Abstract
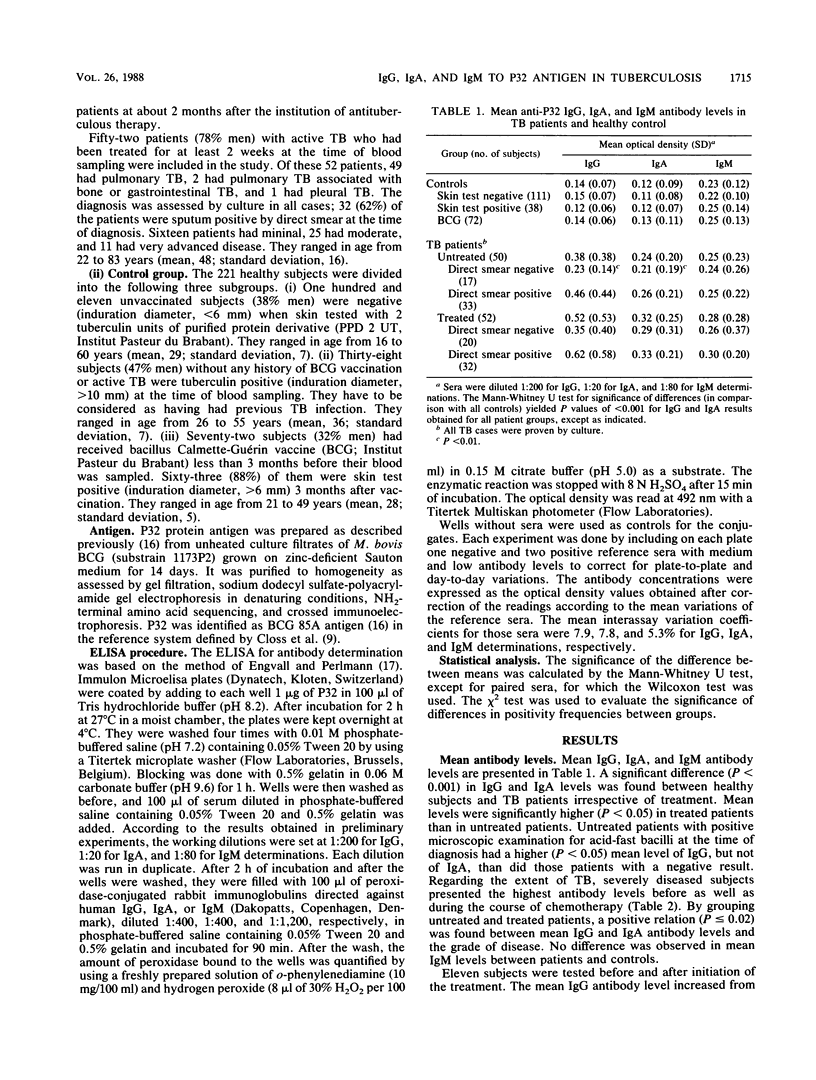
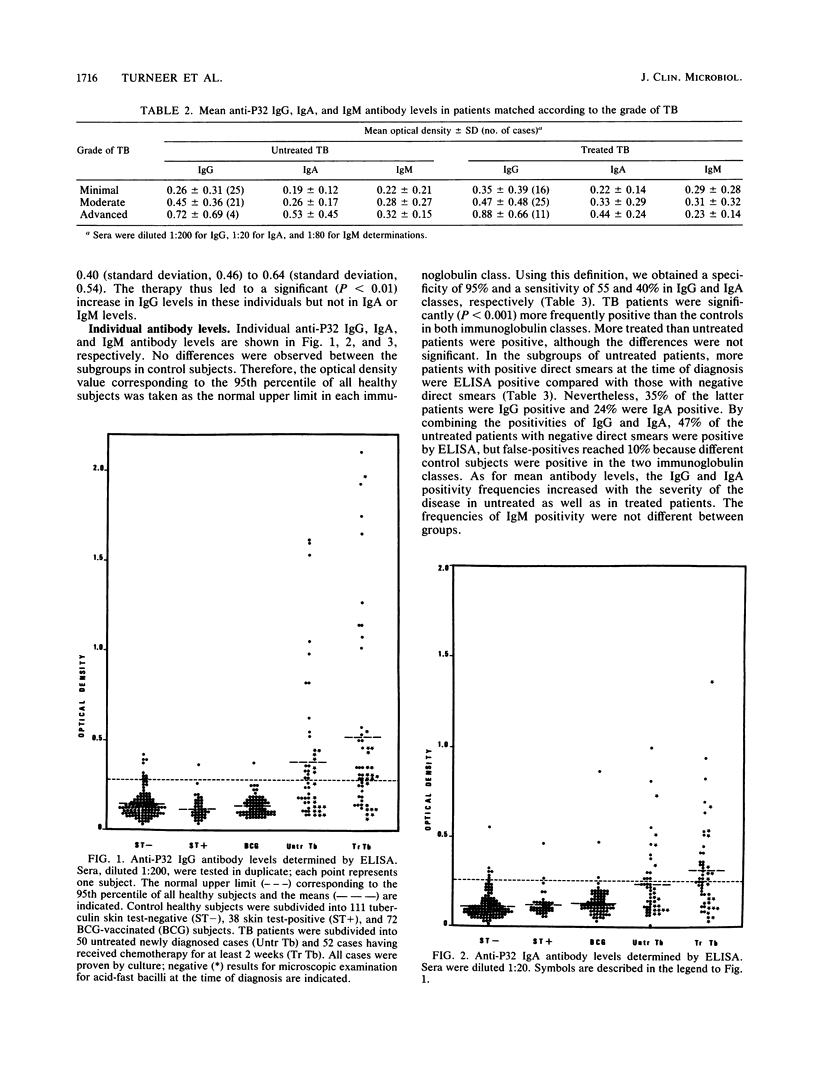
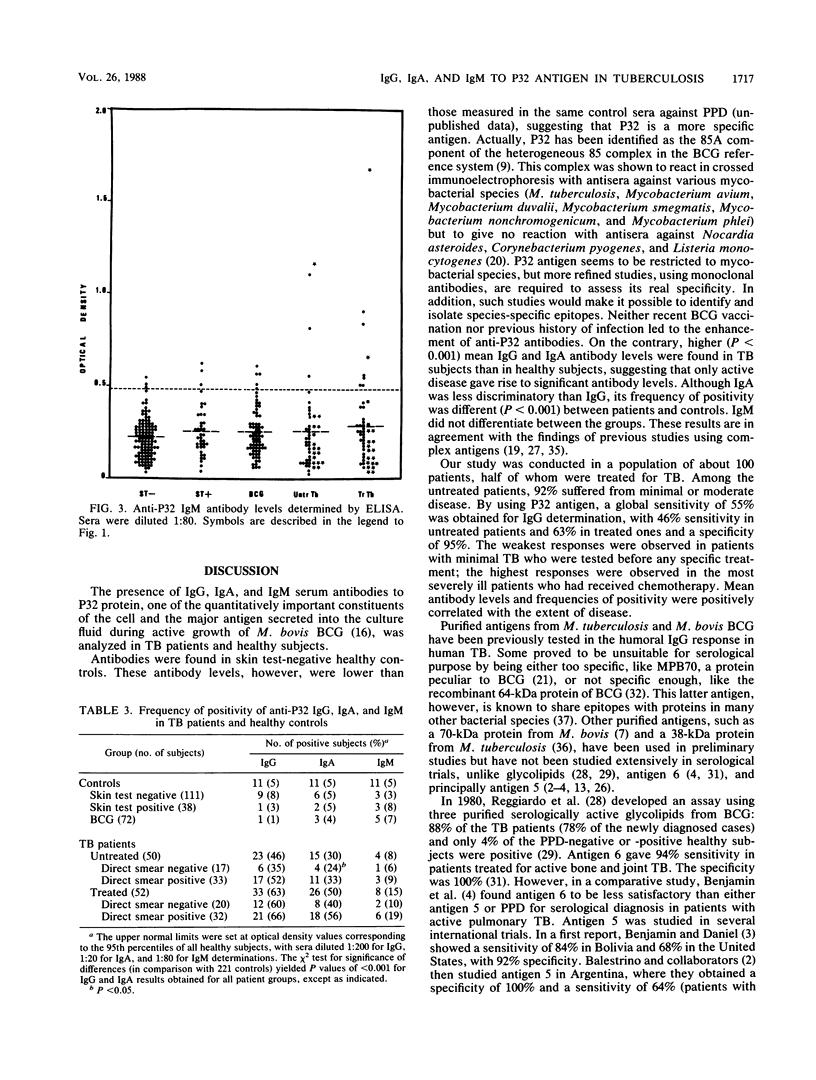
The P32 protein antigen of Mycobacterium bovis BCG, identified as antigen 85A in the BCG reference system, was used to investigate the humoral immune response in human tuberculosis (TB). Immunoglobulin G (IgG), IgA, and IgM directed against P32 were measured by an enzyme-linked immunosorbent assay. Mean IgG and IgA antibody levels differed significantly (P less than 0.001) between active-TB patients (50 untreated and 52 treated) and healthy control subjects (111 unvaccinated tuberculin negative, 38 unvaccinated tuberculin positive, and 72 recently BCG vaccinated). Mean IgG antibody levels, but not mean IgA antibody levels, were higher (P less than 0.05) in patients with positive microscopic examination for acid-fast bacilli than in patients with negative microscopic examination. A positive relation was found between mean levels and the extent of disease. There was no difference in mean IgM antibody levels between patients and controls. By setting the upper normal limit at the 95th percentile of the 221 healthy subjects, the sensitivities were 46% in untreated and 63% in treated patients for IgG and 30 and 50%, respectively, for IgA. Of the untreated patients, 56% were positive for either IgG or IgA antibodies. Among the untreated patients with negative direct smear, 35% were positive for IgG and 24% were positive for IgA. When both immunoglobulin classes were combined, the serological test was positive in 47% of those patients. Neither naturally acquired tuberculin hypersensitivity nor BCG vaccination affected positivity frequencies in healthy subjects. Only active TB seemed to induce significant anti-P32 antibody levels and to be associated with positivity. A serological test with P32 as the antigen might therefore be helpful for the rapid diagnosis of TB.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balestrino E. A., Daniel T. M., de Latini M. D., Latini O. A., Ma Y., Scocozza J. B. Serodiagnosis of pulmonary tuberculosis in Argentina by enzyme-linked immunosorbent assay (ELISA) of IgG antibody to Mycobacterium tuberculosis antigen 5 and tuberculin purified protein derivative. Bull World Health Organ. 1984;62(5):755–761. [PMC free article] [PubMed] [Google Scholar]
