molecules to target cells/tissues. In the free form, siRNA molecules have a very short half-life in physiological conditions, owing to their vulnerability for degradation by endogeneous nucleases. Therefore, they need macromolecular carriers (vectors) that will not only protect them from degradation in the biological milieu, but also steer them to desired cells/tissues and facilitate their cellular entry. Furthermore, the brain, the target organ for drug addiction therapy, represents a particularly inaccessible organ for siRNA delivery owing to the presence of the blood–brain barrier (BBB), which excludes the brain-specific delivery of 100% of large-molecule neurotherapeutics and more than 98% of all small-molecule drugs (15, 16).
In recent years, gold nanoparticles (GNPs) and nanorods (GNRs) have gained increasing interest as site-specific carriers of various diagnostic and therapeutic agents, primarily owing to their biocompatibility (1, 17–21). Their surfaces can be easily modified to incorporate cationic charges, which will facilitate their stable electrostatic complexation with anionic genetic materials such as siRNA, for the purpose of targeted gene delivery/silencing. Moreover, by exploiting the phenomenon of surface plasmon resonance (SPR) associated with GNPs/GNRs, their complexation with genetic materials and subsequently their delivery and distribution within target tissues can be monitored (22, 23).
In this paper, we introduce a nanotechnology-based, different approach for gene silencing-mediated drug addiction therapy. We demonstrate that GNRs electrostatically complexed with appropriate siRNA molecules (nanoplexes) can be used for modulating the key components of the dopaminergic signaling pathway. Specifically, we confirmed the formation of the nanoplexes from the observed red shift in the localized longitudinal plasmon resonance peak of gold nanorods, and from the restricted electrophoretic mobility of the nanoplexes using gel electrophoresis. The uptake of the nanoplexes into dopaminergic neuronal (DAN) cells in vitro was determined using not only the plasmonic enhanced dark-field imaging of gold nanorods, but also from confocal microscopy and fluorimetric analysis of cell lysates. Moreover, we observed that the delivery of the nanoplex involving siRNA against the DARPP-32 gene in DAN cells resulted in the silencing of not only DARPP-32, but also other key downstream effector molecules of this pathway, such as ERK and protein phosphatase-1 (PP-1), with efficiency greater than that of commercially available reagent (siPORT). Finally, we have demonstrated that these nanoplexes showed significantly higher transmigration efficiency across an in vitro BBB model over that of free siRNA, without compromising the functional integrity of the barrier (24–28). Therefore, these nanoplexes appear to be ideally suited for brain-specific delivery of appropriate siRNA for therapy of drug addiction and other brain diseases.
Results and Discussions
Electrostatic Binding of siRNA with Gold Nanorods to Form Nanoplexes.
The cationic GNRs, prepared by growing multilayers of cationic polyelectrolytes upon cetyltrimethylammonium bromide (CTAB)-coated GNRs, were electrostatically complexed to: (i) fluorescently labeled marker siRNA (siRNA-FAM; siRNAF) for studying their binding with nanoparticles and subsequent cellular uptake and (ii) functional siRNA specific for DARPP-32 (siRNAD). To examine the binding efficiency of the siRNA with the GNRs, we performed agarose gel electrophoresis. The results obtained for siRNA, both free and complexed with GNRs, are shown in Fig. S1 a and b. It is evident that siRNA complexed with nanoparticles (lanes 2, 3, and 4) does not have the same electrophoretic mobility as free siRNA (lane 1), and this restricted mobility is proportional to the amount of GNR complexed with siRNA. Also, we observed a partial quenching in the emission signal from both siRNAF (Fig. S1a) and ethidium bromide stained siRNAD (Fig. S1b) as a result of the complexation with the GNRs. These data indicate the successful complexation of siRNA with GNRs, and the highest loading of siRNA per GNRs can be calculated to be ≈70 pmoles of siRNA per 1 μg of GNR. We also determined the effect of siRNA complexation on the overall size and morphology of the GNRs, using transmission electron microscopy (TEM) indicating that siRNA complexation did not cause any aggregation effect on the GNRs, and we have observed this stability for more than 1 month postcomplexation. Surface charge (zeta potential) measurements of the GNRs yielded zeta potential for nanoparticles without siRNA, a value of +28.6 mV, and for nanoparticles complexed with siRNA, a value of −2.4 mV. These results clearly indicated that the anionic siRNA molecules coat the surface of the otherwise cationic GNRs, leading to a moderately negative net charge of the complex. The moderately negative charge on their surface becomes significant when considering the well-known fact that nanoparticles with neutral or slightly negative surface charge are known to evade capture by the reticuloendothelial system (RES) of the body, and avoid nonspecific interaction with physiological proteins and other biomolecules (29).
