Abstract
IL-23/IL-17-induced neutrophil recruitment plays a pivotal role in rheumatoid arthritis (RA). However, the mechanism of the neutrophil recruitment is obscure. Here we report that prostaglandin enhances the IL-23/IL-17-induced neutrophil migration in a murine model of RA by inhibiting IL-12 and IFN γ production. Methylated BSA (mBSA) and IL-23-induced neutrophil migration was inhibited by anti-IL-23 and anti-IL-17 antibodies, COX inhibitors, IL-12, or IFNγ but was enhanced by prostaglandin E2 (PGE2). IL-23-induced IL-17 production was increased by PGE2 and suppressed by COX-inhibition or IL-12. Furthermore, COX inhibition failed to reduce IL-23-induced neutrophil migration in IL-12- or IFNγ-deficient mice. IL-17-induced neutrophil migration was not affected by COX inhibitors, IL-12, or IFNγ but was inhibited by MK886 (a leukotriene synthesis inhibitor), anti-TNFα, anti-CXCL1, and anti-CXCL5 antibodies and by repertaxin (a CXCR1/2 antagonist). These treatments all inhibited mBSA- or IL-23-induced neutrophil migration. IL-17 induced neutrophil chemotaxis through a CXC chemokines-dependent pathway. Our results suggest that prostaglandin plays an important role in IL-23-induced neutrophil migration in arthritis by enhancing IL-17 synthesis and by inhibiting IL-12 and IFNγ production. We thus provide a mechanism for the pathogenic role of the IL-23/IL-17 axis in RA and also suggest an additional mechanism of action for nonsteroidal anti-inflammatory drugs.
Keywords: antigen-induced arthritis, chemokines, cytokines, rheumatoid arthritis, Th17
Rheumatoid arthritis (RA) is a debilitating chronic autoimmune disease with repeated acute episodes characterized by infiltration of leukocytes, particularly neutrophils, into the synovial and periarticular tissues. The recruited neutrophils contribute to the development of hyperplasia of the synovial tissue, pannus formation, and subsequent cartilage and bone destruction (1–3).
Over the past decade, IL-12-driven T-helper (Th)-1 cells, characterized by IFNγ production, have been implicated in the development of RA (4). However, it was demonstrated recently that mice lacking other components of the IL-12/IFNγ pathway (IL-12p35−/−, IFNγ−/−, IFNγR−/−) are highly susceptible to collagen-induced arthritis (CIA), indicating that these cytokines are not always required for disease induction (5–7). These apparently contradictory findings coupled with the discovery of IL-23, a cytokine that shares a p40 subunit with IL-12 and is distinguished by a p19 subunit, led to the re-evaluation of the relevance of the IL-12/IFNγ axis in RA pathogenesis (8).
The current concept is that the combination of TGF-β and IL-6 induces the differentiation of Th0 cells to Th17 cells (9). IL-23 in conjunction with IL-1 then contributes to the expansion and maintenance of Th17 cells that, once activated, release the cytokines IL-17A, IL-17F, IL-22, TNFα, and IL-6, all of which can induce inflammatory responses (9–12). Consistent with this notion, it was shown that IL-23 gene-targeted mice did not develop clinical signs of CIA and were completely resistant to the development of joint and bone disease, despite normal Th1 cell activation (5). Moreover, mice lacking IL-17 are more resistant to CIA induction and IL-17 is essential for the previously recognized proinflammatory effect of IL-1 in arthritis (13, 14). Clinically, synovial fluid of RA patients exhibits elevated numbers of IL-17-expressing cells and increased levels of cytokines IL-23 and IL-17 (15, 16).
Neutrophil migration induced by the IL-23/IL-17 axis has been implicated in the pathogenesis of RA (17). However, the precise mechanism how the IL-23/IL-17 axis induces neutrophil migration in the articular context is unknown. We have reported previously that intra-articular (tibiofemoral joint) injection of antigen (methylated BSA, mBSA) induced a significant neutrophil migration to the articular cavity in immunized mice compared with non-immunized mice (18). Lipid mediators such as prostaglandins, particularly prostaglandin E2 (PGE2), are commonly found in the synovial fluid of RA patients and clearly are involved in the tissue inflammation observed in these subjects (19, 20). Therefore, we investigated the potential association of PGE2 and IL-23/IL-17 in the neutrophil recruitment observed in the antigen-induced arthritis (AIA). We report here that prostaglandin mediates IL-23-induced neutrophil migration in AIA by enhancing IL-17 synthesis via the down-regulation of IL-12 and IFNγ production. Our study therefore provides a mechanism by which the IL-23/IL-17 axis induces neutrophil migration and hence pathogenesis of articular inflammation and also reveals a pathway of action of nonsteroidal anti-inflammatory drugs.
Results
The IL-23/IL-17 Axis Is Essential for the Neutrophil Migration in Antigen-Induced Arthritis.
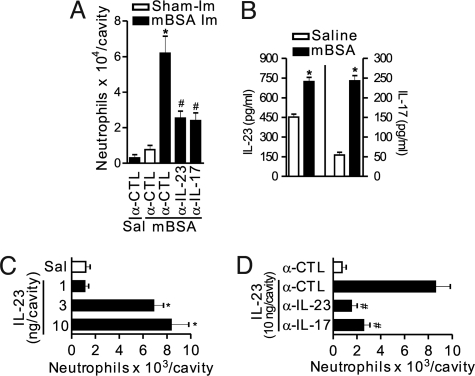
Intra-articular (tibiofemoral joint) injection of mBSA in mBSA-immunized mice induced a significant neutrophil migration to the articular cavity compared with non-immunized mice. The migration peaked 24 h after mBSA challenge (10 μg/articular cavity) (18). mBSA-induced neutrophil recruitment was significantly inhibited by anti-IL-23 or anti-IL-17 antibodies (Fig. 1A). Moreover, elevated levels of IL-23 and IL-17 were found in the knee joints 3 h after mBSA challenge (Fig. 1B). These results indicate a key role of IL-23 and IL-17 in neutrophil recruitment to the knee joints induced by specific antigen challenge.