- Benjamin R. G., Daniel T. M. Serodiagnosis of tuberculosis using the enzyme-linked immunoabsorbent assay (ELISA) of antibody to Mycobacterium tuberculosis antigen 5. Am Rev Respir Dis. 1982 Dec;126(6):1013–1016. doi: 10.1164/arrd.1982.126.6.1013. [DOI] [PubMed] [Google Scholar]
- Benjamin R. G., Debanne S. M., Ma Y., Daniel T. M. Evaluation of mycobacterial antigens in an enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of tuberculosis. J Med Microbiol. 1984 Dec;18(3):309–318. doi: 10.1099/00222615-18-3-309. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Richman L. K., Killion D. J. Distinct H-2-linked Ir genes control both antibody and T cell responses to different determinants on the same antigen, myoglobin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4046–4050. doi: 10.1073/pnas.76.8.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A., Ranadive S. N., Kale M., Bhattacharya S. Antibody-based enzyme-linked immunosorbent assay for determination of immune complexes in clinical tuberculosis. Am Rev Respir Dis. 1986 Aug;134(2):205–209. doi: 10.1164/arrd.1986.134.2.205. [DOI] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Inglis A. S. Immunoreactivity of a 70 kD protein purified from Mycobacterium bovis Bacillus Calmette-Guerin by monoclonal antibody affinity chromatography. J Exp Med. 1986 Sep 1;164(3):695–708. doi: 10.1084/jem.164.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., De Murillo G. L., Sawyer J. A., Griffin A. M., Pinto E., Debanne S. M., Espinosa P., Cespedes E. Field evaluation of enzyme-linked immunosorbent assay for the serodiagnosis of tuberculosis. Am Rev Respir Dis. 1986 Oct;134(4):662–665. doi: 10.1164/arrd.1986.134.4.662. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Debanne S. M. The serodiagnosis of tuberculosis and other mycobacterial diseases by enzyme-linked immunosorbent assay. Am Rev Respir Dis. 1987 May;135(5):1137–1151. doi: 10.1164/arrd.1987.135.5.1137. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Debanne S. M., van der Kuyp F. Enzyme-linked immunosorbent assay using Mycobacterium tuberculosis antigen 5 and PPD for the serodiagnosis of tuberculosis. Chest. 1985 Sep;88(3):388–392. doi: 10.1378/chest.88.3.388. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Oxtoby M. J., Pinto E., Moreno E. The immune spectrum in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1981 May;123(5):556–559. doi: 10.1164/arrd.1981.123.5.556. [DOI] [PubMed] [Google Scholar]
