Abstract
Transcription of Bdnf is controlled by multiple promoters, which drive expression of multiple transcripts encoding for the same protein. Promoter IV contributes significantly to activity-dependent brain-derived neurotrophic factor (BDNF) transcription. We have generated promoter IV mutant mice (BDNF-KIV) by inserting a GFP-STOP cassette within the Bdnf exon IV locus. This genetic manipulation results in disruption of promoter IV-mediated Bdnf expression. BDNF-KIV animals exhibited significant deficits in GABAergic interneurons in the prefrontal cortex (PFC), particularly those expressing parvalbumin, a subtype implicated in executive function and schizophrenia. Moreover, disruption of promoter IV-driven Bdnf transcription impaired inhibitory but not excitatory synaptic transmission recorded from layer V pyramidal neurons in the PFC. The attenuation of GABAergic inputs resulted in an aberrant appearance of spike-timing-dependent synaptic potentiation (STDP) in PFC slices derived from BDNF-KIV, but not wild-type littermates. These results demonstrate the importance of promoter IV-dependent Bdnf transcription in GABAergic function and reveal an unexpected regulation of STDP in the PFC by BDNF.
Keywords: activity-dependent, GABAergic interneuron, knockout mice, parvalbumin, cortical inhibition
Brain-derived neurotrophic factor (BDNF) is a key player in synaptic plasticity and cognitive function. Additionally, impairments in BDNF signaling have been associated with numerous neurological and neuropsychiatric disorders (1, 2). One of the numerous functions of BDNF in the brain is the regulation of GABAergic interneurons in the cerebral cortex (3). Among GABAergic neurons, the BDNF receptor TrkB is more abundantly expressed in parvalbumin (PV)-positive cells than in calbindin (CB)- or calretinin (CR)-positive cells (4). In mice that overexpress BDNF, the maturation of PV interneurons is accelerated (5, 6), whereas BDNF or TrkB deletion reduces the number of cortical PV interneurons (7, 8). Two major types of PV interneurons, the basket cells and the chandelier cells, innervate the somatic and axon initial segments, respectively, of a large number of pyramidal cells. Both cell types exert powerful negative control over pyramidal cells by firing high-frequency, nonadapting (fast-spiking) action potentials (7). These unique features allow PV interneurons to synchronize firing of a network of excitatory neurons (3). PV interneurons also control phasing of excitatory neuron action potentials, thereby influencing spike-timing-dependent (STDP) forms of plasticity (8). Both neuronal synchronization and STDP in the prefrontal cortex (PFC) have been implicated in “executive functions,” such as working memory, rule learning, and planning (9). Although substantial evidence suggests that BDNF regulates the development and/or function of PV interneurons, the mode(s) by which BDNF elicits such regulation remains unclear.
Transcription of the mouse Bdnf gene is controlled by at least 9 distinct promoters (Fig. S1A); each drives the expression of a small, untranslated exon spliced onto a common, final exon (exon IX) with 2 polyadenylation sites (10, 11). Thus, the use of alternative promoters and different polyadenylation sites results in the production of at least 18 unique Bdnf transcripts, which encode the identical pre-pro-BDNF protein. This complex genomic organization allows for precise temporal and stimulus-specific regulation. Although the exact reasons for such a multitude of transcripts is not fully understood, an intriguing idea is that some promoters maintain a basal level of BDNF expression necessary for neuronal survival and differentiation, whereas others mediate activity-dependent BDNF expression involved in synapse development and plasticity (1). In vitro evidence has shown that depolarization of cultured cortical neurons by application of high K+ selectively enhances expression of exon IV via Ca2+-dependent mechanisms (12, 13). In vivo experiments have further demonstrated that transcription from promoters I and IV is most robustly regulated by neuronal activity induced by kainic acid (KA) seizures and fear conditioning (13–15). Moreover, sensory inputs to the visual and barrel cortices appear to preferentially stimulate expression of exon IV-containing BDNF transcripts (16, 17).
Despite a large body of literature demonstrating a strong correlation between neuronal activity, BDNF gene transcription, and cognitive function (1, 18), the functional consequences of disrupting activity-dependent BDNF gene expression in vivo remain unknown. We sought to directly address this issue by generating mice in which promoter IV-driven BDNF transcription is selectively disrupted. We inserted a GFP-STOP cassette within the exon IV locus, thereby halting the translation of BDNF protein derived from exon IV transcript without direct disruption of promoter IV activity. In these mice, activity-dependent BDNF expression in the prefrontal cortex is severely inhibited. Mutant animals exhibit a striking reduction in the number of PV interneurons as well as impaired inhibitory but not excitatory synaptic transmission in the PFC and dramatically altered STDP. Our results have identified a specific role for BDNF promoter IV in GABAergic transmission and cortical STDP.