Fig. 1.
The IL-23/IL-17 axis is essential for the neutrophil migration in antigen-induced arthritis. (A) Neutrophils harvested from articular cavity 24 h after intra-articular injection of mBSA (10 μg/cavity) or its vehicle (saline, Sal) in immunized (mBSA Im, closed bars) or sham-immunized (Sham-Im, open bars) mice treated with a co-injection of IgG control (α-CTL), α-IL-23 (700 ng/cavity), or α-IL-17 (700 ng/cavity) antibodies. (B) The levels of IL-23 and IL-17 in joint homogenate were determined 3 h after challenge with saline or mBSA (10 μg/cavity) in immunized mice. (C and D) Neutrophils harvested from articular cavity 6 h after intra-articular injection of IL-23 (1–10 ng/cavity, closed bars) or saline (Sal, open bar) in mice treated with a co-injection of IgG control (α-CTL), anti-IL-23 (5 μg/cavity), or anti-IL-17 (10 μg/cavity) antibodies. *P < 0.05 vs. saline control group; #P < 0.05 vs. IL-23 or mBSA (immunized) groups. Data are mean ± SEM, n = 5, representative of 3 experiments.
Because the effector functions of IL-23 in the pathogenesis of RA depend on the induction of IL-17 production (21), we examined the role of IL-17 in IL-23-induced neutrophil migration. IL-23 induced a dose-dependent neutrophil migration after intra-articular injection compared with control (intra-articular injection of the vehicle, saline). The maximal response was observed with 10 ng/articular cavity 6 h after IL-23 administration (Fig. 1C) and was inhibited by treatment with anti-IL-23 or anti-IL-17 antibodies (Fig. 1D).
We also investigated the role of IL-23 in neutrophil migration to the peritoneal cavity, a convenient model for assessing cell migration. Neutrophil migration to the peritoneal cavity was induced by IL-23 injected i.p. in a dose-dependent manner. The peak response was observed with 10 ng/peritoneal cavity at 4 h (Fig. S1A) and also was inhibited by anti-IL-23 or anti-IL-17 antibodies (Fig. S1B).
IL-23-Induced Neutrophil Migration Is Prostanoid Dependent.
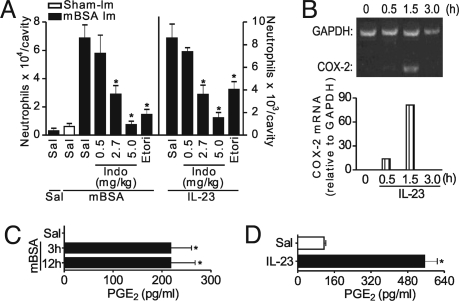
Prostaglandins are found commonly in synovial fluid of RA patients and are clearly involved in the tissue inflammation observed in these individuals (19, 20). Thus, we investigated the potential role of prostaglandins in the neutrophil recruitment observed in AIA. Neutrophil migration induced by mBSA challenge in mBSA-immunized mice was markedly attenuated when the mice were treated with either indomethacin (a nonselective COX inhibitor), dose dependently, or etoricoxib (a COX-2 selective inhibitor) (Fig. 2A). Furthermore, IL-23-induced neutrophil migration to articular and peritoneal cavities also was inhibited by indomethacin (dose dependently) or etoricoxib (Figs. 2A and S1C). The effect of indomethacin was examined on another cytokine, IL-15, which has a significant role in the manifestation of RA and induces neutrophil migration and IL-17 production (22). However, indomethacin treatment did not affect the neutrophil migration induced by either IL-15 or N-formylmethionyl-leucyl-phenylalanine, a nonspecific flogistic stimulus (data not shown), indicating that prostanoids are specifically involved in IL-23-induced neutrophil migration. Notably the range of indomethacin dose that inhibited mBSA- or IL-23-induced neutrophil migration corresponds to the range that inhibits prostanoid production in vivo (23). IL-23 also triggered a significant increase in COX-2 mRNA expression in peritoneal cells 30 min after IL-23 injection. The expression peaked at 1.5 h and returned to baseline levels after 3 h (Fig. 2B). Moreover, elevated levels of PGE2 were found in the knee joints 3 and 12 h after mBSA challenge (Fig. 2C) and in supernatants of cultured lymph node cells stimulated with IL-23 for 36 h (Fig. 2D).
Fig. 2.
IL-23-induced neutrophil migration in AIA is prostanoid dependent. (A) Neutrophils harvested from articular cavity 6 h after intra-articular injection of IL-23 (closed bars in the right side of the panel) in naïve mice or 24 h after intra-articular injection of mBSA (10 μg/cavity) or saline (Sal) in immunized (mBSA Im, closed bars) mice or mBSA in sham-immunized (Sham-Im, open bar) mice treated 30 min before with indomethacin (Indo, 0.5, 2.7, or 5 mg/kg, s.c.) or etoricoxib (Etori, 45 mg/kg, s.c.) or not treated. (B) Expression of COX-2 mRNA in peritoneal cells harvested 0.5, 1.5, and 3 h after IL-23 (10 ng/cavity) injection into mouse peritoneal cavity. (C) The levels of PGE2 in joint homogenate were determined 3 and 12 h after challenge with saline (Sal) or mBSA (10 μg/cavity) in immunized mice. (D) The levels of PGE2 in cultured lymph node cells stimulated with IL-23 (100 ng/ml) or saline (Sal, IL-23 diluent) for 36 h. *P < 0.05 vs. IL-23 or mBSA (immunized) groups. Data are mean ± SEM, n = 5, representative of 3 experiments.
