Abstract
Cognitive shifting is the ability to adapt to changes in the environment. Extensive research has revealed that the prefrontal cortex plays an important role in cognitive shifting. Adult neuroimaging studies have shown that the inferior prefrontal cortex is activated during cognitive shifting tasks. Developmental studies have shown that cognitive shifting changes significantly during preschool years. It is known that 3-year-old children often perseverate to previous mental sets, whereas 5-year-old children do not. Developmental psychologists assume that maturation of the prefrontal cortex plays an essential role in the development of shifting; however, direct supporting evidence is lacking. We used near-infrared spectroscopy and showed that inferior prefrontal activation is associated with successful shifting in young children. We also showed that even preschool children display adult-like inferior prefrontal activation during a simple cognitive shifting task. This report demonstrates the neural origins of cognitive shifting in young children. These results have the potential to contribute to our understanding of cognitive and brain development in both typically and atypically developed children.
Keywords: cognitive development, inferior prefrontal cortex, near-infrared spectroscopy, preschool children, executive function
Cognitive shifting is an important ability that enables individuals to adapt to changes in the environment by switching from one mental set to another. The Wisconsin Card Sorting Test (WCST) is the most widely used method to measure cognitive shifting (1). In this test, participants are required to switch between rules in terms of features, such as shape, color, and number, according to the experimenter's feedback. Extensive research has revealed that the prefrontal cortex plays an important role in cognitive shifting. Patients with prefrontal damage continue adhering to the older rules despite repeated feedback (2–4). Monkeys with prefrontal lesions also experience difficulty in a modified WCST (5, 6). Moreover, recent neuroimaging studies have shown that the inferior (ventrolateral) prefrontal cortex contributes to flexible shifting from one rule to the next (7–11).
In developmental literature, it is well established that young children also experience difficulty with flexible shifting of mental sets. In the Dimensional Change Card Sort (DCCS) task, children are asked to sort cards that have 2 dimensions, such as color and shape. In the preswitch phase, children are asked to sort cards (e.g., red boats, blue rabbits) into trays with target cards (e.g., a blue boat, a red rabbit) according to one rule (e.g., color). In the postswitch phase, children are asked to sort the cards according to a second rule (e.g., shape). Typically, 3-year-old children perseverate to the first rule, whereas 4- and 5-year-old children do not (12–15). The perseverative errors in 3-year-old children are apparently similar to those in patients with prefrontal damage. Therefore, developmental psychologists assume that maturation of the lateral prefrontal cortex may play an essential role in the development of cognitive shifting (12, 13). Extensive behavioral research has supported the assumption. For example, some researchers developed a neuropsychological battery for prefrontal function that is derived from animal lesion and neurologically impaired patients' studies and showed that young children improved their performance on the battery during preschool age (16). In addition, there is some anatomical evidence (17, 18) and electrophysiological evidence (19) stating that the prefrontal cortex develops during preschool years. However, there are no neuroimaging data demonstrating the functional development of the prefrontal cortex during preschool years. Recent neuroimaging studies have shown that even school-aged children were less likely to engage the lateral prefrontal cortex in cognitive control tasks (11, 20) and in working memory tasks (21). Thus, it is still unclear whether the functional development of the prefrontal cortex is associated with significant changes in cognitive shifting in young children.
Here, we report that inferior prefrontal activation is strongly correlated with cognitive shifting in young children. We used a near-infrared spectroscopy (NIRS) technique to monitor cerebral hemodynamics by measuring changes in the attenuation of near-infrared light passing through tissue. Because NIRS is noninvasive and does not require fixing of the body as in functional magnetic resonance imaging (fMRI), it is suitable for brain imaging studies in infants and children (22, 23). The validity of NIRS has been confirmed by studies on prefrontal function in children (24, 25) and by the WCST in adults (26).