The complexation of siRNA with GNRs was confirmed by measuring the plasmonic shift of the GNRs. As shown in Fig. S2, the complexation with siRNA results in an ≈5 nm red shift in the localized longitudinal surface plasmon resonance peak of the GNRs. This shift in the absorption spectra of GNRs is the result of changes in the local refractive indices around the GNRs, following complexation with siRNA molecules. Previously, such a plasmonic red shift was demonstrated by Sokolov et al., following noncovalent complexation of antibodies with gold nanoparticles (23). Such plasmonic shift experiments can serve as a powerful nanotechnology-based characterization tool for studying the interaction of gold nanoparticles/nanorods with biological molecules such as proteins and nucleic acids.
Uptake of Nanoplexes in Neuronal Cells in Vitro, Without any Sign of Cytotoxicity.
The strong orange-red plasmonic scattering associated with GNRs can be used in studying cellular delivery using dark-field microscopy (19, 22). Fig. 1 shows the dark-field images of DAN cells, without (1c) and with (1a) treatment with the GNR-siRNAD nanoplex. The cellular uptake of the nanoplexes can be easily observed from the strong orange-red scattering of the nanoplex treated, as opposed to the untreated, DAN cells. This experiment highlights another advantage of using nanotechnology in the delivery of therapeutics, where in addition to the therapeutic efficacy, the unique properties of the GNRs can be used to determine their uptake in cells using dark-field imaging.
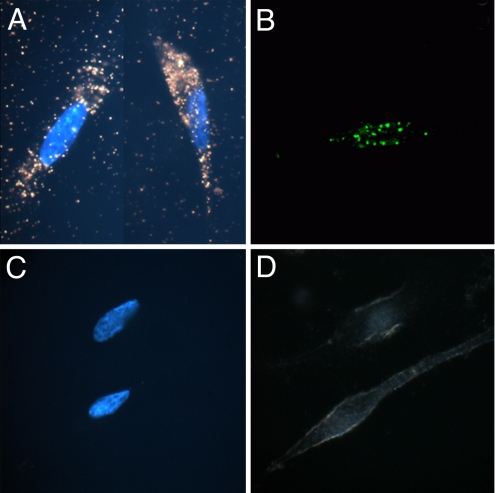
Fig. 1.
Study of GNR-siRNA nanoplex uptake in DAN cells. Dark-field images with Hoechst nuclear staining (A and C), and confocal images (B and D) of DAN cells, following treatment with GNR-siRNA nanoplex (A and B) and free siRNA (C and D). The dark-field images of GNRs corresponding to the longitudinal surface plasmonic enhancement in the red region can be clearly distinguished from the background (A). Confocal images show robust uptake of the siRNAF complexed with GNRs (B), as opposed to free siRNAF (C).
Confocal microscopy was also used to confirm the cellular entry of the nanoplexes in DAN cells, using GNR-siRNAF nanoplex. The confocal images of DAN cells treated with either siRNAF alone (Fig. 1d), or with GNR-siRNAF nanoplex (Fig. 1b), demonstrate strong fluorescence signal from the treated, as opposed to untreated, DAN cells. We also measured fluorescence from cellular lysates following their treatment with siRNAF, either free, or complexed with GNRs or complexed with the commercially available gene silencing agent siPORT (Fig. S3). Our results indicate that while lysates from cells treated with free siRNAF show little or no fluorescence, the fluorescence from lysates of cells treated with GNR-siRNAF is approximately 3 times higher than that obtained from cells treated with siPORT-siRNAF indicating that the intracellular delivery efficiency of siRNA using GNRs is superior to that using this commercially available gene silencing agent.
We also investigated the potential cytotoxic effects of these nanocomplexes, and found that the DAN cells treated with GNR-siRNA maintained 98% viability, even after 1 week posttreatment (Fig. S4). From these data, it can be concluded that these nanocomplexes are not toxic to cells and can be safely used for further biological studies.