PGE2 Enhances Neutrophil Migration by Inhibiting the IL-12/IFNγ Pathway.
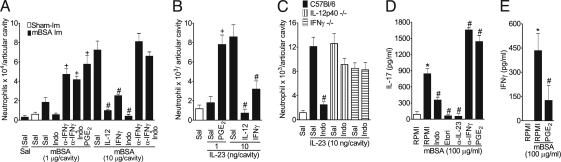
We then investigated the mechanisms by which prostanoids mediate IL-23-induced neutrophil migration. Intra-articular co-administration of low doses of mBSA (1 μg) or IL-23 (1 ng) with PGE2 induced a significant neutrophil migration into the knee joint at levels comparable to the maximal response to mBSA (10 μg) or IL-23 (10 ng) (Fig. 3 A and B), suggesting that PGE2 can enhance the IL-23-induced neutrophil migration observed in AIA. Moreover, consistent with previous reports that the IL-12/IFNγ axis can antagonize Th17-induced events (24, 25), we found that IL-12 and IFNγ inhibited neutrophil migration induced by high doses of mBSA or IL-23 (Fig. 3 A and B). The doses of IL-12 or IFNγ used alone were not able to induce significant neutrophil migration (data not shown). Confirming the inhibitory effect of the IL-12/IFN-γ axis on IL-23/IL-17-induced neutrophil migration, we showed that anti-IFNγ antibody treatment exacerbated the neutrophil migration induced by the low dose of mBSA, an effect that was not limited by indomethacin treatment. Indomethacin treatment also did not affect the neutrophil migration induced by either the high dose of mBSA in mice treated with anti-IFNγ antibody (Fig. 3A) or the high dose of IL-23 in IL-12−/− or IFNγ−/− mice (Fig. 3C). Moreover, indomethacin, etoricoxib, and anti-IL-23 inhibited IL-17 production, whereas anti-IFNγ and PGE2 exacerbated IL-17 production. Furthermore, PGE2 inhibited the production of IFNγ by lymph node cells from immunized mice when cultured with mBSA in vitro (Fig. 3 D and E).
Fig. 3.
PGE2 enhances IL-23-induced neutrophil recruitment by increasing IL-17 synthesis via suppressing the IL-12/IFNγ axis. (A) Neutrophils harvested from the articular cavity 24 h after intra-articular injection of mBSA (1 or 10 μg/cavity) or saline (Sal) in immunized (mBSA-Im, closed bars) or mBSA (10 μg/cavity) in sham-immunized (Sham-Im, open bar) mice treated with a co-injection of PGE2 (30 pg/cavity), IL-12 (10 pg/cavity), IFNγ (100 pg/cavity), or α-IFNγ antibody (700 ng/cavity). Some mice were pretreated 30 min earlier with indomethacin (Indo, 5 mg/kg, s.c.), as indicated. (B and C) Neutrophils harvested from articular cavity 6 h after intra-articular injection (1 or 10 ng/cavity) or saline (open bar) in wild-type, IL-12p40−/−, or IFNγ−/− mice. Some mice were treated with a co-injection of PGE2 (1 pg/cavity), IL-12 (0.1 ng/cavity), or IFNγ (1 ng/cavity) or were pretreated with indomethacin (Indo, 5 mg/kg, s.c. 30 min earlier). (D and E) Lymph node cells harvested from immunized mice were cultured with or without mBSA (100 μg/ml), indomethacin (Indo, 50 μg/ml), etoricoxib (Etori, 50 μg/ml), PGE2 (1 μM), α-IFNγ (10 μg/ml), or α-IL-23 (100 ng/ml) for 36 h. IL-17 and IFNγ concentrations in culture supernatants were determined by ELISA. *P < 0.05 vs. medium (RPMI) controls; #P < 0.05 vs. mBSA (10 μg/cavity; immunized), mBSA (100 μg/ml), IL-23 (10 ng/cavity) or IL-23 (100 ng/ml); +P < 0.05 vs. IL-23 (1 ng/cavity) or mBSA (1 μg/cavity; immunized). Data are mean ± SEM, n = 5, representative of at least 2 experiments.
Extending these observations, we also demonstrated that the i.p. co-administration of low doses of IL-23 (1 ng) and PGE2 induced significant neutrophil migration to the peritoneal cavity compared with the injection of these mediators alone. Moreover, IL-12 inhibited the neutrophil migration induced by co-administration of PGE2 and IL-23. Furthermore, IL-12 or IFNγ inhibited the neutrophil migration induced by an effective high dose of IL-23 (Fig. S2A). In agreement with these observation, IL-23-induced neutrophil migration into the peritoneal cavity was not inhibited by indomethacin in IL-12−/− or IFNγ−/− mice (Fig. S2B). Finally, we showed that although indomethacin and IL-12 inhibited IL-17 production by IL-23-stimulated peritoneal cells, PGE2 enhanced IL-17 and inhibited IFNγ production (Fig. S2 C and D; see method in SI Text). Together, these results therefore demonstrate that PGE2 potentiates IL-23-induced neutrophil migration by enhancing IL-17 production through the inhibition of the IL-12/IFNγ pathway.