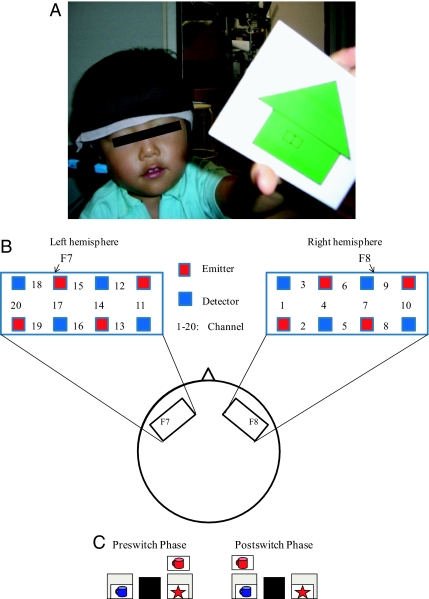
Three-year-old children and 5-year-old children underwent 4 sessions of the DCCS task, whereas adults underwent 5 sessions. The brain activation during the DCCS task was examined using a multichannel NIRS system that covered the region of interest (ROI) (Fig. 1A). We measured changes in the hemoglobin concentration and its oxygenation level (oxy-Hb) in the inferior prefrontal areas. The ROI was located at around F7/8 on the International 10/20 system (Fig. 1B), which corresponds to Brodmann areas (BAs) 45/47 (27). Previous studies in adults have shown that these areas are activated during cognitive shifting tasks (7–11). The spatial resolution of NIRS is relatively low; therefore, channels (ch) 6, 7, and 9 (right inferior prefrontal area) and ch 15, 17, and 18 (left inferior prefrontal area) roughly correspond to F8 and F7, respectively. We separately analyzed brain activation during the preswitch and postswitch phases (Fig. 1C). The brain activation during each phase was compared with the activation during the control phase, during which adults were instructed to sit still and children were given control tasks. In the control task, children were asked to sort blank cards into an extra tray.
Fig. 1.
Experimental settings. (A) A child with an NIRS probe. (B) The NIRS probe was attached to the inferior prefrontal area. Each channel consisted of 1 emitter optode and 1 detector optode. The ROI was located near F7/8, which corresponds to ch 15, 17, and 18 and to ch 6, 7, and 9, respectively. (C) An example of preswitch and postswitch phases.
Results
Behavioral Results.
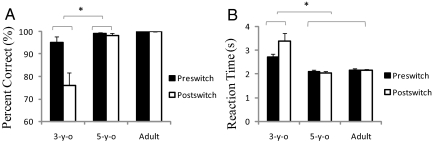
The behavioral results revealed that the adults and 5-year-old children performed almost perfectly during both the preswitch and postswitch phases. On the other hand, the 3-year-old children exhibited difficulty during the postswitch phase (Fig. 2A). The percentage of correct responses was analyzed using a 2 (3-year old vs. 5-year old) × 2 (preswitch vs. postswitch) mixed ANOVA, and we observed a significant interaction [F (1, 99) = 6.438, P < 0.02]. Furthermore, the 3-year-old children required more time to sort the cards during the postswitch phase than during the preswitch phase, whereas the 5-year-old children and adults did not (Fig. 2B). We conducted a 3 (3-year old vs. 5-year old vs. adults) × 2 (preswitch vs. postswitch) mixed ANOVA and found a significant interaction of the reaction time [F (1, 123) = 3.6, P < 0.04].
Fig. 2.
Behavioral results. Percentage of correct responses (A) and reaction time (B) for each age group during the preswitch and postswitch phases. Error bars indicate SE. ∗, P < 0.05.
NIRS Results.
We measured changes in the oxy-Hb in the inferior prefrontal areas. The results of the NIRS recordings revealed that the adults' bilateral inferior prefrontal cortex exhibited significant activation during both the preswitch and postswitch phases [supporting information (SI) Fig. S1]. We observed significant increases in oxy-Hb in the right and left inferior prefrontal areas (ch 6, 7, and 9 and ch 15, 17, and 18, respectively) during the preswitch and postswitch phases compared with the control phase (Student's t test, P < 0.002). Similar brain areas were also activated in the 5-year-old children (Fig. S2). In the right (ch 6 and 7) and left (ch 15 and 17) prefrontal areas, the mean change in oxy-Hb was significantly higher during the preswitch and postswitch phases than during the control phase (P < 0.002).
