Abstract
In response to early or developmental lesions, responsiveness of sensory cortex can be converted from the deprived modality to that of the remaining sensory systems. However, little is known about capacity of the adult cortex for cross-modal reorganization. The present study examined the auditory cortices of animals deafened as adults, and observed an extensive somatosensory conversion within as little as 16 days after deafening. These results demonstrate that cortical cross-modal reorganization can occur after the period of sensory system maturation.
Keywords: aging, cross-modal reorganization, hearing loss, plasticity, reorganization
The study of neural plasticity has revealed the considerable vulnerability of the developing brain to altered sensory experience (1). This vulnerability is particularly evident when the loss of peripheral sensory input results in the cross-modal reorganization of cortex. In their classic study, Rauschecker and Korte (2) showed that the ectosylvian visual area responded vigorously to auditory and/or somatosensory stimuli in cats visually deprived from birth. Similar developmental studies have been performed in a variety of species and their common finding was that cortical areas normally devoted to processing one sensory modality were converted to the other sensory systems following early visual deprivation (3–8) or early deafening (9–13, but see 14).
In contrast to these established developmental effects, little is known about the potential for cross-modal reorganization from lesions occurring after the sensory systems have matured. The few animal studies of adult cross-modal reorganization have described far less robust effects and collectively appear inconclusive. Blinding of adult rabbits resulted in somatosensory innervation of primary visual cortex near its border with somatosensory cortex (15). However, in deafened auditory cortex (16), visual responses were observed in the primary auditory area of cats deafened at 1 week of age, but not when cats were deafened at a later stage. In humans, regions of multi-sensory cortex become more visually active after late-onset deafness (17), which is not surprising given the presence of visual inputs before the hearing loss.
Despite these limited and equivocal results regarding late-onset cross-modal reorganization, there is considerable evidence that the adult cortex is capable of reacting to loss of peripheral input (18, 19). Investigations of adult subjects that received peripheral insults such as digit amputation (20), focal retinal lesions (21, 22), and frequency-specific cochlear lesions (23) revealed that the initially silenced cortical locus eventually developed responses similar to those of unaffected neighboring neurons (18, 24). Although these effects occurred within the same modality, the fact that intra-modal reorganization had occurred is consistent with the likelihood that the sensory cortices of adults are indeed mutable.
Hearing impairment is one of the most prevalent neurological disorders in humans, affecting 16% of adults in the United States (25). Despite the prevalence of this form of sensory loss, the possible cross-modal effects have largely been inferred from clinical observations. For example, the longer the duration of deafness before receiving a cochlear implant, the lower the probability that the implant will be effective (26–28), and this phenomenon, although dependent on cochlear nerve viability, appears to be affected by the level of cortical cross-modal reorganization that occurred in the interim (27). Furthermore, because vision influences hearing in numerous perceptual effects, it is reasonable to expect that adult hearing loss would uncover visual effects (e.g., 17). However, the nature and magnitude of cross-modal reorganization in subjects with adult hearing loss has not been experimentally examined to our knowledge. One well studied animal model of auditory system is that of the ferret (e.g., 29). In this species, the period of auditory development has been established, such that auditory spatial selectivity, tonotopic organization, and binaural properties reach maturity in auditory cortical areas by approximately 60 d of age (30, 31). Therefore, to evaluate the potential for cross-modal reorganization in animals with mature sensory systems, the present investigation examined neuronal responses in auditory cortex in ferrets deafened as adults.
Results
Ferrets (n = 6) were deafened at approximately 152 d of age for a duration of approximately 76 d. A total of 69 recording penetrations were made in “auditory” cortex, with neurons sampled at 225 recording sites. In each case, recordings made in the auditory cortices of deafened animals were devoid of acoustically driven responses but consistently revealed neurons responsive to somatosensory stimulation (84% ± 4%; average ± SEM). As illustrated in Fig. 1, recording sites corresponding to A1 (primary auditory cortex) and anterior auditory field were activated by somatosensory stimulation primarily on the face, head, and neck. This somatosensory responsivity also extended into adjoining auditory cortical fields. The few non-somatosensory sites that were encountered were unresponsive to all sensory stimulation and were usually found at the outer borders of the auditory cortices.
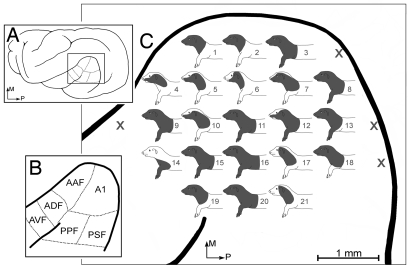
Fig. 1.