IL-17-Mediated Neutrophil Migration Depends on TNFα, Leukotrienes, and CXC Chemokines.
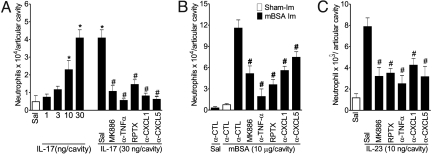
Next we addressed the mechanisms involved in IL-17-induced neutrophil migration to the articular cavity. Intra-articular injection of IL-17 induced a dose-dependent neutrophil migration peaking at 30 ng/articular cavity 6 h after IL-17 administration (Fig. 4A). IL-17-induced neutrophil recruitment was not inhibited by indomethacin, etoricoxib, IL-12, or IFNγ treatment (Fig. S3), suggesting that PGE2, IL-12, and IFNγ regulate IL-23-induced neutrophil migration upstream of IL-17 production. However, the neutrophil migration induced by IL-17, mBSA, or IL-23 was inhibited by treatment of the mice with MK886, anti-TNFα, repertaxin (RPTX), anti-CXCL1, or anti-CXCL5 (Fig. 4). These results indicate that TNFα, leukotrienes, and CXC chemokines act downstream of IL-17 mediating mBSA-induced neutrophils.
Fig. 4.
IL-17 mediates neutrophil migration via TNFα, leukotrienes, and CXC chemokines (CXCL1 and CXCL-5). (A and C) Neutrophils harvested from articular cavity 6 h after intra-articular injection of IL-17 (1–30 ng/joint), IL-23 (10 ng/cavity), or saline (Sal, open bars) in mice treated with a co-injection of α-TNFα serum (5 μl/cavity), α-CXCL1 (700 ng/cavity), or α-CXCL5 (700 ng/cavity) antibodies. Some mice also were treated 30 min before with repertaxin (RPTX, 30 mg/kg, s.c.) or 1 h before with MK886 (1 mg/kg, by gavage). (B) Neutrophils harvested from articular cavity 24 h after intra-articular injection of mBSA (10 μg/cavity) or saline in immunized mice (mBSA Im, closed bars) or mBSA (10 μg/cavity) in sham-immunized (Sham-Im, open bar) mice treated with a co-injection of IgG control (α-CTL), α-TNFα serum (5 μl/cavity), α-CXCL1 (700 ng/cavity), or α-CXCL5 (700 ng/cavity) antibodies or 1 h before with MK886 (1 mg/kg, by gavage). *P < 0.05 vs. saline control; #P < 0.05 vs. IL-23, IL-17, or mBSA (immunized) groups. Data are mean ± SEM, n = 5, representative of 3 experiments.
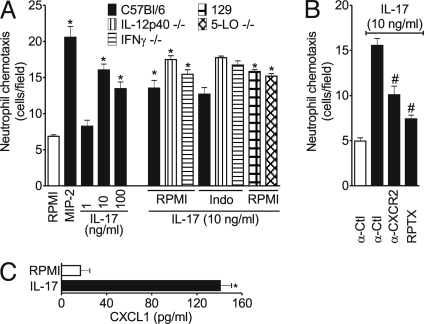
To strengthen this conclusion further, we performed neutrophil chemotaxis in vitro. Fig. 5 shows that IL-17 induced neutrophil chemotaxis in a dose-dependent manner and was not affected by the presence of indomethacin. Furthermore, IL-17 induced chemotaxis of neutrophils from IL-12p40−/−, IFNγ−/−, or 5-LO−/− mice, and the chemotaxis also was not affected by indomethacin (Fig. 5A). In contrast, the IL-17-induced neutrophil chemotaxis was inhibited significantly by anti-CXCR2 antibody or repertaxin (Fig. 5B). In addition, IL-17 induced CXCL1 production by neutrophils in vitro (Fig. 5C). Thus, IL-17 induces the production of CXC chemokines, which act in an autocrine manner to induce neutrophil chemotaxis.
Fig. 5.
IL-17-mediated neutrophil chemotaxis depends on CXC chemokines released by these cells. (A) Neutrophils were harvested from bone marrow of mice (IL-12p40−/−, IFNγ−/− and their control C57BL/6, or 5-LO−/− and their control 129S1/SvImJ [129]), treated for 30 min with indomethacin (Indo, 50 μg/ml). Chemotaxis was determined in a microwell chamber initiated by stimulation for 1 h with IL-17 (1–100 ng/ml), MIP-2 (positive control, 20 ng/ml) or RPMI (vehicle). (B) Neutrophils were harvested from bone marrow of C57BL/6 mice and treated for 30 min with IgG control (α-Ctl, 30 μg/ml), anti-CXCR2 antibodies (30 μg/ml), or repertaxin (RPTX, 30 μg/ml). Chemotaxis was determined in a microwell chamber initiated by stimulation for 1 h with IL-17 (50 ng/ml) or RPMI (vehicle). (C) The levels of CXCL1 in culture supernatants of neutrophils harvested from bone marrow of C57BL/6 mice stimulated or not with IL-17 (50 ng/ml) for 1 h. *P < 0.05 vs. RPMI; #P < 0.05 vs. IL-17. Data are mean ± SEM, representative of at least 3 independent experiments. Data presented in the RPMI control group are representative for the basal migration of all mouse strains.