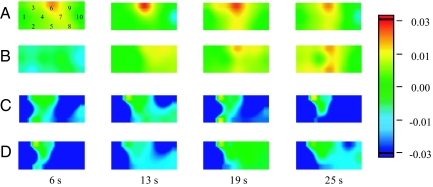
We analyzed 3-year-old children separately according to whether they committed perseverative errors during at least 1 of the 4 assigned sessions. Children were considered to commit perseverative errors when they continued to follow the preswitch rule during the postswitch phases in all the trials. We initially examined the brain activation of children who did not perseverate (pass group, n = 9) (Fig. 3 A and B). In the pass group, significant oxy-Hb increases were observed in the right (ch 6 and 9) and left (ch 15) inferior prefrontal areas during the preswitch phase compared with the control phase (P < 0.002). During the postswitch phase, children in the pass group exhibited significant changes in oxy-Hb in the right (ch 6) and left (ch 17) prefrontal areas. The results were similar to those of the adults and 5-year-old children. On the other hand, children who perseverated (perseverate group, n = 6) exhibited quite a different pattern. These children exhibited no significant oxy-Hb increases in either the right or left inferior prefrontal area during both preswitch and postswitch phases (Fig. 3 C and D). Rather, they showed significant decreases in oxy-Hb at some channels during both phases (see SI Text).
Fig. 3.
A 3-year-old child in the pass group (A, B) and a 3-year-old child in the perseverate group (C, D) during the DCCS task. Averaged data during the task phase for a typical subject are shown at 6, 13, 19, and 25 s after task onset (0 s). The numbers (1–10) indicate the channel of the NIRS probe. Preswitch phase (A) and postswitch phase (B) in the pass group. Preswitch phase (C) and postswitch phase (D) in the perseverate group.
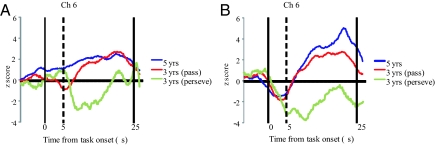
Next, we compared the changes in oxy-Hb in the ROI in the case of the perseverate group with those in the case of the pass group. The results revealed that children in the pass group exhibited greater changes in oxy-Hb in both the right (ch 6 and 9) and left (ch 15) inferior prefrontal areas during the preswitch phase and in the right inferior prefrontal area (ch 6) during the postswitch phase than those in the perseverate group (P < 0.002) (Fig. 4). Importantly, children in the pass group did not exhibit greater oxy-Hb changes in the regions outside the ROI than those in the perseverate group (see SI Text). Finally, we examined whether individuals in each group exhibited consistent patterns of inferior prefrontal activation (Table S1). During the preswitch phase, 2 of 6 children in the perseverate group exhibited significant oxy-Hb changes compared with the control phase in the right inferior prefrontal area. During the postswitch phase, 2 other children in the group exhibited significant changes. However, none of children in the perseverate group exhibited significant oxy-Hb changes in the right inferior prefrontal cortex during both the preswitch and postswitch phases. Thus, no children in the group exhibited sustained right inferior prefrontal activation during the DCCS task. On the other hand, 6 of 9 children in the pass group exhibited the sustained activation. We found a significant difference in sustained prefrontal activation between the pass group and the perseverate group [Fisher's exact test (two-tailed), P < 0.03]. The group and individual comparisons suggest that sustained right inferior prefrontal activation might be crucial for successful cognitive shifting in young children.
Fig. 4.
Temporal changes in the oxy-Hb concentration in the right inferior prefrontal area (ch 6) during the experiment. The preswitch phase (A) and postswitch phase (B), respectively, are shown. Data of the group mean in 5-year-old children (blue line), 3-year-old children in the pass group (red line), and 3-year-old children in the perseverate group (green line) are shown.
Discussion
The present study provides neuroimaging data demonstrating the functional development of the prefrontal cortex during preschool years. Five-year-old children were found to exhibit adult-like prefrontal activation. These results were in contrast to previous neuroimaging evidence stating that school-aged children failed to engage the right inferior prefrontal cortex (11, 20). The difference in results may be attributable to the difference in strategies used by children and adults. A previous study suggested that during relatively complex tasks, children used a different strategy compared with adults and that children thus exhibited different brain activation (20). However, the DCCS task is quite simple, and children may adopt the same strategy as adults. Alternatively, the differences in imaging methods may explain the discrepancy between the previous studies and the present study. It has been suggested that participating in an fMRI experiment can be challenging for children (e.g., large noisy equipment) (28). This indicates that the fMRI environment probably affected the children's brain activation.