Cross-modal reorganization of auditory cortex in a ferret deafened as an adult. (A) The location of the auditory cortex (box) on lateral view of the ferret brain; (B) auditory cortical areas (after 32). (C) An enlarged view of the auditory cortices in an adult-deafened ferret (175 DPN at deafening, 74 d deaf) shows each recording site indicated by the numbered somatosensory receptive field that was mapped at that site (X, unresponsive). All auditory areas tested [corresponding to A1, anterior auditory field (AAF), and portions of anterior dorsal field (ADF) and posterior pseudo-sylvian field (PPF)] were responsive to somatosensory stimulation, primarily on the head. All recordings were made at 1,000 μm depth. (AVF, anterior ventral field; PSF, posterior suprasylvian field.)
Fig. 2 shows that somatosensory responses in auditory cortex of deafened ferrets occurred across the full thickness of the cortical mantle. As depicted in the response histograms, somatosensory responses were robust and repeatable and showed reliable response latency. Receptive fields within a given penetration often had similar distributions on the body surface and different penetrations revealed neurons with different receptive fields, but evidence for a global somatotopy was not observed. As shown in Fig. 1, penetrations 1 and 2 exhibited the same receptive field distribution as that identified at penetration 19, despite being at widely different medial-lateral locations. Similarly, the receptive field at site 3 was virtually the same as those at sites 8, 9, 11, 13, 16, and 20. Instead of somatotopy, the trend was for the representation of rostral parts of the body, with most (92% ± 7%) neurons demonstrating receptive fields on the head. Also, most (92% ± 5%) somatosensory responses showed bilaterally symmetrical receptive fields. Response force thresholds (measured using calibrated von Frey hairs) correlated primarily with cutaneous activation, either through hair (63% ± 12%) or skin (26% ± 8%) receptors; only 11% ± 6% were activated via deep receptors.
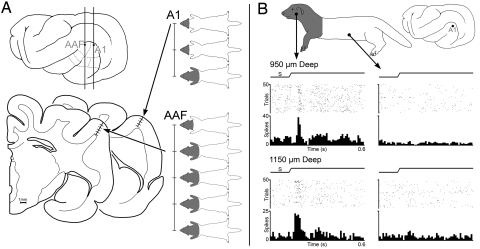
Fig. 2.
Somatosensory responses from auditory cortex in adult-deafened ferrets. (A). The lateral view of the ferret brain shows the location of the sections (Bottom Left) containing the recording penetrations in areas A1 and anterior auditory field (AAF) from an adult ferret 64 days post-deafening. Recording penetrations sampled neurons at 250 μm intervals (for space reasons, the examples shown are at 500-μm intervals) with corresponding receptive fields indicated on ferret body plots. For each penetration, receptive fields were on the head and were uniformly bilateral, and somatosensory responses spanned the full cortical thickness. (B) Recordings made from an adult ferret at 16 days post-deafening. Two representative neurons (950 and 1,150 μm deep) from area A1 (marked on lateral view of brain) exhibited somatosensory receptive fields on the head, neck, and forelimb. The peristimulus-time histograms show that repeated, electronically triggered tactile stimulation within the receptive field (cheek) evoked vigorous responses in both neurons, but stimulation outside the receptive field (torso) failed to elicit responses. (Tactile stimulus, ramp labeled S; raster 1 dot represents 1 spike; each row represents 1 trial; histogram, 10-ms time bins.)
In an additional adult animal examined after only 16 d of deafness, somatosensory responses similar to those described earlier were recorded in A1 (Fig. 2B). In this animal, 8 penetrations recorded 35 somatosensory neurons that were activated by hair receptors, responded at a reliable latency, and exhibited bilateral somatosensory receptive fields primarily on the head.
These findings in adult-deafened animals were in stark contrast to those recorded from hearing ferrets (n = 4) of the same age. In hearing animals, almost all of the recording sites (n = 100; 10 penetrations) were vigorously responsive to auditory stimuli (96% ± 3%), none were activated by somatosensory or visual stimuli, and few were unresponsive (4% ± 3%). At these recording sites, the possibility of sub-threshold cross-modal inputs was quantitatively examined for 111 auditory-responsive neurons using separate auditory, visual, and somatosensory stimuli, as well as their combinations (auditory-visual and auditory-somatosensory). Although this paradigm has been successful in identifying sub-threshold multi-sensory effects in a variety of cortices and species (33–35), the vast majority of auditory neurons (93%; 103 of 111) failed to reveal any significant non-auditory influences (Fig. 3A). Significant sub-threshold cross-modal effects were observed in only a few neurons (7%; 8 of 111), when the auditory stimulus was combined with either the somatosensory stimulus (2 facilitated; 2 suppressed) or visual stimulus (1 facilitated; 3 suppressed). In fact, most neurons showed similar auditory and combined-modality responses (as plotted in Fig. 3 B and C), and nearly equal proportions of the population fell slightly above or below the line of unity. Furthermore, the population average response to the auditory [mean of 3.4 ± 0.2 spikes/trial (SEM)], auditory-somatosensory (3.3 ± 0.2 spikes/trial) and auditory-visual (3.3 ± 0.2 spikes/trial) combinations were not significantly different (P > 0.05; Fig. 3D). Collectively, these results indicate that cross-modal effects were rare at the neuronal level and undetectable at the population level in the auditory cortices of hearing adults.