Results
Disruption of Promoter IV-Driven Expression of BDNF Transcripts and Protein.
BDNF promoter IV is best known for its role in mediating activity-dependent BDNF transcription (13). We generated promoter IV-specific knockout mice (BDNF-KIV) to investigate the functional role of transcription from this promoter. To avoid a compensatory increase of BDNF transcription from other promoters, we avoided a direct knockout of exon IV, but instead inserted the GFP gene followed by multiple stop codons into the exon IV locus (Fig. 1 and Fig. S1A). In these mice, neuronal activity leads to production of GFP protein in lieu of BDNF. This particular design does not interfere with promoter IV-mediated transcription per se, but it does prevent translation of BDNF protein from exon IV. To determine whether our design effectively disrupted promoter IV-driven BDNF expression, we analyzed whether KA failed to induce exon IV transcript levels in BDNF-KIV. KA has been demonstrated to robustly elevate levels of BDNF IV transcript in many brain regions (14). Quantitative analyses of all transcripts by using real-time RT-PCR revealed a marked increase in exon IV-IX transcripts in the frontal cortex (FC) of wild-type mice (WT), but these transcripts are not detected in BDNF-KIV 3 h after KA administration (Fig. 2A Upper). In contrast, exon IV-GFP primers detected a dramatic increase in newly generated, exon IV-GFP transcript in BDNF-KIV but not in WT (Fig. 2A Lower). GFP knockin at the exon IV locus did not alter transcription from any of the alternative promoters tested (Fig. S1B). This included promoter IV itself (Fig. 2A), where an IV-GFP transcript was produced instead of the IV-IX transcript in the BDNF-KIV brain.
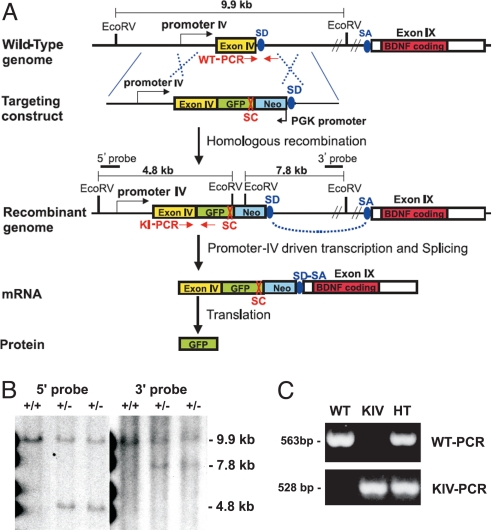
Fig. 1.
Generation of BDNF-KIV mice. (A) Strategy to generate promoter IV-specific knockin mice (BDNF-KIV). The targeting construct contained sequences corresponding to Bdnf promoter, exon IV, GFP, and a neomycin cassette (Neo) driven by a PGK promoter in the antisense orientation. The recombinant genome generates an mRNA that translates GFP in lieu of BDNF protein. EcoRV cleavage sites, the positions of the 5′ and 3′ probes for Southern blot analysis, and locations of genotyping primers as well as splicing donor sites (SD) and splicing acceptor sites (SA) are shown. (B) Southern blot analysis of EcoRV digests of genomic DNA prepared from the F1 generation of mice. For the WT allele, both 5′ and 3′ probes detected a 9.9-kb fragment. For the BDNF-KIV allele, the 5′ probe detected 4.8-kb fragments, and the 3′ probe detected 7.8-kb fragments. (C) PCR analysis of genomic DNA. A 563-bp fragment and a 528-bp fragment were amplified from genomic DNA isolated from WT and mutant mice, respectively. KIV indicates BDNF-KIV−/− mice; HT, BDNF-KIV+/− mice.
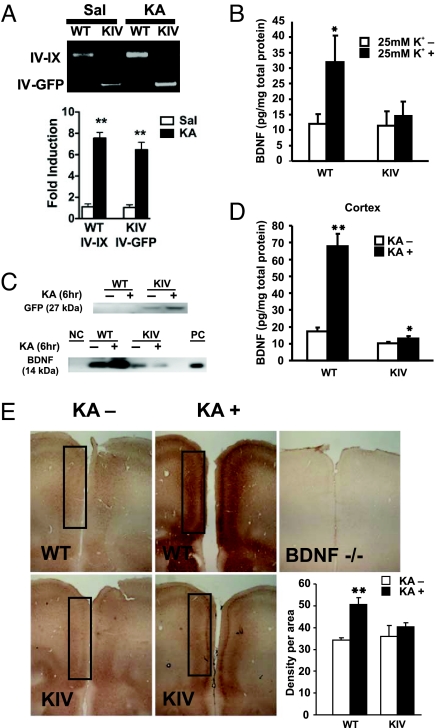
Fig. 2.