Discussion
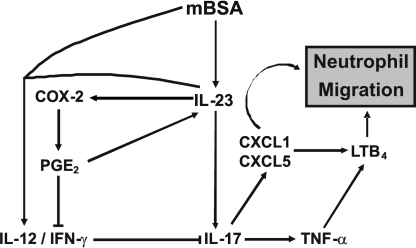
Tissue destruction and bone erosion in RA is mediated, at least in part, by proteolytic enzymes and free radicals released by infiltrating neutrophils into the joints during repeated acute episodes (1–3, 26). The role of IL-23 and IL-17 in the recruitment of neutrophils leading to the pathogenesis of RA is supported by substantial experimental and clinical evidence (5, 14). However, the underlying mechanism for IL-23/IL-17-mediated neutrophil migration was unknown. In the present study, we demonstrate that prostanoids are key mediators of the IL-23/IL-17 axis-induced neutrophil recruitment. In mBSA-induced neutrophil migration in immunized mice, we propose that IL-23 plays a pivotal role in mediating this inflammatory event through 2 interdependent mechanisms:(i) induction of the chemotactic IL-17, which mediates the production of other chemotactic mediators (CXC chemokines, TNFα, and leukotriene B4 [LTB4]) and(ii) indirectly, by suppressing the IL-12/IFNγ pathway, via the production of COX-2-derived prostaglandins and hence further enhancing IL-17 synthesis (Fig. 6).
Fig. 6.
Schematic representation of IL-23-induced neutrophil migration in antigen (mBSA)-induced arthritis. Lines terminating in blunt ends indicate inhibition. During antigen-induced neutrophil migration to the joints, IL-23 is released early and, together with several cytokines, stimulates IL-17 production that induces the release of TNFα, the CXC chemokines (CXCL1 and CXCL5), and LTB4, which together contribute to neutrophil recruitment by inducing locomotion and the expression of adhesion molecules. IL-23 elicited during antigen challenge also can induce COX2 expression leading to the production of PGE2, which contributes to neutrophil recruitment by enhancing the IL-23-induced production of IL-17 through the impairment of the IL-12/IFNγ axis. PGE also can induce IL-23 production via dendritic cells (40), thus providing an IL-23-COX2-PGE-IL-23 amplification circuit, perpetuating neutrophil migration and inflammation.
Reinforcing the relevance of the IL-23/IL-17 axis for RA pathogenesis, we demonstrated that IL-23 is pivotal to neutrophil recruitment to the joint cavity in mBSA-induced arthritis through the induction of IL-17 release. Confirming the involvement of IL-17 in the chemotactic activity of IL-23, IL-23-induced neutrophil migration in both joint and peritoneal cavities depends on IL-17 production. The mBSA experimental model of arthritis in mice exhibits a delayed-type hypersensitivity response with histopathological features akin to those observed in human RA (27). Generalizing our findings, we investigated the ability of IL-23 to induce neutrophil migration into the peritoneal cavity, which is a convenient experimental model to address the mechanisms involved in the multiple steps responsible for neutrophil migration induced by a range of stimuli (28, 29).
Prostaglandins, particularly PGE2, as well as COX-2 and COX-1 (enzymes involved in prostaglandin synthesis) are found in the synovial tissue of RA patients and experimental models of RA (30–32). Although some in vitro studies suggest an anti-inflammatory role for PGE2 (33), most reports implicate PGE2 as an important pro-inflammatory agent in RA (34–36). It was previously shown that PGE2 exacerbates CIA as a result of increased IL-23 and IL-6 expression by dendritic cells (37). We now demonstrate that locally produced COX-2-derived prostaglandins are essential for mBSA- and IL-23-induced IL-17 production and neutrophil recruitment into joints and peritoneal cavities. Inhibition of COX-2 blocked, whereas exogenous PGE2 enhanced, mBSA- or IL-23-induced neutrophil migration. Moreover, we observed that IL-23 is able to increase COX-2 mRNA and PGE2 production. Our results are consistent with a recent report that IL-1, a cytokine that plays a pivotal role in RA development (13, 38), is required for the synergistic effect of PGE2 and IL-23 in promoting human Th17 expansion and the subsequent IL-17 release (39).
IL-12 and IFNγ block the polarization of Th17 cells in experimental RA (5–7, 24). Here we demonstrate that IL-12 and IFNγ inhibit neutrophil migration induced by either mBSA or IL-23 and that IL-12 limits IL-23-induced IL-17 production. Thus, it is possible that the source of IL-17 that mediates the mBSA-induced neutrophil migration is Th17 cells. However, we could not discard gamma-delta T cells, because this subset of T cells also is an important source of IL-17 and is associated with the exacerbation of CIA (40). We examined whether the effect of COX-2-derived prostaglandins in IL-23-mediated neutrophil migration is caused by the inhibition of IL-12/IFNγ production and, as a consequence, abrogation of the inhibitory effect of the IL-12/IFNγ-axis on IL-17 production. COX inhibitors were ineffective in reducing mBSA-induced neutrophil migration to the articular cavity of mice treated with anti-IFNγ antibodies. Similarly, these COX inhibitors had no effect on the IL-23-induced neutrophil migration into joints and peritoneal cavities in IL-12- or IFNγ-deficient mice. Moreover, the ability of PGE2 to enhance IL-23-induced neutrophil migration was prevented by exogenous administration of IL-12. These results identify another important role for PGE2 in the context of RA pathogenesis as an autocrine mediator in amplifying and perpetuating chronic RA. We did not identify the PGE2 receptor that operates this PGE2 effect. However, it is possible that EP2/EP4 signaling participates, because these receptors mediate the ability of PGE2 to enhance the release IL-17 (41).