- Daniel T. M. The immunology of tuberculosis. Clin Chest Med. 1980 May;1(2):189–201. [PubMed] [Google Scholar]
- De Bruyn J., Huygen K., Bosmans R., Fauville M., Lippens R., Van Vooren J. P., Falmagne P., Weckx M., Wiker H. G., Harboe M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987 May;2(5):351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Grange J. M., Gibson J., Nassau E., Kardjito T. Enzyme-linked immunosorbent assay (ELISA): a study of antibodies to Mycobacterium tuberculosis in the IgG, IgA and IgM classes in tuberculosis, sarcoidosis and Crohn's disease. Tubercle. 1980 Sep;61(3):145–152. doi: 10.1016/0041-3879(80)90003-3. [DOI] [PubMed] [Google Scholar]
- Grange J. M. The humoral immune response in tuberculosis: its nature, biological role and diagnostic usefulness. Adv Tuberc Res. 1984;21:1–78. [PubMed] [Google Scholar]
- Harboe M., Mshana R. N., Closs O., Kronvall G., Axelsen N. H. Cross-reactions between mycobacteria. II. Crossed immunoelectrophoretic analysis of soluble antigens of BCG and comparison with other mycobacteria. Scand J Immunol. 1979;9(2):115–124. doi: 10.1111/j.1365-3083.1979.tb02713.x. [DOI] [PubMed] [Google Scholar]
- Harboe M., Nagai S. MPB70, a unique antigen of Mycobacterium bovis BCG. Am Rev Respir Dis. 1984 Mar;129(3):444–452. doi: 10.1164/arrd.1984.129.3.444. [DOI] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Kaplan M. H., Chase M. W. Antibodies to mycobacteria in human tuberculosis. I. Development of antibodies before and after antimicrobial therapy. J Infect Dis. 1980 Dec;142(6):825–834. doi: 10.1093/infdis/142.6.825. [DOI] [PubMed] [Google Scholar]
- Lenzini L., Rottoli P., Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977 Feb;27(2):230–237. [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang Y. M., Daniel T. M. Enzyme-linked immunosorbent assay using Mycobacterium tuberculosis antigen 5 for the diagnosis of pulmonary tuberculosis in China. Am Rev Respir Dis. 1986 Dec;134(6):1273–1275. doi: 10.1164/arrd.1986.134.5.1273. [DOI] [PubMed] [Google Scholar]
- Radin R. C., Zeiss C. R., Phair J. P. Antibodies to purified protein derivative in different immunoglobulin classes in the diagnosis of tuberculosis in man. Int Arch Allergy Appl Immunol. 1983;70(1):25–29. doi: 10.1159/000233268. [DOI] [PubMed] [Google Scholar]