Blockade of promoter IV expression of BDNF mRNA and protein. (A) Kainic acid (KA) induces promoter IV-driven transcription in WT and KIV. Quantitative RT-PCR was performed to analyze mRNA expression levels in the FC 3 h after KA administration. (Upper) Representative agarose gels showing the RT-PCR results. (Lower) Quantitative PCR results. KA-induced increases in transcript levels were obtained by normalizing to the mean value of saline (Sal) controls. KA treatment leads to increased levels of IV-IX transcripts in WT mice, and to IV-GFP transcripts in KIV mice. No IV-IX transcripts were detected in BDNF-KIV mice. (n = 3 each genotype). In this and all other figures, error bars are SEM. Unless indicated otherwise, Student's t test was used. **, P < 0.01. (B) ELISA quantification showing BDNF protein levels in cortical neurons cultured for 7 days after a 3-h treatment with high K+ (25 mM). Note that the K+-induced increase in BDNF protein is abolished in cortical neurons derived from KIV mice (*, P = 0.649). (C) Western blot showing BDNF (Lower) and GFP (Upper) FC protein levels 6 h after vehicle or KA administration. NC indicates negative control; BDNF−/− brain. PC indicates positive control; recombinant BDNF. Note that KA enhanced GFP expression in BDNF-KIV but not in WT mice, and it enhanced expression of BDNF expression in WT mice but not KIV mice. (D) Inhibition of KA-induced expression of BDNF protein in FC of BDNF-KIV in vivo. ELISA was used to measure BDNF protein levels from FC 6 h after vehicle or KA administration (i.p.) (n = 3 pairs of brains). KA induced less increase in BDNF protein in KIV (P = 0.123). (E) Immunohistochemical staining of BDNF protein in mPFC before and 6 h after KA administration. KO indicates PFC section from BDNF−/− mice as a negative control. Immunoreactive signals in the boxed area were quantified (Lower Right). Note that the increase in BDNF protein in mPFC is significantly attenuated in BDNF-KIV (n = 3). KA induced no increase in BDNF protein in KIV (P = 0.208).
To test whether insertion of the GFP-STOP cassette prevents the promoter IV-driven expression of BDNF protein, we performed biochemical analyses in cultured neurons. Cortical neurons from WT and BDNF-KIV were cultured in serum-free medium for 7 days. Cells were treated with high K+ (25 mM) and harvested 3 h later for detection of BDNF protein by ELISA. BDNF protein levels dramatically increased in cortical cultures from WT (Fig. 2B). In contrast, treatment with high K+ failed to enhance BDNF protein expression in cultured cortical neurons derived from BDNF-KIV (Fig. 2B). These results are consistent with previous data showing that high K+ selectively increases expression of BDNF exon IV transcript in cultured cortical neurons (13).
Next, we determined whether KA-induced expression of BDNF protein is impaired in vivo. It has been shown that BDNF mRNA is highly expressed in the FC of rodents, monkeys, and humans (19–21), and that this expression is largely mediated by promoters I and IV (14, 22). Western blotting revealed an increase in the expression of GFP (27 kDa) in the cortex 6 h after administration of KA to BDNF-KIV (Fig. 2C). In WT, BDNF (14 kDa) but not GFP was detected (Fig. 2C). These results were confirmed by using quantitative ELISA, which revealed that KA dramatically increased BDNF protein levels in WT (422%, P < 0.01) but not in BDNF-KIV (131%, P = 0.123; Fig. 2D). The basal level of BDNF protein in the BDNF-KIV cortex appeared to decrease, but it did not reach statistical significance compared with WT (KIV/WT = 58.6%, P = 0.0558; Fig. 2D).
We next used immunohistochemistry to determine the distribution of BDNF protein in sections from the forebrain. Under basal conditions, a BDNF-specific antibody detected moderate levels of BDNF immunoreactivity in sections from PFC areas in both WT and BDNF-KIV, but not in BDNF null (BDNF−/−) mice (Fig. 2E Upper Right). Treatment with KA for 6 h induced a widespread increase in BDNF protein in WT (Fig. 2E Upper). In contrast, levels of BDNF did not increase significantly after KA administration in the BDNF-KIV (Fig. 2E Lower). Semiquantitative analysis of scanned images revealed a significant increase in BDNF immunoreactivity in medial PFC (mPFC) in WT, but not in BDNF-KIV (Fig. 2E Lower Right). Hence, insertion of GFP into exon IV significantly decreased activity-dependent expression of BDNF protein in the mPFC.
Regulation of GABAergic Function in mPFC.