Recent studies demonstrated that IL-17 induces the release by joint structural and resident cells (e.g., chondrocytes, synovial fibroblasts, and macrophages) of several inflammatory mediators, including cytokines (e.g., TNFα) and chemokines (e.g., CXCL1 and CXCL5), which may recruit neutrophils (42, 43). Here we identify the mediators downstream of IL-17 participating in the neutrophil migration to the joint cavities in AIA. We demonstrate that IL-17 induces a dose-dependent neutrophil migration into the tibiofemoral joints of mice. The neutrophil migration thus induced is not affected by COX inhibitors (indomethacin and etoricoxib) or by IL-12 or IFNγ. These results confirm that prostaglandin and the IL-12/IFNγ axis interferes with mBSA-induced neutrophil migration upstream of the IL-17 effect. We then demonstrate that IL-17-induced neutrophil migration to the knee joint is dependent on leukotrienes, TNFα, CXCL1, and CXCL5.
Neutrophil migration in vitro induced by a chemotactic mediator depends mainly on the concentration gradient, whereas neutrophil migration in vivo is complex and requires, besides the concentration gradient, the activation of endothelial cells and the consequent expression of adhesion molecules (44). Therefore, neutrophil migration induced by IL-17 may not be totally via release of CXC chemokines, TNFα, and LTB4 but also could depend on a direct effect of IL-17 on neutrophils. Here we demonstrate that IL-17 can induce neutrophil chemotaxis in vitro. However, this event is dependent on the release of CXC chemokines, suggesting that in vitro IL-17 stimulates the release of CXC chemokines by neutrophils that, acting through an autocrine manner, induce neutrophil chemotaxis.
Together, our results demonstrate that during antigen-induced neutrophil migration to the joints, IL-23 is released early and stimulates IL-17 production. IL-17 production induces the release of TNFα, the CXC chemokines (CXCL1 and CXCL5), and LTB4, which together contribute to neutrophil recruitment by inducing locomotion and the expression of adhesion molecules (Fig. 6). IL-23 elicited during antigen challenge also can induce COX2 expression leading to the production of PGE2, which contributes to neutrophil recruitment by enhancing the production of IL-23-induced IL-17 through the impairment of the IL-12/IFNγ axis. These findings reveal a mechanism of nonsteroidal anti-inflammatory drugs used for RA treatment and suggest the possible use of such drugs in other diseases in which neutrophil migration along the IL-23/IL-17 axis is involved.
Materials and Methods
Animals and Reagents.
Male and female BALB/c, C57BL/6, and 129S1/SvImJ wild types and IL-12p40 (C57BL/6)-, IFNγ (C57BL/6)- and 5-Lipoxigenase (5-LO, 129-Alox5tm1Fun)-deficient mice weighing 18–22 g were used. The mice were housed in the animal facility of the School of Medicine of Ribeirão Preto, Brazil and received water and food ad libitum. The study protocol was approved by the local Animal Ethics Committee. The following materials were obtained from the sources indicated: IL-23, IL-17, IL-12, IFNγ, MIP-2, anti-IL-23, anti-IL-17, anti-IFNγ, anti-CXCL1, anti-CXCL5, anti-CXCR2, and IgG control from R&D Systems; mBSA, Freund's adjuvant, PGE2, and indomethacin from Sigma-Aldrich; etoricoxib from Merck Sharp & Dohme; sheep anti-TNFα serum (H92/B5) was a gift from Dr. S. Poole (National Institute for Biological Standards and Control, London, United Kingdom).
Induction of Experimental Arthritis.
BALB/c mice were sensitized (s.c.) with 500 μg of mBSA in an emulsion containing 0.1 ml PBS and 0.1 ml complete Freund's adjuvant. Booster injections of mBSA in incomplete Freund's adjuvant were given 7 and 14 d after the first immunization. Non-immunized (sham) mice were given similar injections but without mBSA. Arthritis was induced in the immunized mice 21 d after the initial injection by intra-articular injection of mBSA (10 μg/cavity), and the neutrophil migration was evaluated as described in the next section.
In Vivo Neutrophil Migration.
Neutrophil migration was assessed 4 h after i.p. injection of IL-23 (3–30 ng/cavity) or 6 h after intra-articular injection of IL-23 (1–10 ng/cavity) and IL-17 (1–30 ng/cavity) in naive BALB/c, C57BL/6, IL-12p40−/−, and IFNγ−/− mice or 24 h after intra-articular mBSA challenge (1 or 10 μg/cavity) in immunized or sham-immunized BALB/c mice. These mice were treated with co-injections of 1 of the following: IL-12, IFNγ, anti-TNFα serum, anti-IL-23, anti-IL-17, anti-IFNγ, anti-CXCL1, anti-CXCL5 30 min before injection indomethacin (a COX inhibitor, s.c.), etoricoxib (a COX-2 inhibitor, s.c.), or repertaxin (an allosteric CXCR1/2 antagonist, s.c) or with MK886 (a 5-lipoxygenase-activating protein inhibitor, gavage) 1 h before injection. The doses used are indicated in the figure legends. At the end of the experiment, mice were killed, and the cells present in the peritoneal or articular cavities were harvested by washing cavities with PBS/EDTA. Total and differential cell counts were performed, and the results were presented as the number (mean ± SEM) of neutrophils per cavity.
Culture of Lymph Node Cells and Neutrophils.