Knockdown of DARPP-32 Gene Following Treatment with GNR-siRNAD Nanoplexes.
DAN cells were incubated with the GNR-siRNAD nanoplexes and the efficiency of gene silencing was determined by measuring the percentage inhibition of the expression of DARPP-32 using quantitative real-time (Q)-PCR and Western blot. Results from these experiments show that significant inhibition (>80%) of DARPP-32 expression was produced in the DAN cells (Fig. 2) and this knockdown was observed up to 1 week posttransfection. In comparison to the commercial agent siPORT, it was found that the GNR-siRNAD nanoplexes have superior gene silencing efficacy at all of the time points posttransfection, particularly at the onset (24 h posttransfection) of the gene silencing process. The efficient silencing of the DARPP-32 gene using GNR-mediated siRNA delivery was further confirmed by investigating the downregulation of the DARPP-32 protein using Western blot analysis (Fig. 3). Our results show ≈67% knockdown of protein expression DARPP-32 with GNR-siRNAD nanoplexes after 120 h posttransfection, higher than that obtained using the siPORT-siRNAD complex (≈30% knockdown). Thus the superior gene silencing efficacy of these nanoplexes over that of the commercial agent siPORT-siRNA complexes have been validated using 2 independent methods. This enhanced gene silencing efficiency of nanoplexes over siPORT-siRNA complex directly correlates with the enhanced cellular delivery of siRNA using the GNRs over that using siPORT (Fig. S3).
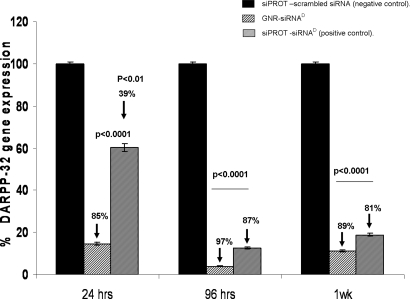
Fig. 2.
DARPP-32 gene silencing efficiency of GNR-siRNAD nanoplex in DAN cells. Quantitative real-time PCR (Q-PCR) data showing a >80% suppression of DARPP-32 gene expression in DAN cells that were transfected with the GNR-siRNAD nanoplex. Relative expression of mRNA species was calculated using the comparative CT method. Data are the mean ± SD of 3 separate experiments done in duplicate. Statistical significance was determined using ANOVA based on comparison between the siPORT-siRNAD (positive control), GNR-siRNAD nanoplex, and the siPORT with scrambled siRNA (negative control). A significant decrease in DARPP-32 gene expression was observed up to 1 week posttransfection, with maximum suppression observed at 96 h posttransfection.
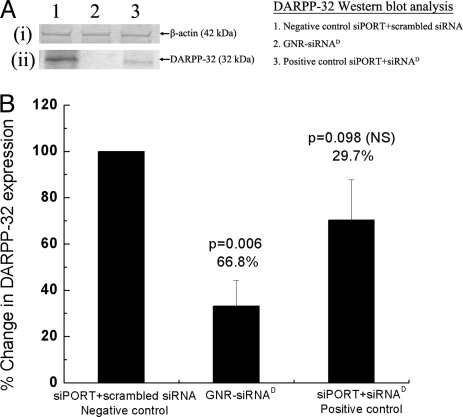
Fig. 3.
Effect of the GNR-siRNAD nanoplex on DARPP-32 protein expression in DAN cells. Western blot analysis of DARPP-32 protein from lysates of DAN cells following their treatment in vitro with GNR-siRNAD nanoplexes (lane 2) and both siPORT positive control (lane 3) and negative control (lane 1), at 120 h posttransfection. Panel A shows data from a representative Western blot experiment showing (i) no change in b-actin (42-kDa band) protein expression and (ii) a significant decrease in DARPP-32 (32-kDa band) protein expression, in cells treated with the GNR-siRNAD nanoplex (lane 2), as opposed to cells treated with negative control (siPORT-scrambled siRNA, lane 1). Panel B is a graphical representation of the densitometric analysis of the DARPP-32 protein band showing percentage decrease in the DARPP-32 protein expression in cells treated with GNR-siRNAD and siPORT-siRNAD, when compared with that in cells treated with siPORT-scrambled siRNA (negative control, 100% DARPP-32 expression). Data shown are mean ± SD of results from 3 separate experiments, and statistical significance was determined using ANOVA. Results show a significant decrease in DARPP-32 protein expression in the GNR-siRNAD-treated cells (66.8% decrease), significantly higher than that obtained from siPORT-siRNAD-treated cells (29.7% decrease).