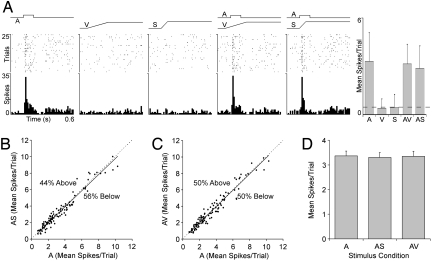
Fig. 3.
Responses of auditory neurons from hearing adult ferret to auditory, visual, somatosensory, and multi-sensory stimulation. (A) Responses of a typical neuron in A1 of a hearing adult to auditory (square-wave labeled A, contralateral white noise, 75 dB SPL, 100 ms), visual (ramp labeled V, light bar moved across the visual field), somatosensory (ramp labeled S, tactile probe indented skin on contralateral cheek), and combined stimulation (AV and AS) are shown in the rasters (1 dot represents 1 spike; each row represents 1 trial) and histograms (10-ms time bins). As summarized in the bar graph (mean spikes/trial ± SD; spontaneous activity indicated by dashed line), the neuron responded vigorously to the auditory stimulus, with no response to the visual or somatosensory stimulus. Because the response to the auditory stimulus alone was not statistically different from that of the combined stimuli conditions (AV or AS), this auditory neuron did not show subthreshold cross-modal effects. For the population of auditory neurons examined (n = 111), B and C plot the responses to the auditory stimulus alone (A; x axis) to those evoked by the combined stimuli (AS or AV; y axis). In both graphs, most responses fell on or near the line of unity (dashed), with similar numbers either slightly above or below. (D) Bar graph shows the population response average (mean spikes/trial ± SEM) to the auditory stimulus alone (A), auditory-somatosensory combined stimuli (AS), and auditory-visual combined stimuli (AV), which were not significantly different (P > 0.05).
To assess if the somatosensory conversion of auditory cortex in adult-deafened animals might be a result of ingrowth of new connections from somatosensory structures, tracer injections (biotinylated dextran amine) were made into A1 of adult-deafened (≈76 d, n = 4; opposite hemisphere from recordings) and hearing ferrets (n = 4). The results are depicted in Fig. 4. Given the large volume of auditory cortical tissue converted to somatosensory responsiveness in the deafened animals, it was expected that a large projection from the head representation of S1 (and/or SII) would be revealed. However, labeled neurons that project to A1 in adult-deafened ferrets originated largely in the same areas of auditory cortex as they did in the hearing animals. Similarly, labeled thalamic neurons that project to A1 in adult-deafened ferrets originated almost exclusively in the medial geniculate nucleus of the thalamus; none were observed in the somatosensory ventrobasal complex.
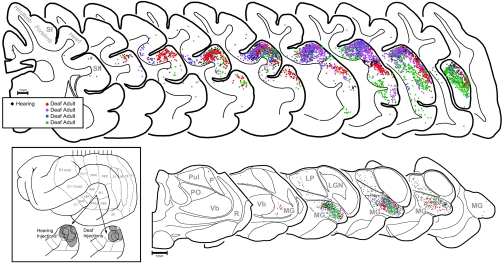
Fig. 4.
Anatomical tracer injected into A1 revealed the same pattern of connections in both adult-deafened (≈76 d duration) and hearing ferrets. (Inset) Lateral view of ferret cortex with expanded views of the auditory cortices depicting the tracer deposits (gray) for hearing and deafened animals. (Top) Serially arranged coronal sections containing somatosensory and auditory cortices. Each dot represents 1 labeled neuron (black dots represent 1 hearing adult ferret; colored dots represent 4 deafened adult ferrets). The distribution of black and colored dots is essentially co-extensive; areas of somatosensory cortex (S1; SII) are largely devoid of label. No labeled neurons were identified in sections anterior or posterior to those depicted. (Bottom) Serially arranged sections through thalamus with the auditory (MG, medial geniculate), somatosensory (Vb, ventrobasal), visual (LGN, lateral geniculate; Pul, pulvinar), and non-specific (PO, posterior; LP, lateral posterior) nuclei depicted. Each dot represents 1 labeled neuron (black dots, hearing; colored dots, deafened). The distribution of black and colored dots is essentially co-extensive within the MG; somatosensory thalamus (Vb) is devoid of label in both conditions.