Our results indicated that inferior prefrontal activation determined whether young children displayed perseveration. The difference between the children in the pass group and those in the perseverate group was associated with the activation of the right inferior prefrontal cortex during both preswitch and postswitch phases. Given this differentiation, we propose that sustained right inferior prefrontal activation across the preswitch and postswitch phases may be responsible for the development of cognitive shifting during the DCCS task. Intuitively, it appears that prefrontal activation during the postswitch phase is strongly related to the shifting. Adult neuroimaging studies have demonstrated that the inferior prefrontal areas are significantly activated when participants are required to switch rules (7–10). However, in the current study, the right inferior prefrontal regions of 2 children in the perseverative group were activated during the postswitch phase. Consequently, prefrontal activation during the postswitch phase alone may not be sufficient for successful cognitive shifting in children. On the basis of this result, we speculate that the inferior prefrontal regions should be “prepared” during the preswitch phase for successful cognitive shifting during the postswitch phase. Activation during the preswitch phase may be related to the representation of the task structure (e.g., rule system) (29), and this activation may prepare the inferior prefrontal regions for rule switching during the postswitch phase.
Our results confirmed that the inferior prefrontal cortex contributes to cognitive shifting not only in adults but in young children. Furthermore, our results may shed light on the relation between prefrontal activation and perseverative behaviors. Developmental psychologists suggest that functional development of the prefrontal cortex may help young children to improve their perseverative behaviors (12, 13). This speculation is based on the results of the WCST in patients with prefrontal damage (2–4). The present results support this speculation. It is difficult, however, to assess whether the underlying mechanism in young children's perseveration is similar to the mechanism in prefrontal-damaged patients, because the damaged areas in these patients were not uniform. Nevertheless, our results, coupled with neuropsychological studies, suggest that the prefrontal cortex may be responsible for perseverative tendencies.
The present study might also contribute to our understanding of developmental disorders, such as attention-deficit hyperactivity disorder (ADHD). Recently, it has been demonstrated that children with ADHD experience difficulty with a modified DCCS task (30) and that some of the patients with ADHD displayed weaker prefrontal activation in a stop-signal task (31). In addition, recent neuroanatomical research suggests that children with ADHD exhibit a marked delay in the maturation of prefrontal areas (32). These studies suggest that patients with ADHD may have functional and anatomical deficits in the prefrontal cortex. However, there have been few brain imaging studies of young children with ADHD. Thus, our functional developmental approach, along with the anatomical evidence, may contribute to determining when and how children with ADHD exhibit the earlier neural symptoms.
It is also important to consider the limitations of the present study. First, the procedure in adults was slightly different from that in children, which may make direct comparison between them difficult (for details, see SI Text). Second, other brain regions as well as inferior prefrontal areas may contribute to the development of cognitive shifting. Adult brain imaging studies have shown that other brain areas, such as the dorsolateral prefrontal cortex and parietal cortex, are significantly activated during cognitive shifting tasks (8, 9). Third, the present study did not clarify the reason why the inferior prefrontal regions were not activated in children in the perseverate group. Given the recent anatomical (17, 18) and structural MRI evidence (33, 34) that neuronal circuits in the prefrontal cortex might not be fully formed in preschool-aged children, it is likely that the children could not activate the regions. We can address the issue by examining the brain activation of the inferior prefrontal areas using various tasks, including both cognitive shifting and other tasks.
Experimental Procedures
Participants.
Ten right-handed healthy adults [aged between 21 and 31 years, 25.8 ± 2.9 (mean ± SD); 6 women], 11 right-handed 5-year-old children [aged between 61 and 74 months, 68.3 ± 3.0 (mean ± SD); 4 girls], and 15 right-handed 3-year-old children [aged between 37 and 47 months, 41.3 ± 3.8 (mean ± SD); 8 girls] participated in this study. Additionally, one 5-year-old child and two 3-year-old children participated but failed to complete the experiment. Adult subjects provided informed consent for the study. For the children, parents provided written informed consent and were informed verbally of the purpose of the study and the safety of the NIRS experiment. The experiments were approved by the local ethics committee.
Behavioral Tasks.
Laminated cards (3.5 × 7.0 cm) were used as stimuli. The stimuli had 2 dimensions: shape and color. The DCCS task included target cards and test cards; target cards matched test cards in one dimension but did not match in the other dimension (e.g., a red star, a blue cup, red cups, blue stars). The present experiments included 5 pairs of target and test cards, each of which was different in shape and color. There were 5 pairs of target trays, each of which contained target cards. At each session, a different tray with a different set of cards was used. For the children's control task, blank cards and an extra tray were used. The extra tray was placed between 2 target trays. The entire experiment was videotaped.