Knockdown of Effector Molecules (PP-1 and ERK) Downstream of DARPP-32 Following Treatment with GNR-siRNAD Nanoplexes.
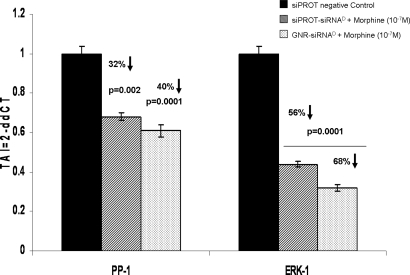
To evaluate whether the GNR-mediated DARPP-32 gene silencing will have further biological effects, we investigated the effect of DARPP-32 silencing on effector molecules such as PP-1 and ERK, which are downstream to DARPP-32 in the opiate signaling pathway. Here, using Q-PCR analysis, we measured the levels of PP-1 and ERK mRNA from DAN cells, which were treated first with the various transfection reagents for 24 h, then with morphine (10−7 M) overnight. The results (Fig. 4) demonstrate a substantial knockdown of both PP-1 (≈40%) and ERK (≈68%) genes using the GNR-siRNAD nanoplexes, which are superior to that obtained using siPORT-siRNAD. These data indicate that silencing of DARPP-32 by GNR-conjugated siRNAD resulted in a significant decrease in the gene expression of downstream effector molecules, therefore showing that the DARPP-32 gene has functional relevance as an important signaling intermediate in the molecular mechanism underlying the development of drug addiction.
Fig. 4.
Modulation of gene expression of effector molecules downstream of DARPP-32 (PP-1 and ERK-1) in siRNAD transfected DAN cells. Q-PCR data show a significant decrease in both PP-1 and ERK-1 gene expression in DAN cells following treatment with GNR-siRNAD nanoplexes. Data are the mean ± SD of 3 separate experiments performed in duplicate. Statistical significance was determined using ANOVA based on comparison between the GNR-siRNAD and siPORT- siRNAD (positive control) samples with the siPORT-scrambled siRNA (negative control).
Using immunofluorescent staining, we also investigated the effect of the stimulator morphine (drug of abuse), and the antagonist GNR-siRNAD nanoplex, on the expression of DARPP-32 and the downstream effector protein ERK-1 in DAN cells. We found that in the morphine-treated DAN cells, the expression of DARPP-32 is enhanced when compared to the untreated control (Fig. 5 a and c, respectively). Subsequent treatment of the morphine-treated DAN cells with GNR-siRNAD nanoplexes resulted in a reduction of the DARPP-32 levels to background signal (Fig. 5d). A similar reduction of ERK was also observed in the morphine-treated DAN cells following treatment with GNR-siRNAD nanoplexes (Fig. 5 e and f, respectively). These results indicate that morphine treatment enhances the DARPP-32 levels in the DAN cells and GNR mediated transfection of siRNAD reduces not only the levels of DARPP-32 expression, but also the expression of its downstream effector molecule ERK-1. These results further highlight the potential of GNRs to serve as a siRNA delivery vehicle for functional gene silencing.
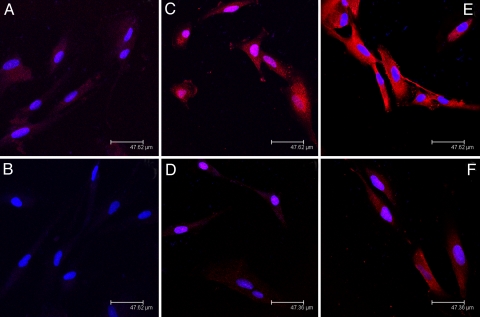
Fig. 5.