Discussion
In adult ferrets deafened after the established age for auditory cortical maturity (30, 31), we observed extensive cross-modal reorganization of the areas corresponding to auditory cortices. This cross-modal reorganization was characterized by a consistent somatosensory conversion in neuron responsiveness such that, after approximately 76 d of deafness, 84% of neurons sampled in auditory cortex now responded to somatosensory stimulation. These somatosensory responses demonstrated features specialized for that modality. Responses were activated best by the displacement of hairs or by light touch on delimited areas of the body surface (i.e., exhibited a receptive field). Somatosensory responses occurred across all cortical laminae and predominantly exhibited bilateral receptive fields on the head without an obvious somatotopy. This cross-modal reorganization was observed consistently in each animal and across all of the auditory cortical regions examined.
Despite reports of visual inputs to auditory cortex in hearing ferrets (32) or early-deafened humans (10, 12), studies of early-onset deafness (11, 13) have also observed somatosensory (and visual) reorganization in auditory cortex. Moreover, somatosensory responses were found in visual cortical areas of adult rabbits following late-onset blindness (15). These findings, coupled with the present observations, indicate that the somatosensory modality may be as relevant to cross-modal reorganization as the visual modality.
It has been suggested that the mechanisms underlying cross-modal reorganization may be similar to those responsible for intra-modal reorganization of adult sensory maps following peripheral sensory loss (36). These mechanisms include changes in synaptic effectiveness (e.g., unmasking) of preexisting connections and/or the development of new connections via axonal sprouting (for review, see ref. 19). Accordingly, the cross-modal conversion of responses in adult-deafened auditory cortex might be the result of unmasking sub-threshold somatosensory inputs that were already present in hearing cortex, or as an amplification of the subtle signals that produce somatosensory evoked oscillations in neurons in hearing auditory cortex (37). However, by using techniques that have proven successful in identifying sub-threshold cross-modal processing in other cortical structures (33–35), the present experiment failed to identify sub-threshold somatosensory inputs to hearing auditory cortex in all but a small fraction of the sample (<4%). Furthermore, tracer studies failed to reveal cross-modal connections that could underlie sub-threshold effects, although these same techniques have been adequate in doing so in other structures (34, 38). These observations, however, do not rule out the possibility that somatosensory inputs to normal auditory cortex might arrive from non-specific areas.
Alternatively, the establishment of new intracortical connections via axonal sprouting has been observed in relation to cortical reorganization induced in adulthood (15, 21, 39). The remodeling of a deactivated cortical area via axonal sprouting occurs progressively over a period of weeks to months, such that the neurons nearest to the source are the first to become responsive to the replacement modality (15). However, several clues suggest that it is unlikely that the cross-modal conversion observed in the present study resulted from newly formed cortico-cortical connections. First, within 16 d of deafness, cross-modal reorganization was observed in A1 at distances >4 mm from the nearest somatosensory representation. Second, anatomical connections between auditory cortex and S1/SII somatosensory regions were not observed in the deafened animals. Furthermore, no connections between deaf auditory cortex and somatosensory thalamus were identified. Thus, these results do not support the notion that somatosensory reorganization of the auditory cortex following late-onset deafness was caused by axonal sprouting of new intracortical or thalamocortical connections.
Although neither unmasking of previously sub-threshold inputs nor sprouting of new connections appear to be strong candidates for the mechanism of cross-modal reorganization following late-onset deafness, the possibility remains that the cross-modal effects observed in cortex were manifest from changes that occurred at a distant, sub-cortical locus. It is well established in hearing animals that somatosensory afferents converge on auditory neurons at various sub-cortical levels within the auditory system, including the cochlear nucleus and inferior colliculus (40, 41). In hearing animals, dorsal cochlear nucleus neurons respond to somatosensory stimulation of the pinna, vibrissa, neck, and forelimb (42), and neurons in the external nucleus of the inferior colliculus respond to displacement of hairs and light touch on the bilateral or contralateral body surface, with no apparent somatotopic organization (43–45). These somatosensory response properties observed in sub-cortical auditory areas of hearing animals are remarkably similar to those of the somatosensory neurons found in the deafened auditory cortex. Furthermore, a significant increase in cross-modal somatosensory responsiveness in the dorsal cochlear nucleus has been observed after just 2 weeks of hearing loss in adults (46). In addition to unmasking of existing inputs to sub-cortical auditory structures, axonal sprouting and ingrowth of new connections may also occur at these locations. In fact, such a scenario has been demonstrated for intra-modal somatosensory reorganization following limb de-afferentation in adults, in which axonal sprouting in the medulla, where afferents from the trigeminal nucleus grow into the deprived cuneate nucleus, result in massive somatosensory cortical remodeling (47). Therefore, it is possible that deafness-induced changes in somatosensory processing at the sub-cortical level could account for the massive redistribution of that information manifested as cortical cross-modal reorganization. Furthermore, given that the auditory pathways are highly crossed in their projections from brainstem to cortex, deafness-induced somatic inputs to the first station of this relay (40, 46) could generate a large proportion of bilateral receptive fields at higher levels, as was observed.