Anatomical and histological examinations did not show obvious abnormalities in the BDNF-KIV brains (Fig. S2). A major function of cortical BDNF is developmental and functional regulation of GABAergic interneurons (3). We performed a series of immunofluorescence stainings to examine whether elimination of promoter IV-mediated BDNF transcription affects GABAergic interneurons. We initially focused on parvalbumin (PV)-positive, fast-spiking interneurons in the mPFC (Fig. 3A), because these neurons are believed to be a major target of BDNF regulation (4). The number of PV interneurons, as revealed by an antibody against PV, was significantly reduced (Fig. 3B). Quantitative analysis of mPFC sections showed a 66.6% reduction in the number of PV interneurons in the mPFC of BDNF-KIV (Fig. 3C). However, it is unclear which of the 2 PV interneuron subtypes, basket or chandelier, was affected in the BDNF-KIV. Moreover, there was no decrease in the number of PV interneurons in the adjacent motor cortex (MC) of the same sections (Fig. 3 B and D). The total number of GABAergic interneurons in the mPFC, as determined by GAD67 expression, remained the same between genotypes (Fig. 3C). Furthermore, there was a small decrease (13%) in the number of calbindin (CB)-positive interneurons (Fig. S3 A and C) and an increase in calretinin (CR)-positive interneurons (Fig. S3 B and C) in BDNF-KIV mPFC. Because a majority of the GABAergic neurons in the cortex are PV interneurons, and only a small fraction of cortical interneurons are CR cells (<17%) (23), reduction in PV interneurons in mPFC represents a major cellular phenotype of the BDNF-KIV. It should be noted that because additional markers that specifically label PV interneurons in the mouse are lacking, we were unable to definitively determine whether promoter IV-derived BDNF regulates the survival, and hence the decrease in the number of PV-positive GABAergic interneurons or, rather, the level of PV expression.
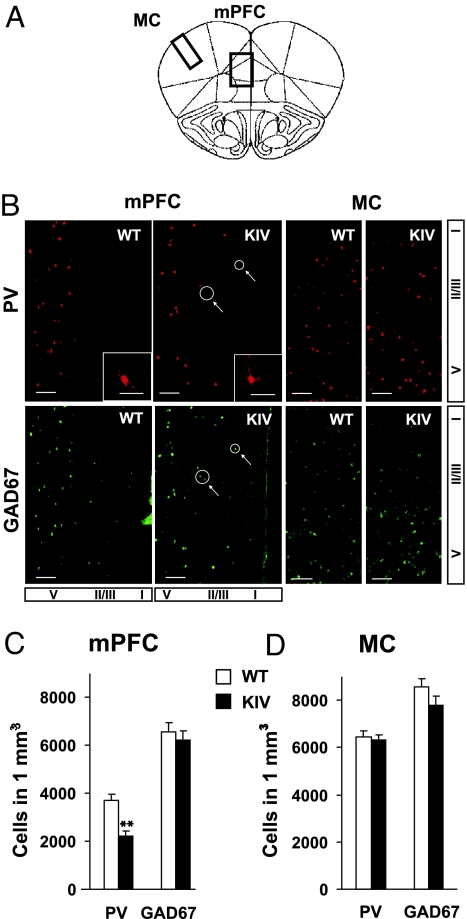
Fig. 3.
Reduction of PV interneurons in the PFC. (A) Schematic diagram showing the locations of mPFC and motor cortex (MC) where the GABAergic neurons were analyzed. Areas of MC and mPFC shown in B are highlighted by the rectangular boxes. (B) Examples of immunostaining of PV and GAD67 in mPFC and MC (bregma, 2.0 mm). Arrows denote example cells immunopositive for GAD67 but immunonegative for PV. (C and D) Quantitative analysis of the number of PV-positive and GAD67-positive neurons in PFC and MC. There was a significant reduction in the number of PV-positive neurons in the mPFC (**, P < 0.01), but not in the MC (P = 0.912), of BDNF-KIV, compared with littermate WT mice. There was no difference in GAD67-positive neurons both in the PFC (P = 0.524) and the MC (P = 0.281). n = 5 pairs of mice, each with 10 sections.
To assess the impact of the change in GABAergic interneurons on excitatory neurons, we performed whole-cell recordings of layer V pyramidal cells, based on their morphology and firing properties, in acute mPFC slices. Sequential depolarization steps induced an increase in the number of action potentials with adaptive properties characteristic of pyramidal neurons in both BDNF-KIV and WT mice (Fig. S4). Resting membrane potential, latency to action potential firing, as well as action potential threshold and amplitude were not different between genotypes (Table S1). Moreover, voltage-clamp recordings revealed no change in the amplitude and frequency of spontaneous excitatory postsynaptic currents (sEPSCs) (Fig. 4 A and B). We next analyzed the properties of spontaneous inhibitory postsynaptic currents (sIPSCs) in layer V pyramidal neurons. In contrast to sEPSCs, both amplitude and frequency of sIPSCs, which were recorded in the presence of the AMPA receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX) and NMDA receptor antagonist amino-5-phosphonovaleric acid (APV), were markedly reduced in all neurons from mutant mPFC slices (Fig. 4 C and D). The mean amplitude of sIPSCs was 33.4 ± 4.1 pA in BDNF-KIV neurons but 51.3 ± 5.6 pA in WT controls (P < 0.05, Fig. 4D Left). The mean sIPSC frequency in BDNF-KIV was reduced to about half of the WT value (WT: 19.0 ± 3.8 Hz; KIV: 8.9 ± 1.3 Hz; P < 0.05; Fig. 5D Right). Glutamate receptor function, as revealed by rise time and decay times, was not affected by genotype. These results, along with the immunohistochemistry data, suggest that ablation of promoter IV-driven BDNF expression alters the number of PV-positive interneurons, which could lead to the observed impairment of functional GABAergic inputs to layer V pyramidal neurons in the mPFC.
