Abstract
We compared the epidemiological markers of 13 Staphylococcus epidermidis strains isolated from an adult inpatient during a febrile episode and 23 S. epidermidis strains isolated during a presumptive outbreak of nosocomial infection in a neonatal ward. The total DNA restriction endonuclease analysis (REA) was processed along with the following conventional markers: biotyping, serotyping, phage typing, antibiotic susceptibility profiles, and plasmid profiles. The REA method was reproducible, giving stable results both in vitro and in vivo. For the hospitalized adult patient, the conventional markers of the 13 strains were concordant and the restriction profiles were identical. Five restriction groups were demonstrated during the course of the outbreak. Within two of the groups, the identities of all of the markers were used to verify whether all of the isolates belonged to the same cell clone. In a third group, combined analysis of the conventional markers and REA had to be used to demonstrate isolate similarity. On the other hand, in another group, none of the markers were similar; interpretation was not easy. An epidemiological study of S. epidermidis infections in hospitals must take into account all of the epidemiological markers: biotypes, serotypes, phage types, antibiograms, plasmid profiles, and REA.
Full text
PDF



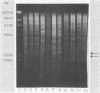
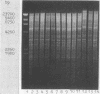
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Karchmer A. W., Vishniavsky N., Johnston J. L. Plasmid-pattern analysis for the differentiation of infecting from noninfecting Staphylococcus epidermidis. J Infect Dis. 1984 Jun;149(6):913–920. doi: 10.1093/infdis/149.6.913. [DOI] [PubMed] [Google Scholar]
- Archer G. L., Vishniavsky N., Stiver H. G. Plasmid pattern analysis of Staphylococcal epidermidis isolates from patients with prosthetic valve endocarditis. Infect Immun. 1982 Feb;35(2):627–632. doi: 10.1128/iai.35.2.627-632.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsotti O., Renaud F., Freney J., Benay G., Decoret D., Dumont J. Rapid isolation of DNA from Actinomyces. Ann Inst Pasteur Microbiol. 1987 Sep-Oct;138(5):529–536. doi: 10.1016/0769-2609(87)90038-x. [DOI] [PubMed] [Google Scholar]
- Bes M., Brun Y., Fleurette J. Nouveaux bactériophages de Staphylococcus epidermidis: évaluation de leur intérêt épidémiologique. Ann Microbiol (Paris) 1984 Sep-Oct;135B(2):165–176. [PubMed] [Google Scholar]
- Bjorvatn B., Lund V., Kristiansen B. E., Korsnes L., Spanne O., Lindqvist B. Applications of restriction endonuclease fingerprinting of chromosomal DNA of Neisseria meningitidis. J Clin Microbiol. 1984 Jun;19(6):763–765. doi: 10.1128/jcm.19.6.763-765.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury W. C., Murray R. G., Mancini C., Morris V. L. Bacterial chromosomal restriction endonuclease analysis of the homology of Bacteroides species. J Clin Microbiol. 1985 Jan;21(1):24–28. doi: 10.1128/jcm.21.1.24-28.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun Y., Fleurette J., Forey F. Micromethod for biochemical identification of coagulase-negative staphylococci. J Clin Microbiol. 1978 Nov;8(5):503–508. doi: 10.1128/jcm.8.5.503-508.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coagulase-negative staphylococci. J Med Microbiol. 1986 Dec;22(4):285–295. doi: 10.1099/00222615-22-4-285. [DOI] [PubMed] [Google Scholar]
- Collins D. M., De Lisle G. W. DNA restriction endonuclease analysis of Mycobacterium bovis and other members of the tuberculosis complex. J Clin Microbiol. 1985 Apr;21(4):562–564. doi: 10.1128/jcm.21.4.562-564.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D. M., De Lisle G. W., Gabric D. M. Geographic distribution of restriction types of Mycobacterium bovis isolates from brush-tailed possums (Trichosurus vulpecula) in New Zealand. J Hyg (Lond) 1986 Jun;96(3):431–438. doi: 10.1017/s0022172400066201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey R. C., Baldwin J. N. Relatedness of tetracycline resistance plasmids among species of coagulase-negative staphylococci. Antimicrob Agents Chemother. 1985 Feb;27(2):234–238. doi: 10.1128/aac.27.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne J., Brun Y., el Solh N., Delorme V., Mouren C., Bes M., Fleurette J. Characterization of clinically significant isolates of Staphylococcus epidermidis from patients with endocarditis. J Clin Microbiol. 1988 Apr;26(4):613–617. doi: 10.1128/jcm.26.4.613-617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Ruff E. A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabbaz R. F., Kaper J. B., Moody M. R., Schimpff S. C., Tenney J. H. Molecular epidemiology of group JK Corynebacterium on a cancer ward: lack of evidence for patient-to-patient transmission. J Infect Dis. 1986 Jul;154(1):95–99. doi: 10.1093/infdis/154.1.95. [DOI] [PubMed] [Google Scholar]