Regardless of the mechanism, the finding that the adult auditory system extensively remodels its cortical representations to process signals from the body surface indicates that deafened auditory cortex recovers the function of receiving and processing patterned input signals. Whether a reorganized brain of this type can actually make functional or perceptual use of the re-routed somatosensory information is not known, but it certainly increases the complexity of the problems facing efforts to re-activate a de-afferented auditory system with neuroprosthetic implants.
Materials and Methods
All procedures were performed in compliance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health publication 86–23), the National Research Council's Guidelines for Care and Use of Mammals in Neuroscience and Behavioral Research (2003), and the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Deafening.
Ferrets aged 112 to 198 days postnatal (DPN; mean = 152 ± 32; n = 7) were deafened, under isoflurane anesthesia, using a single co-administration of kanamycin (300 mg/kg, s.c.) and ethacrynic acid (25 mg/kg, i.v.; following protocol of refs. 48–50). At approximately 2 weeks (n = 7) and 9 weeks (n = 6) following deafening, auditory brainstem responses were assessed for each ear separately. The auditory stimulus was a calibrated click (2,000–3,000 trials each, 0.1-ms square-wave click, rarefaction), delivered through a speaker positioned directly in front of one ear. Sub-dermal recording leads were positioned over the right and left mastoid processes, at mid-cranium and on mid-back. Evoked electrical activity was signal averaged and threshold response levels were determined for each ear of each animal. Only those animals with a bilateral hearing threshold of >90 dB hearing level were included in the subsequent experiments. Bilateral auditory brainstem responses were also tested on hearing animals (threshold ≈15 dB hearing level).
Electrophysiology.
Ten weeks after the deafening procedure, 6 deaf ferrets were surgically prepared for electrophysiological recording (mean 76 ± 9 d deafness duration; range, 64–92 d at an average 226 ± 35 DPN; range, 183–275 DPN). A single deaf adult ferret (age, 186 DPN) was prepared after 2 weeks of deafness. Four age-matched hearing controls were also used (mean, 199 ± 4 DPN; range, 197–205 DPN). Under pentobarbital anesthesia (40 mg/kg i.p.) and aseptic conditions, a craniotomy was made over the left auditory cortices and a head-support device implanted. Following recovery, the recording experiment occurred 2 to 4 d after implantation.
Electrophysiological recordings were initiated by anesthetizing the animal (35 mg/kg ketamine and 2 mg/kg acepromazine i.m.) and securing the implanted well to a supporting bar. The animal was intubated through the mouth and ventilated with expired CO2 monitored and maintained at ≈4.5%. Fluids, supplemental anesthetic agents (8 mg/kg/h ketamine; 0.5 mg/kg/h acepromazine) and a muscle relaxant (to prevent spontaneous movements; pancuronium bromide 0.2 mg/kg/h i.p.) were continuously infused. The implanted well was opened and the recording electrode, either a glass-insulated tungsten electrode or a silicon multi-channel electrode, was inserted into auditory cortex.
Neuronal activity was amplified and routed to a PC. To reduce sampling bias during single-unit recording penetrations, neurons were studied at 250-μm intervals. Neurons were identified by their spontaneous activity and their responses to an extensive battery of manually presented auditory (clicks, claps, whistles, and hisses), visual (flashed or moving light or dark stimuli), somatosensory (air puffs, strokes and taps using brushes and von Frey hairs), and manual (pressure and joint rotation) stimuli. Thus, at each location, the sensory response modality of the neuron (auditory, visual, somatosensory, multi-sensory, unresponsive) was identified and tabulated and the sensory receptive field(s) were mapped and graphically recorded.