Adult participants underwent 5 consecutive test sessions. One session consisted of a rest phase (control 1), a preswitch phase, a second rest phase (control 2), and a postswitch phase. Participants were given no instructions during the rest phases and provided with the rules only during the preswitch and postswitch phases. During the preswitch phase, participants were asked to sort the cards according to the first rule (e.g., color). During the postswitch phase, participants were asked to sort the cards according to the second rule (e.g., shape). The rule order (color vs. shape first) was constant across the 5 sessions for each participant, although the order was counterbalanced across participants. The preswitch and postswitch phases were 20 s each, and each rest phase was 25 s. Each participant underwent 8 trials on average during the preswitch and postswitch phases.
Five-year-old and 3-year-old children underwent 4 consecutive test sessions. One session for children consisted of a control phase (control 1), a preswitch phase, a second control phase (control 2), and a postswitch phase. During the control phase, children were provided with blank cards and asked to place these cards into an extra tray. This phase was conducted to examine whether a child's movements alone evoked any response and reduced motion artifacts during the experiment. During the preswitch and postswitch phases, children were given detailed instructions regarding the rules (e.g., “This is a shape game. All of the stars go here, and all of the cups go there.”) and asked to sort the cards. The rule order was the same as in adult studies. In the children's experiment, the preswitch and postswitch phases were 25 s each, which included the time required to provide instructions concerning the rules (first 5 s in each phase), and each control phase was 25 s. The 5-year-old children underwent 8 trials on average, and the 3-year-old children underwent 6 trials during the preswitch and postswitch phases.
The percentage of correct responses and the reaction time were analyzed. The reaction time was defined as the average time taken by the participants to sort 1 test card. The reaction time was analyzed with the videotapes.
NIRS Recordings and Analysis.
NIRS measurements were performed throughout the experiment. A multichannel NIRS unit operating at wavelengths of 780, 805, and 830 nm (OMM-1080S; Shimadzu) was used to measure temporal changes in the concentrations of oxy-Hb, deoxyhemoglobin, and total hemoglobin. One NIRS probe included 8 optodes that comprised 10 channels. Each probe was placed on the inferior prefrontal areas of each hemisphere. Each channel consisted of 1 emitter optode and 1 detector optode located 2 cm apart. The sampling rate at each channel was ≈10 Hz. To compare the children's brain activation directly with that of adults, we used the same NIRS system for both children and adults.
The ROI was located near F7/8 of the International 10/20 system, which corresponds to BA 45/47 (27). The spatial resolution of NIRS is relatively low (for details, see SI Text); therefore, ch 6, 7, and 9 (right inferior prefrontal areas) and ch 15, 17, and 18 (left inferior prefrontal areas) were considered to correspond to F8 and F7, respectively.
In NIRS experiments, when participants make quick head motions, it can cause sharp changes in hemoglobin signals in the NIRS system. The test sessions with motion artifacts as revealed by the video recordings and NIRS data were discarded, and ≈9% of the data from the 5-year-old children and 5% from the 3-year-old children were excluded from the analyses. Among the 3 NIRS parameters measured, the concentration of oxy-Hb was found to be the most sensitive to changes in regional cerebral blood flow, and this provided the strongest correlation with the blood oxygen level-dependent signal (35, 36). Thus, a change in the oxy-Hb concentration was considered to be the best indicator of brain activity. On the basis of previous studies (22, 23), the raw data were converted into z scores. The z score was calculated using the mean value and the SD of the oxy-Hb concentration changes during the rest phase (for adults) and control phase (for children). Consequently, the mean value and SD during the rest and control phases were, respectively, changed to z scores of 0 and 1 in every channel.
We analyzed the preswitch and postswitch phases (0–20 s) in adults, and the last 20 s during the preswitch and postswitch phase (5–25 s) in 5-year-old and 3-year-old children; children were given instructions regarding the rules during the first 5 s of the task phases. The average changes in oxy-Hb during the preswitch and postswitch phases were calculated for all channels and each subject. The significance of the differences between the changes in oxy-Hb for the baseline (the last 5 s of the rest phase in adults and the control phase in children) and task (preswitch or postswitch) was determined by a two-tailed Student's t test for each channel. The preswitch phase was compared with control 1, and the postswitch phase was compared with control 2. Because multiple comparisons were conducted, we applied a strict 0.002 alpha level of significance. Moreover, we classified the 3-year-old children into the pass group and the perseverate group depending on whether the children exhibited perseverative errors during at least 1 of the 4 test sessions. The difference between the pass group and the perseverate group for each channel was compared using a Student's t test (P < 0.002).