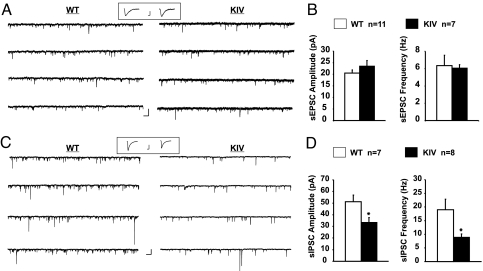
Fig. 4.
Impairment of GABAergic inhibition in mPFC layer V neurons in BDNF-KIV. (A and C) Sample voltage-clamp recordings of sEPSCs (A) and sIPSCs (C) in a layer V pyramidal neuron in an mPFC slice taken from a WT or BDNF-KIV animal. (Scale bars: 50 pA, 200 ms.) In C, recordings were performed in the presence of DNQX and APV. Representative average trace of (A) sEPSC (scale bars: 10 mV, 4 ms) or (C) sIPSC (scale bars: 20 mV, 4 ms) recorded from mPFC slices isolated from WT and BDNF-KIV mice (Inset). (B and D) Amplitude (Left) and frequency (Right) of sEPSCs (B) and sIPSCs (D) in layer V pyramidal neurons from BDNF-KIV slices, compared with those from WT littermates. WT and KIV exhibit similar sEPSC amplitude (P = 0.27) and frequency (P = 0.85).
Fig. 5.
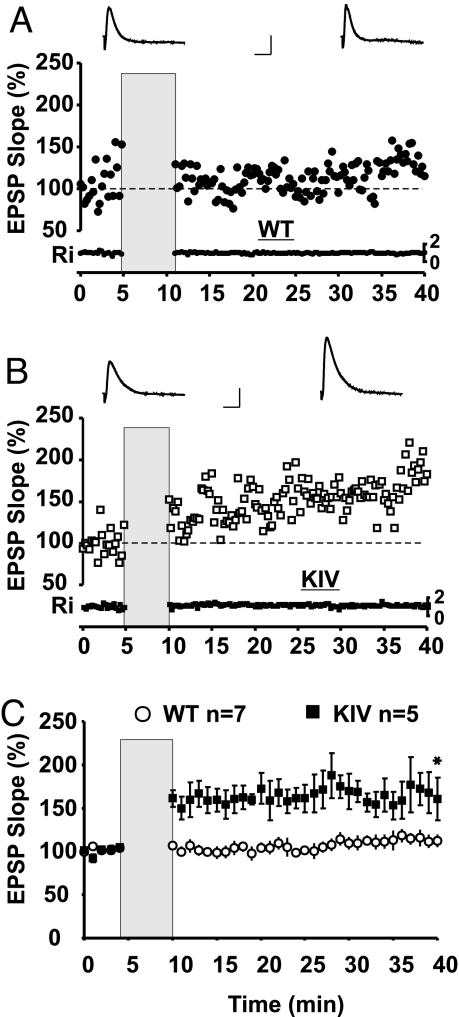
Impairment of STDP in mPFC layer V neurons in BDNF-KIV. (A and B) Example showing STDP in a layer V pyramidal neuron recorded from (A) a WT or (B) a BDNF-KIV mPFC slice. Sample traces before (red) and after (black) multiple pairings of presynaptic evoked EPSPs with postsynaptic spikes are shown above. Input resistance (Ri) was monitored in all experiments (shown below each plot). Gray box indicates the pairing period. (Scale bars: 5 mV, 20 ms.) (C) Summary plot depicting robust STDP in BDNF-KIV slices but not in WT littermates. *, Significant difference between WT and BDNF-KIV groups at that time point (40 min after pairing).
Deficits in Spike-Timing-Dependent Potentiation in mPFC.