In 4 hearing adults, additional sensory tests were performed on auditory cortical neurons to quantify sub-threshold somatosensory or visual effects. Given the uniform responses of neurons in the deafened animals, only selected neurons from the deafened animals were tested quantitatively. Quantitative sensory tests consisted of computer-triggered auditory, visual, and somatosensory stimuli presented alone and in combination. Free-field auditory cues were electronically generated white-noise bursts (100 ms, 75 dB SPL) from a hoop-mounted speaker 44 cm from the head (45° azimuth/0° elevation). Projected onto a translucent hemisphere (92 cm diameter), visual cues were bars of light whose size, direction, velocity, and amplitude were independently controlled. An electronically driven, modified shaker with independently programmable amplitude and velocity settings was used to indent the skin or deflect hairs within the tactile receptive field. When no receptive field could be identified, standard visual and somatosensory stimuli were presented: a large light bar (5 × 15°) was moved across the contralateral visual field from nasal to temporal at 200°/s, and the tactile probe was positioned on the contralateral cheek. During the combined-modality presentations, the visual stimulus preceded the auditory and somatosensory cues by 50 ms (51). Each stimulus presentation was separated by 3 to 7 s, and each condition was presented 25 times. Neuronal responses were digitized (rate >25 kHz), and individual waveforms were templated and routed to a computer for analysis. For each waveform (i.e., single neuron), a peristimulus-time histogram was constructed for each of the test conditions from which the response (mean spikes per trial) was measured. To determine if sub-threshold multi-sensory effects were present, the response to auditory stimulus alone was compared statistically (paired t test, P < 0.05) with the responses during the combined stimuli conditions (auditory-somatosensory and auditory-visual conditions) (33–35).
Using a 32-site silicon multichannel electrode (≈1 MΩ impedance; 4 shanks × 8 sites; 200 μm separation between sites; NeuroNexus Technologies), single-unit extracellular recordings were performed on an adult ferret deafened for only 16 d (170 DPN at time of deafening). For each recording site, neuronal activity was amplified (Medusa 16-channel pre-amps; Tucker-Davis Technologies), displayed on an oscilloscope, and played on an audio monitor. Using similar techniques as those described earlier, manual and computer-triggered stimuli were presented to determine neuron responsiveness. After mapping the somatosensory receptive field for each responsive neuron, the computer-triggered somatosensory stimulus was delivered repeatedly (50 trials, separated by 3–7 s). Neuronal responses from each of the recording sites were digitized at 25 kHz using a Tucker-Davis neurophysiology workstation (System III) and routed to a computer for analysis, as described earlier.
The depth of each neuron within a penetration was noted and, in a data table, correlated with its various measures of sensory responsiveness. Several recording penetrations were made in each animal and their location was plotted on a digital photograph of the cortical surface. Each recording penetration using a glass-insulated tungsten electrode was marked at its terminus with a small electrolytic lesion (0.3 mA for 0.5 s) to facilitate its histological reconstruction. At the conclusion of the recording experiment, the animal was euthanized and the brain was fixed, blocked, and serially sectioned (50 μm). The sections were processed using standard histological procedures, and a projecting microscope was used to make scaled reconstructions of the recording penetrations. Auditory cortical fields were approximated using sulcal landmarks according to the criteria of Bizley et al. (29).
Neuroanatomy.
The cortical connectivity of 4 adult ferrets deaf for approximately 76 d (opposite hemisphere from recording in same animals) and 4 hearing adult ferrets was examined. Under pentobarbital anesthesia (40 mg/kg i.p.) and aseptic surgical conditions, a craniotomy was made over auditory cortex. Biotinylated dextran amine (3 k molecular weight, 10% in citrate buffer) was pressure-injected (0.8–1.5 μL volume) into the A1 region. After a period of 3 to 9 d, the animal was euthanized and the brain processed to visualize the transported tracer. Serial, coronal sections (50 μm thick) were digitized using Neurolucida software (MBF Biosciences). Digitized plots included the tissue outline, gray-white border, and the location of retrogradely labeled neuronal cell bodies. Sections were graphically arranged and neural regions were identified using accepted landmarks (29, 52).
Acknowledgments.