Supplementary Material
Acknowledgments.
We greatly thank G. Matsuda, R. Matsunaka, T. Imai, and I. Shinohara for their help in conducting the study. We also thank the parents and children who participated in the study. This research was supported by a Japan Society for the Promotion of Science Research Fellowship for Young Scientists (to Y.M.) and by Grant 18200018 from the Japan Society for the Promotion of Science Research (to K.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809747106/DCSupplemental.
References
- 1.Grant DA, Berg EA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- 2.Milner B. Effects of different brain lesions on card sorting. Arch Neurol (Chicago) 1963;9:90–100. [Google Scholar]
- 3.Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- 4.Owen AM, et al. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain. 1993;116:1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- 5.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 6.Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of lateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318:987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- 7.Konishi S, et al. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci. 1998;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- 8.Monchi O, et al. Wisconsin card sorting revisited: Distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- 10.Nakahara K, Hayashi T, Konishi S, Miyashita Y. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- 11.Crone EA, et al. Brain regions mediating flexible rule use during development. J Neurosci. 2006;26:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelazo PD, Müller U, Frye D, Marcovitch S. The development of executive function in early childhood. Monogr Soc Res Child Dev. 2003;68:138–151. doi: 10.1111/j.0037-976x.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 13.Kirkham NZ, Cruess L, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Dev Sci. 2003;6:449–476. [Google Scholar]
- 14.Müller U, et al. The role of negative priming in the Dimensional Change Card Sort task. Child Dev. 2006;77:395–412. doi: 10.1111/j.1467-8624.2006.00878.x. [DOI] [PubMed] [Google Scholar]
- 15.Moriguchi Y, Lee K, Itakura S. Social transmission of disinhibition in young children. Dev Sci. 2007;10:481–491. doi: 10.1111/j.1467-7687.2007.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- 17.Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford Univ Press; 2002. pp. 466–503. [Google Scholar]
- 18.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 19.Rueda MR, Rothbart MK, Saccamanno L, Posner MI. Training, maturation and genetic influences on the development of executive attention. Proc Natl Acad Sci USA. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunge SA, et al. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;3:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crone EA, et al. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci USA. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda G, Hiraki K. Sustained decrease in oxygenated hemoglobin during video games in the dorsal prefrontal cortex: A NIRS study of children. NeuroImage. 2006;29:706–711. doi: 10.1016/j.neuroimage.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Shimada S, Hiraki K. Infant's brain responses to live and televised action. NeuroImage. 2006;32:930–939. doi: 10.1016/j.neuroimage.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 24.Schroeter ML, Zysset S, Wahl MM, von Cramon DY. Prefrontal activation due to Stroop interference increases during development—An event-related fNIRS study. NeuroImage. 2004;23:1317–1325. doi: 10.1016/j.neuroimage.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Tsujimoto S, et al. Prefrontal cortical activation associated with working memory in adults and preschool children: An event-related optical topography study. Cereb Cortex. 2004;14:703–712. doi: 10.1093/cercor/bhh030. [DOI] [PubMed] [Google Scholar]
- 26.Sumitani S, et al. Activation of the prefrontal cortex during the Wisconsin Card Sorting Test as measured by multichannel near-infrared spectroscopy. Neuropsychobiology. 2006;53:70–76. doi: 10.1159/000091722. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto M, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Kotsoni E, Byrd D, Casey BJ. Special considerations for functional magnetic resonance imaging of pediatric populations. J Magn Reson Imaging. 2006;23:877–886. doi: 10.1002/jmri.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunge S, Zelazo PD. A brain-based account of the development of rule use in childhood. Cur Dir in Psychol Sci. 2006;15:118–121. [Google Scholar]
- 30.Mulas F, et al. Shifting-related brain magnetic activity in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;59:373–379. doi: 10.1016/j.biopsych.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 31.Bush G, Vaela EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: A review and suggested future directions. Biol Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 32.Shaw P, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giedd JN, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 34.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshi K, Kobayashi N, Tamura M. Interpretation of near-infrared spectroscopy signals: A study with a newly developed perfused rat brain model. J Appl Physiol. 2001;90:1657–1662. doi: 10.1152/jappl.2001.90.5.1657. [DOI] [PubMed] [Google Scholar]
- 36.Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. NeuroImage. 2002;10:327–338. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.