GABAergic inhibition has been shown to suppress STDP in the mPFC, and this effect is thought to be important for mPFC-mediated cognitive function (8). To determine the functional consequence of impairment in GABAergic transmission in the mPFC of BDNF-KIV, we induced STDP by repeated pairings of extracellular stimulation of presynaptic glutamatergic input (i.e., layer II/III) with a single postsynaptic action potential evoked by current injection to layer V pyramidal neurons. In adult WT, STDP was not induced by this repetitive pairing protocol (Fig. 5A). Mean slope at 30 min after applying the pairing protocol was 112% ± 6% (Fig. 5C). However, in BDNF-KIV littermates, which exhibited a partial reduction in GABAergic transmission (Fig. 4D), pairing of presynaptic stimulation with subsequent postsynaptic action potential resulted in robust potentiation of synaptic strength that lasted longer than 30 min (mean slope 161% ± 24% of baseline; Fig. 5 B and C).
Because BDNF has been shown to enhance NMDA currents in cultured hippocampal neurons (24), we tested whether the unmasking of spike-timing-induced long-term potentiation was due to enhanced NMDA receptor function. When NMDA receptor-mediated synaptic currents were pharmacologically isolated in BDNF-KIV, the amplitude and current-voltage relationship of the evoked synaptic currents were not different from those evoked in WT (Fig. S5A). In addition, action potential waveform evoked by the pairing protocol was similar in both genotypes, indicating that the action potential amplitude and threshold were not altered by genetic elimination of promoter IV-mediated BDNF transcription (Fig. S5B). Furthermore, a reduction in GABAergic transmission by bicuculline also unmasked STDP in WT slices, mimicking the effect of promoter IV mutation (Fig. S5 C and D). Statistical analysis indicates a significant difference in STDP with (Fig. S5D) and without (Fig. 5D, open circles) bicuculline treatment in WT slices. Thus, promoter IV-mediated BDNF transcription, through regulation of GABAergic inhibition, elicits profound effects on STDP in the mPFC.
Discussion
Since the discovery that Bdnf expression is regulated by multiple promoters (10, 11), the most extensive efforts to characterize the mechanisms regulating Bdnf transcription have been directed toward promoter IV because of its significant contribution to activity-dependent transcription of Bdnf (1, 18). Despite extensive characterization of the molecular mechanisms underlying promoter IV-driven transcription, specific functions for BDNF derived from transcription of promoter IV remains elusive because of a lack of tools with which to selectively inhibit promoter IV-driven BDNF expression. By inserting a GFP-STOP cassette within the BDNF exon IV locus, we have generated a knockin line of mice in which promoter IV-driven Bdnf transcription is selectively blocked. Using this line of animals, we have made a number of interesting observations. We revealed changes in the number of GABAergic interneurons, particularly those expressing PV, in the mPFC of BDNF-KIV animals. We also showed a selective decrease in GABAergic but not glutamatergic synaptic transmission, leading to an abnormal appearance of STDP in layer V pyramidal neurons in the mPFC. Although it remains possible that BDNF regulates PV interneurons indirectly, our results underscore the importance of promoter IV-driven Bdnf expression in regulation of GABAergic transmission and cortical synaptic plasticity.
BDNF-KIV mice exhibited impairments in GABAergic transmission as well as STDP in the mPFC. These changes are most likely due to the deficits in PV interneurons, because a decrease in IPSCs cannot be caused by an increase in CR cells, and the small decrease in CB cell numbers is less likely to reduce IPSCs significantly. Several lines of evidence suggest that promoter IV-driven BDNF expression controls the development of PV interneurons, as opposed to eliciting rapid modulation of GABAergic synaptic transmission. Overexpression of BDNF in transgenic mice promotes PV interneuron maturation, resulting in precocious development of visual acuity and an earlier termination of the critical period in the visual cortex (6). Conversely, deletion of Bdnf in mice (BDNF−/−) results in a marked decrease in PV interneurons and a small decrease in CB interneurons in visual, barrel, and prefrontal cortices (25–27). Further, acute application of BDNF generally inhibits rather than enhances GABAergic transmission in various brain regions (28). Interestingly, we found that it is the promoter IV-driven expression of Bdnf, rather than BDNF per se, that is important for the development of PV interneurons. We also showed that disruption of promoter IV activity attenuated IPSCs. Thus, promoter IV-dependent Bdnf gene expression is more likely to facilitate formation of neuronal circuits by controlling development of GABAergic interneurons, with a minimal role in acute suppression of GABAergic transmission.
The reduction in PV-positive cell counts observed in BDNF-KIV mPFC could be due to a loss of PV interneurons or a decrease in PV expression. Several studies support the latter interpretation. BDNF−/− mice exhibit significantly lower numbers of PV-immunoreactive interneurons at postnatal day (P)15 but not at P28, suggesting a developmental delay rather than death of these neurons (25). If the PV-expressing cells in WT mice account for approximately half of the total GABAergic neurons, and they are reduced by one third in BDNF-KIV (Fig. 3C), there should be a one-sixth reduction of total GABAergic cells. The reduction in total GABAergic neurons in BDNF-KIV that we saw was small and did not reach statistically significant levels, suggesting that the difference in PV+ cells is due to decreased expression of PV. However, to firmly distinguish between the two possibilities, we would need an additional marker that specifically labels PV interneurons. A selective reduction in PV signal but not the other PV markers would suggest a selective decrease in PV expression. Kv3.1 is the only marker that has been reported as specific for rat PV interneurons. Unfortunately, antibodies against rat Kv3.1 did not work well in our hands to detect mouse PV interneurons.