We thank Dr. R. K. Shepherd for advice on ototoxic procedures, and Drs. S. Shapiro and A. Rice for their technical assistance. This work was supported by National Institutes of Health grant NS39460.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Rauschecker JP, Korte M. Auditory compensation for early blindness in cat cerebral cortex. J Neurosci. 1993;13:4538–4548. doi: 10.1523/JNEUROSCI.13-10-04538.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyvarinen J, Carlson S, Hyvarinen L. Early visual deprivation alters modality of neuronal responses in area 19 of monkey cortex. Neurosci Lett. 1981;26:239–243. doi: 10.1016/0304-3940(81)90139-7. [DOI] [PubMed] [Google Scholar]
- 4.Izraeli R, et al. Cross-modal neuroplasticity in neonatally enucleated hamsters: structure, electrophysiology and behaviour. Eur J Neurosci. 2002;15:693–712. doi: 10.1046/j.1460-9568.2002.01902.x. [DOI] [PubMed] [Google Scholar]
- 5.Kahn DM, Krubitzer L. Massive cross-modal cortical plasticity and the emergence of a new cortical area in developmentally blind mammals. Proc Natl Acad Sci USA. 2002;99:11429–11434. doi: 10.1073/pnas.162342799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piche M, et al. Auditory responses in the visual cortex of neonatally enucleated rats. Neuroscience. 2007;145:1144–1156. doi: 10.1016/j.neuroscience.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 7.Yaka R, Yinon U, Rosner M, Wollberg Z. Pathological and experimentally induced blindness induces auditory activity in the cat primary visual cortex. Exp Brain Res. 2000;131:144–148. doi: 10.1007/s002219900295. [DOI] [PubMed] [Google Scholar]
- 8.Yaka R, Yinon U, Wollberg Z. Auditory activation of cortical visual areas in cats after early visual deprivation. Eur J Neurosci. 1999;11:1301–1312. doi: 10.1046/j.1460-9568.1999.00536.x. [DOI] [PubMed] [Google Scholar]
- 9.Auer ET, Jr, Bernstein LE, Sungkarat W, Singh M. Vibrotactile activation of the auditory cortices in deaf versus hearing adults. Neuroreport. 2007;18:645–648. doi: 10.1097/WNR.0b013e3280d943b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finney EM, Fine I, Dobkins KR. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001;4:1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- 11.Hunt DL, Yamoah EN, Krubitzer L. Multisensory plasticity in congenitally deaf mice: how are cortical areas functionally specified? Neuroscience. 2006;139:1507–1524. doi: 10.1016/j.neuroscience.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Lambertz N, Gizewski ER, de Greiff A, Forsting M. Cross-modal plasticity in deaf subjects dependent on the extent of hearing loss. Brain Res Cogn Brain Res. 2005;25:884–890. doi: 10.1016/j.cogbrainres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Levanen S, Jousmaki V, Hari R. Vibration-induced auditory-cortex activation in a congenitally deaf adult. Curr Biol. 1998;8:869–872. doi: 10.1016/s0960-9822(07)00348-x. [DOI] [PubMed] [Google Scholar]
- 14.Kral A, Schroder JH, Klinke R, Engel AK. Absence of cross-modal reorganization in the primary auditory cortex of congenitally deaf cats. Exp Brain Res. 2003;153:605–613. doi: 10.1007/s00221-003-1609-z. [DOI] [PubMed] [Google Scholar]
- 15.Newton JR, Sikes RW, Skavenski AA. Cross-modal plasticity after monocular enucleation of the adult rabbit. Exp Brain Res. 2002;144:423–429. doi: 10.1007/s00221-002-1087-8. [DOI] [PubMed] [Google Scholar]
- 16.Rebillard G, Carlier E, Rebillard M, Pujol R. Enhancement of visual responses on the primary auditory cortex of the cat after an early destruction of cochlear receptors. Brain Res. 1977;129:162–164. doi: 10.1016/0006-8993(77)90980-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Truy E, Mamou G, Sappey-Marinier D, Giraud AL. Visual speech circuits in profound acquired deafness: a possible role for latent multimodal connectivity. Brain. 2007;130:2929–2941. doi: 10.1093/brain/awm230. [DOI] [PubMed] [Google Scholar]
- 18.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 19.Xerri C. Imprinting of idiosyncratic experience in cortical sensory maps: neural substrates of representational remodeling and correlative perceptual changes. Behav Brain Res. 2008;192:26–41. doi: 10.1016/j.bbr.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Merzenich MM, et al. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- 22.Kaas JH, et al. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990;248:229–231. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- 23.Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- 24.Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- 26.Blamey P, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurootol. 1996;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, et al. PET evidence of neuroplasticity in adult auditory cortex of postlingual deafness. J Nucl Med. 2003;44:1435–1439. [PubMed] [Google Scholar]