Popliteal and inguinal lymph node cells (5 × 105 cells/well) in 250 μl of RPMI-1640 were incubated in a 96-well plates for 36 h in the presence or absence of mBSA (100 μg/ml), IL-23 (100 ng/ml), indomethacin (50 μg/ml), PGE2 (1 μM), etoricoxib (50 μg/ml), anti-IL-23 antibodies (100 ng/ml), or anti-IFNγ antibodies (10 μg/ml). The culture supernatants then were harvested, and IL-17, IFNγ, and PGE2 concentrations were measured. Bone marrow neutrophils (1 × 106 cells/well), purified as described previously (45), were incubated in 250 μl of RPMI in 96-well plates for 1 h in the presence or absence of IL-17 (50 ng/ml). The CXCL1 concentrations were measured in supernatants.
Joint Homogenates.
The knee joints were dissected out, frozen with liquid nitrogen, crushed in a mortar and pestle, and then solubilized in PBS. The homogenates then were centrifuged at 10,000 × g for 10 min, and the supernatants were used for detection of IL-23, IL-17, PGE2, and CXCL1.
Measurement of IL-23, IL-17, IFNγ, CXCL1, and PGE2.
IL-23, IL-17, IFNγ, and CXCL1 concentrations were assessed by ELISA using paired antibodies (detection limits, 11 pg/ml, R&D Systems). The concentrations of PGE2 were assessed by RIA (Amersham Biosciences).
RT-PCR Assays.
COX-2 mRNA assay was performed by RT-PCR as previously described (29). Briefly, mice were killed 0.5, 1.5, or 3 h after IL-23 i.p. injection, and the total peritoneal cells were harvested. Total cellular RNA from peritoneal cells was extracted using the TRIzol reagent (Gibco BRLLife Technologies) according to the directions of the manufacturer. The primers used were COX-2 (sense: 5′-AGC CTT CTC CAA CCT CTC CTA-3′; antisense: 5′-CAC CTC TCC ACC AAT GAC CT3′), and GAPDH (sense: 5′-GCC ATC AAC GAC CCC TTC ATT G-3′; anti-sense: 5′-TGC CAG TGA GCT TCC CGT TC-3′). The expression of GAPDH mRNA was used as loading control in all samples. Densitometry analysis of scanned images was carried out using the Gel Pro-Analyzer 3.1 (MediaCybernetics) image analysis software. The integrated optical density (IOD) was determined for each labeled band, and the data were expressed as the ratio of the IOD of COX-2 and GAPDH.
Neutrophil Chemotaxis.
Bone marrow neutrophils were purified as previously described (45), and the chemotaxis was performed using a 48-well microchamber (Neuro Probe). The stimuli and negative control were added to the lower chambers. A 5-μm pore polycarbonate membrane (Neuro Probe) was placed between the upper and lower chambers, and 5 × 104 cells previously treated for 30 min with indomethacin (50 μg/ml), anti-CXCR2 antibodies (30 μg/ml), or repertaxin (30 μM) or not treated were added to the top chambers. Cells were allowed to migrate into the membrane for 1 h at 37 °C, 5% CO2. Following incubation, the membrane was washed in PBS, fixed in methanol, and stained using the Diff-Quik system (Dade Behring). Each well-associated membrane area was scored using light microscopy to count the intact cells present in 5 random fields. The results are expressed as the number of neutrophils per field.
Statistical Analysis.
Data are presented as means ± SEM and are representative of 2 or 3 separate experiments. The means from different treatments were compared by ANOVA with Tukey's correction. Statistical significance was set at P < 0.05.
Supplementary Material
Acknowledgments.
We thank Giuliana Bertozi, Fabíola Mestriner, Ana Kátia dos Santos, Diva M. A. Souza, and Ingrid F. Metzger for their excellent technical assistance. This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo, Pronex, Fundação de Amparo à Pesquisa do Estado do Amazonas, Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior and Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil), The Wellcome Trust, and the Medical Research Council (United Kingdom).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812782106/DCSupplemental.
References
- 1.Liew FY, McInnes IB. A fork in the pathway to inflammation and arthritis. Nat Med. 2005;11(6):601–602. doi: 10.1038/nm0605-601. [DOI] [PubMed] [Google Scholar]
- 2.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167(3):1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 3.Mohr W, Wessinghage D. The relationship between polymorphonuclear granulocytes and cartilage destruction in rheumatoid arthritis. Zeitschrift für Rheumatologie. 1978;37(3–4):81–86. [PubMed] [Google Scholar]
- 4.McIntyre KW, et al. Reduced incidence and severity of collagen-induced arthritis in interleukin-12-deficient mice. Eur J Immunol. 1996;26(12):2933–2938. doi: 10.1002/eji.1830261219. [DOI] [PubMed] [Google Scholar]
- 5.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manoury-Schwartz B, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997;158(11):5501–5506. [PubMed] [Google Scholar]
- 7.Chu CQ, Song Z, Mayton L, Wu B, Wooley PH. IFNgamma deficient C57BL/6 (H-2b) mice develop collagen induced arthritis with predominant usage of T cell receptor Vbeta6 and Vbeta8 in arthritic joints. Ann Rheum Dis. 2003;62(10):983–990. doi: 10.1136/ard.62.10.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 9.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 10.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson NS, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 12.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakae S, et al. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171(11):6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 15.Kageyama Y, et al. Etanercept reduces the serum levels of interleukin-23 and macrophage inflammatory protein-3 alpha in patients with rheumatoid arthritis. Rheumatology International. 2007;2:137–143. doi: 10.1007/s00296-007-0388-4. [DOI] [PubMed] [Google Scholar]