Spike-timing-dependent plasticity gradually diminishes in mPFC as a result of developmental strengthening of GABAA receptor-mediated inhibition (8, 29). In young mPFC slices (P14–P23), pairing presynaptic stimulation of layer II/III with postsynaptic firing of a layer V pyramidal neuron results in robust STDP (8). In contrast, adult mPFC slices exhibit little STDP, but inhibition of GABAergic transmission by bicuculline can unmask it (Fig. S5D). Nicotine, which promotes PFC-mediated cognitive functions, increases the threshold for STDP by enhancing GABAergic transmission (8). We now show that a consequence of reduced sIPSCs in the mPFC of BDNF-KIV is to relieve the constraint imposed by GABA-mediated transmission to allow STDP. Thus, promoter IV-driven BDNF expression may influence PFC-mediated behaviors through GABAergic “gating” of STDP.
Promoter IV-driven BDNF expression is important for both long-term development of neuronal circuits as well as synaptic plasticity in the adult. Ongoing spontaneous neuronal activity during development is very likely a major factor that drives promoter IV-dependent BDNF expression. Consequently, lower “baseline levels” of BDNF could result from reduced BDNF expression driven by spontaneous activity. Thus, it is likely that blockade of promoter IV-driven Bdnf transcription may have both acute and chronic consequences. The impairment in PV interneuron development may result in reduced sIPSCs, because these phenotypes can be observed without acute enhancement of activity. Deficits in GABAergic transmission are likely to be the main reason for an abnormal appearance of STDP. Given that fear memory extinction may induce BDNF-IV transcription, it is conceivable that this acute expression of BDNF may also be involved in the memory extinction process. Future studies should address these possibilities. In summary, these results provide new insights into the role of BDNF promoter IV in GABAergic function and STDP in the PFC. The BDNF-KIV mice may be a useful model to study the function of activity-dependent BDNF expression in other brain regions. Finally, our findings may have implications in psychiatric diseases involving GABAergic dysfunction, such as schizophrenia and PTSD.
Materials and Methods
A mouse genomic bacterial artificial chromosome (BAC) library (Genome Systems) was screened by using a cDNA probe against the BDNF exon IV region. A 120-kb BAC clone (118F10) containing the promoter IV region was obtained. The clone was subcloned for the construct with BDNF promoter IV region with 5′-flanking region (3.3 kb) and 3′-flanking regions (4.7 kb). A GFP gene including multiple stop codons was inserted after exon IV. The GFP sequence is followed by a neomycin resistance cassette, which is driven by the PGK promoter in the reverse direction (Fig. 1A Upper). When integrated into the genome, promoter IV drives the expression of a GFP-BDNF fusion transcript, which in turn leads to translation of GFP instead of BDNF protein because of the stop codon in between (Fig. 1A Lower). The construct was transfected into 129/sv ES cells (R1) by electroporation and selected by Geneticin (Invitrogen) (G418) resistance. Several clones were selected after screening 200 ES colonies by Southern blotting with genomic DNA digested with EcoRV using 5′ and 3′ probes. The 5′ probe was a 421-bp fragment obtained by enzyme digestion with EcoRI and EcoRV, and the 3′ probe was generated by PCR with the following primer pairs: sense, 5′-CTTCAGAAAGTTATGGACCC-3′; and antisense, 5′-GTGAACCTTTGGGGAAAACT-3′ (Fig. 1B). After an EcoRV digestion, the 5′ probe detected a 4.8-kb band, and the 3′ probe detected a 7.8-kb band in ES cells that underwent homologous recombination, whereas both probes could detect a 9.9-kb fragment in the wild-type genomic DNA (Fig. 1B). Two ES cell clones with recombinant alleles were injected into surrogate female mice with blastocysts of C57BL/6J mice. The resulting chimeric mice were crossed with C57BL/6 to produce F1 mice. F1 mice were screened for germ-line transmission by PCR and confirmed by Southern blotting. The positive F1 mice were crossed to generate F2 homozygous for BDNF promoter IV knockin mice. Male heterozygous were backcrossed with C57BL/6 females for 5–7 generations. A PCR protocol was developed to distinguish between WT allele (563 bp) and the mutant allele (528 bp) with the following primer sets (Fig. 1C): WT, 5′-TGGAGCCCTCTCGTGGAC-3′ and 5′-CCTCTCCGGAGTGTGCCTAA-3′; and BDNF-KIV, 5′-TGGAGCCCTCTCGTGGAC-3′ and 5′-AAGCACTGCACGCCGTAGGTCA-3′. Amplification was carried out for 35 cycles, and each cycle was consisted of the following steps: 94 °C for 30 s, 62 °C for 30 s, and 72 °C for 30 s. Animals were kept in a temperature-controlled environment with a 12-h light/12-h dark cycle. All experiments were conducted according to National Institutes of Health animal use guidelines.