Immunofluorescent studies on neuronal cells. Immunofluorescent staining for DARPP-32 (A–D) and ERK-1 (E and F) in transfected and nontransfected DAN cells. (A) DARPP-32 expression in nontransfected DAN cells. (B) DARPP-32 expression in GNR-siRNAD nanoplex transfected DAN cells. (C) Morphine-treated (10−7 M) but nontransfected DAN cells. (D) Morphine-treated (10−7 M) DAN cells, transfected with GNR-siRNAD. (E) Morphine-treated (10−7 M) untransfected DAN cells showing ERK-1 expression. (F) Morphine-treated (10−7 M) GNR-siRNAD tranfected DAN cells showing ERK-1 expression. Our results show (A) morphine treatment enhances the DARPP-32 levels, and (B) GNR-mediated transfection of siRNA against DARPP-32 reduces not only the levels of DARPP-32 expression but also the expression of its downstream effector molecule ERK-1 in DAN cells.
Transmigration of GNR-siRNAF Nanoplexes Across an in Vitro Model of BBB.
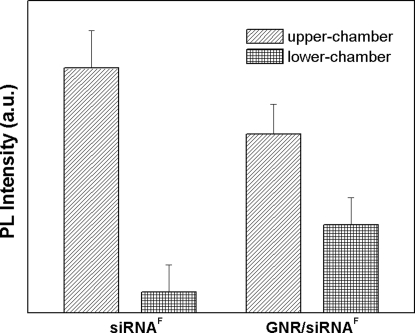
Because brain is the target organ for the application of DARPP-32 gene silencing and the BBB is a major impediment toward the brain-specific noninvasive delivery of therapeutic agents, we used an established in vitro BBB model to determine the ability of GNR-siRNA nanoplexes to cross the BBB (16, 24, 30, 31). Our results show that 4 h after incubation of GNR- siRNAF in the upper chamber (venous side) of the BBB model, nearly 40% of the original fluorescence can be recovered in the lower chamber (brain side) of the model. Using similar conditions with free siRNAF, only 1% of the original fluorescence was recovered in the lower chamber (Fig. 6). This result shows that the transmigration efficiency of siRNA across BBB increases significantly following complexation with GNRs. Furthermore, no change was observed in the transendothelial electrical resistance (TEER) values of the BBB, before (214 ± 2.52 ohm/cm2) and after (211.33 ± 2.80 ohm/cm2) treatment with the GNR-siRNA nanoplexes. Because TEER values indicate the functional integrity of the BBB, the above data show that the GNR-siRNAF nanoplexes did not cause any functional damage to the BBB while traversing through this barrier (Table S1).
Fig. 6.
Transmigration of GNR-siRNAF nanoplex across the brain blood–barrier model. Fluorescence signal distribution collected from BBB model in upper chamber (blood end) and lower chamber (brain end) of an in vitro BBB model, following treatment with free siRNAF and GNR-siRNAF nanoplex. Results show enhanced transmigration efficiency of the GNR-siRNAF nanoplex when compared to that of free siRNAF.
In subsequent experiments, we evaluated the ability of the GNR-siRNAD nanoplex to induce DARPP-32 gene knockdown in DAN cells in the lower chamber of the BBB model. This was performed using multiple doses of GNR-siRNAD, which were added to the upper chamber every 24 h for a period of 7 days. After 7 days, the DAN cells in the bottom of the chamber showed a significant suppression (50–60%) of the DARPP-32 gene expression as determined by Q-PCR. This study indicates that the GNR-siRNAD nanoplex retains its function after crossing the BBB.
Conclusions
Our study presents a novel approach that combines the therapeutic potential of gene silencing technology with the imaging and site-specific delivery potential of nanotechnology using GNRs. The ability of these nanoplexes to silence not only the target gene DARPP-32, but also the effector genes ERK and PP-1, with efficiency surpassing that obtained using the commercial agent siPORT, underscores the potential of gold nanorods as efficient carriers of siRNA molecules for gene silencing. The GNR nanoplex-mediated modulation of key components of the dopaminergic signaling pathway, along with the observation of its facile transport across the in vitro model of BBB, is expected to lead to exciting new nanotechnology-based treatment options against drug abuse, via the modulation of both transcriptional and posttranslational events in the brain involved in the addictive behavior.