- 16.Hwang SY, Kim HY. Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Mol Cell. 2005;19(2):180–184. [PubMed] [Google Scholar]
- 17.Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: Lessons from animal models. Arthritis Research & Therapy. 2005;7(1):29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grespan R, et al. CXCR2-specific chemokines mediate leukotriene B(4)-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis. Arthritis Rheum. 2008;58(7):2030–2040. doi: 10.1002/art.23597. [DOI] [PubMed] [Google Scholar]
- 19.Stichtenoth DO, et al. Microsomal prostaglandin E synthase is regulated by proinflammatory cytokines and glucocorticoids in primary rheumatoid synovial cells. J Immunol. 2001;167(1):469–474. doi: 10.4049/jimmunol.167.1.469. [DOI] [PubMed] [Google Scholar]
- 20.Akaogi J, Nozaki T, Satoh M, Yamada H. Role of PGE2 and EP receptors in the pathogenesis of rheumatoid arthritis and as a novel therapeutic strategy. Endocrine, Metabolic and Immune Disorders Drug Targets. 2006;6(4):383–394. doi: 10.2174/187153006779025711. [DOI] [PubMed] [Google Scholar]
- 21.Paradowska A, Maslinski W, Grzybowska-Kowalczyk A, Lacki J. The function of interleukin 17 in the pathogenesis of rheumatoid arthritis. Archivum Immunologiae et Therapiae Experimentalis. 2007;5(5):329–334. doi: 10.1007/s00005-007-0032-8. [DOI] [PubMed] [Google Scholar]
- 22.Yoshihara K, et al. IL-15 exacerbates collagen-induced arthritis with an enhanced CD4+ T cell response to produce IL-17. Eur J Immunol. 2007;37(10):2744–2752. doi: 10.1002/eji.200737229. [DOI] [PubMed] [Google Scholar]
- 23.Shigeta J, Takahashi S, Okabe S. Role of cyclooxygenase-2 in the healing of gastric ulcers in rats. J Pharmacol Exp Ther. 1998;286(3):1383–1390. [PubMed] [Google Scholar]
- 24.Chu CQ, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56(4):1145–1151. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- 25.Wynn TA. T(H)-17: A giant step from T(H)1 and T(H)2. Nat Immunol. 2005;6(11):1069–1070. doi: 10.1038/ni1105-1069. [DOI] [PubMed] [Google Scholar]
- 26.Hollingsworth JW, Siegel ER, Creasey WA. Granulocyte survival in synovial exudate of patients with rheumatoid arthritis and other inflammatory joint diseases. Yale J Biol Med. 1967;39:289–296. [PMC free article] [PubMed] [Google Scholar]
- 27.Brackertz D, Mitchell GF, Vadas MA, Mackay IR, Miller JF. Studies on antigen-induced arthritis in mice. II. Immunologic correlates of arthritis susceptibility in mice. J Immunol. 1977;118(5):1639–1644. [PubMed] [Google Scholar]
- 28.Witowski J, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000;165(10):5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 29.Ramos CD, et al. Neutrophil recruitment in immunized mice depends on MIP-2 inducing the sequential release of MIP-1alpha, TNF-alpha and LTB(4) Eur J Immunol. 2006;36(8):2025–2034. doi: 10.1002/eji.200636057. [DOI] [PubMed] [Google Scholar]
- 30.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martel-Pelletier J, Pelletier JP, Fahmi H. Cyclooxygenase-2 and prostaglandins in articular tissues. Seminars in Arthritis and Rheumatism. 2003;33(3):155–167. doi: 10.1016/s0049-0172(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 32.Siegle I, et al. Expression of cyclooxygenase 1 and cyclooxygenase 2 in human synovial tissue: Differential elevation of cyclooxygenase 2 in inflammatory joint diseases. Arthritis Rheum. 1998;41(1):122–129. doi: 10.1002/1529-0131(199801)41:1<122::AID-ART15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Harizi H, Gualde N. The impact of eicosanoids on the crosstalk between innate and adaptive immunity: The key roles of dendritic cells. Tissue Antigens. 2005;65(6):507–514. doi: 10.1111/j.1399-0039.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 34.Portanova JP, et al. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184(3):883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110(5):651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers LK, et al. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 2000;43(12):2687–2693. doi: 10.1002/1529-0131(200012)43:12<2687::AID-ANR8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Sheibanie AF, Khayrullina T, Safadi FF, Ganea D. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum. 2007;56(8):2608–2619. doi: 10.1002/art.22794. [DOI] [PubMed] [Google Scholar]
- 38.Daver JM. The pivotal role of interleukin-1 in the critical manifestations of rheumatoid arthritis. Rheumatology. 2003;42(Suppl2):ii3–ii10. doi: 10.1093/rheumatology/keg326. [DOI] [PubMed] [Google Scholar]
- 39.Chizzolini C, et al. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112(9):3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roark CL, et al. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol. 2007;179(8):5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181(1):721–735. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116(5):1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruddy MJ, Shen F, Smith JB, Sharma A, Gaffen SL. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: Implications for inflammation and neutrophil recruitment. J Leukocyte Biol. 2004;76(1):135–144. doi: 10.1189/jlb.0204065. [DOI] [PubMed] [Google Scholar]
- 44.Wyble CW, Desai TR, Clark ET, Hynes KL, Gewertz BL. Physiologic concentrations of TNFalpha and IL-1beta released from reperfused human intestine upregulate E-selectin and ICAM-1. J Surg Res. 1996;63(1):333–338. doi: 10.1006/jsre.1996.0271. [DOI] [PubMed] [Google Scholar]
- 45.Pinho V, et al. Tissue- and stimulus-dependent role of phosphatidylinositol 3-kinase isoforms for neutrophil recruitment induced by chemoattractants in vivo. J Immunol. 2007;179(11):7891–7898. doi: 10.4049/jimmunol.179.11.7891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.