The size and shape of brains from BDNF-KIV appeared normal (Fig. S2A). Histological examination of BDNF-KIV brains by Nissl staining did not reveal gross morphological abnormality (Fig. S2B). Detailed examination of the Nissl-stained sections indicated that cytoarchitectural organization and cell density in the hippocampus and PFC (Fig. S2C) in BDNF-KIV were almost identical to those of WT.
See SI Methods for descriptions of other methods.
Supplementary Material
Acknowledgments.
We thank K. Nakazawa, K. Christian, P. Ernfors, and A. Morozov for thoughtful comments; D. Abebe, V. Senetorov, and S. Speransky for technical support; Q. Sun for assistance in immunohistochemistry; and P. Ernfors and S. Linnarsson for providing the genomic construct. This work is supported by the Intramural Research Programs of the National Institute of Child Health and Human Development and the National Institute of Mental Health. K.S. is supported in part by the Japan Society for the Promotion of Science, and N.H.W. is supported by fellowships from the Alberta Heritage Foundation for Medical Research, the Natural Sciences and Engineering Research Council, and the Canadian Institutes of Health Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811431106/DCSupplemental.
References
- 1.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 3.Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: From development to cognitive disorders. Neuroscientist. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- 4.Gorba T, Wahle P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur J Neurosci. 1999;11:1179–1190. doi: 10.1046/j.1460-9568.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 5.Aguado F, et al. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- 6.Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 7.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 8.Couey JJ, et al. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Liu QR, et al. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067:1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Pruunsild P, et al. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90:397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shieh PB, et al. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 13.Tao X, et al. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 14.Metsis M, Timmusk T, Arenas E, Persson H. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proc Natl Acad Sci USA. 1993;90:8802–8806. doi: 10.1073/pnas.90.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauterborn JC, et al. Differential effects of protein synthesis inhibition on the activity-dependent expression of BDNF transcripts: Evidence for immediate-early gene responses from specific promoters. J Neurosci. 1996;16:7428–7436. doi: 10.1523/JNEUROSCI.16-23-07428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanda S, Mack KJ. Multiple promoters direct stimulus and temporal specific expression of brain-derived neurotrophic factor in the somatosensory cortex. Brain Res Mol Brain Res. 1998;62:216–219. doi: 10.1016/s0169-328x(98)00242-3. [DOI] [PubMed] [Google Scholar]
- 17.Pattabiraman PP, et al. Neuronal activity regulates the developmental expression and subcellular localization of cortical BDNF mRNA isoforms in vivo. Mol Cell Neurosci. 2005;28:556–570. doi: 10.1016/j.mcn.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Greer PL, Greenberg ME. From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Huntley GW, Benson DL, Jones EG, Isackson PJ. Developmental expression of brain derived neurotrophic factor mRNA by neurons of fetal and adult monkey prefrontal cortex. Brain Res Dev Brain Res. 1992;70:53–63. doi: 10.1016/0165-3806(92)90103-4. [DOI] [PubMed] [Google Scholar]
- 20.Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: Effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci. 2001;14:135–144. doi: 10.1046/j.1460-9568.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- 21.Webster MJ, Weickert CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Res Dev Brain Res. 2002;139:139–150. doi: 10.1016/s0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]
- 22.Murray KD, Hayes VY, Gall CM, Isackson PJ. Attenuation of the seizure-induced expression of BDNF mRNA in adult rat brain by an inhibitor of calcium/calmodulin-dependent protein kinases. Eur J Neurosci. 1998;10:377–387. doi: 10.1046/j.1460-9568.1998.00019.x. [DOI] [PubMed] [Google Scholar]
- 23.Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- 24.Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto T, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itami C, Kimura F, Nakamura S. Brain-derived neurotrophic factor regulates the maturation of layer 4 fast-spiking cells after the second postnatal week in the developing barrel cortex. J Neurosci. 2007;27:2241–2252. doi: 10.1523/JNEUROSCI.3345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Saito H, Matsuki N. Inhibition of GABAa synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meredith RM, Floyer-Lea AM, Paulsen O. Maturation of long-term potentiation induction rules in rodent hippocampus: Role of GABAergic inhibition. J Neurosci. 2003;23:11142–11146. doi: 10.1523/JNEUROSCI.23-35-11142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.