A major goal for the treatment of drug addiction is to prevent physiological withdrawal symptoms. This current study, using GNR-mediated siRNA delivery to silence DARPP-32 gene expression by neuronal cells, is a highly innovative, new approach to the treatment of drug addiction and other addictive behaviors. Thus, nanotherapy, combined with more traditional psychological and sociological methods, may yield a more effective therapeutic approach for the this problem that, to date, has been so resistant to treatment. However, there are several pharmaceutical challenges to effective siRNA delivery and activity in vivo, which must be overcome for achieving therapeutically relevant gene silencing (32–35). Although the study demonstrates the efficacy of this use of GNRs as an effective transport platform for siRNA, in vitro, their efficacy in vivo remains to be determined.
In our studies, we have observed a number of encouraging results, which suggests the in vivo success of our approach. Firstly, the overall charge of the nanoplexes is sparsely negative, which is ideal for their avoidance of nonspecific interaction with physiological proteins and other biomolecules. Next, they affect extremely high efficiency of gene silencing in a sustained manner, which is superior to that of commercially available reagents for the same. Finally, their ability to transverse the in vitro BBB and maintain their functionality further bolsters the prospects of their success in vivo. The ability of the GNR-siRNA nanoplex to cross the blood–brain barrier suggests that they can be administered by a peripheral i.v. route. Lastly, we do not envision this as continuous therapy for the treatment of addiction. Rather, DARPP-32 gene silencing can be used initially to break the cycle of addiction by blocking reward and motivational systems and conditioned responses. Although issues of efficient delivery, specificity, and safety remain, siRNA nanotherapeutics holds great promise as a “partner” in the therapeutic treatment of drug addiction. Furthermore, this nanotechnology-based gene silencing approach also may have a role in broader applications for the treatment of numerous other brain specific diseases/disorders.
Methods
Sequence of siRNA.
The siRNA sequences for DARPP-32 (accession no. AF464196) are: sense- ACA CAC CAC CUU CGC UGA AAG CUG U; antisense- ACA GCU UUC AGC GAA GGU GGU GUG U. The appropriate scrambled control siRNA sequences are: sense- ACA CCC AUC CUC GGU AAG ACA CUG U; antisense- ACA GUG UCU UAC CGA GGA UGG GUG U.
Synthesis of the GNRs and GNR-siRNA Nanoplexes.
Cetyltrimethylammonium bromide (CTAB), hydrogen tetrachloroaurate (III) trihydrate (HAuCl4*3H2O), silver nitrate (AgNO3), L-ascorbic acid, glutaraldehyde (50% aqueous solution), and sodium borohydride were purchased from Aldrich. All chemicals were used as received. HPLC-grade water was used in all of the experiments. Stock solutions of sodium borohydride and L-ascorbic acid were freshly prepared for each new set of experiments. Poly (3,4-ethylenedioxythiophene)/poly(styrenesulfate) (PEDT/PSS) and poly(diallyldimethyl ammonium chloride) (PDDAC) were obtained from Polysciences. The GNRs were prepared by the seed-mediated growth method in CTAB solution, followed by their sequential coating with two successive layers of polyelectrolytes, (i) the negatively charged PEDT/PSS and (ii) the positively charged PDDAC, as described previously (22). The cationic GNR were then electrostatically attached to an appropriate amount of siRNA in OptiMEM media (Invitrogen), resulting in the formation of the nanoplexes (SI Text).
Supplementary Material
Acknowledgments.
This study was supported by grants from the National Cancer Institute Grants CA119397, and CA104492, the Kaleida Health Cameron Troup Foundation, the John R. Oishei Foundation, and the Chemistry and Life Sciences Division of the Air Force Office of Scientific Research. Support from the Center of Excellence in Bioinformatics and Life Sciences at the University at Buffalo is also acknowledged.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901715106/DCSupplemental.