- Kristiansen B. E., Sørensen B., Bjorvatn B., Falk E. S., Fosse E., Bryn K., Frøholm L. O., Gaustad P., Bøvre K. An outbreak of group B meningococcal disease: tracing the causative strain of Neisseria meningitidis by DNA fingerprinting. J Clin Microbiol. 1986 Apr;23(4):764–767. doi: 10.1128/jcm.23.4.764-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen B. E., Sørensen B., Simonsen T., Spanne O., Lund V., Bjorvatn B. Isolates of Neisseria meningitidis from different sites in the same patient: phenotypic and genomic studies, with special reference to adherence, piliation, and DNA restriction endonuclease pattern. J Infect Dis. 1984 Sep;150(3):389–396. doi: 10.1093/infdis/150.3.389. [DOI] [PubMed] [Google Scholar]
- Kristiansen B. E., Sørensen B., Spanne O., Bjorvatn B. Restriction fingerprinting and serology in a small outbreak of B15 meningococcal disease among Norwegian soldiers. Scand J Infect Dis. 1985;17(1):19–24. doi: 10.3109/00365548509070415. [DOI] [PubMed] [Google Scholar]
- Kuijper E. J., Oudbier J. H., Stuifbergen W. N., Jansz A., Zanen H. C. Application of whole-cell DNA restriction endonuclease profiles to the epidemiology of Clostridium difficile-induced diarrhea. J Clin Microbiol. 1987 Apr;25(4):751–753. doi: 10.1128/jcm.25.4.751-753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Plorde J. J., Gordon K. P., Hargiss C., McClure J., Schoenknecht F. D., Condie F., Tenover F. C., Tompkins L. S. Instability of antibiotic resistance in a strain of Staphylococcus epidermidis isolated from an outbreak of prosthetic valve endocarditis. J Infect Dis. 1985 Jul;152(1):50–58. doi: 10.1093/infdis/152.1.50. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Jr, Lin F. Y., Morrison C. B., Gross R. J., Khabbaz R., Maher K. O., Rowe B., Israel E., Libonati J. P. Molecular epidemiology of neonatal meningitis due to Citrobacter diversus: a study of isolates from hospitals in Maryland. J Infect Dis. 1986 Sep;154(3):409–414. doi: 10.1093/infdis/154.3.409. [DOI] [PubMed] [Google Scholar]
- Parisi J. T., Hecht D. W. Plasmid profiles in epidemiologic studies of infections by Staphylococcus epidermidis. J Infect Dis. 1980 May;141(5):637–643. doi: 10.1093/infdis/141.5.637. [DOI] [PubMed] [Google Scholar]
- Patel R., Kvach J. T., Mounts P. Isolation and restriction endonuclease analysis of mycobacterial DNA. J Gen Microbiol. 1986 Feb;132(2):541–551. doi: 10.1099/00221287-132-2-541. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N., Mills S. D., Bradbury W. C. Application of serotyping and chromosomal restriction endonuclease digest analysis in investigating a laboratory-acquired case of Campylobacter jejuni enteritis. J Clin Microbiol. 1983 Dec;18(6):1427–1428. doi: 10.1128/jcm.18.6.1427-1428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillet J., Orta B. Recherche sur l'analyse sérologique des staphylocoques coagulase négatifs. Etude de deux sérotypes. Ann Inst Pasteur (Paris) 1970 Aug;119(2):193–205. [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987 Apr;25(4):675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjold S. A., Quie P. G., Fries L. A., Barnham M., Cleary P. P. DNA fingerprinting of Streptococcus zooepidemicus (Lancefield group C) as an aid to epidemiological study. J Infect Dis. 1987 Jun;155(6):1145–1150. doi: 10.1093/infdis/155.6.1145. [DOI] [PubMed] [Google Scholar]
- Thakker-Varia S., Jenssen W. D., Moon-McDermott L., Weinstein M. P., Dubin D. T. Molecular epidemiology of macrolides-lincosamides-streptogramin B resistance in Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987 May;31(5):735–743. doi: 10.1128/aac.31.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiermann A. B., Handsaker A. L., Moseley S. L., Kingscote B. New method for classification of leptospiral isolates belonging to serogroup pomona by restriction endonuclease analysis: serovar kennewicki. J Clin Microbiol. 1985 Apr;21(4):585–587. doi: 10.1128/jcm.21.4.585-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ketel R. J., ter Schegget J., Zanen H. C. Molecular epidemiology of Legionella pneumophila serogroup 1. J Clin Microbiol. 1984 Sep;20(3):362–364. doi: 10.1128/jcm.20.3.362-364.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]