- 28.Proops DW, et al. Outcomes from adult implantation, the first 100 patients. J Laryngol Otol Suppl. 1999;24:5–13. [PubMed] [Google Scholar]
- 29.Bizley JK, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cereb Cortex. 2005;15:1637–1653. doi: 10.1093/cercor/bhi042. [DOI] [PubMed] [Google Scholar]
- 30.Mrsic-Flogel TD, Schnupp JW, King AJ. Acoustic factors govern developmental sharpening of spatial tuning in the auditory cortex. Nat Neurosci. 2003;6:981–988. doi: 10.1038/nn1108. [DOI] [PubMed] [Google Scholar]
- 31.Mrsic-Flogel TD, Versnel H, King AJ. Development of contralateral and ipsilateral frequency representations in ferret primary auditory cortex. Eur J Neurosci. 2006;23:780–792. doi: 10.1111/j.1460-9568.2006.04609.x. [DOI] [PubMed] [Google Scholar]
- 32.Bizley JK, Nodal FR, Bajo VM, Nelken I, King AJ. Physiological and anatomical evidence for multisensory interactions in auditory cortex. Cereb Cortex. 2007;17:2172–2189. doi: 10.1093/cercor/bhl128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allman BL, Meredith MA. Multisensory processing in “unimodal” neurons: cross-modal subthreshold auditory effects in cat extrastriate visual cortex. J Neurophysiol. 2007;98:545–549. doi: 10.1152/jn.00173.2007. [DOI] [PubMed] [Google Scholar]
- 34.Dehner LR, Keniston LP, Clemo HR, Meredith MA. Cross-modal circuitry between auditory and somatosensory areas of the cat anterior ectosylvian sulcal cortex: a ‘new’ inhibitory form of multisensory convergence. Cereb Cortex. 2004;14:387–403. doi: 10.1093/cercor/bhg135. [DOI] [PubMed] [Google Scholar]
- 35.Meredith MA, Allman BL. Subthreshold multisensory processing in cat auditory cortex. Neuroreport. 2009;20:126–131. doi: 10.1097/WNR.0b013e32831d7bb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nat Rev Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- 37.Lakatos P, Chen CM, O'Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–292. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clemo HR, Sharma GK, Allman BL, Meredith MA. Auditory projections to extrastriate visual cortex: connectional basis for multisensory processing in ‘unimodal’ visual neurons. Exp Brain Res. 2008;191:37–47. doi: 10.1007/s00221-008-1493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- 40.Dehmel S, Cui YL, Shore SE. Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. Am J Audiol. 2008;17:S193–S209. doi: 10.1044/1059-0889(2008/07-0045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shore SE, Zhou J. Somatosensory influence on the cochlear nucleus and beyond. Hear Res 216- 2006;217:90–99. doi: 10.1016/j.heares.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Kanold PO, Young ED. Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. J Neurosci. 2001;21:7848–7858. doi: 10.1523/JNEUROSCI.21-19-07848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aitkin LM, Dickhaus H, Schult W, Zimmermann M. External nucleus of inferior colliculus: auditory and spinal somatosensory afferents and their interactions. J Neurophysiol. 1978;41:837–847. doi: 10.1152/jn.1978.41.4.837. [DOI] [PubMed] [Google Scholar]
- 44.Aitkin LM, Kenyon CE, Philpott P. The representation of the auditory and somatosensory systems in the external nucleus of the cat inferior colliculus. J Comp Neurol. 1981;196:25–40. doi: 10.1002/cne.901960104. [DOI] [PubMed] [Google Scholar]
- 45.Robards MJ. Somatic neurons in the brainstem and neocortex projecting to the external nucleus of the inferior colliculus: an anatomical study in the opossum. J Comp Neurol. 1979;184:547–565. doi: 10.1002/cne.901840308. [DOI] [PubMed] [Google Scholar]
- 46.Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci. 2008;27:155–168. doi: 10.1111/j.1460-9568.2007.05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain N, Florence SL, Oi HX, Kaas JH. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc Natl Acad Sci USA. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore CM, Vollmer M, Leake PA, Snyder RL, Rebscher SJ. The effects of chronic intracochlear electrical stimulation on inferior colliculus spatial representation in adult deafened cats. Hear Res. 2002;164:82–96. doi: 10.1016/s0378-5955(01)00415-4. [DOI] [PubMed] [Google Scholar]
- 49.Shepherd RK, Martin RL. Onset of ototoxicity in the cat is related to onset of auditory function. Hear Res. 1995;92:131–142. doi: 10.1016/0378-5955(95)00211-1. [DOI] [PubMed] [Google Scholar]
- 50.Xu SA, Shepherd RK, Chen Y, Clark GM. Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear Res. 1993;70:205–215. doi: 10.1016/0378-5955(93)90159-x. [DOI] [PubMed] [Google Scholar]
- 51.Wallace MT, Meredith MA, Stein BE. Integration of multiple sensory modalities in cat cortex. Exp Brain Res. 1992;91:484–488. doi: 10.1007/BF00227844. [DOI] [PubMed] [Google Scholar]
- 52.Manger PR, Masiello I, Innocenti GM. Areal organization of the posterior parietal cortex of the ferret (Mustela putorius) Cereb Cortex. 2002;12:1280–1297. doi: 10.1093/cercor/12.12.1280. [DOI] [PubMed] [Google Scholar]