References
- 1.Prasad PN. Introduction to Biophotonics. New York: Wiley; 2004. [Google Scholar]
- 2.Kang H, DeLong R, Fisher MH, Juliano R. Tat-conjugated PAMAM dendrimers as delivey agents for antisense and siRNA oligonucleotides. J Pharm Res. 2005;22(12):2099–2106. doi: 10.1007/s11095-005-8330-5. [DOI] [PubMed] [Google Scholar]
- 3.Farokhzad OC, Langer R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Adv Drug Delivery Rev. 2006;58(14):1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug Chem. 2008;19(7):1327–1338. doi: 10.1021/bc800148t. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 6.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: The DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 7.Greengard P, et al. The DARPP-32/protein phosphatase-1 cascade: A model for signal integration. Brain Res Rev. 1998;26(2–3):274–284. doi: 10.1016/s0165-0173(97)00057-x. [DOI] [PubMed] [Google Scholar]
- 8.Gould TD, Manji HK. DARPP-32: A molecular switch at the nexus of reward pathway plasticity. Proc Natl Acad Sci USA. 2005;102(2):253–254. doi: 10.1073/pnas.0408700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svenningsson P, et al. DARPP-32: An integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 10.Calabresi P, et al. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J Neurosci. 2000;20(22):8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nairn AC, et al. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47(S1):14–23. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Valjent E, Caboche J, Vanhoutte P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: A molecular substrate for learning and memory? Vol 23. NY: Humana Press; 2001. pp. 83–99. [DOI] [PubMed] [Google Scholar]
- 13.Valjent E, Hervé D, Girault JA. Drugs of abuse, protein phosphatases, and ERK pathway. Med Sci (Paris) 2005;21(5):453–454. doi: 10.1051/medsci/2005215453. [DOI] [PubMed] [Google Scholar]
- 14.Valjent E, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102(2):491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardridge WM. shRNA and siRNA delivery to the brain. Adv Drug Deliv Rev. 2007;59(2–3):141–152. doi: 10.1016/j.addr.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deli MA, Abrahám C, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: Physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25(1):59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60(11):1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomed. 2007;2(5):681–693. doi: 10.2217/17435889.2.5.681. [DOI] [PubMed] [Google Scholar]
- 20.Stone JW, Sisco P, Goldsmith EC, Baxter SC, Murphy CJ. Using gold nanorods to probe cell-induced collagen deformation. Nano Lett. 2007;7(1):116–119. doi: 10.1021/nl062248d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf B Biointerfaces. 2008;66(2):274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Ding H, et al. Gold nanorords coated with multilayer polyelectrolyte as contrast agent for multimodal imaging. J Phys Chem C. 2007;34(111):12552–12557. [Google Scholar]
- 23.Sokolov K, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63(9):1999–2004. [PubMed] [Google Scholar]
- 24.XuG, et al. Bioconjugated quantum rods as targeted probes for efficient transmigration across an in vitro blood-brain barrier. Bioconjug Chem. 2008;19(6):1179–1185. doi: 10.1021/bc700477u. [DOI] [PubMed] [Google Scholar]
- 25.Persidsky YSM, et al. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158(7):3499–3510. [PubMed] [Google Scholar]
- 26.Mahajan SD, et al. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 2008;1203:133–148. doi: 10.1016/j.brainres.2008.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan SD, et al. Tight junction regulation by morphine and HIV-1 Tat modulates blood–brain barrier permeability. J Clin Immunol. 2008;28(5):528–541. doi: 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 28.Persidsky Y, Gendelman H. Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia. J Leukoc Biol. 1997;62(1):100–106. doi: 10.1002/jlb.62.1.100. [DOI] [PubMed] [Google Scholar]
- 29.Davis SS. Biomedical applications of nanotechnology–implications for drug targeting and gene therapy. Trends Biotechnol. 1997;15(6):217–224. doi: 10.1016/S0167-7799(97)01036-6. [DOI] [PubMed] [Google Scholar]
- 30.Tähti H, Nevala H, Toimela T. Refining in vitro neurotoxicity testing–the development of blood-brain barrier models. Altern Lab Anim. 2003;31(3):273–276. doi: 10.1177/026119290303100309. [DOI] [PubMed] [Google Scholar]
- 31.Terasaki T, et al. ) New approaches to in vitro models of blood-brain barrier drug transport. Drug Discov Today. 2003;8(20):944–954. doi: 10.1016/s1359-6446(03)02858-7. [DOI] [PubMed] [Google Scholar]
- 32.Akhtar S, Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: Potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev. 2007;59:164–182. doi: 10.1016/j.addr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Bumcrot DM, Benter M, Koteliansky V, Sah DW. RNAi therapeutics: A potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2:711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhn R, Streif S, Wurst W. RNA interference in mice. Handb Exp Pharmacol. 2007;178:149–176. doi: 10.1007/978-3-540-35109-2_7. [DOI] [PubMed] [Google Scholar]
- 35.de Fougerolles AV, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: A progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.