- Reggiardo Z., Vazquez E. Comparison of enzyme-linked immunosorbent assay and hemagglutination test using mycobacterial glycolipids. J Clin Microbiol. 1981 May;13(5):1007–1009. doi: 10.1128/jcm.13.5.1007-1009.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiardo Z., Vazquez E., Schnaper L. ELISA tests for antibodies against mycobacterial glycolipids. J Immunol Methods. 1980;34(1):55–60. doi: 10.1016/0022-1759(80)90224-0. [DOI] [PubMed] [Google Scholar]
- Samuel A. M., Ashtekar M. D., Ganatra R. D. Significance of circulating immune complexes in pulmonary tuberculosis. Clin Exp Immunol. 1984 Nov;58(2):317–324. [PMC free article] [PubMed] [Google Scholar]
- Stroebel A. B., Daniel T. M., Lau J. H., Leong J. C., Richardson H. Serologic diagnosis of bone and joint tuberculosis by an enzyme-linked immunosorbent assay. J Infect Dis. 1982 Aug;146(2):280–283. doi: 10.1093/infdis/146.2.280. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljanen M. K., Eskola J., Tala E. Enzyme-linked immunosorbent assay (ELISA) for antibodies to purified protein derivative of tuberculin (PPD). IgM-, IgA- and IgG- anti-PPD antibodies in active pulmonary tuberculosis. Eur J Respir Dis. 1982 May;63(3):257–262. [PubMed] [Google Scholar]
- Young D., Kent L., Rees A., Lamb J., Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986 Oct;54(1):177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiss C. R., Kalish S. B., Erlich K. S., Levitz D., Metzger E., Radin R., Phair J. P. IgG antibody to purified protein derivative by enzyme-linked immunosorbent assay in the diagnosis of pulmonary tuberculosis. Am Rev Respir Dis. 1984 Nov;130(5):845–848. doi: 10.1164/arrd.1984.130.5.845. [DOI] [PubMed] [Google Scholar]
- van Eden W., Elferink B. G., Hermans J., de Vries R. R., van Rood J. J. Role of HLA class II products in proliferative T-lymphocyte responses to PPD. Evidence of a regulatory influence associated with MB1. Scand J Immunol. 1984 Dec;20(6):503–510. doi: 10.1111/j.1365-3083.1984.tb01032.x. [DOI] [PubMed] [Google Scholar]
- van Eden W., de Vries R. R., Stanford J. L., Rook G. A. HLA-DR3 associated genetic control of response to multiple skin tests with new tuberculins. Clin Exp Immunol. 1983 May;52(2):287–292. [PMC free article] [PubMed] [Google Scholar]



